Abstract
The adult heart has been reported to have an extensive lymphatic system, yet the development of this important system during cardiogenesis is still largely unexplored. The nuclear-localized transcription factor Prox-1 identified a sheet of Prox-1-positive cells on the developing aorta and pulmonary trunk in avian and murine embryos just prior to septation of the four heart chambers. The cells coalesced into a branching lymphatic network that spread within the epicardium to cover the heart. These vessels eventually expressed the lymphatic markers LYVE-1, VEGFR-3, and podoplanin. Before the Prox-1-positive cells were detected in the mouse epicardium, LYVE-1, a homologue of the CD44 glycoprotein, was primarily expressed in individual epicardial cells. Similar staining patterns were observed for CD44 in avian embryos. The proximity of these LYVE-1/CD44-positive mesenchymal cells to Prox-1-positive vessels suggests that they may become incorporated into the lymphatics. Unexpectedly, we detected LYVE-1/PECAM/VEGFR-3-positive vessels within the embryonic and adult myocardium which remained Prox-1/podoplanin-negative. Lymphatic markers were surprisingly found in adult rat and embryonic mouse epicardial cell lines, with Prox-1 also exhibiting nuclear-localized expression in primary cultures of embryonic avian epicardial cells. Our data identified three types of cells in the embryonic heart expressing lymphatic markers: (1) Prox-1-positive cells from an extracardiac source that migrate within the serosa of the outflow tract into the epicardium of the developing heart, (2) individual LYVE-1-positive cells in the epicardium that may be incorporated into the Prox-1-positive lymphatic vasculature, and (3) LYVE-1-positive cells/vessels in the myocardium that do not become Prox-1-positive even in the adult heart.
Keywords: lymphatics, heart, Prox-1, LYVE-1, VEGFR-3, epicardium
Introduction
The main functions of the lymphatics include the maintenance of the fluid balance of the internal environment, the absorption of fat from the small intestine, and the trafficking of antigen-presenting cells from tissues to lymph nodes in immune surveillance (Randolph et al, 2005; Baluk et al, 2007). In addition, the lymphatics serve as one of the major routes by which tumor cells can metastasize to distant organs. Prox-1 (Prospero-related homeobox 1), podoplanin, LYVE-1 (Lymphatic Vascular Endothelial Hyaluronan Receptor-1), and VEGFR-3 (Vascular Endothelial Growth Factor Receptor-3) are expressed in lymphatic endothelial cells and have been used as lymphatic markers in previous studies (Kaipainen et al, 1995; Banerji et al, 1999; Breiteneder-Geleff et al, 1999; Wigle and Oliver, 1999). While these lymphatic markers are not totally specific to lymphatic endothelial cells (Rishi et al, 1995; Wetterwald et al, 1996; Wigle et al, 1999; Partanen et al, 2000; Sosa-Pineda et al, 2000; Mouta Carreira et al, 2001; Schoppmann et al, 2002; Hamrah et al, 2003; Gittenberger-de Groot et al, 2006), they play a significant role in lymphatic development and when used in combination, have been useful in identifying lymphatic precursors and lymphatic endothelial cells during development.
There are two major theories on the origin of lymphatic endothelial cells. In 1902, Florence Sabin proposed that the lymphatics had a venous origin by suggesting that lymphatic endothelial cells bud off the cardinal vein (Sabin, 1902; Sabin, 1904; Sabin, 1909). Her theory was referred to as the centrifugal model. In 1910, Huntington and his colleagues countered that lymphatic endothelial cells originate from mesenchyme, giving rise to the centripetal model (Huntington and McClure, 1910; Kampmeier, 1912). Recent studies in the field appear to support both hypotheses (Kaipainen et al, 1995; Kukk et al, 1996; Schneider et al, 1999; Wigle and Oliver, 1999; Papoutsi et al, 2001; Wigle et al, 2002; Parsons-Wingerter et al, 2006; Yaniv et al, 2006). In mice, Prox-1 is expressed in a subset of LYVE-1-positive endothelial cells in the anterior cardinal vein at ED (Embryonic Day) 9.5–10 (Wigle and Oliver, 1999; Oliver and Detmar, 2002; Oliver, 2004). These cells also express other lymphatic markers such as VEGFR-3. The Prox-1-positive lymphatic precursor cells then migrate away to form the primary lymph sacs. The interaction of the mesenchymal growth factor VEGF-C and VEGFR-3 plays an important role in the proliferation and migration of these lymphatic precursor cells from the cardinal veins (Veikkola et al, 2001; Karkkainen et al, 2004).
Knocking out the gene for Prox-1 in the mouse embryo resulted in the complete absence of a lymphatic system in all tissues and death at ED 14.5 (Wigle and Oliver, 1999). In Prox-1-null mice, the budding of lymphatic endothelial progenitor cells from the cardinal vein was arrested at ED 11.5. These cells did not up-regulate lymphatic markers such as VEGFR-3, but did continue to express blood-specific genes such as CD34. There was no obvious alteration in heart function in the Prox-1 knockout mice as assessed by the circulation of injected ink. However, no further physiological assays have been carried out. In addition, adenoviral transduced expression of Prox-1 in cultured blood vessel endothelial cells re-programmed the cells to express a lymphatic endothelial cell phenotype with a higher expression of lymphatic markers and a decreased expression of blood-specific markers (Hong et al, 2002; Petrova et al, 2002). Therefore, Prox-1 appears to be essential for the development of the lymphatic system, but is not required for the development of the blood vasculature (Wigle and Oliver, 1999; Wigle et al, 2002).
Comparatively less is known about the functions of the highly O-glycosylated integral membrane protein podoplanin and LYVE-1, a membrane glycoprotein that is a receptor for the extracellular glycosaminoglycan hyaluronan (Banerji et al, 1999; Prevo et al, 2001; Edward et al, 2005). LYVE-1-deficient mice are healthy and fertile, with no visible defects in their lymphatic system (Gale et al, 2007). On the other hand, podoplanin knockout mice acquire defects in the patterning of their lymphatic vessels, but not their blood vessels. Eventually, they develop edema and die of respiratory failure at birth (Schacht et al, 2003). In VEGF-C knockout mice, the Prox-1-positive lymphatic precursor cells fail to migrate away from the cardinal veins, and may disappear through apoptosis. Thus, the development of the lymphatic system is brought to a halt. However, the application of VEGF-C and VEGF-D, both of which bind to VEGFR-3, rescued the sprouting and migration of the lymphatic progenitor cells (Karkkainen et al, 2004). VEGFR-3 knockout mice die at approximately ED 9.5 before the initial formation of the lymphatics in normal development (Dumont et al, 1998; Jussila and Alitalo, 2002). This early death precludes analysis of the role of the receptor in the development of the lymphatic system using this model. Thus, the roles of lymphatic markers in lymphangiogenesis, even that of Prox-1 that is the most extensively studied, are still not completely understood.
Despite the extensive nature and importance of the lymphatic system in the adult heart (Shimada et al, 1989; Shimada et al, 1990), the developmental steps of the lymphatics in the embryonic heart have not been described in detail. In a recent study (Wilting et al, 2007), Prox-1 expression was examined at later stages of embryonic cardiac development after morphogenesis was largely complete (from Hamburger-Hamilton (HH) Stage 35 onwards). These investigators also conducted quail-chick grafting experiments and concluded that grafts of the proepicardial organ, an outgrowth of the dorsal body wall that eventually gives rise to the epicardium and the precursors of the coronary arteries (Mikawa et al, 1996; Munoz-Chapuli et al, 2002; Perez-Pomares et al, 2002), do not give rise to the bulk of lymphatic endothelial cells within the heart. In their studies, LYVE-1 was also used to demarcate the lymphatic vessels in ED 11.5–13.5 mice but no comparison was made between Prox-1 and LYVE-1 expression at these stages. In our study, we analyzed the expression pattern of Prox-1 at younger stages in the avian embryo than in previous studies; that is, from HH (Hamburger and Hamilton, 1951) Stage 24 to 40, stages which span the most active phases of morphogenesis and coronary vessel vasculogenesis. We also analyzed the expression of various lymphatic markers in the mouse heart, including Prox-1, LYVE-1, VEGFR-3, and podoplanin, at different developmental stages for comparison, since lymphatic markers are not truly lymphatic-specific. Our primary objective was to confirm lymphatic vessel identity in the mouse heart by investigating the expression patterns of two primary lymphatic markers Prox-1 and LYVE-1 throughout development (ED 9.5 to adult). Two epicardial cell lines (Eid et al, 1994; Austin et al, 2008) and quail embryonic epicardial cell primary cultures were also used to augment our in vivo findings.
Results
Prox-1 stained a branching lymphatic network in the avian embryonic heart that first appeared at HH Stage 26
In order to analyze lymphatic development within the embryonic heart, we stained quail hearts ranging from HH Stage 24 to HH Stage 40 for the lymphatic marker Prox-1, with co-localization for the endothelial precursor marker QH-1 that labels both lymphatic and blood vessel precursors as has been shown in the chorioallantoic membrane (Parsons-Wingerter et al, 2006). These stages were chosen because they span a time in development when vasculogenesis is active in the epicardium, leading to the formation of the first definitive blood vessels including the coronaries. At HH Stage 24, after the heart has just looped and developed distinct atrial chambers, QH-1-positive cells were found at the base of the outflow tract and were also scattered over the atrium and ventricle in an equidistant pattern, but there were no distinctly detectable Prox-1-positive cells on the epicardial surface of the embryonic heart (Figure 1 A, B). However, in sections, Prox-1-positive cells were found in the atrial and ventricular myocardium at this stage (Supplementary Figure 1 A), as well as in the lining and mesenchyme of the forming endocardial cushions (Supplementary Figure 1 B). One or two Prox-1-positive cells were also found in the ventricular epicardium in sections at this stage. Between HH Stages 26–28, with the septation of the outflow tract into the aorta and pulmonary trunk, large, irregularly shaped regions of Prox-1-positive/QH-1-positive cells started to appear along the great vessels with the greatest concentration appearing at the cranial region (Figure 1 C, D).
Figure 1. Lymphatic markers in the early quail embryonic heart.
No cells with nuclear-localized Prox-1 immunostaining (green) were detected on the epicardial surface of the HH stage 24 heart (A), but QH-1-positive (red) cells were present on the atria, ventricles, and the base of the outflow tract (B). Nuclear-localized Prox-1-positive/QH-1-positive cells on the great vessels first appeared in the embryonic heart at HH Stage 26 (C, D). No Prox-1-positive cells were detected on the ventricular and atrial surfaces of the heart at this stage (not shown). At = atrium, OFT = outflow tract, V = ventricle. Bars = 100 um.
At HH Stages 29–30, after the heart had completed septation into four chambers, we observed several Prox-1-positive/QH-1-positive vessels that ran down the length of the great vessels, including the aorta and pulmonary artery, and formed a widespread, branching network at their base and over the surface of the ventricles (Figure 2 A–B). At these stages, Prox-1-positive cells were also located in the endocardium and mesenchyme of the aortic and atrioventricular valves (Supplementary Figure 1 C). In all of the valves, Prox-1 appeared to be expressed specifically in cells of the fibrosa layer, otherwise known as the arterial aspect of the valvular cusp which is composed primarily of collagen fibers (Gross and Kugel, 1931; Schoen, 2005). Prox-1 stained both atrial and ventricular cardiac myocytes as well, but the nuclear staining intensity in the cardiac myocytes was lower than that exhibited by the lymphatic vessels or the cells lining the valves. Due to the distribution patterns, it would appear that Prox-1 may play roles in different cell types during cardiac development in addition to contributing to the formation of the cardiac lymphatic vessels. At HH Stages 38–40, when cardiac morphogenesis is largely complete, the Prox-1-positive vessels encircling the great vessels were larger and well developed, but those covering the ventricles were obscured by the thickness of the epicardium. Clusters of Prox-1-positive cells were also apparent at regular intervals along the lymphatics of the great vessels, which may be indicative of lymphatic valve formation (Figure 2 C). At these later stages, Prox-1-positive/QH-1-positive vessels were also found surrounding the posterior interventricular blood vessel running within the epicardial connective tissue of the interventricular sulcus on the posterior aspect of the embryonic heart (Figure 2 D). Thus, the avian embryonic heart acquired an extensive lymphatic network throughout cardiac morphogenesis (Figure 3) during the embryonic stages when coronary vessel development is actively proceeding.
Figure 2. Lymphatic markers in the septated quail embryonic heart.
Prox-1-positive (green)/QH-1-positive (red) lymphatic vessels began to form a branching network over the great vessels (A) and ventricles (B) in the HH Stage 29–30 heart. The individual QH-1-positive (red) cells (A, arrow) adjacent to the lymphatic vessels immunostained with a greater intensity than cells within the vessels. Clusters of Prox-1-positive cells (C, arrow) were observed along lymphatic vessels of the HH Stage 38 heart. At this stage, lymphatic vessels (D, white arrow) were also found on the posterior aspect of the heart surrounding major blood vessels (D, red arrow). Bars = 100 um.
Figure 3. Diagram of the progressive development of the Prox-1-positive lymphatic network in the avian embryonic heart from HH Stage 24 to 38.

Green coloring represents Prox-1-positive cells and vessels. Dotted pink lines indicate presumed pathways for the development of the ventricular lymphatics at HH Stage 38. The quail epicardium becomes opaque at later stages and obscures Prox-1 staining. For reference, septation of the heart is complete by HH Stage 30 and the coronary arteries connect to the aortic lumen by HH Stage 32.
A LYVE-1-positive vascular network found within the mouse myocardium as early as ED 9.5 did not stain for Prox-1
Mouse heart sections were labeled for additional lymphatic markers such as LYVE-1 and VEGFR-3 in order to compare with Prox-1 localization, since commercially available antibodies for LYVE-1 and VEGFR-3 do not work in chicken or quail tissues. At ED 9.5–10.5, Prox-1 was found only in cardiac myocytes, while LYVE-1 and VEGFR-3 were expressed in cells/vessels within the myocardium (Figure 4 A–D). The LYVE-1-positive cells did not stain for the cardiac myocyte marker MF20 or the lymphatic marker podoplanin (Figure 4 E–H) but they did express the endothelial cell marker CD31/PECAM (Figure 4 I–K).
Figure 4. Lymphatic markers in the ED 9.5 mouse heart.

(A) Prox-1 was found in cardiac myocytes in the ventricle while LYVE-1 (B) and VEGFR-3 (C) were expressed in cells/vessels on the endocardial side of the myocardium at the leading edge of the nascent interventricular septum. (D) is a high magnification view of the Prox-1-positive cardiac myocytes in the boxed region in (A). Sections in A–C were 7 microns thick and immediately adjacent to each other. The LYVE-1-positive cells/vessels did not co-localize with the cardiac myocyte marker MF20 (E, F) or with the lymphatic marker podoplanin (G, H), but they did stain for the endothelial cell marker CD31/PECAM (I–K). The overlay image (K) for the LYVE-1 and CD31 staining revealed that only a subset of the CD31-positive cells were LYVE-1-positive. Double-positive cells are yellow. Bars = 100 um.
Prox-1-positive vessels appearing at ED 13.5 in the mouse heart did not initially co-localize with LYVE-1 staining
At ED 13.5, Prox-1 labeled the nuclei of cells organized into vessels running along the aorta and pulmonary trunk (Figure 5 A), but there was no co-localization with LYVE-1 which was expressed in myocardial vessels as well as individual cells found in the epicardium of the heart (Figure 5 B–D). Some Prox-1-negative/LYVE-1-positive cells were found scattered in close vicinity of the Prox-1-positive lymphatic vessels (Figure 5 A–B). Expression patterns for LYVE-1, which is homologous to the hyaluronan receptor CD44, in the ED 13.5 mouse embryo heart were similar to staining patterns observed for CD44 in the HH Stage 30 chick embryo heart. CD44 was present in cells within the chicken epicardium and myocardium, with certain cells found next to Prox-1-positive vessels, but no co-localization was seen at this stage (Figure 5 E–G). Examination of semi-thin (0.5–1 micron) resin embedded mouse tissue sections (results not shown) and confocal microscopy (Figure 6) revealed LYVE-1-positive cells to be within the mesothelial layer of the epicardium and the subepicardial mesenchyme of the ED 13.5 mouse embryo heart, as well as in the myocardium.
Figure 5. Prox-1 and LYVE-1/CD44 were initially not co-localized in the mouse or chick embryo heart.
(A) In the ED 13.5 mouse heart, Prox-1 (green) was expressed in the lymphatic vessels found between the aorta and pulmonary trunk while LYVE-1 (green; homologous to CD44) was found in epicardial cells on the great vessels (B), a myocardial network of vessels (C), as well as in cells in the ventricular epicardium (D). Cardiac myocytes were stained for MF20 (red) in (B–D). In the HH Stage 30 chick heart, CD44 (LYVE-1 homolog) was also expressed in epicardial cells (E, green) close to Prox-1-positive cells (red) on the great vessels, cells in the myocardium (F), and ventricular epicardial cells (G). In chick sections at this stage, Prox-1 (red) was found in the lymphatics and cardiac myocytes and did not co-localize with CD44 staining (E–G). Ao = aorta, PT = pulmonary trunk. Bars = 100 um.
Figure 6. Confocal microscopy of LYVE-1-positive cells (green) in the ED 13.5 mouse heart (441X magnification).

Cardiac myocytes were stained for MF20 (red) and DAPI (blue) was used for nuclear visualization. LYVE-1 was expressed in (A) mesothelial cells of the epicardium (arrowhead), and cells within the (B) subepicardial mesenchyme and (C) myocardium. Note that the cell indicated in (C) is LYVE-1-positive but MF20-negative. EM = epicardial mesothelium, Myo = myocardium, SM = subepicardial mesenchyme.
Prox-1 co-localized with LYVE-1 in the epicardial lymphatics at ED 15 in mouse
In the ED 15 mouse heart, the Prox-1-positive lymphatic vessels had begun to extend from the great vessels down towards the ventricles, while the LYVE-1-positive cells were present in large numbers on the great vessels, atria and ventricles (Figure 7 A–B). Several of the LYVE-1-positive cells appeared to be coalescing into a tubular network and, in the sections, showed co-localization with Prox-1 staining in the lymphatics encircling the great vessels (Figure 7 C–F). The LYVE-1-positive cells found on the ventricles and within the myocardium remained Prox-1-negative (Figure 7 E–F).
Figure 7. Lymphatic markers in the ED 15 mouse heart.
(A) In the immunostained whole mount hearts, Prox-1 was expressed in lymphatic vessels along the great vessels and the base of the heart. (B) LYVE-1-positive cells (white arrow) were found on the great vessels, atria and ventricles with some cells arranged in a network (black arrow). Immunostained sections revealed co-localization of Prox-1 (C) and LYVE-1 (D) staining in the lymphatics on the great vessels but individual LYVE-1-positive cells at the atrioventricular junction (F, white arrowhead) did not stain for Prox-1 (E). (C) and (D) are alternate sections; (E) and (F) are alternate sections. Arrows in (C) and (D) indicate the same region, and arrows in (E) and (F) indicate the same region. At = atrium, PT = pulmonary trunk, V = ventricle. Bars = 100 um.
The epicardial lymphatics in the adult mouse heart stained for 4 lymphatic markers
In the adult mouse heart, the epicardial lymphatics, such as those at the atrioventricular junction and apex of the heart, stained positive for the four lymphatic markers: Prox-1, LYVE-1, VEGFR-3 and podoplanin (Figure 8 A–H). These epicardial vessels also expressed the endothelial marker CD31 (Supplementary Figure 2 A). However, the myocardial network of vessels and cells expressed only LYVE-1 and VEGFR-3 and were negative for Prox-1 and podoplanin (Figure 8 I–L). A subset of the myocardial LYVE-1-positive vessels also expressed CD31, while other LYVE-1-positive cells within the myocardium were negative for CD31 (Supplementary Figure 2 A–B). Our findings from the mouse studies suggest that there are three different types of cells/vessels in the heart with lymphatic phenotype (Figure 9).
Figure 8. Lymphatic markers in the adult mouse heart.

(A–D) Prox-1, VEGFR-3, LYVE-1, and podoplanin were expressed in the lymphatic vessels (arrows) at the atrio-ventricular junction of the adult mouse heart (frontal sections). (E–H) All four lymphatic markers were also expressed in the epicardial lymphatic vessels covering the ventricles (arrows). (I–L) Prox-1 and podoplanin were not expressed in the VEGFR-3-positive/LYVE-1-positive cells and vessels (arrows) found within the ventricular myocardium. V = ventricle. Bars = 100 um.
Figure 9. Diagram of the three different types of cells/vessels with lymphatic phenotype in the mouse heart arranged according to location.
These include (1) Prox-1-positive cells migrating into the heart via the great vessels, (2) LYVE-1-positive cells already present in the epicardium of the heart, and (3) LYVE-1-positive cells found within the myocardium.
Primary cultures of epicardial cells and epicardial cell lines expressed lymphatic markers
For comparison, two epicardial cell lines and cultured embryonic epicardial cells were also analyzed for expression of lymphatic markers. Primary cultures of quail epicardial cells (QECs) were isolated from hearts at stages when there were no apparent Prox-1-positive cells found in the epicardium in sections. Some of these isolated cells in culture were QH-1-positive but unexpectedly many expressed Prox-1 in both the nucleus and cytoplasm (Figure 10 A–C). An adult rat epicardial cell line (ARECs) was found to express three lymphatic markers: Prox-1, LYVE-1, and VEGFR-3, but not in the subcellular localization reported for adult lymphatic endothelial cells (Figure 10 D–F). Prox-1 was found at low levels homogeneously throughout the cytoplasm of all the cells, while LYVE-1 staining was present both in the cytoplasm and in a pattern suggesting its presence in the plasma membranes in the majority of the cells. VEGFR-3 was present in the perinuclear region in most of the cells. In the embryonic mouse epicardial cells (EMECs), a subset of cells were observed to express all three lymphatic markers, and the subcellular localization for each marker was the same as for the ARECs (Figure 10 G–I).
Figure 10. Lymphatic markers in vitro.
(A) Prox-1 expression assessed by immunostaining in quail epicardial cells (QECs) which were also labeled for QH-1 (B) and mounted with DAPI for nuclear visualization (C). (A–C) are the same field of view. (D–F) Expression of lymphatic markers (green) in the adult rat epicardial cells (ARECs). Inserts in (E, F) are confocal images of VEGFR-3 and LYVE-1-expression in the ARECs. (G–I) Expression of lymphatic markers (red) in the embryonic mouse epicardial cells (EMECs). DAPI = blue staining. Bar = 100 um.
Prox-1 may have phosphoisoforms like its Drosophila homolog Prospero
Western blot analysis of heart tissue extracts from HH Stage 30 quail and chicken embryos, post-hatched chickens, ED 15 mouse embryos, postnatal mice, and adult mice revealed 2 Prox-1-positive protein bands (Figure 11 A). In fact, for the embryonic mouse samples, the two bands of the Prox-1 doublet were equally intense but in the postnatal and adult mice, the upper band grew steadily weaker in intensity in comparison to the lower band. Furthermore, only one Prox-1 band was detected in the extracts from the ARECs and EMECs (Figure 11 A). The single Prox-1 band for the two cell lines had a molecular weight of 83 kDa and was consistent with the upper band of the doublet that appeared in the other samples. This difference may be due to the fact that the whole heart extracts consisted primarily of myocardial tissue, while the cell lines were purely epicardial cells. Anti-Prox-1 also detected 2 bands in Western blots of lens extracts from HH Stage 30 quail embryos (Figure 11 A). In previous studies, Prox-1 appeared as a single thick band for lens extracts from other species (Del Rio-Tsonis et al, 1999). This was also the case in our preliminary trials with the quail lens extract but with further dilution of the protein concentrations, 2 bands were resolved with the upper band being very faint. Avian embryonic heart tissue was also treated with an alkaline phosphatase and analyzed using Western Blotting in comparison to control samples that had not been exposed to the enzyme. In the dephosphorylated samples, the upper band for Prox-1 was greatly reduced after 1½ hours of phosphatase treatment (Figure 11 B). Srinivasan et al (1998) found that Prospero, the Drosophila homolog of Prox-1, also had several phosphoisoforms. In Western Blots, the upper band for Prospero (220 kDa) was reduced significantly with phosphatase treatment, analogous to our findings with Prox-1. We suggest therefore that Prox-1 has a phosphorylated form, and that phosphorylation of this transcription factor may be associated with its activation or a change in its subcellular localization and/or function as is the case for Prospero.
Figure 11. Prox-1 Western Blots.
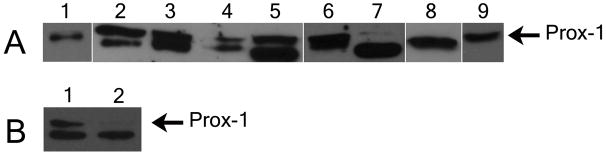
(A) Samples included HH Stage 30 quail lens extract (lane 1), HH Stage 30 quail embryonic hearts (lane 2), HH Stage 30 chicken embryonic hearts (lane 3), post-hatched chicken hearts (lane 4), postnatal mouse hearts (lane 5), ED 15 mouse embryo hearts (lane 6), adult mouse heart tissue (lane 7), adult rat epicardial cells (lane 8), and embryonic mouse epicardial cells (lane 9). (B) Control HH Stage 30 quail heart samples (lane 1) were run alongside dephosphorylated samples (lane 2) for a Western Blot. The upper band of the Prox-1 doublet almost completely disappeared with phosphatase treatment.
Discussion
Lymphatic markers that are currently used are not completely specific to lymphatic endothelial cells, thereby complicating efforts to map the lymphatics in various organs and tissues. For example, in the heart, Prox-1 is found in the lymphatics, as well as in cardiac myocytes and valvular cells (Wilting et al, 2007 and our studies). According to Kaipainen et al (1995), VEGFR-3 is found in venous and lymphatic endothelia in the early mouse embryo. From about ED 14.5 onwards however, VEGFR-3 appeared to be expressed only in the lymphatic endothelium. Nevertheless, in our studies, we have used several lymphatic markers (Prox-1, LYVE-1, VEGFR-3 and podoplanin) in the developing and adult mouse heart in order to confirm the specificity of the lymphatic staining.
We detected two sets of cells in the epicardium expressing lymphatic markers. One group was positive for nuclear-localized Prox-1 and appeared as a sheet of cells in the serosa of the future aorta and pulmonary trunk that migrated down into the epicardium of the heart. These Prox-1-positive cells expressed QH-1 in the quail, but did not initially express CD44 in the chick embr yo or LYVE-1 (homologous to CD44) in the mouse embryo. Therefore, if the Prox-1-positive cells are originating from the cardinal vein and from the lymph sacs, they have lost LYVE-1 expression. A down-regulation of LYVE-1 expression in lymphatic precursors has not been previously reported. In the mouse embryo, the Prox-1-positive/LYVE-1-negative cells then organized into a network of lymphatic vessels that eventually became positive for LYVE-1 and maintained their Prox-1 positivity. Thus, LYVE-1 seems to be up-regulated once these Prox-1-positive cells have organized into vessels. The functional significance of this pattern of LYVE-1 expression is not known.
The other set of epicardial cells that we detected were Prox-1-negative/LYVE-1-positive cells and were present prior to the appearance of the Prox-1-positive cells migrating over the aorta and pulmonary trunk into the heart. The Prox-1-negative/LYVE-1-positive cells were equidistantly scattered over the entire heart except for the distal portion of the OFT and reminiscent in their location to CD44-positive cells in the chick embryo heart or to QH-1-positive cells in the quail embryo heart prior to septation. However, despite the similarity in staining patterns of the epicardial cells for each of these markers, we cannot at this time conclude that these markers are all expressed by the same set of cells. At later stages in the mouse (ED 15), we saw Prox-1-positive/LYVE-1-positive cells that appeared to be organized into a network of vessels at the base of the heart with Prox-1-negative/LYVE-1-positive cells scattered nearby. In the adult, the epicardium was populated by cells/vessels expressing both LYVE-1 and nuclear-localized Prox-1. These findings led us to the question “Does the subset of Prox-1-negative/LYVE-1-positive cells in the embryonic epicardium get incorporated into the Prox-1-positive/LYVE-1-positive vessels that we later observed in this layer and become Prox-1-positive themselves or do they have another fate altogether not related to the lymphatics?” The LYVE-1-positive cells in the epicardium of the heart may undergo a fate similar to what was observed in a study conducted by Buttler et al (2006). In their murine model, LYVE-1-positive mesenchymal cells were found to also express the leukocytic marker CD45. However, once some of these LYVE-1-positive cells were incorporated into the developing dermal lymphatics, they down-regulated CD45 expression, and up-regulated PECAM and Prox-1 expression. The cells therefore down-regulated their leukocyte phenotype and up-regulated lymphendothelial characteristics. Similarly, in the quail chorioallantoic membrane, it appeared that scattered Prox-1-negative/QH-1-positive cells were incorporated into Prox-1-positive/QH-1-positive lymphatic vessels and became Prox-1-positive once connected to the vessel (Parsons-Wingerter et al, 2006).
QH-1 is known to detect quail angioblasts, hemangioblasts, and precursors of the blood vessel endothelial cells (Reese et al, 2002; Wada et al, 2003; Tomanek, 2005; Tomanek et al, 2006). QH-1 also labels lymphatic precursors in the chorioallantoic membrane (CAM) more intensely than it labels blood vascular endothelial cells (Parsons-Wingerter et al, 2006). This raises the question of how many of the QH-1-positive cells in the embryonic epicardium are precursors of blood vessel endothelial cells and whether any could be destined to become lymphatic endothelial cells. In our studies, the overall pattern of LYVE-1-positive cells in the epicardium of the embryonic mouse heart at ED 13.5 is reminiscent of the pattern of QH-1-positive cells found in quail embryo hearts at HH Stage 24. These findings raise the possibility that the QH-1-positive cells scattered over the epicardium in the quail embryonic heart represent precursor cells that can become blood or lymphatic endothelial cells. Unfortunately, it is not possible to support this idea using immunohistological methods at this time because QH-1 only recognizes quail cells and currently available LYVE-1 antibodies do not recognize quail LYVE-1. LYVE-1 expression by itself may not indicate that the mouse epicardial cells will become lymphatic precursors because LYVE-1 is also expressed in the endothelial lining of liver sinusoids and in vessels within the spleen (Mouta Carreira et al, 2001). The significance of LYVE-1 expression in epicardial cells requires further study.
A third set of cells noted on the endocardial side of the myocardium at an early stage in the mouse expressed LYVE-1, VEGFR-3 and PECAM, but not Prox-1 or podoplanin. Furthermore, cells with similar positive protein expression for LYVE-1 and VEGFR-3 populate the thickness of the adult myocardium. The identity and function of these subsets of cells in the embryo and the adult require further investigation.
Thus, it appears that there are three types of cells with lymphatic phenotype in the mouse heart which include: (1) Prox-1-positive cells that migrate into the heart within the epicardium of the great vessels to eventually express other lymphatic markers such as LYVE-1, VEGFR-3, and podoplanin; (2) LYVE-1-positive cells in the ventricular epicardium, a subset of which eventually co-localize with Prox-1 expression in the developing epicardial lymphatics; and (3) LYVE-1-positive/VEGFR-3 positive cells within the myocardium that remain Prox-1-negative throughout development, perhaps serving as a ‘pool’ of cells that have not reached terminal lymphatic differentiation but could potentially be recruited for future lymphangiogenesis.
Surprisingly, both the adult rat epicardial cells (ARECs) and embryonic mouse epicardial cells (EMECs) expressed three lymphatic markers but in a different subcellular localization than in mature lymphatics. The mature lymphatics express LYVE-1 and VEGFR-3 on the cell surface and nuclear-localized Prox-1. Most of the ARECs and EMECs displayed intense immunostaining for LYVE-1 primarily in the cytoplasm and VEGFR-3 in the perinuclear region, with low levels of Prox-1 in the cytoplasm. A subset of cells in primary cultures of quail embryo epicardial cells also exhibited nuclear-localized Prox-1 expression. We confirmed the presence of Prox-1 in the cultured cells by Western blot analysis. These findings are intriguing for several reasons. First, the diffuse pattern and low intensity of the Prox-1 staining in the cell lines raises the possibility that the protein could be expressed in the same manner in the epicardial cells of the embryonic quail, chicken and mouse heart but was undetected by immunostaining because of the weak cytoplasmic staining and the thin profile of these squamous epithelial cells in histological sections. If we extrapolate from the mouse lens system (Duncan et al, 1999), where Prox-1 is translocated from the cytoplasm to the nucleus of the lens fiber during terminal differentiation, then the diffuse cytoplasmic staining of the epicardial cells may reflect their undifferentiated state. The fact that the ARECs and EMECs expressed three lymphatic markers albeit at low levels and not in the subcellular location exhibited by mature lymphatic endothelial cells raises the possibility that these cells may be poised to differentiate into lymphatic endothelial cells, when exposed to an appropriate stimulus.
Materials and Methods
Animals
Chicken (Gallus gallus; Squire Valleevue Farm, Hunting Valley, OH) and quail (Coturnix coturnix japonica; Boyd’s Bird Company, Pullman, WA) embryos were incubated in an egg incubator (GQF Mfg Co., Savannah, GA) with a rocking apparatus and dissected out at Hamburger-Hamilton (HH) Stages 24–40 (Hamburger and Hamilton, 1951). Mice were obtained from Dr. Radhika Atit (Case Western Reserve University, OH). The adult rat epicardial cell line (Eid et al, 1994) was obtained from Dr. David Bader (Vanderbilt University, TN). The embryonic mouse epicardial cell line (Austin et al, 2008) was obtained from Drs. Austin and Barnett (Vanderbilt University, TN).
Antibodies
The antibodies against Prox-1 (polyclonal rabbit anti-human, 5 ug/ml; Research Diagnostics Inc., Concord, MA), LYVE-1 (polyclonal rabbit anti-mouse, 10 ug/ml; Angio-Proteomie, Boston, MA), VEGFR-3 (polyclonal rabbit anti-mouse, 4 ug/ml; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), podoplanin (monoclonal hamster anti-mouse, 5 ug/ml, eBioscience, San Diego, CA), CD44 (monoclonal mouse anti-chicken, 125 ug/ml; US Biological Inc., Swampscott, MA), and CD31/PECAM (rat anti-mouse, 10 ug/ml; BP Pharmingen, San Jose, CA) were used according to the manufacturer’s protocol. The endothelial precursor marker QH-1 (mouse anti-quail, 1:2000) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA.
Immunostaining of Whole Hearts and Sections
Intact hearts from staged embryonic quail embryos were dissected and fixed in 4% paraformaldehyde. The hearts were incubated in the primary antibodies anti-Prox-1 and anti-QH-1 overnight. The hearts were then incubated with the appropriate fluorescent secondary antibodies (Molecular Probes, Eugene, OR) and observed under the stereoscope. Cryosections of quail and chicken embryonic hearts were examined for Prox-1 immunofluorescence as well. ED 9.5–15 mouse hearts and adult mouse hearts were also sectioned and stained for Prox-1, LYVE-1, podoplanin, and VEGFR-3. The cardiac myocyte marker MF20/titin was used for co-localization. Stained sections were mounted with DAPI for nuclear visualization, and examined under the Nikon DIAPHOT 200 fluorescence microscope. Images were captured with the digital camera and QCapture Pro software.
Primary Cultures
Quail embryonic hearts at HH Stage 21 were harvested and placed on Matrigel-coated coverslips to allow the epicardial cells to grow out onto the matrix. The hearts were removed after 24 hours and the quail epicardial cells (QECs) were maintained in culture at 37°C and 5% CO2 in M199 media ((Mediatech Inc., Manassas, VA) supplemented with 10% FBS. The coverslips were then rinsed in PBS, fixed in 4% paraformaldehyde, and stained for Prox-1. After being mounted with DAPI for nuclear visualization, the coverslips were examined under the Nikon DIAPHOT 200 fluorescence microscope. Images were captured with the digital camera and QCapture Pro software.
Cell Culture
The adult rat epicardial cells (ARECs) were maintained in culture at 37° and 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM) media with sodium pyruvate (Mediatech Inc., Manassas, VA) and supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA). The embryonic mouse epicardial cells (EMECs) were maintained in culture at 33° and 5% CO2 in DMEM media without sodium pyruvate (Mediatech Inc., Manassas, VA) and supplemented with 10% FBS. The ARECs and EMECs were seeded onto poly-L-lysine-coated coverslips and collagen-coated coverslips respectively and once they were confluent, the coverslips were rinsed in PBS, fixed in 4% paraformaldehyde, and stained for Prox-1, LYVE-1, and VEGFR-3. The coverslips were then mounted with DAPI for nuclear visualization, and examined under the Nikon DIAPHOT 200 fluorescence microscope. Images were captured with the digital camera and QCapture Pro software.
Western Blotting
Tissue samples were sonicated in RIPA lysis buffer (with protease inhibitors) and assayed for protein concentration. The sample lysate was then electrophoresed on an 8% SDS-PAGE gel and transferred onto a PVDF membrane (Millipore, Bedford, MA). Membranes were blocked in a solution of 5% milk/TBS-T and incubated with the primary antibody Prox-1 overnight. The monoclonal antibody anti-β-actin was used as a loading control. After washing, the blots were incubated with the appropriate HRP-linked secondary antibodies (Cell Signaling, Beverly, MA) and signals were then detected using an enhanced chemiluminescence detection system (Pierce Chemical Co., Rockford, IL).
Phosphatase Assay
HH Stage 30 quail heart samples were exposed to a calf intestinal alkaline phosphatase enzyme (NE Biolabs, Ipswich, MA) for 1½ hours at 37°C in 1X NE Reaction Buffer. The enzyme was then inactivated with the addition of 50 mM EDTA. Dephosphorylated samples were run on a Western Blot alongside control samples that were not enzyme-treated.
Supplementary Material
At HH Stage 24, nuclear-localized Prox-1 expression was found in the ventricular myocardium (A) and the endocardial cushions (B). At HH Stage 30, Prox-1 was also found in the cells lining the various cardiac valves, including the aortic valve (C). Bars = 100 um.
In the adult mouse heart, LYVE-1 and CD31 co-stained the epicardial lymphatics (A, arrows) at the apex of the heart and vessels within the ventricular myocardium (B, arrow). There were also cells in the myocardium that were positive for LYVE-1 expression but did not stain for CD31 (A, arrowhead). Bar = 100 um.
Acknowledgments
This work was supported by an award from the American Heart Association (0715153B to GK) and grants from the National Institutes of Health R01 ES013507, HL65314, HL0775436, HL083048, HL085708 and NASA GRC IR&D 04-54.
Confocal microscopy was performed in the Pediatric Imaging Center at Rainbow Babies & Children’s Hospital in Cleveland, Ohio. We are grateful to Dr. Midori Hitomi with the Department of Pathology at University Hospitals in Cleveland, Ohio, for her help in obtaining the semi-thin sections. We are thankful for Dr. Susann Brady-Kalnay’s advice (Department of Molecular Biology and Microbiology, Case Western Reserve University, Cleveland, Ohio) for the phosphatase experiments. We also thank Robert Thompson, Edward Clark, and David Sedmera for stimulating discussion and directing us to as well as providing us with key papers.
References
- 1.Austin AF, Compton LA, Love JD, Brown CB, Barnett JV. Primary and immortalized mouse epicardial cells undergo differentiation in response to TGFbeta. Dev Dyn. 2008;237(2):366–376. doi: 10.1002/dvdy.21421. [DOI] [PubMed] [Google Scholar]
- 2.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald D. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204(10):2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144(4):789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154(2):385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttler K, Kreysing A, von Kaisenberg CS, Schweigerer L, Gale N, Papoutsi M, Wilting J. Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev Dyn. 2006;235(6):1554–1562. doi: 10.1002/dvdy.20737. [DOI] [PubMed] [Google Scholar]
- 6.Del Rio-Tsonis K, Tomarev SI, Tsonis PA. Regulation of Prox 1 during lens regeneration. Invest Ophthalmol Vis Sci. 1999;40(9):2039–2045. [PubMed] [Google Scholar]
- 7.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 8.Duncan MK, Cui W, Oh DJ, Tomarev SI. Prox1 is differentially localized during lens development. Mech Dev. 2002;112:195–198. doi: 10.1016/s0925-4773(01)00645-1. [DOI] [PubMed] [Google Scholar]
- 9.Edward M, Gillan C, Micha D, Tammi RH. Tumour regulation of fibroblast hyaluronan expression: a mechanism to facilitate tumour growth and invasion. Carcinogenesis. 2005;26(7):1215–1223. doi: 10.1093/carcin/bgi064. [DOI] [PubMed] [Google Scholar]
- 10.Eid H, de Bold ML, Chen JH, de Bold AJ. Epicardial mesothelial cells synthesize and release endothelin. J Cardiovasc Pharmacol. 1994;24:715–720. doi: 10.1097/00005344-199424050-00005. [DOI] [PubMed] [Google Scholar]
- 11.Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, Thurston G, Jackson DG. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27(2):595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gittenberger-de Groot AC, Mahtab EA, Hahurij ND, Wisse LJ, Deruiter MC, Wijffels MC, Poelmann RE. Nkx2.5-negative myocardium of the posterior heart field and its correlation with podoplanin expression in cells from the developing cardiac pacemaking and conduction system. Anat Rec (Hoboken) 2007;290(1):115–122. doi: 10.1002/ar.20406. [DOI] [PubMed] [Google Scholar]
- 13.Gross L, Kugel M. Topographic anatomy and histology of the valves in the human heart. Am J Pathol. 1931;7:445–473. [PMC free article] [PubMed] [Google Scholar]
- 14.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 15.Hamrah P, Chen L, Zhang Q, Dana MR. Novel expression of vascular endothelial growth factor receptor VEGFR-3 and VEGF-C on corneal dendritic cells. Am J Pathol. 2003;163:57–68. doi: 10.1016/S0002-9440(10)63630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 17.Huntington G, McClure C. The anatomy and development of the jugular lymph sac in the domestic cat (Felis domestica) Am J Anat. 1910;10:177–311. [Google Scholar]
- 18.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82:673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- 19.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92(8):3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kampmeier O. The development of the thoracic duct in the pig. Am J Anat. 1912;13:401–745. [Google Scholar]
- 21.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 22.Klika E, Antalikova L, Rychter Z, Jelinek R. Incept and manner of development of the lymph vessel of the chick embryo heart. Lymphology. 1971;5(4):137–148. [PubMed] [Google Scholar]
- 23.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3387. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 24.Mikawa T, Gourdie RG. Pericadial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 25.Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61(22):8079–8084. [PubMed] [Google Scholar]
- 26.Munoz-Chapuli R, Gonzalez-Iriarte M, Carmona R, Atencia G, Macias D, Perez-Pomares JM. Cellular precursors of the coronary arteries. Tex Heart Inst J. 2002;29:243–249. [PMC free article] [PubMed] [Google Scholar]
- 27.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes and Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 28.Oliver G. Lymphatic vasculature development. Nature Rev Immunol. 2004;4:35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- 29.Papoutsi M, Tomarev SI, Eichamann A, Prols F, Christ B, Wilting J. Endogenous origin of the lymphatics in the avian chorioallantoic membrane. Dev Dyn. 2001;222(2):238–251. doi: 10.1002/dvdy.1187. [DOI] [PubMed] [Google Scholar]
- 30.Parsons-Wingerter P, McKay TL, Leontiev D, Vickerman MB, Condrich TK, Dicorleto PE. Lymphangiogenesis by blind-ended vessel sprouting is concurrent with hemangiogenesis by vascular splitting. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(3):233–247. doi: 10.1002/ar.a.20309. [DOI] [PubMed] [Google Scholar]
- 31.Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, Stacker SA, Achen MG, Alitalo K. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14(13):2087–2096. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002;46:1005–1013. [PubMed] [Google Scholar]
- 33.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 35.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 36.Reese DE, Mikawa T, Bader DM. Development of the coronary vessel System. Circ Res. 2002;91:761–768. doi: 10.1161/01.res.0000038961.53759.3c. [DOI] [PubMed] [Google Scholar]
- 37.Rishi AK, Joyce-Brady M, Fisher J, Dobbs LG, Floros J, VanderSpek J, Brody JS, Williams MC. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol. 1995;167(1):294–306. doi: 10.1006/dbio.1995.1024. [DOI] [PubMed] [Google Scholar]
- 38.Rychter Z, Jelinek R, Klika E, Antalikova L. Development of the lymph bed in the wall of the chick embryo heart. Physiol bohemoslov. 1971;20(6):533–539. [PubMed] [Google Scholar]
- 39.Sabin F. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am J Anat. 1902;1:367–391. [Google Scholar]
- 40.Sabin F. On the development of the superficial lymphatics in the skin of the pig. Am J Anat. 1904;3:183–195. [Google Scholar]
- 41.Sabin F. The lymphatic system in human embryos, with a consideration of the morphology of the system as a whole. Am J Anat. 1909;9:43–91. [Google Scholar]
- 42.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. Embo J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider M, Othman-Hassan K, Christ B, Wilting J. Lymphangioblasts in the avian wing bud. Dev Dyn. 1999;216(4–5):311–319. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<311::AID-DVDY1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 44.Schoen FJ. Cardiac valves and valvular pathology: update on function, disease, repair, and replacement. Cardiovasc Pathol. 2005;14:189–194. doi: 10.1016/j.carpath.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161(3):947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sedmera D, Reckova M, DeAlmeida A, Coppen SR, Kubalak SW, Gourdie RG, Thompson RP. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat Rec A Discov Mol Cell Evol Biol. 2003;274(1):773–777. doi: 10.1002/ar.a.10085. [DOI] [PubMed] [Google Scholar]
- 47.Shimada T, Noguchi T, Takita K, Kitamura H, Nakamura M. Morphology of lymphatics of the mammalian heart with special reference to the architecture and distribution of the subepicardial lymphatic system. Acta Anat (Basel) 1989;136(1):16–20. doi: 10.1159/000146791. [DOI] [PubMed] [Google Scholar]
- 48.Shimada T, Morita T, Oya M, Kitamura H. Morphological studies of the cardiac lymphatic system. Arch Histol Cytol. 1990;53(Suppl):115–126. doi: 10.1679/aohc.53.suppl_115. [DOI] [PubMed] [Google Scholar]
- 49.Sosa-Pineda B, Wigle JT, Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Genet. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan S, Peng CY, Nair S, Skeath JB, Spana EP, Doe CQ. Biochemical analysis of Prospero protein during asymmetric cell division: cortical Prospero is highly phosphorylated relative to nuclear Prospero. Dev Bio. 1998;204:478–487. doi: 10.1006/dbio.1998.9079. [DOI] [PubMed] [Google Scholar]
- 51.Tomanek RJ, Hansen HK, Dedkov EI. Vascular patterning of the quail coronary system during development. Anat Rec Discov Mol Cell Evol Biol. 2006;288(9):989–999. doi: 10.1002/ar.a.20365. [DOI] [PubMed] [Google Scholar]
- 52.Tomanek RJ. Formation of the coronary vasculature during development. Angiogenesis. 2005;8:273–284. doi: 10.1007/s10456-005-9014-9. [DOI] [PubMed] [Google Scholar]
- 53.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. Signaling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. Embo J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wada AM, Willet SG, Bader D. Coronary vessel development: a unique form of vasculogenesis. Arterioscler Thromb Vasc Biol. 2003;23:2138–2145. doi: 10.1161/01.ATV.0000098645.38676.CC. [DOI] [PubMed] [Google Scholar]
- 55.Wetterwald A, Hoffstetter W, Cecchini MG, Lanske B, Wagner C, Fleisch H, Atkinson M. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18(2):125–32. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 56.Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Gen. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- 57.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 59.Wilting J, Buttler K, Schulte I, Papoutsi M, Schweigerer L, Manner J. The proepicardium delivers hemngiobalsts but not lymphangioblasts to the developing heart. Dev Biol. 2007;305(2):451–459. doi: 10.1016/j.ydbio.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 60.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nature Medicine. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
At HH Stage 24, nuclear-localized Prox-1 expression was found in the ventricular myocardium (A) and the endocardial cushions (B). At HH Stage 30, Prox-1 was also found in the cells lining the various cardiac valves, including the aortic valve (C). Bars = 100 um.
In the adult mouse heart, LYVE-1 and CD31 co-stained the epicardial lymphatics (A, arrows) at the apex of the heart and vessels within the ventricular myocardium (B, arrow). There were also cells in the myocardium that were positive for LYVE-1 expression but did not stain for CD31 (A, arrowhead). Bar = 100 um.








