Abstract
The effective radiation dose of human from natural sources is about 2.4 mSv/y and the dose limit for radiation workers is 20 mSv/y. Ramsar, a city in Iran, has been the subject of concern in the last forty years for a high level of radiation measured in some spots as high as 260 mSv/y. Carcinogenesis is one of the most studied effects of radiation especially in high doses. Recent studies showed that the high level of natural radiation received by inhabitants of this area, paradoxically don’t have significant health effect. Natural killer (NK) cells and cytotoxic T cells are the most important cells in tumor immune surveillance and CD107a is a widely expressed intracellular protein located in the lysosomal/endosomal membrane. CD107a transiently located on the cell membrane can be used as a marker of CD8 + T cell degranulation following stimulation. It is also expressed, to a lower extent, on activated NK cells. In this study, 60 healthy people were selected randomly and their consent obtained and confounding factors such as sex, age, life-styles was matched then the count of activated NK and CD8 + cells was compared in high and normal background radiation areas inhabitants of Ramsar. After filling the questionnaire and measurement of background radiation, blood samples of 30 healthy people from each region were analyzed immediately by means of flowcytometry. The leukocytes and their subsets were not significantly different between two groups and the count of active cells was higher in control group. The result shows that the changes in immune system occur due to radiation and maybe it is as a result of higher radiosensitivity of activated cells.
Keywords: CD107a, cytotoxic T cell, high background radiation area, natural killer cell, Ramsar
Introduction
Treg (T) cells can be broadly classified as cytotoxic CD8 + T cells, which can directly kill an antigen-expressing cell or cytokine-secreting CD4 + T cells. The CD4 + T-cell (T helper) response can elicit both immune stimulatory or immune inhibitory effects. T helper type 1 (Th1) lymphocytes (LYM) secrete interleukin (IL)-2, interferon (INF)-γ, and lymphotoxin-a and stimulate type 1 immunity, which is characterized by intense phagocytic activity. But, Th2 cells secrete IL-4, IL-5, IL-9, IL-10, and IL-13 and stimulate type 2 immunity, which is characterized by high antibody titers. Type 1 and type 2 immunity are not strictly synonymous with cell-mediated and humoral immunity, because Th1 cells also stimulate moderate levels of antibody production, whereas Th2 cells actively suppress phagocytosis.[1] Specific CD4 + T-helper cell phenotypes are crucial for the expansion and persistence of tissue-destructive CD8 + T cells.[2] Natural killer (NK) cells are a subset that plays a central role in the innate immune response to tumors and infections.[3] NK cells are an important component of the innate immune responses they are able to lyse tumor cells or virally infected cells without prior antigen sensitization.[3] Cytotoxic T cells destroy virally infected cells and tumor cells, and are also implicated in transplant rejection. These cells are also known as CD8 + T cells since they express the CD8 glycoprotein at their surface. These cells recognize their targets by binding to antigen associated with major histocompatibility complex (MHC) class I, which is present on the surface of nearly every cell of the body. Through IL-10, adenosine and other molecules secreted by regulatory T cells, the CD8 + cells can be inactivated to an anergic state, which prevent autoimmune diseases such as experimental autoimmune encephalomyelitis.[4]
There are a few areas in the world that have high natural background radiation. Among these areas, Ramsar, a coastal city in Iran, has the highest dose level.[5,6] This high dose radiation is mainly from Radium-226 and its progenies coming from hot springs eventually spreading in the environment.[7] The effective dose received by the inhabitants of Ramsar, especially in Talesh Mahalleh, is even many times greater than the dose limit for radiation workers (260 mSv/y versus 20 mSv/y).[8–10] According to international commission on radiological protection recommendation, the maximum effective dose to public at large should be < 1 mSv/y.[8] Many studies about radiation doses and health indices of inhabitants of Ramsar are conducted. In the study of their health status and frequency of cancer by Monfared et al., they have reported that although the radiation doses received by these individuals is many times greater than residents of normal background radiation areas (NBRAs) and the cytogenetic studies showed that there is higher chromosomal aberration in the LYM of the residents of high background radiation area (HBRA) of Ramsar,[11] the frequency of cancer in these people, was not higher than other people living in NBRAs of Ramsar.[12–14] According to immune surveillance theory, one of the important roles of immune system is to identify and to kill tumor cells. CD8 + T cells and NK cells lyse tumor cells after activation.[15]
Lysosomal-associated membrane protein-1 (LAMP-1 or CD107a) has been described as a marker of CD8 + T cell degranulation following stimulation and Recently, Galit Alter et al., described CD107a as marker of NK cell functional activity using multi-parameter flowcytometry.[3] CD107a is significantly upregulated on the surface of NK cells following stimulation with MHC devoid targets. Additionally, CD107a expression correlates with both cytokine-secretion and NK cell-mediated lysis of target cells.[3]
In this study, we have counted activated CD8 + T cells and NK cells and our hypothesis was that HBRA residents exhibit a similar immunological change observed in acutely and heavily exposed people. The higher number of activated cells in HBRA residents could indicate that high level of radiation dose received by these individuals could induce tumor cells and activated NK and CD8 + cells, eradicate these tumors. Thus it can be as evidence for the relationship between activation of immune system and cancer prevention, and immunological implication in radiation carcinogenesis.
Materials and Methods
Thirty healthy residents of HBRA and 30 healthy residents of NBRA were selected randomly and a questionnaire were filled by interview. The age and sex was matched and pregnant women, recently operated, and individuals suffering from an illness or taking medications were excluded from the study.
The people who were living for at least 10 years in this area considered as study population. Radiation dose rates of their homes were measured by calibrated dosimeter (Gratez A, Germany). To estimate the annual effective dose that is the total dose resulting from exposures of ionizing radiation over a year (μSv/y), the dose rate (nSv/h) was multiplied by occupancy factor. The occupancy factor was the proportion of exposure time multiplied by 4380 (365 days × 24 h).
Two mililiter fresh peripheral blood of them was taken in sterile conditions. The samples carried to laboratory (Tooba Lab., Sari, Iran) in K3EDTA tubes and the cell count was performed automatic hematology analyzer (sysmex kx-21, Japan).
Twenty microliter of each sample was stained with 10 μL of CD107a-PE (Exbio, Czech Republic) and 10 μL CD45-PE (Partec, Germany) monoclonal antibody for 15 min in dark. Then we added buffer 1 and 2 according to catalog for detection of all CD45 + cells (Partec, Germany). This method is simplified volumetric flowcytometry that allows feasible and accurate determination of CD45 + cells that don’t need to lyse and washing buff er. Afterward, the samples were shaken and stimulated CD107a + cells were counted by flowcytometer (Partec Pas, Germany). This study was approved by the accredited Ethics Committee of Babol University of Medical sciences, Babol, Iran.
The statistical analysis of results was done by SPSS 16. The results of HBRA and NBRA inhabitants were compared by independent sample t test and P < 0.05 assumed significant.
Results
The radiation dose rate at HBRA was significantly more than NBRA [Table 1]. Annual effective doses of HBRA group were between 4511 and 57000 and its mean was 9770 μSv/y. On the other hand NBRA group received 570-3020 μSv/y and the mean of their effective dose was 1207 μSv/y.
Table 1.
Radiation dose rate of habitats of individuals (nSv/h), their ages (year) and the habitance duration of them (years), difference between doses is significant

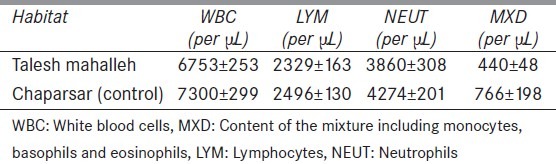
The difference in counts of white blood cells (WBC), content of the mixture (MXD), neutrophils and LYM between two groups was not significant [Table 2].
Table 2.
White blood cells, mixture, Neutrophils, lymphocytes counts (per μL) in two regions. None of the differences are statistically significant
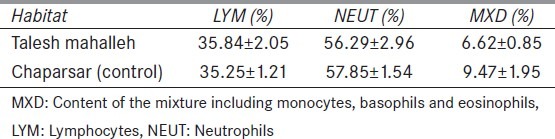
The difference between MXD, neutrophils and LYM percentages of two groups was not significant [Table 3].
Table 3.
The difference between mixture, neutrophils and lymphocytes percentages of two groups. None of the differences are statistically significant
The number of CD107a + cells in NBRA group was higher in comparison with HBRA inhabitants [Figure 1].
Figure 1.
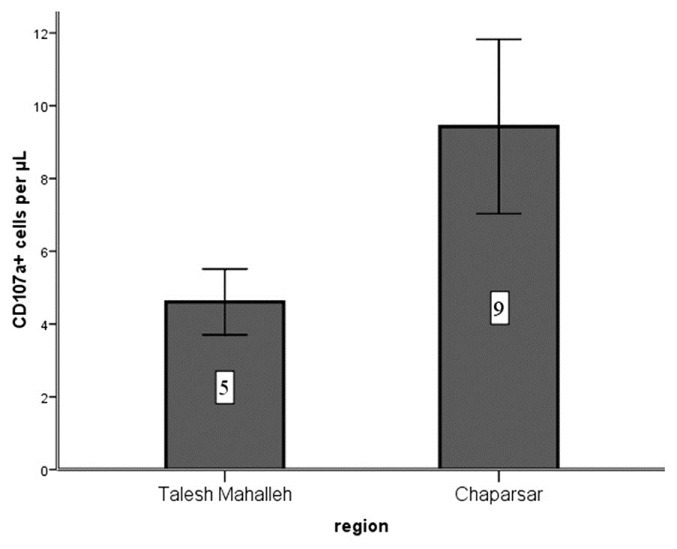
The number of CD107a+ cells per μL (P < 0.005)
Discussions
There are a lot of studies that have evaluated the radiation effects on the immune system of animals.[16,17] Studies showed that following a single dose of 100 and 200 mGy, increase in cytotoxic activity of NK cells is observable 24 until 72 h later.[18–21]
Fuggetta et al. have shown impaired NK activity in vitro after gamma irradiation (20 Gy).[22] INF-β (200 IU/mL) was able to completely reverse this inhibitory radiation induced effect, but was not able to modify the number of CD16 + and CD56 + cells that died by apoptosis after irradiation. Concerning in vivo radiation exposure, no changes in NK cell activity were found in 1341 atomic bombing survivors residing in Hiroshima.[23] Koike et al. evaluated the immune status in children with goiter living in highly contaminated area of Gomel. They found increased serum levels of Immunoglobulin G (IgG), IgM and IgE and depressed NK cell activity.[24] Although IFN-γ and IL-2 enhanced the cytotoxicity of these NK cells, the response to IFN-γ was still below control values.
Attar et al. have studied the immune responses to infection and inflammation of the exposed residents of Ramsar.[25] Their results showed that the total serum antioxidant level in the exposed people was significantly lower than control and the exposed individuals also had higher LYM-induced IL-4 and IL-10 production, and lower IL-2 and IFN-γ production. In addition, neutrophil Nitroblue Tetrazolium, phagocytosis, and locomotion were higher in the exposed group. In contrast, LYM proliferation in response to phytohemagglutinin was unaffected. The authors conclude that the immune system of individuals exposed to high dose ionizing radiation has adapted to its environment by shifting from a Type 1 to a Type 2 response to promote anti-inflammation. This may be because inflammatory Type 1 responses generate more free radicals than Type 2 responses, in addition to the free radicals generated as a result of high environmental radiation. Thus, the serum total antioxidant level in the exposed residents was lower than the unexposed group.
Zakeri and Kariminia have evaluated hormone levels associated with immune responses of inhabitants in HBRAs of Ramsar and their study demonstrated a decreased level of dehydroepiandrosterone (DHEA) and increased level of cortisol in the elderly HBRA group which might be possibly associated with higher cumulative doses. There was a higher incidence of hypothyroidism in the HBRA group, mostly in elder group. A significant increase of CD69 expression on CD4 + T LYM was found in the young HBRA group. Low levels of DHEA in the elder group were correlated to their increased IgE levels.[26]
Former study reported a significant increase in CD4+% and CD8+% in HBRA group. But the absolute counts of CD4 + and CD8 + and CD4+/CD8 + ratio was not significantly different between HBRA and NBRA residents of Ramsar.[27]
Mechanisms for putative tumor suppression or other mechanisms defending against tumor induction and development include the DNA repair, apoptosis, terminal differentiation, phenotypic suppression and radioprotectors should be considered. Altogether, these mechanisms will reduce the probability that a specifically damaged target cell will progress to frank malignancy.[28]
We recommend the measurements of the proportions or counts of the other T-cell subsets including T helper 1 (Th1), Th2, Th17 and regulatory T cells to determine other aspects of the immune involvement. The ratios of Th1/Th2 and Th17/Tregs that could reflect altered states of immune responses against foreign antigens or tumor antigens and the proportions or counts of peripheral leucocytes including monocytes and polymorph nuclear leucocytes, which also modify or even suppress antitumor immunity also, are important.
Conclusion
Our results showed that although the effective radiation doses received by inhabitants of HBRA of Ramsar were significantly higher than normal, the count of CD107a + cells is lower than NBRA inhabitants. This maybe as the result of higher radiosensitivity of active cells and shows that these cells are present and immune surveillance are active there. Other measured parameters, WBC and MXD, neutrophil, LYM counts and percentages were not significantly different between two groups.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Spellberg B, Edwards JE., Jr Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 2.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373:673–83. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114:1198–208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation.Annex B: Exposures from natural radiation sources. UNSCEAR REPORT. 2000;1:83–156. [Google Scholar]
- 6.Ghiassi-nejad M, Mortazavi SM, Cameron JR, Niroomand-rad A, Karam PA. Very high background radiation areas of Ramsar, Iran: Preliminary biological studies. Health Phys. 2002;82:87–93. doi: 10.1097/00004032-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Mortazavi SM. Ramsar Hot springs: How safe is to live in an environment with high level of natural radiation. Nippon Genshiryoku Kenkyujo JAERI. Conf 2005:194–204. [Google Scholar]
- 8.ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103, Ann ICRP 37 (2-4) 2007 doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Mortazavi SMJ, Ghiassi-nejad M, Niroomand-rad A, Karam PA, Cameron JR. How should governments address high levels of natural radiation and radon? Lessons from the Chernobyl nuclear accident. Risk. 2002;13:31–45. [Google Scholar]
- 10.Karam PA, Mortazavi SMJ, Ghiassi-nejad M, Ikushima T, Cameron JR, Niroomand-rad A. ICRP evolutionary recommendations and the reluctance of the members of the public to carry out remedial work against radon in some high-level natural radiation areas. Int Congr. 2002;1236:35–7. [Google Scholar]
- 11.Mortazavi SMJ, Ghiassi-Nejad M, Ikushima T, Assaie R, Heidary A, Varzegar A, et al. Are the inhabitants of high background radiation areas of Ramsar more radioresistant? Scope of the Problem and the Need for Future Studies. Iran J Radiol. 2003;(1,2):37–44. [Google Scholar]
- 12.Monfared AS, Jalali F, Sedaghat S, Mansoorizade E, Jarrahi A, Hajiahmadi M, et al. Natural background radiation areas in Ramsar-Iran.Can inhabitants feel safe? Int J Low Radiat. 2006;3:171–7. [Google Scholar]
- 13.Mortazavi SMJ, Ghiassi-Nejad M, Karam PA, Ikushima A, Niroomand-rad A, Cameron JR. Cancer incidence in areas with elevated levels of natural radiation. Int J Low Radiat. 2006;2:20–7. [Google Scholar]
- 14.Monfared AS, Hajian K, Hosseini R, Nasir A. Association between Local External Gamma Rays and Frequency of Cancer in Babol-Iran. Dose Response. 2010;8:368–77. doi: 10.2203/dose-response.09-011.Monfared. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas AK, Lichtman AH, Pillai S. Cellular And Molecular Immunology. 6th ed. Philadelphia: Saunders Elsevier; 2007. Introduction to the Immune System; pp. 3–74. [Google Scholar]
- 16.Cheda A, Wrembel-Wargocka J, Lisiak E, Nowosielska EM, Marciniak M, Janiak MK. Single low doses of X rays inhibit the development of experimental tumor metastases and trigger the activities of NK cells in mice. Radiat Res. 2004;161:335–40. doi: 10.1667/rr3123. [DOI] [PubMed] [Google Scholar]
- 17.Nowosielska EM, Wrembel-Wargocka J, Cheda A, Lisiak E, Janiak MK. Low-level exposures to ionising radiation modulate the anti-tumour activity of murine NK cells. Nukleonika. 2005;50:S21–4. [Google Scholar]
- 18.Liu SZ, Han ZB, Liu WH. Changes in lymphocyte reactivity to modulatory factors following low dose ionizing radiation. Biomed Environ Sci. 1994;7:130–5. [PubMed] [Google Scholar]
- 19.Liu XD, Ma SM, Liu SZ. Effects of 0.075 Gy x-ray irradiation on the expression of IL-10 and IL-12 in mice. Phys Med Biol. 2003;48:2041–9. doi: 10.1088/0031-9155/48/13/315. [DOI] [PubMed] [Google Scholar]
- 20.Liu SZ, Jin SZ, Liu XD, Sun YM. Role of CD28/B7 costimulation and IL-12/IL-10 interaction in the radiation-induced immune changes. BMC Immunol. 2001;2:8. doi: 10.1186/1471-2172-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju GZ, Liu SZ, Li XY, Liu WH, Fu HQ. Effect of high versus low dose radiation on the immune system. In: Hagen U, Harder D, Jung H, Streffer C, editors. Radiation Research 1895-1995, Proc of the Tenth Int Congress of Radiation Research. Wilrzburg, Germany: 1995. pp. 709–14. [Google Scholar]
- 22.Fuggetta MP, Tricarico M, Starace G, Pepponi R, Bonmassar E. Interferons antagonize gamma-ray-induced depression of natural immunity. Int J Radiat Oncol Biol Phys. 1998;40:953–60. doi: 10.1016/s0360-3016(97)00908-5. [DOI] [PubMed] [Google Scholar]
- 23.Bloom ET, Akiyama M, Korn EL, Kusunoki Y, Makinodan T. Immunological responses of aging Japanese A-bomb survivors. Radiat Res. 1988;116:343–55. [PubMed] [Google Scholar]
- 24.Koike K, Yabuhara A, Yang FC, Shiohara M, Sawai N, Sugenoya A, et al. Frequent natural killer cell abnormality in children in an area highly contaminated by the Chernobyl accident. Int J Hematol. 1995;61:139–45. doi: 10.1016/0925-5710(95)00353-t. [DOI] [PubMed] [Google Scholar]
- 25.Attar M, Molaie Kondolousy Y, Khansari N. Effect of high dose natural ionizing radiation on the immune system of the exposed residents of Ramsar Town, Iran. Iran J Allergy Asthma Immunol. 2007;6:73–8. [PubMed] [Google Scholar]
- 26.Zakeri F, Kariminia A. Hormone levels associated with immune responses among inhabitants in HBRAs of Ramsar-Iran. Int Congr. 2005;1276:199–200. [Google Scholar]
- 27.Borzoueisileh S, Monfared AS, Abediankenari S, Mostafazadeh A, Khosravifarsani M, Amiri M, et al. The comparison of CD4/CD8 ratio among high and normal background radiation areas in Ramsar-Iran. Int J Low Radiat. 2011;8:329–37. [Google Scholar]
- 28.Biological effects of low doses of ionizing radiation: A fuller picture. Vienna: IAEA Bulletin; 1994. International Atomic Energy Agency; pp. 37–45. [Google Scholar]


