Abstract
Blood pressure variability based on office measurement predicts outcome in selected patients. We explored whether novel indices of blood pressure variability derived from the self-measured home blood pressure predicted outcome in a general population. We monitored mortality and stroke in 2421 Ohasama residents (Iwate Prefecture, Japan). At enrollment (1988–1995), participants (mean age, 58.6 years; 60.9% women; 27.1% treated) measured their blood pressure at home, using an oscillometric device. In multivariable-adjusted Cox models, we assessed the independent predictive value of the within-subject mean systolic blood pressure (SBP) and corresponding variability as estimated by variability independent of the mean, difference between maximum and minimum blood pressure, and average real variability. Over 12.0 years (median), 412 participants died, 139 of cardiovascular causes, and 223 had a stroke. In models including morning SBP, variability independent of the mean and average real variability (median, 26 readings) predicted total and cardiovascular mortality in all of the participants (P≤0.044); variability independent of the mean predicted cardiovascular mortality in treated (P=0.014) but not in untreated (P=0.23) participants; and morning maximum and minimum blood pressure did not predict any end point (P≥0.085). In models already including evening SBP, only variability independent of the mean predicted cardiovascular mortality in all and in untreated participants (P≤0.046). The R2 statistics, a measure for the incremental risk explained by adding blood pressure variability to models already including SBP and covariables, ranged from <0.01% to 0.88%. In a general population, new indices of blood pressure variability derived from home blood pressure did not incrementally predict outcome over and beyond mean SBP.
Keywords: blood pressure variability, variability independent of the mean index, average real variability, general population, home blood pressure, risk factors
Current guidelines propose out-of-office blood pressure measurement as the standard in the diagnosis and management of hypertension.1-3 Self-measurement of the blood pressure at home offers several of the well-recognized advantages of the more complex approach of ambulatory monitoring.4-6 The greater number of readings and the minimization of the white coat effect, observer bias, and measurement error all contribute to better diagnostic accuracy compared with office blood pressure measurement. Similar to visit-to-visit variability in clinic blood pressure,7 multiple home blood pressure measurements8-10 provide information on day-to-day blood pressure variability in the relatively controlled home environment.
We demonstrated previously that the within-subject SD of blood pressure predicted mortality.10 However, the SD is highly dependent on the mean.7,11 Rothwell et al7,11 therefore proposed blood pressure variability independent of the mean (VIM) as a new index, which might be a better predictor of cardiovascular outcome over and beyond blood pressure level. The aim of the present study was to test the hypothesis that new indices of blood pressure variability,7,11 derived from the self-measured home blood pressure in a general population instead of the office blood pressure in selected hypertensive or high-risk patients,7,11,12 would predict outcome.
Methods
Study Design
As described in detail elsewhere,5 from 1988 until 1995, we contacted 4969 individuals who resided in 4 districts of Ohasama, a rural community in Iwate Prefecture, Japan, and who were ≥35 years old. Residents were not eligible if they were not at home during normal working hours (n=1057) or if they were hospitalized (n=166) or incapacitated (n=94). Of the remaining 3652 residents, 3090 (84.6%) participated in baseline and follow-up examinations. The study complies with the Declaration of Helsinki, and the study protocol was approved by the institutional review board of Tohoku University School of Medicine and by the Ohasama Town Government Department of Health. Participants gave written informed consent. We excluded 669 participants from analysis because they had not measured their home blood pressure (n=218), had obtained <5 morning or evening readings (n=322), or because at enrollment they had a history of stroke (n=129). Thus, the number of participants statistically analyzed totaled 2421.
Data Collection
Physicians and public health nurses instructed participants how to measure their home blood pressure using a validated oscillometric device (OMRON HEM 401C, Omron Healthcare Co Ltd, Kyoto, Japan).13 Participants were asked to record their blood pressure for 4 weeks after ≥2 minutes of rest in the morning within 1 hour after awakening and, if applicable, before taking their blood pressure–lowering medications, and in the evening just before going to bed.3 The first reading obtained on each occasion was used for analysis.
At public health centers, study nurses measured anthropometric characteristics. Body mass index was weight in kilograms divided by the square of height in meters. The nurses also administered questionnaires inquiring into each participant’s medical history, intake of medications, and smoking and drinking habits. Venous blood samples were analyzed for total cholesterol and blood glucose. Diabetes mellitus was a fasting or random blood glucose level of ±7.0 or 11.1 mmol/L or the use of insulin or oral antidiabetic drugs.14 Hypercholesterolemia was a serum level of ≥5.68 mmol/L or use of lipid-lowering agents. Previous cardiovascular disease included coronary heart disease, atrial fibrillation, and heart failure.
Follow-Up and Risk Assessment
We ascertained outcome until December 31, 2004, based on the Ohasama residents’ registration cards, which in Japan provide access to social security and retirement benefits; the National Japanese Mortality Registry of causes of death; the Stroke Registration System of Iwate Prefecture; and questionnaires sent every 4 years to each participant. End points were all-cause and cardiovascular mortality and stroke events. Cardiovascular mortality included deaths from cerebrovascular and cardiac causes. Stroke events included, in addition to fatal events, nonfatal stroke but not transient ischemic attack. End points were adjudicated by checking the medical charts at Ohasama Hospital, where >90% of participants had regular health checkups.
Statistical Analysis
For database management and statistical analysis, we used the SAS system, version 9.3 (SAS Institute Inc, Cary, NC). We limited our analyses to systolic blood pressure, because this is the overriding risk factor in middle-aged and older people.15 We used all of the available home readings of the self-measured blood pressure. We analyzed the morning and evening blood pressures separately, because previous studies showed that they have a different prognostic meaning.5 In sensitivity analyses, we used 5 home readings in the morning and 5 in the evening (10 readings) according to European population studies16 and the Japanese randomized trial.17
For descriptive purposes, we computed the within-participant blood pressure variability from the SD and coefficient of variation. We based our analyses on VIM, difference between maximum minus minimum blood pressure (MMD), and average real variability (ARV). Coefficient of variation is the within-participant SD divided by the within-participant mean. VIM7,11 is SD divided by the mean to the powerx. Power x is modeled as SD = a × meanx and was derived by nonlinear regression analysis as implemented in the PROC NLIN procedure of the SAS package. ARV is the average of the absolute differences between consecutive day blood pressure measurements.18,19
We compared means and proportions using the standard normal z test for large samples and the Fisher exact test, respectively. Kruskal-Wallis test was used to assess differences between quartiles of VIM. We plotted incidence rates by fourths of the blood pressure variability distribution while standardizing rates for sex and age by the direct method. In multivariable-adjusted Cox regression, we derived standardized relative hazard ratios that expressed the change in risk associated with 1-SD increase in mean blood pressure or in variability. Covariables were sex, age, body mass index, heart rate, smoking and drinking, diabetes mellitus, total cholesterol, history of cardiovascular disease, and treatment with antihypertensive drugs. For 608 participants without known alcohol use, we interpolated drinking status based on sex and age (continuous). We checked the proportional hazards assumption by testing the interaction terms between follow-up duration and the variable of interest. Finally, we applied the generalized R2 statistic20 to assess the additional risk explained in Cox regression by adding the indices of blood pressure variability to models already including the mean systolic blood pressure level and covariables. The formula for the calculation of generalized R2 is as follows:
where n is the number of participants, ln L(X2) and ln L(X1) are the log likelihood statistics of the full model and the basic model, respectively, and χ2 is the likelihood ratio chi-square.
Results
Baseline Characteristics
Of the 2421 participants, 656 (27.1%) were treated with antihypertensive drugs at baseline, 475 (19.6%) were current smokers, 219 (9.1%) had diabetes mellitus, and 635 (26.2%) had hypercholesterolemia. The median number of days with self-measured blood pressure readings available for analysis was 26 (interquartile range, 21 to 28) in the morning and 26 (interquartile range, 22 to 28) in the evening. The self-measured systolic blood pressure was 2.15 mm Hg (95% confidence interval [CI], 1.88–2.42 mm Hg; P<0.0001) higher in the morning than evening. In patients on antihypertensive drug treatment, this difference was to 3.55 mm Hg (95% CI, 2.91–4.18 mm Hg; P<0.0001). For the morning blood pressure, the VIM formulas were (123.8^1.22) × SD/(mean^1.22) in all participants, (119.9^1.12) × SD/(mean^1.12) in 1765 untreated subjects, and (134.6^0.78) × SD/(mean^0.78) in 656 patients on antihypertensive drug treatment. For the evening blood pressure, the corresponding formulas were (121.7^1.15) × SD/(mean^1.15), (118.2^1.08) × SD/(mean^1.08) and (131.1^0.73) × SD/(mean^0.73), respectively.
Table 1 lists the baseline characteristics of the 2421 participants by sex-specific quartiles of VIM based on systolic blood pressure in the morning. The average morning blood pressure was similar across VIM quartiles (P=0.61), whereas the other indices of variability significantly increased with higher VIM (P<0.0001). These trends were similar across quartiles of VIM based on systolic blood pressure in the evening (Table S1, available in the online-only Data Supplement). The proportion of participants on antihypertensive drug treatment increased (P<0.0001) from low to high VIM (Table 1 and Table S1). All of the indices of variability were significantly (P<0.0001) higher in 656 treated compared with 1765 untreated participants (Table S2). The morning and evening blood pressures were lower (P<0.0001) in women than in men (Table S2). Although heart rate in women was similar to that in men (P=0.15), women had a significantly lower heart rate than men did in the evening (P<0.0001; Table S2). Coefficient of variation, VIM, and MMD were higher in women than in men in the morning (P≤0.0039), whereas in the evening these indices were similar in both sexes (P≥0.089; Table S2).
Table 1. Baseline Characteristics of Participants by Fourths of the Distribution of Overall Systolic Variability Independent of the Mean in the Morning.
| Characteristic | Categories of Systolic Blood Pressure Variability | P Value | |||
|---|---|---|---|---|---|
| Limits, units | |||||
| Women | 0.79–6.78 | 6.78–8.61 | 8.61–10.5 | 10.5–22.3 | |
| Men | 0.86–6.32 | 6.32–7.81 | 7.83–9.67 | 9.67–24.6 | |
| Number of participants, % | |||||
| All participants in category | 604 | 606 | 606 | 605 | |
| Antihypertensive treatment | 118 (19.5) | 142 (23.4) | 181 (29.9)* | 215 (35.5)* | <0.0001 |
| Smokers | 123 (20.4) | 122 (20.1) | 124 (20.5) | 106 (17.5) | 0.52 |
| Drinking alcohol | 111 (26.1) | 129 (27.9) | 125 (26.2) | 106 (23.6) | 0.52 |
| Diabetes mellitus | 45 (7.5) | 66 (10.9)* | 53 (8.8) | 55 (9.1) | 0.22 |
| Cardiovascular disease | 11 (1.8) | 18 (3.0) | 15 (2.5) | 26 (4.3) | 0.068 |
| Mean (SD) of characteristic | |||||
| Age, y | 55.2 (12.0) | 56.6 (11.3) | 59.6 (12.2)† | 62.8 (12.5)† | <0.0001 |
| Body mass index, kg/m2 | 23.9 (2.9) | 23.6 (2.8) | 23.2 (2.9) | 23.1 (2.9) | <0.0001 |
| Serum total cholesterol, mmol/L | 5.00 (0.79) | 5.03 (0.91) | 5.02 (0.80) | 4.94 (0.85) | 0.42 |
| Systolic blood pressure, mm Hg | 123.7 (15.0) | 123.2 (14.2) | 124.0 (15.4) | 124.5 (15.1) | 0.61 |
| Diastolic blood pressure, mm Hg | 75.9 (10.1) | 75.0 (9.4) | 74.2 (9.7) | 73.4 (10.1) | <0.0001 |
| Heart rate, beats per minute | 68.0 (7.6) | 68.0 (7.3) | 67.2 (7.9) | 66.8 (8.0) | 0.0012 |
| Mean (SD) of variability index | |||||
| SD, mm Hg | 5.3 (1.3) | 7.4 (1.2)† | 9.2 (1.5)† | 12.4 (2.9)† | <0.0001 |
| Coefficient of variation, % | 4.3 (0.9) | 6.0 (0.5)† | 7.4 (0.6)† | 9.9 (1.8)† | <0.0001 |
| Maximum-minimum difference, mm Hg | 20.7 (6.1) | 29.2 (6.3)† | 35.9 (7.8)† | 46.8 (12.6)† | <0.0001 |
| Average real variability, mm Hg | 5.6 (1.6) | 7.7 (1.7)† | 9.5 (2.1)† | 12.6 (3.5)† | <0.0001 |
Values are number of participants (%) or arithmetic mean (SD). Blood pressure variability is based on self-measurement in the morning on 5 days within 1 hour after awakening and before taking antihypertensive medications in treated participants. Drinking status was unavailable in 608 participants. P denotes the significance of the linear trend across categories of systolic blood pressure level.
P<0.05, significance of the difference with the adjacent lower fourth.
P<0.0001, significance of the difference with the adjacent lower fourth.
Incidence of Mortality and Morbidity
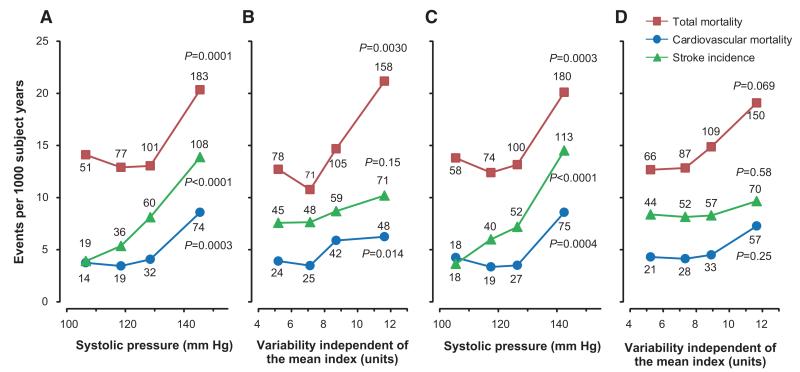
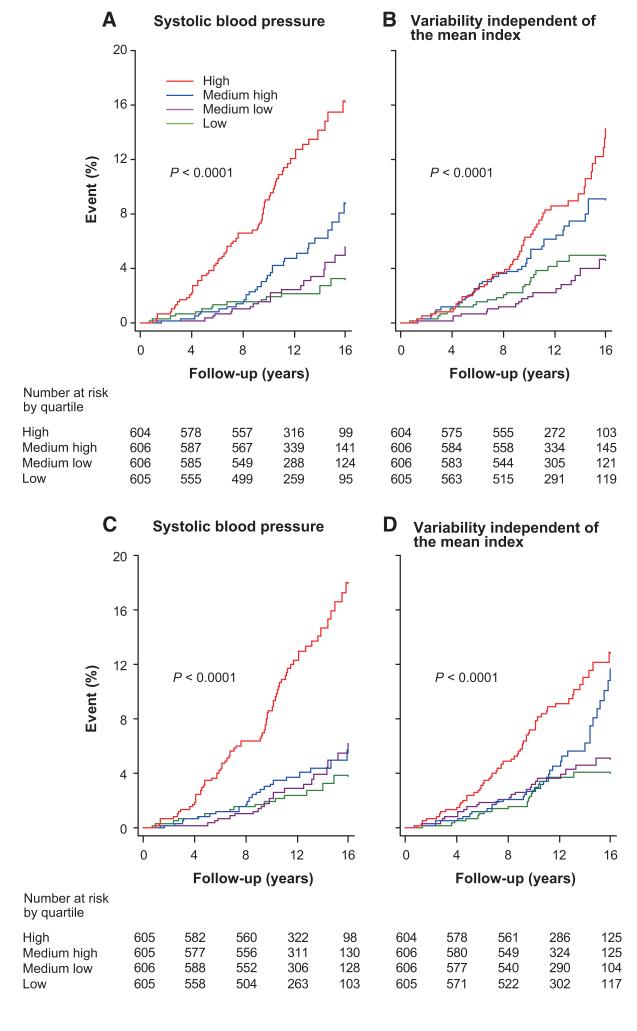
Over a median follow-up of 12.0 years (interquartile range, 9.9–15.4; maximum, 16.9 years), 412 participants died, 139 of cardiovascular causes (33.7%), and 223 stroke events occurred. All of the end points occurred at higher rates (P<0.0001) in treated compared with untreated participants (Table S3). Figure 1 shows the sex- and age-standardized rates across quartiles of the mean level and VIM of the morning systolic blood pressure. Total and cardiovascular mortality and stroke increased (P≤0.0004) with higher levels of mean blood pressure in the morning and evening. Total and cardiovascular mortality (P≤0.014) but not stroke (P=0.15) increased with higher morning VIM, whereas none of the studied end points was associated with evening VIM (P≥0.069). As shown by Kaplan-Meier survival estimates across quartiles of systolic blood pressure in the morning or evening, the mean level and VIM were consistent and significant (P<0.0001) predictors of cardiovascular mortality for all of the participants (Figure 2).
Figure 1.
Incidence of total and cardiovascular mortality and stroke by quartiles of the mean level (A and C) and variability independent of the mean (B and D) of systolic blood pressure measured at home in the morning (A and B) or evening (C and D) in 2421 participants. Rates given as end points per 1000 person-years were standardized for sex and age by the direct method. The number of end points contributing to the rates is presented. The P values refer to the significance for linear trend across the quartiles.
Figure 2.
Kaplan-Meier survival function estimates for cardiovascular mortality by quartiles of the mean level (A and C) and variability independent of the mean (B and D) of systolic blood pressure measured at home in the morning (A and B) or evening (C and D) in 2421 participants. The P values refer to the significance of the log-rank test across quartiles.
Multivariable-Adjusted Analyses
Morning Blood Pressure
In multivariable-adjusted models (Table 2), the morning systolic blood pressure predicted total and cardiovascular mortality and stroke in all (P≤0.021) and untreated (P≤0.018) participants and stroke in patients on antihypertensive treatment (P=0.0008). In models including mean systolic blood pressure, VIM and ARV predicted total and cardiovascular mortality in all of the participants (P≤0.044). VIM also predicted total mortality in untreated participants (P=0.019) and cardiovascular mortality in treated patients (P=0.014). However, these indices did not predict any cardiovascular end point in untreated participants (P≥0.11). The R2 statistics for adding morning VIM or ARV to models including mean blood pressure (Table 2) ranged from 0.08% to 0.88%. MMD added to models including the morning systolic blood pressure did not predict future events (Table 2; P≥0.085).
Table 2. Adjusted Standardized Hazard Ratios for End Points in Relation to the Mean and Overall Variability of Systolic Pressure in the Morning.
| Full Model |
||||||||
|---|---|---|---|---|---|---|---|---|
| Basic Model, Mean SBP |
VIM |
MMD |
ARV |
|||||
| End Point (n) | RH (95% CI) | R2 (%) | RH (95% CI) | R2 (%) | RH (95% CI) | R2 (%) | RH (95% CI) | R2 (%) |
| All participants | ||||||||
| Total mortality (412) | 1.14 (1.02–1.27)* | 22.9 | 1.15 (1.04–1.26)† | 0.30 | 1.06 (0.96–1.17) | 0.06 | 1.10 (1.00–1.21)* | 0.16 |
| CV mortality (139) | 1.30 (1.09–1.56)† | 10.7 | 1.26 (1.07–1.49)† | 0.31 | 1.14 (0.97–1.34) | 0.10 | 1.22 (1.04–1.42)* | 0.25 |
| Stroke (223) | 1.43 (1.23–1.66)§ | 8.3 | 1.14 (1.00–1.30) | 0.15 | 1.12 (0.98–1.28) | 0.12 | 1.13 (1.00–1.27) | 0.14 |
| Untreated participants | ||||||||
| Total mortality (231) | 1.17 (1.03–1.34)* | 20.1 | 1.17 (1.03–1.33)* | 0.30 | 1.11 (0.98–1.27) | 0.15 | 1.12 (0.99–1.26) | 0.17 |
| CV mortality (68) | 1.38 (1.09–1.74)† | 7.9 | 1.16 (0.92–1.46) | 0.08 | 1.10 (0.88–1.39) | 0.04 | 1.19 (0.96–1.46) | 0.14 |
| Stroke (113) | 1.35 (1.12–1.63)† | 6.8 | 1.11 (0.91–1.34) | 0.06 | 1.08 (0.90–1.31) | 0.04 | 1.12 (0.94–1.35) | 0.09 |
| Treated participants | ||||||||
| Total mortality (181) | 1.06 (0.91–1.23) | 23.9 | 1.12 (0.97–1.30) | 0.33 | 1.01 (0.87–1.17) | <0.01 | 1.09 (0.94–1.27) | 0.20 |
| CV mortality (71) | 1.20 (0.95–1.52) | 13.5 | 1.34 (1.06–1.69)* | 0.88 | 1.15 (0.91–1.45) | 0.21 | 1.24 (0.99–1.55) | 0.50 |
| Stroke (110) | 1.39 (1.15–1.68)‡ | 5.7 | 1.17 (0.97–1.41) | 0.39 | 1.15 (0.95–1.38) | 0.31 | 1.14 (0.96–1.36) | 0.32 |
Systolic blood pressure (SBP) level and variability are based on self-measurement in the morning for median 26 days (interquartile range, 21–28) within 1 hour after awakening and before taking antihypertensive medications in treated participants. VIM, MMD, and ARV indicate variability independent of the mean, the difference between maximum and minimum blood pressure, and average real variability. CV indicates cardiovascular. The basic model accounts for sex, age, body mass index, heart rate, smoking and drinking, serum cholesterol, and diabetes mellitus, and history of CV disease. Full models include the aforementioned covariables and both mean SBP and an index of SBP variability. Hazard ratios (HRs) given with 95% confidence intervals (CIs) express the risk associated with a 1-SD increase in the explanatory variables: in all participants, 14.9 mm Hg for level of blood pressure and 2.9 units, 12.8 mm Hg, and 3.5 mm Hg for VIM, MMD, and ARV, respectively. The R2 statistic is a measure for the risk prediction provided by the basic model including mean SBP and the additive contribution of the indexes of variability.
P<0.05, significance of the hazard ratios.
P<0.01, significance of the hazard ratios.
P<0.001, significance of the hazard ratios.
§P<0.0001, significance of the hazard ratios.
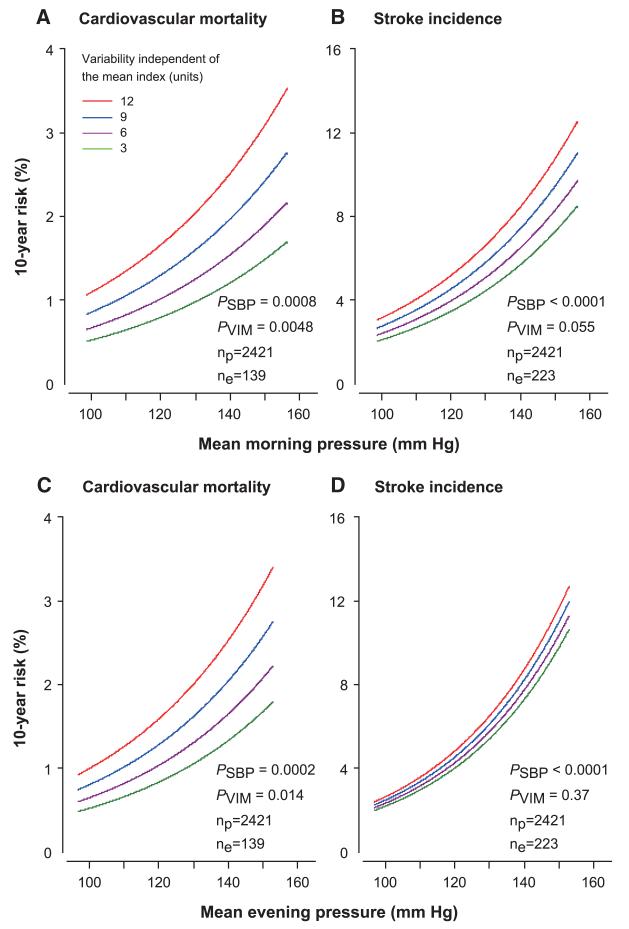
Figure 3 shows the multivariable-adjusted 10-year risk of a cardiovascular death in relation to the mean level and VIM of morning and evening systolic blood pressures in all of the participants. Figures S1 and S2 show similar information by treatment status. Morning systolic blood pressure was a consistent predictor of stroke and cardiovascular mortality (P≤0.0049) with the exception of cardiovascular mortality in treated participants (P=0.082).
Figure 3.
Absolute 10-year risk of cardiovascular mortality (A and C) and stroke incidence (B and D) in relation to the mean level of systolic blood pressure measured at home in the morning (A and B) or evening (C and D) in 2421 participants. The analyses were standardized to the distributions (mean or ratio) of sex, age, body mass index, heart rate, smoking and drinking, total cholesterol, diabetes mellitus, history of cardiovascular diseases, and treatment with antihypertensive drugs. In each panel, mean systolic blood pressure along the horizontal axis (SBP) covers the 2.5th to 97.5th percentile interval. Four continuous lines represent the risk independently associated with variability independent of the mean (VIM) equal to 3, 6, 9, and 12 units.P values are for the independent effect of SBP (PSBP) and VIM (PVIM). np and ne indicate the number of participants at risk and the number of events.
Evening Blood Pressure
The evening systolic blood pressure predicted all of the end points (P≤0.044). In models already including the evening systolic blood pressure, VIM predicted cardiovascular mortality in all (P=0.014) and untreated (P=0.46) participants, whereas MMD and ARV did not significantly (P≥0.057) refine risk stratification (Table 3). The R2 statistics for adding evening VIM, MMD, or ARV to models including mean blood pressure ranged from <0.01% to 0.27% (Table 3).
Table 3. Adjusted Standardized Hazard Ratios for End Points in Relation to the Mean and Overall Variability of Systolic Pressure in the Evening.
| Full Model |
||||||||
|---|---|---|---|---|---|---|---|---|
| Basic Model Mean SBP |
VIM |
MMD |
ARV |
|||||
| End Point (n) | RH (95% CI) | R2 (%) | RH (95% CI) | R2 (%) | RH (95% CI) | R2 (%) | RH (95% CI) | R2 (%) |
| All participants | ||||||||
| Total mortality (412) | 1.16 (1.05–1.29)† | 23.0 | 1.08 (0.98–1.19) | 0.09 | 1.00 (0.91–1.10) | <0.01 | 1.05 (0.95–1.16) | 0.04 |
| CV mortality (139) | 1.37 (1.15–1.63)‡ | 10.9 | 1.23 (1.04–1.44)* | 0.24 | 1.15 (0.98–1.34) | 0.12 | 1.15 (0.98–1.35) | 0.12 |
| Stroke (223) | 1.54 (1.34–1.78)§ | 8.9 | 1.06 (0.93–1.21) | 0.03 | 1.04 (0.91–1.18) | 0.01 | 1.05 (0.92–1.19) | 0.02 |
| Untreated participants | ||||||||
| Total mortality (231) | 1.14 (1.01–1.30)* | 20.0 | 1.09 (0.96–1.25) | 0.10 | 1.01 (0.89–1.15) | <0.01 | 1.10 (0.96–1.25) | 0.11 |
| CV mortality (68) | 1.40 (1.12–1.76)† | 7.9 | 1.26 (1.01–1.59)* | 0.22 | 1.12 (0.90–1.40) | 0.06 | 1.24 (0.99–1.55) | 0.19 |
| Stroke (113) | 1.49 (1.25–1.79)§ | 7.2 | 1.03 (0.86–1.24) | 0.01 | 1.00 (0.84–1.20) | <0.01 | 1.03 (0.86–1.24) | <0.01 |
| Treated participants | ||||||||
| Total mortality (181) | 1.17 (1.00–1.36)* | 24.3 | 1.05 (0.91–1.21) | 0.07 | 1.00 (0.87–1.14) | <0.01 | 0.99 (0.86–1.14) | <0.01 |
| CV mortality (71) | 1.31 (1.03–1.67)* | 13.9 | 1.16 (0.93–1.47) | 0.25 | 1.16 (0.93–1.43) | 0.27 | 1.04 (0.83–1.30) | 0.01 |
| Stroke (110) | 1.46 (1.20–1.76)‡ | 6.2 | 1.09 (0.91–1.31) | 0.13 | 1.08 (0.90–1.29) | 0.10 | 1.07 (0.90–1.28) | 0.09 |
Systolic blood pressure (SBP) level and variability are based on self-measurement for median 26 days (interquartile range, 22–28) before going to bed. VIM, MMD, and ARV indicate variability independent of the mean, the difference between maximum and minimum blood pressure, and average real variability. CV indicates cardiovascular. The basic model accounts for sex, age, body mass index, heart rate, smoking and drinking, serum cholesterol, and diabetes mellitus, and history of CV disease. Full models include the aforementioned covariables and both mean SBP and an index of SBP variability. Hazard ratios (HRs) given with 95% confidence interval (CIs) express the risk associated with a 1-SD increase in the explanatory variables: in all participants, 14.5 mm Hg for level of blood pressure and 2.8 units, 12.6 mm Hg, and 3.5 mm Hg for VIM, MMD, and ARV, respectively. The R2 statistic is a measure for the risk prediction provided by the basic model including mean SBP and the additive contribution of the indices of variability.
P<0.05, significance of the hazard ratios.
P<0.01, significance of the hazard ratios.
P<0.001, significance of the hazard ratios.
P<0.0001, significance of the hazard ratios.
Additional Analyses
The interaction terms between treatment status and the indices of variability were all not significant (P≥0.06). Results based on 5 home readings were confirmatory for morning (Table S4) and evening (Table S5) blood pressures.
Discussion
Our study addressed the incremental value of newly proposed indices11 in the prediction of mortality and cardiovascular events over and beyond the blood pressure level. The key findings were as follows: (1) morning VIM and ARV, independent of blood pressure level, predicted total and cardiovascular mortality in all of the participants; (2) being treated with blood pressure–lowering drugs underlayed the predictive value of morning VIM; (3) in untreated participants, these indices of variability did not predict cardiovascular mortality; (4) none of the variability indices predicted stroke incidence; and (5) for all or cause-specific fatal combined with nonfatal outcomes, the incremental predictive value of VIM, MMD, and ARV over and beyond blood pressure level was tiny based on the viewpoint of R2 statistics. These findings must be interpreted within the context of the overall available evidence.
To our knowledge, only 2 previous population studies10,21 reported on the refinement of prognosis by considering variability of the self-measured blood pressure in addition to blood pressure level and other risk factors. In 2008, we assessed blood pressure variability from the self-measured blood pressure in the Ohasama population, using the within-subject SD of the morning systolic blood pressure over 26 days (median) of self-measurement.10 Over 11.9 years of follow-up, 462 participants died, the underlying cause being cardiovascular in 168, noncardiovascular in 294, stroke in 83, and cardiac in 85 participants.10 In multivariable-adjusted Cox models also including blood pressure level, a 1-SD increase in the between-subject variability was associated with higher risk of any death (hazard ratio, 1.18 [95% CI, 1.07–1.31]), cardiovascular death (hazard ratio, 1.20 [95% CI, 1.02–1.40]), noncardiovascular mortality (hazard ratio, 1.18 [95% CI, 1.04–1.34]), and stroke mortality (hazard ratio, 1.38 [95% CI, 1.12–1.72]) but not cardiac mortality hazard ratio (hazard ratio, 1.02 [95% CI, 0.89–1.29]). The association of blood pressure variability with noncardiovascular mortality was difficult to interpret but might reflect reverse causality, subclinical disease leading to greater variability. More recently, the Finn-Home investigators noticed 130 deaths and 179 cardiovascular events among 1866 Finns followed up for 7.8 years. In multivariable-adjusted Cox models, day-to-day variability of the self-measured systolic blood pressure in the morning, estimated from the within-participant SD over 7 days, predicted total mortality and cardiovascular events. The hazard ratios expressing incremental risk for a 1-SD between-subject increment in variability (3.93 mm Hg) were ≈1.17 (95% CI, 1.00–1.30; P=0.03) and 1.21 (95% CI, 1.08–1.40; P=0.006), respectively.21 Day-to-day variability in the evening systolic blood pressure was not predictive (P≥0.11).21
Our current findings confirm that variability of the self-measured systolic blood pressure in the morning predicts total10 and cardiovascular10,21 mortality. In addition to being based on novel indices of blood pressure variability relatively independent of the mean,7,11 our current report breaks new grounds in various ways. First, the previous Ohasama report10 evaluated only fatal outcomes. In our current report, we demonstrated that neither morning nor evening blood pressure variability refined the risk stratification for fatal combined with nonfatal outcomes based on blood pressure level. In the Finn-Home study,21 evening variability of systolic blood pressure (SD, 3.94 mm Hg) neither predicted total mortality (hazard ratio, 1.17 [95% CI, 0.96–1.35]; P=0.11) nor cardiovascular events (hazard ratio, 1.08 [95% CI, 0.92–1.26]; P=0.27). Second, as in previous studies,10,21 we adjusted for antihypertensive treatment in the whole study population. In addition, we also ran analyses stratified by treatment status, which showed that the association between cardiovascular mortality and morning blood pressure variability was in fact confined to patients on antihypertensive drug treatment. Third, cerebrovascular disease causes increased blood pressure variability by impairing baroreflexes22 and interfering with the central regulation of blood pressure by the autonomous nervous system. To avoid confounding by reverse causality, we excluded patients with a history of stroke at enrollment.23 Fourth, although the morning systolic blood pressure was an independent predictor of cardiovascular mortality, the incremental prognostic value over and beyond blood pressure level was small with R2 statistics in the whole study population of ≤0.31% and ≤0.88% in the patients on antihypertensive drug treatment. Previous population studies10,21 did not report R2 statistics. Finally, Muntner et al24 selected a subsample (n=2174) of the participants in the Third National Health and Examination Survey, who took part in 3 consecutive study visits, at which the office blood pressure was measured. For various reasons, they excluded 1218 participants from analysis. Among the remaining 956 subjects (44.0%), 240 died. In multivariable-adjusted analyses, hazard ratios for all-cause mortality associated with an SD of systolic blood pressure of 4.80 to 8.34 mm Hg and ≥8.35 mm Hg versus <4.80 mm Hg were 1.57 (95% CI, 1.07–2.18) and 1.50 (95% CI, 1.03–2.18), respectively.24 However, the P value for trend across these tertiles was marginal (0.064). Modeled as a continuous variable, blood pressure variability did not predict mortality.24 Thus, although large epidemiologic studies might pick up incremental prognostic accuracy provided by blood pressure variability, this presumed risk factor remains weak and is unlikely to be clinically meaningful in individual subjects.
A key finding in our current study was that morning blood pressure variability was a predictor of cardiovascular mortality in patients on antihypertensive drug treatment but was not prognostically informative in untreated participants. Redon et al25 enrolled 15 618 hypertensive patients in an observational study. Patients recorded their blood pressure at home for 2 weeks. Blood pressure control was a self-measured blood pressure <135 mm Hg systolic and 85 mm Hg diastolic. At baseline (day 1 of the first week), control rates of the home blood pressure were 8.5%, 11.3%, and 9.9% in the morning, at lunchtime, and in the evening, respectively; at follow-up (day 4 of the second week), these rates were 31.8%, 42.2%, and 36.4%, respectively. In our current study, the systolic difference between morning and evening blood pressure averaged 2.15 mm Hg in the whole study population and 3.55 mm Hg in patients on antihypertensive drug treatment. Patients on treatment had to measure their blood pressure before taking their medication in the morning. In 1992, 21.3% of the Ohasama patients were on a once-daily treatment regimen, and 70.9% were taking nifedipine, nicardipine, or diltiazem in sustained-release formulations,26 which have a duration of action of <12 hours.27 To what extent these findings and the observations of Redon et al25 explain why blood pressure variability was predictive in the morning, but not in the evening, remains to be elucidated. Rothwell et al11 reported that amlodipine reduced visit-to-visit blood pressure variability partly by its long half-life. On the other hand, a meta-analysis by the same group, using interindividual variance as a surrogate for within-individual variability, put forward that calcium channel blockers reduced blood pressure variability independent of drug half-life.12
The present study must be interpreted within the context of its potential limitations. First, our study population consisted of residents from a specific Japanese rural district. Although our results are in partial agreement with the Finn-Home study,21 our current might not be generalizable to Western populations, in whom not stroke but myocardial infarction is the overriding cardiovascular complication associated with blood pressure. In the Ohasama study, stroke caused 18.0% of total mortality, whereas in Finn-Home21 this proportion was only 6.2%. Ischemic heart disease accounted for 7.1% and 22.3%, respectively. Second, in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT),11 within-patient visit-to-visit variability was lower in the amlodipine than in the atenolol group among 19 257 randomized patients. The lower stroke risk in the amlodipine group (0.78 [95% CI, 0.67–0.90]) was partly attenuated by adjusting for mean systolic blood pressure during follow-up (0.84 [95% CI, 0.72–0.98]) and was diminished by also adjusting for VIM of clinic systolic blood pressure (0.96 [95% CI, 0.82–1.12]).11 For coronary events, the ASCOT findings were similar.11 In the current study, we only obtained detailed information on antihypertensive drug class in 366 treated patients, of whom 48.7% were taking calcium channel blockers and 17.5% β-blockers. In view of the incomplete information on drug class and the smaller sample size and observational nature of our current study, we could not stratify our analyses of 656 treated participants by drug class. Third, we based our main analyses on all of the available home blood pressure readings and only involved a single blood pressure reading in the morning and evening over a period of median 26 days. This is more than other European studies; for example, 5 consecutive home blood pressure readings in a European population study16 and 7 days in the Finn-Home study.6 Little information is currently available on how many days are required to capture blood pressure variability in a reliable way. However, sensitivity analyses making use of the home blood pressure over 5 days produced confirmatory results (Tables S3 and S4). Finally, the R2 statistic is not a perfect measure of the variation explained by Cox models. R2 values can be compared within but not across studies because of the dependence on censoring. Nevertheless, a measure of explained variance is crucial for the correct interpretation of the prognostic vale of a risk factor. P values of hazard ratios do not suffice to compare indicators of risk.
Perspectives
In the general population, blood pressure variability derived from home blood pressure does not substantially refine risk profiling over and beyond the blood pressure level. Being on antihypertensive drug treatment seems to be the main driver of the significant associations between cardiovascular mortality and blood pressure variability. Our findings also suggest, at variance with current guidelines,1,3 that blood pressure variability, although showing up for selected fatal end points, might be too weak a predictor to be clinically meaningful. In risk stratification, clinicians should concentrate on what really matters, that is, blood pressure level, the predominant modifiable risk factor, which is reversible by adequate medical treatment.
Supplementary Material
Novelty and Significance.
What Is New?
In multivariable Cox models, home morning variability independent of the mean and average real variability predicted total and cardiovascular mortality in all participants independent of home morning blood pressure level.
In untreated participants, these indices of variability did not predict cardiovascular mortality.
None of the variability indices predicted stroke incidence.
The R2 statistics showed the incremental risk explained by adding blood pressure variability to models already including systolic blood pressure and covariables ranged from <0.01% to 0.88%.
What Is Relevant?
In risk stratification, clinicians should concentrate on what really matters, that is, blood pressure level, the predominant modifiable risk factor, which is reversible by adequate medical treatment.
Summary
In a general population, new indices of blood pressure variability derived from home blood pressure did not incrementally predict outcome over and beyond the blood pressure level. Being on antihypertensive drug treatment seems to be the main driver of the significant associations between cardiovascular mortality and blood pressure variability.
Acknowledgments
We are grateful to the residents and staff members in Ohasama and staff members of the Hanamaki City Government, Ohasama Hospital, and Iwate Prefectural Stroke Registry for their valuable support on the Ohasama study project.
Sources of Funding
This work was supported by the Grants for Scientific Research (22590767, 22790556, 23249036, 23390171, 23790242, and 24390084) and a Health Labor Sciences Research Grant (H23-Junkankitou [Seishuu]-Ippan-005) from the Ministry of Health, Labor, and Welfare, Japan; the Japan Arteriosclerosis Prevention Fund; and the Grant from the Central Miso Research Institute (Tokyo, Japan). The European Union (HEALTH-2011.2.4.2-2-EU-MASCARA and ERC Advanced Researcher 2011-294713-EPLORE) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (Brussels, Belgium; G.0734.09) gave support to the Studies Coordinating Center in Leuven, Belgium. Omron Healthcare gave research support to K. Asayama, H. Metoki, and Y. Imai. K. Asayama received research support from Japan Research Foundation for Clinical Pharmacology. The funding sources had no role in study design, data extraction, data analysis, data interpretation, or writing of the report.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.111.00138/-/DC1.
References
- 1.Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, Kario K, Lurbe E, Manolis A, Mengden T, O’Brien E, Ohkubo T, Padfield P, Palatini P, Pickering T, et al. ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–1526. doi: 10.1097/HJH.0b013e328308da66. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, et al. The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 3.Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim-Mitsuyama S, Kimura G, Matsubara H, et al. Japanese Society of Hypertension Committee The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009) Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- 4.Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Kikuya M, Ito S, Satoh H, Hisamichi S. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16:971–975. doi: 10.1097/00004872-199816070-00010. [DOI] [PubMed] [Google Scholar]
- 5.Asayama K, Ohkubo T, Kikuya M, Obara T, Metoki H, Inoue R, Hara A, Hirose T, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. Prediction of stroke by home “morning” versus “evening” blood pressure values: the Ohasama study. Hypertension. 2006;48:737–743. doi: 10.1161/01.HYP.0000240332.01877.11. [DOI] [PubMed] [Google Scholar]
- 6.Niiranen TJ, Hänninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension. 2010;55:1346–1351. doi: 10.1161/HYPERTENSIONAHA.109.149336. [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 8.Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Factors affecting the variability of home-measured blood pressure and heart rate: the Finn-home study. J Hypertens. 2010;28:1836–1845. doi: 10.1097/HJH.0b013e32833b6c8a. [DOI] [PubMed] [Google Scholar]
- 9.Kato T, Kikuya M, Ohkubo T, Satoh M, Hara A, Obara T, Metoki H, Asayama K, Hirose T, Inoue R, Kanno A, Totsune K, Hoshi H, Satoh H, Imai Y. Factors associated with day-by-day variability of self-measured blood pressure at home: the Ohasama study. Am J Hypertens. 2010;23:980–986. doi: 10.1038/ajh.2010.94. [DOI] [PubMed] [Google Scholar]
- 10.Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045–1050. doi: 10.1161/HYPERTENSIONAHA.107.104620. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Poulter NR, Sever PS. ASCOT-BPLA and MRC Trial Investigators. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 12.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 13.Imai Y, Abe K, Sekino H. Evaluation of automatic devices for blood pressure measurement. In: Koshikawa S, nagasawa T, editors. Annual Review Nephrology. 1992. 1991:55-61. [Google Scholar]
- 14.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 15.Staessen J, Amery A, Fagard R. Isolated systolic hypertension in the elderly. J Hypertens. 1990;8:393–405. doi: 10.1097/00004872-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Kuznetsova T, Staessen JA, Kawecka-Jaszcz K, Babeanu S, Casiglia E, Filipovsky J, Nachev C, Nikitin Y, Peleskã J, O’Brien E. Quality control of the blood pressure phenotype in the European Project on Genes in Hypertension. Blood Press Monit. 2002;7:215–224. doi: 10.1097/00126097-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Asayama K, Ohkubo T, Metoki H, Obara T, Inoue R, Kikuya M, Thijs L, Staessen JA, Imai Y. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertens Res. 2012;35:1102–1110. doi: 10.1038/hr.2012.125. [DOI] [PubMed] [Google Scholar]
- 18.Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–511. doi: 10.1097/01.hjh.0000160205.81652.5a. [DOI] [PubMed] [Google Scholar]
- 19.Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, et al. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. doi: 10.1161/HYPERTENSIONAHA.109.140798. [DOI] [PubMed] [Google Scholar]
- 20.Gillespie B. Use of generalized R-squared in Cox regression. APHA Scientific Session and Event Listing. 2006 [Google Scholar]
- 21.Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home Study. Hypertension. 2012;59:212–218. doi: 10.1161/HYPERTENSIONAHA.111.178657. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TG, James M, Youde J, Panerai R, Potter J. Cardiac baroreceptor sensitivity is impaired after acute stroke. Stroke. 1997;28:1671–1676. doi: 10.1161/01.str.28.9.1671. [DOI] [PubMed] [Google Scholar]
- 23.McLaren A, Kerr S, Allan L, Steen IN, Ballard C, Allen J, Murray A, Kenny RA. Autonomic function is impaired in elderly stroke survivors. Stroke. 2005;36:1026–1030. doi: 10.1161/01.STR.0000160748.88374.ce. [DOI] [PubMed] [Google Scholar]
- 24.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 25.Redon J, Bilo G, Parati G, SURGE Steering Committee Home blood pressure control is low during the critical morning hours in patients with hypertension: the SURGE observational study. Fam Pract. 2012;29:421–426. doi: 10.1093/fampra/cmr121. [DOI] [PubMed] [Google Scholar]
- 26.Chonan K, Hashimoto J, Ohkubo T, Tsuji I, Nagai K, Kikuya M, Hozawa A, Matsubara M, Suzuki M, Fujiwara T, Araki T, Satoh H, Hisamichi S, Imai Y. Insufficient duration of action of antihypertensive drugs mediates high blood pressure in the morning in hypertensive population: the Ohasama study. Clin Exp Hypertens. 2002;24:261–275. doi: 10.1081/ceh-120004230. [DOI] [PubMed] [Google Scholar]
- 27.Imai Y, Abe K, Munakata M, Sasaki S, Minami N, Sakuma H, Hashimoto J, Watanabe N, Sakuma M, Sekino H. Effect of slow release nifedipine tablets in patients with essential hypertension. Arzneimittelforschung. 1992;42:1434–1438. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.