Abstract
Background
Selection of patients with renal artery stenosis (RAS) likely to improve glomerular filtration rate (GFR) after percutaneous transluminal renal angioplasty (PTRA) is difficult. We examined basal hemodynamic and inflammatory factors linked to improved stenotic kidney (STK) function after PTRA in swine RAS.
Methods and Results
Fifteen pigs after 6 weeks of hemodynamically-significant RAS were studied before and 4 weeks after technically-successful PTRA+stenting. STK and contralateral kidney (CLK) hemodynamics and function were evaluated by multi-detector computed-tomography before and after acetylcholine challenge. Single-kidney deoxyhemoglobin (R2*, reciprocal to blood relaxation) and energy-dependent tubular function were assessed using blood-oxygen-level-dependent magnetic resonance imaging (BOLD-MRI) before and after furosemide. Baseline renal vein (RV) and inferior vena cava (IVC) levels of inflammatory markers were measured, and their gradient and net release calculated. Baseline parameters were compared to normal (n=7) and sham RAS (n=7) pigs, and correlated with the change in STK-GFR after revascularization (ΔGFR). Four weeks after PTRA blood pressure was normalized in all animals, but STK-GFR improved in 10/15 (ΔGFR=+22.0±8.5mL/min). ΔGFR correlated inversely with basal STK-GFR, renal release of inflammatory markers, and medullary R2* response to furosemide, but directly with GFR response to acetylcholine. Basal CLK-GFR directly correlated with ΔGFR.
Conclusions
Low basal STK-GFR with preserved response to acetylcholine may predict benefit from revascularization in RAS, while renal inflammation and robust STK-R2* responses to furosemide (possibly reflecting avid tubular oxygen consumption) are associated with less favorable outcomes. These tools may be useful for identification of patients likely to improve renal function after revascularization.
Keywords: renal hypertension, hemodynamics, inflammation, cytokines
Renal artery stenosis (RAS), an important cause of secondary hypertension in the elderly population1, is frequently detected incidentally in patients with widespread peripheral artery disease or those undergoing coronary angiography2, 3. RAS may ultimately lead to end-stage renal disease, and represents a sizeable fraction of new patients entering dialysis programs in the United-States4. Furthermore, patients with RAS have increased risk of death for cardiovascular events including myocardial infarction, stroke, and heart failure5.
Renal revascularization by percutaneous transluminal renal angioplasty (PTRA) and stenting has been recommended commonly for patients with RAS, but recovery of glomerular filtration rate (GFR) after revascularization is inconsistent. Recent prospective randomized controlled trials comparing PTRA combined with medical therapy to medical therapy alone identified no differences in blood pressure and renal function at follow-up between the two treatment groups6, 7. This might reflect partly the difficulty in selecting patients whose renal function is likely to improve following revascularization. Considering the variable efficacy, cost, and potential complications of PTRA8, identification of good candidates for revascularization beforehand is critical.
Pre-treatment characteristics of the stenotic kidney (STK) likely determine its recovery potential better than the function of total renal mass, which the contralateral kidney (CLK) may mask. Renal flow and functional reserve (responses to vasoactive challenge) may provide indices of STK viability. The degree of histopathological STK tissue damage correlates with clinical outcomes in RAS patients9, and might be partially mediated by inflammation, which magnifies parenchymal damage in RAS10. Thus, assessment of post-stenotic kidney inflammation may constitute an important tool for evaluating RAS patients before vascular intervention.
The current study was therefore designed to test the hypothesis that STK inflammation and reduced functional reserve are associated with attenuated renal functional recovery after PTRA in a well-established swine model of RAS. For this purpose, we correlated basal hemodynamics, function, oxygenation, inflammation, and functional reserve in the STK and CLK with the change in STK-GFR after revascularization (ΔGFR).
Methods
Experimental design
Twenty-nine female domestic pigs (40–55 kg) were studied after approval of the institutional Animal Care and Use Committee. At baseline, we induced RAS in 22 animals, while 7 others (normal) underwent a sham procedure. Six weeks later, single-kidney function, oxygenation, and inflammatory marker release were measured, followed immediately by either a sham procedure (7 normal and 7 sham-RAS), or PTRA+stenting (15 RAS+PTRA). Single-kidney function was then measured again 4 weeks later.
Induction of RAS
Pigs were anesthetized with intramuscular telazol (5 mg/kg) and xylazine (2 mg/kg), intubated, and anesthesia maintained with intravenous ketamine (0.2 mg/kg/min) and xylazine (0.03 mg/kg/min). The left femoral artery was catheterized, followed by a heparin bolus (5000U). Under fluoroscopic guidance, a 7mm balloon catheter wrapped with an irritant copper-coil was placed into the proximal-middle right renal artery of RAS pigs and inflated to high pressure. The balloon was then deflated and removed, leaving the coil embedded in the vessel wall, as previously shown11. Normal animals underwent a sham procedure. A telemetry transducer (Data Sciences International, Arden Hills, MN) was implanted in the femoral artery to continuously measure mean arterial pressure (MAP) until study completion. Animals were then allowed to recover.
Measurement of kidney oxygenation
Six weeks later, animals were similarly induced, and anesthesia maintained with inhaled 1–2% isoflurane. Blood-oxygen-level-dependent 3T magnetic resonance imaging (BOLD-MRI) scanning (Signa Echo Speed; GE Medical Systems, Milwaukee, WI) was performed in the normal, sham-RAS, and 9 of the 15 RAS+PTRA pigs, to assess renal oxygenation, as described12–15. For data analysis (4–6 slices/kidney), regions of interest (ROI) were manually traced in the cortex and medulla, and their average MR signal computed. The BOLD signal (relaxivity index R2*) was then measured, which is directly proportional to the local concentration of deoxyhemoglobin12. Following baseline acquisition, furosemide (0.05 ml/kg IV) was administered, flushed with saline, and BOLD measurements repeated 15 min later. The degree of change in R2* in response to furosemide was calculated. These pigs underwent sample collection, measurement of kidney function, and PTRA or sham 2–3 days later.
Sample collection
Prior to PTRA or sham, all pigs were again anesthetized, and under fluoroscopic guidance, catheters advanced into the STK and CLK renal vein (RV) to collect samples. Blood samples were also collected from the inferior vena cava (IVC) for measurement of plasma renin activity (PRA) and creatinine. Samples were centrifuged and plasma aliquots stored at −80°C until assay. IVC and RV levels of e-Selectin (Biotang, P4988), tumor necrosis-factor (TNF)-α (Invitorgen, Cat# KSC3011), interleukin (IL)-1β (R&D DY681), IL-10 (Invitrogen, Cat# KSC0101), IL-17 (Kingfisher Biotech, Cat# VS0260S-002), monocyte chemoattractant-protein (MCP-1), and interferon (IF)-γ (Kingfisher, VS0259S-002) were measured by ELISA. Then, given lack of arterio-venous gradient of inflammatory cytokines across the renal circulation16,17(implying comparable arterial and IVC levels), we estimated cytokine gradient (RV-IVC) and net renal release (gradient × renal blood flow (RBF)) for each measured analyte, as recently shown17. In addition, urine samples were collected and micro-albumin concentration quantified by ELISA (Bethyl Laboratories, Texas).
Measurement of kidney function
Single-kidney volume, regional perfusion, RBF, and GFR were then evaluated using multi-detector computerized-tomography (MDCT, SOMOTOM Definition-64; Siemens, Forcheim, Germany), and again after a 10-min suprarenal arterial infusion of acetylcholine (Ach, 5 mg/kg/min). Ach induces endothelium-dependent microvascular vasodilation and diuresis, thereby increasing RBF and GFR18. MDCT images were analyzed with Analyze™ (Biomedical Imaging Resource, Mayo Clinic, MN). Tissue time-attenuation curves obtained in ROI selected from the aorta, renal cortex, and medulla were fitted by curve-fitting algorithms to obtain measures of renal function19. Cortical and medullary volumes were calculated by planimetry, and RBF as the sum of the products of cortical and medullary perfusions and volumes. GFR was calculated from the cortical proximal-tubular curve20. The degree of stenosis was assessed as the decrease in renal arterial luminal diameter and area, at its most stenotic compared to a disease-free segment, in images acquired at 6mm slice thickness and 3mm overlap, reconstructed with BioF convolution kernel. The stenosis length was measured at high-magnification using a computer-caliper program.
Revascularization
Immediately after MDCT scanning, 15 RAS animals underwent PTRA+stenting using a balloon catheter mounted with a standard tantalum stent. The balloon was inflated to high pressure, and then deflated and removed, 11, 21, 22. Renal artery patency was confirmed with repeat angiography. The other animals underwent a sham procedure with selective renal angiography.
Four weeks after revascularization, the pigs were similarly anesthetized, and MDCT studies repeated. We have previously demonstrated the reproducibility of single-kidney assessment of renal hemodynamics and function using MDCT over 1-month intervals18.
Statistical analysis
Statistical analysis was performed using JMP software package version 8.0 (SAS Institute, Cary, NC). We used the Shapiro-Wilk test to evaluate deviation from normality. Comparisons within groups were performed using paired Student’s t-test and among groups using ANOVA with Bonferroni correction and unpaired t-test. For data without a Gaussian distribution, comparisons within and among the groups used non-parametric tests (Wilcoxon and Kruskal Wallis, respectively). P-values <0.05 were considered significant.
Response to revascularization was evaluated by the change in MAP (ΔMAP) and STK-GFR (ΔGFR). Then, RAS+PTRA animals were classified as having improved (STK-GFR increased by ≥1ml/min or by>15% versus baseline), deteriorated (STK-GFR decreased by ≥1ml/min or by>15%), or remained stable (STK-GFR changed by <1ml/ml and <15%) GFR after revascularization, as described23. A simple linear regression analysis was used to determine correlations between basal parameters and ΔGFR.
Results
Systemic baseline parameters and PTRA
Table 1 shows baseline parameters in normal, sham-RAS, and RAS+PTRA pigs with subsequent improved or deteriorated STK-GFR. Six weeks after induction, all RAS groups achieved similar hemodynamically significant decreases in the diameter and area of the stenotic renal artery (60–99%, p=0.94, ANOVA), and equivalent length suggested comparable stenosis morphology. Basal MAP and serum creatinine levels were similarly elevated in RAS pigs compared to normal, while urinary albumin and PRA were not different among the groups.
Table 1.
Baseline parameters (mean±SD) in normal, sham-treated pigs with unilateral renal artery stenosis (RAS), and RAS pigs that improved or deteriorated stenotic kidney glomerular filtration rate (STK-GFR) after revascularization.
| Normal | Sham RAS |
Improved STK-GFR |
Deteriorated STK-GFR |
|
|---|---|---|---|---|
| Body weight (Kg) | 46.4±3.6 | 49.1±4.0 | 43.7±5.4 | 47.4±3.0 |
| Decrease in lumen diameter (%) | 0 | 69.7±14.7* | 73.4±13.4* | 68.1±5.0* |
| Area of stenosis (mm2) | 0 | 89.8±10.2* | 90.8±15.0* | 89.9±11.1* |
| Length of stenosis (mm) | 0 | 6.4 ±1.8* | 6.6±2.0* | 6.5±1.8* |
| Systolic blood pressure (mmHg) | 113.0±8.2 | 144.6±24.2* | 146.5±20.2* | 156.2±4.5* |
| Diastolic blood pressure (mmHg) | 85.3±6.9 | 116.0±24.1* | 104.9±24.6* | 118.4±20.8* |
| Mean Arterial Pressure (mmHg) | 94.6±6.8 | 125.5±23.9* | 118.7±22.4* | 124.4±19.5* |
| Serum creatinine (mg/dl) | 1.2±0.1 | 1.8±0.3* | 1.8±0.2* | 1.9±0.4* |
| Urinary albumin (µg/ml) | 13.9±8.9 | 25.3±14.8 | 23.4±27.8 | 23.7±12.2 |
| PRA IVC (ng/ml/hr) | 0.26±0.09 | 0.22±0.21 | 0.33±0.49 | 0.25±0.20 |
| PRA STK (ng/ml/hr) | 0.27±0.10 | 0.24±0.11 | 0.22±0.17 | |
| PRA CLK (ng/ml/hr) | 0.20±0.08 | 0.27±0.32 | 0.13±0.18 |
SD: standard deviation; PRA: plasma renin activity; IVC: inferior vena cava; STK: stenotic kidney; CLK: contralateral kidney.
p<0.05 vs. Normal,
p<0.05 vs. Sham RAS,
p<0.05 vs. Improved STK-GFR (none observed for # and † comparisons).
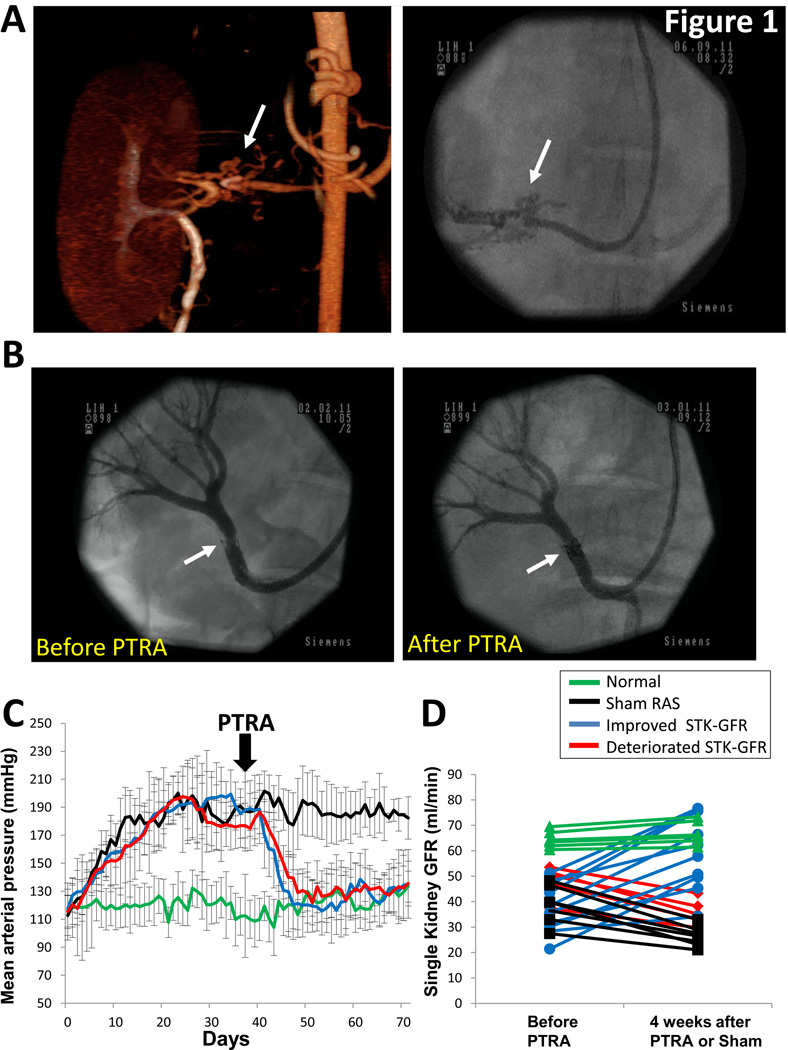
Subsequent PTRA was technically successful in all animals (0% stenosis 4 weeks later, Figure 1B). MAP remained elevated in sham-RAS (p<0.05 vs. normal), but decreased to baseline levels in PTRA-treated pigs (Figure 1C).
Figure 1.
3D computed-tomography image of the stenotic kidney (left) and renal angiography (right) showing peri-stenosis collateral formation (A, arrows), underscoring its chronic nature. Representative renal angiography from a pig with renal artery stenosis (RAS) showing successful restoration of renal artery patency after revascularization (B). Mean arterial pressure measured by telemetry similarly decreased in pigs with improved or deteriorated STK-GFR after percutaneous transluminal renal angioplasty (PTRA) (C). Single-kidney glomerular filtration rate (GFR) at baseline and 4 weeks after revascularization or sham (D).
Baseline STK-GFR was lower in sham-RAS (38.7±7.3ml/min) and RAS+PTRA (51.6±16.7ml/min) compared to normal. Ten of 15 pigs improved STK-GFR after PTRA (ΔGFR=+22.0±8.5ml/min, p<0.0001 vs. baseline), while in the other 5 GFR deteriorated (ΔGFR=−12.1±3.4ml/min, p=0.001 vs. baseline) (Figure 1D); none sustained stable STK-GFR. STK-GFR decreased in sham RAS pigs (ΔGFR=−12.4±4.3, p<0.05 vs. normal). No significant change in single-kidney GFR was observed in sham normal animals (ΔGFR=+2.6±1.3ml/min, p=0.36).
Renal function
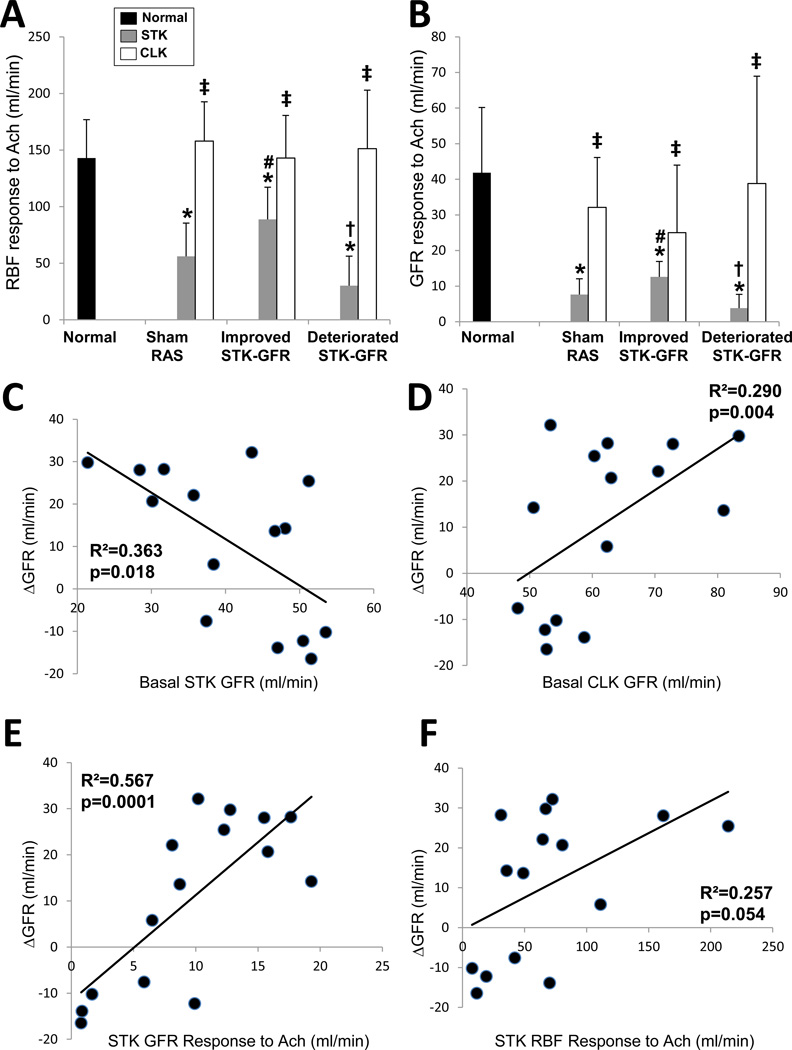
Basal STK cortical volume, perfusion, and RBF were lower in RAS compared to normal, but not different among RAS groups (Table 2). They were also lower than their own CLK, except for cortical perfusion in pigs with deteriorated STK-GFR after PTRA. Notably, basal STK-GFR was lower in sham-RAS and pigs in which STK-GFR subsequently improved compared to those with subsequently deteriorated STK-GFR. Yet, those with subsequently improved STK-GFR showed more robust RBF and GFR responses to Ach compared to Sham-RAS and to those with subsequently deteriorated STK-GFR (Figure 2A–B). Contrarily, CLK-GFR was reduced in pigs with deteriorated STK-GFR compared to the other two RAS groups, but neither CLK-GFR nor RBF responses to Ach were different among the groups. Notably, Bonferroni–adjusted basal STK-RBF, STK-GFR, and their responses to Ach remained significantly different among these groups (all p<0.0001).
Table 2.
Single-kidney baseline renal functional characteristics and release of inflammatory mediators (mean±SD) in pigs with unilateral renal artery stenosis (RAS) before percutaneous transluminal renal angioplasty (PTRA) or sham.
| Normal | Sham RAS |
Improved STK-GFR |
Deteriorated STK-GFR |
||||
|---|---|---|---|---|---|---|---|
| STK | CLK | STK | CLK | STK | CLK | ||
| Renal function | |||||||
| Cortical Volume (cc) | 91.4±8.6 | 48.4±13.8* | 93.4±3.7‡ | 48.7±8.2* | 91.0±12.3‡ | 50.5±12.4* | 90.9±8.9‡ |
| Medullary Volume (cc) | 18.0±7.1 | 21.1±3.4 | 19.9±4.2 | 22.3±6.3 | 19.2±3.8 | 19.9±8.3 | 18.6±3.3 |
| Cortical Perfusion (ml/min/cc tissue) | 4.4±1.2 | 3.2±0.7* | 4.4±0.8‡ | 3.4±0.4* | 4.4±1.3‡ | 3.2±0.7* | 4.1±1.7 |
| Medullary Perfusion (ml/min/cc tissue) | 3.5±1.4 | 2.4±0.5 | 2.7±0.5 | 2.4±1.5 | 3.6±1.6 | 3.3±1.3 | 4.8±2.5 |
| § RBF (ml/min) | 595.9±125.3 | 370.8±34.7* | 651.4±86.4‡ | 335.5±133.2* | 673.9±56.8‡ | 357.3±73.6* | 609.3±88.6‡ |
| § GFR (ml/min) | 64.2±5.8 | 38.7±7.3* | 63.8±2.4‡ | 37.5±9.7* | 66.0±10.8‡ | 47.9±6.3*#† | 53.2±3.8*#†‡ |
| Renal oxygenation (BOLD-MRI) | |||||||
| Cortical R2* (1/s) | 16.4±1.1 | 17.1±1.5 | 17.8±2.3 | 17.4±2.3 | 17.9±0.9 | 16.7±0.6 | 17.7±0.9 |
| Medullary R2* (1/s) | 20.1±1.7 | 27.2±2.4* | 22.7±4.9‡ | 30.0±8.6* | 23.0±2.8‡ | 26.7±7.3* | 22.1±1.0‡ |
| Inflammatory markers: net release | |||||||
| § e-Selectin (µg/ml/min) | −7.5±4.4 | 6.7±3.5* | −3.9±1.0‡ | 2.5±3.9*# | −11.2±4.9#‡ | 9.0±2.1*† | −0.2±8.2†‡ |
| § TNF-α (µg/ml/min) | −4.6±2.0 | 15.5±5.0* | −9.3±9.2‡ | 8.3±13.4*# | −6.7±9.4‡ | 31.4±15.*† | −5.3±3.7‡ |
| § MCP-1 (µg/ml/min) | −12.5±9.4 | 34.5±11.0* | −3.3±3.4‡ | 8.3±6.9*# | −18.6±10.9#‡ | 37.9±25.3*† | −2.8±10.3†‡ |
| IL-1β (µg/ml/min) | −1.7±0.8 | 2.2±1.0* | −4.6±2.4‡ | 3.4±2.8* | −1.8±7.2‡ | 4.6±2.2* | −3.8±5.7‡ |
| IL-17 (µg/ml/min) | −0.8±0.9 | 0.7±1.8* | −0.5±0.8‡ | 0.4±0.4* | −0.2±0.1‡ | 0.5±0.4* | −0.3±0.1‡ |
| IF-γ (pg/ml/min) | −5.1±7.5 | 3.8±7.1* | −3.9±3.5‡ | 1.3±1.6* | −3.0±1.7‡ | 3.1±4.4* | −3.8±2.3‡ |
| IL-10 (µg/ml/min) | 0.7±0.5 | −0.9±0.8* | 0.3±1.0‡ | −0.3±0.4*# | 0.4±0.8‡ | −0.8±0.09*† | 0.3±0.2‡ |
SD: standard deviation, STK: stenotic kidney, CLK: contralateral kidney, BOLD-MRI: blood-oxygen-level-dependent magnetic resonance imaging, TNF: tumor necrosis factor, MCP: monocyte-chemoattractant protein, IL: interleukin, IF: interferon, RBF: renal blood flow, GFR: glomerular filtration rate. Raw p-values:
p<0.05 vs. Normal,
p<0.05 vs. improved (between groups),
p<0.05 vs. Sham RAS,
p<0.05 vs. STK (within groups).
p<0.003 (one-way ANOVA Bonferroni-adjusted).
Figure 2.
A: Renal blood flow (RBF, A) and glomerular filtration rate (GFR, B) responses to acetylcholine (Ach). Basal stenotic-kidney (STK)-GFR inversely correlated with ΔGFR (C), while basal contralateral-kidney (CLK)-GFR directly correlated with ΔGFR (D). RBF and GFR responses to Ach directly correlated with ΔGFR (E–F). The height of the bar represents the mean and the extension-line the standard deviation. Raw p-values: *p<0.05 vs. Normal, †p<0.05 vs. improved STK-GFR, #p<0.05 vs. Sham RAS, ‡p<0.05 vs. STK (within groups).
Correlations between baseline renal function and ΔGFR
ΔGFR correlated inversely with basal STK-GFR (Figure 2C), but directly with basal CLK-GFR (Figure 2D), STK-RBF and GFR responses to Ach (Figure 2E–F). No correlations were found between ΔGFR and either CLK-RBF or CLK-GFR responses to Ach (both, p>0.05).
Renal oxygenation and response to furosemide
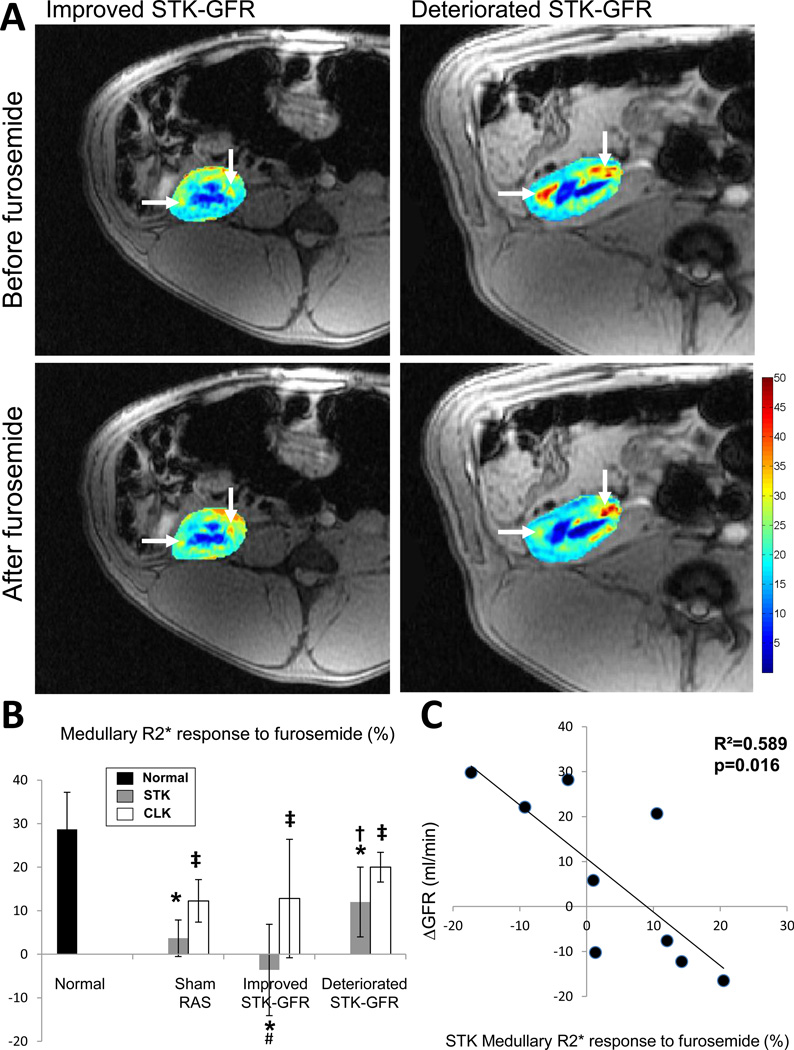
STK and CLK cortical R2* values were similar in all groups. Medullary R2* values were higher in all STK compared to normal and CLK, suggesting decreased oxygenation, but did not differ among RAS groups (Table 2). Basal STK medullary R2* responses to furosemide were reduced in STK compared to normal and CLK, but were more attenuated in pigs with subsequent improved STK-GFR compared to sham RAS and to pigs with deteriorated STK-GFR after revascularization (p=0.03, Figure 3A–B). CLK medullary R2* values and their responses to furosemide were comparable between RAS groups. Furthermore, basal STK (but not CLK) medullary R2* response to furosemide inversely correlated with ΔGFR (Figure 3C).
Figure 3.
Representative BOLD-MRI images (reflecting the level of deoxyhemoglobin) from pigs with improved (left) or deteriorated (right) STK-GFR before (top) and after (bottom) intravenous furosemide. Hypoxic regions (red) decreased after furosemide in pigs with deteriorated, but remained unchanged in pigs that improved STK-GFR after revascularization. B: Medullary R2* response to furosemide. C: Basal stenotic kidney medullary R2* response to furosemide inversely correlated with ΔGFR (n=9). The height of the bar represents the mean and the extension-line the standard deviation. Raw p-values: *p<0.05 vs. Normal, †p<0.05 vs. improved STK-GFR, #p<0.05 vs. Sham RAS, ‡p<0.05 vs. STK (within groups).
Renal inflammation
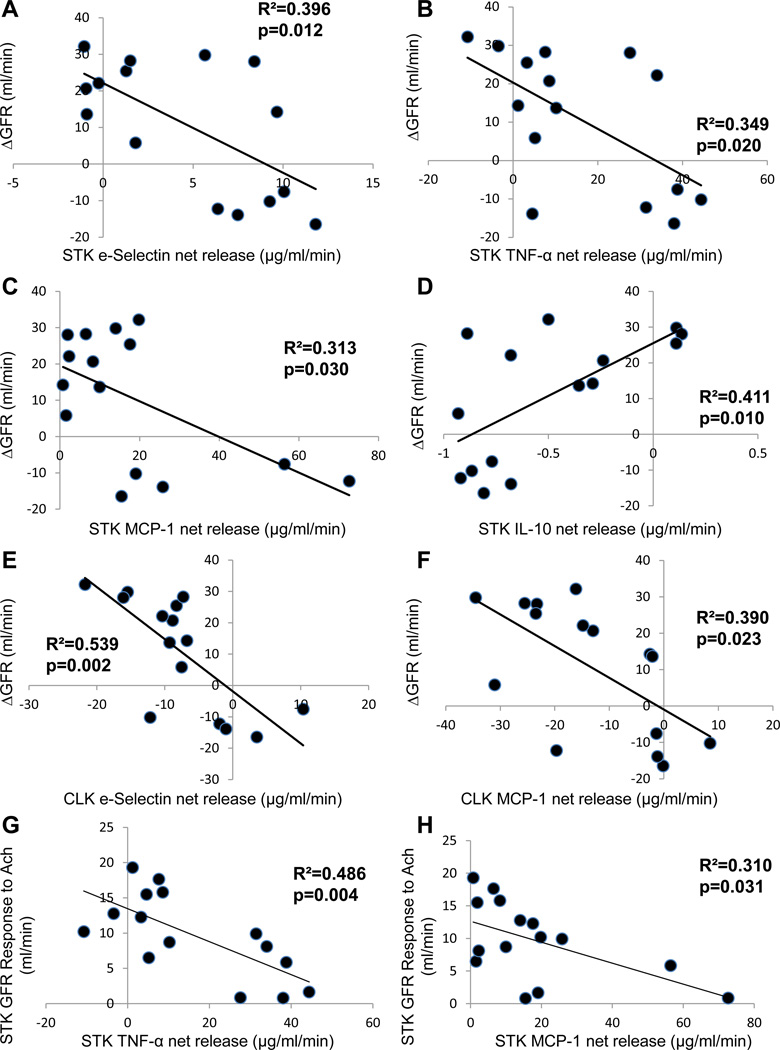
STK net release of e-Selectin, TNF-α, MCP-1, IL-1 β, IL-17, and IF-γ were elevated, while release of the anti-inflammatory IL-10 was reduced in all RAS groups compared with normal and CLK (Table 2). Moreover, STK release of e-Selectin, TNF-α, and MCP-1 were lower and IL-10 higher in pigs with improved STK-GFR compared to sham-RAS and deteriorating RAS. CLK release of only e-Selectin and MCP-1 was higher in pigs with deteriorated STK-GFR after revascularization compared to those that improved. Bonfferoni-adjusted STK-net release of e-Selectin, TNF-α, and MCP-1 remained highly significant (all p<0.0001).
ΔGFR correlated inversely with basal STK release of e-Selectin, TNF-α, and MCP-1 (Figure 4A–C), directly with STK IL-10 release (Figure 4D), and inversely with CLK release of e-Selectin and MCP-1 (Figure 4E–F). Furthermore, STK-GFR responses to Ach inversely correlated with STK net release of TNF-α and MCP-1 (Figure 4G–H). However, no correlation was found between either STK or CLK release of IL-1β, IL-17 or IF-γ and ΔGFR.
Figure 4.
ΔGFR inversely correlated with stenotic kidney (STK) net release of the inflammatory biomarkers e-Selectin, tumor necrosis factor (TNF)-α, and monocyte-chemoattractant protein (MCP)-1 (A–C), but directly correlated with release of the anti-inflammatory IL-10 (D). Similarly, contralateral kidney (CLK) release of e-Selectin and MCP-1 inversely correlated with ΔGFR (E–F). STK release of TNF-α, and MCP-1inversely correlated with GFR responses to acetylcholine (Ach, G–H).
Finally, ΔMAP did not correlate with the stenosis characteristics, serum creatinine levels, or with STK or CLK basal hemodynamics, oxygenation, or inflammatory marker release.
Discussion
This study suggests that preserved endothelial function associated with lower basal STK-GFR may be linked to preserved recovery capacity of renal function after revascularization in RAS. Furthermore, lower release of inflammatory markers from both kidneys is associated with more favorable functional outcomes after PTRA. Contrarily, robust STK BOLD-MRI responses to furosemide (possibly reflecting ongoing tubular oxygen consumption) are associated with blunted improvement of renal function.
RAS remains an important cause of chronic renal disease for which therapeutic strategies remain controversial. In the current study, four weeks after revascularization blood pressure was restored to normal levels in all animals, whereas renal function was improved in only two thirds, underscoring the greater efficacy of PTRA to mitigate renovascular hypertension than STK dysfunction, as we previously showed11, 21. This variability of renal response to PTRA in our otherwise homogeneous group of animals extends previous clinical observations that renal outcomes after revascularization differ between RAS patients with similar demographics and degree of stenosis24–26. Similar heterogeneous adaptive reactions occur in pigs in development of collateral coronary circulation after experimental myocardial infarction27.
Because revascularization often shows little additive benefit over medical therapy for improvement in renal function for entire groups of patients6, 7, identification of basal parameters to predict which specific patients would respond to PTRA could be invaluable. Consequently, several clinical studies have sought predictive factors that could differentiate PTRA “responders” from “non-responders”. For instance, resistance index values <80 by Doppler ultrasound were associated with greater improvement in renal function after revascularization26. In our study, morphological characteristics of the stenotic lesion (length, decrease in luminal diameter or area) did not distinguish pigs with different PTRA response, consistent with previous reports that parenchymal injury predicts PTRA success better than the degree of proximal narrowing28. Indeed, selection of patients for revascularization based on angiographic parameters primarily does not guarantee improvement in renal function6, 7, 29–31. A low32 or rapidly-declining GFR33 before revascularization are associated with favorable renal outcomes in RAS patients. Alas, a recent meta-regression analysis of prospective studies could not identify consistent clinical predictors of favorable renal outcome after revascularization34.
Because rapid deterioration in renal function33 predicts favorable outcomes after PTRA, progressive parenchymal damage may reflect the hemodynamic significance of the stenosis. Our findings extend previous observations, and emphasize that while lower basal GFR is associated with a measurable improvement after revascularization, basal RBF and GFR reserve to Ach in these kidneys needs to be preserved. STK-RBF, STK-GFR, and their responses to Ach emerged as dominant hemodynamic determinants of ΔGFR. In agreement, attenuated endothelium-dependent vasodilation blunts improvement following percutaneous coronary interventions35. In our study, medullary volume and perfusion did not correlate with renal response to PTRA, possibly due to compensatory mechanisms that ensure their preservation in chronic renovascular disease36. Interestingly, STK-GFR, but not serum creatinine levels, negatively correlated with response to PTRA, implying greater sensitivity of selective STK assessment to ultimate recovery. This is likely secondary to the compensatory effect of the CLK, which was supported by a modest direct correlation between basal CLK-GFR and ΔGFR.
BOLD-MRI allows assessment of renal oxygen content in the cortex and medulla. Furthermore, their responses to furosemide offer the opportunity to assess the functional tubular mass, as blunted changes in medullary R2* imply reduced prevailing oxygen consumption related to tubular sodium transport. We observed that basal intra-renal oxygenation (R2*) did not predict response to PTRA. Similarly, basal R2* was similar in human RAS kidneys that improved after PTRA and those that did not, although their ratio with STK-GFR better-predicted renal functional outcome, possibly because STK-GFR was somewhat lower in subjects with subsequently improved STK-GFR23. Indeed, the combination of R2* and STK-GFR might be a powerful index of renal recovery potential. Interestingly, we found that blunted response to furosemide was associated with better renal function after intervention, as were robust responses to Ach. In some respects, the ability to vasodilate in response to acetylcholine appears contradictory to the reduced tubular furosemide response. This ostensible discrepancy between the responses to vascular and tubular functional challenges perhaps attributable to the decreased basal STK-GFR in pigs that subsequently improved after PTRA, which in turn decreased the basal filtrate volume and response to furosemide. This post-stenotic “hibernating” kidney37 decreases its workload and oxygen consumption38, thus adequate recruitment of this protective mechanism may portend better outcomes. Therefore, while blunted responses to Ach (endothelial-dysfunction) reflect microvascular injury and are associated with little benefit from revascularization, decreased medullary R2* responses to furosemide might imply decreased tubular oxygen consumption that preserved tubular viability.
Recent data indicate that renal inflammation is associated with deleterious processes in the stenotic kidney, like fibrosis and microvascular damage17, 39–41. Indeed, our study illustrates that higher basal renal release of inflammatory markers was associated with poorer PTRA outcomes in RAS. STK-net release of e-Selectin, TNF-α, and MCP-1 as appear to be key determinants of improvement in GFR after revascularization. These cytokines, possibly secreted by inflammatory cells, may also impair endothelial functional reserve in the STK and its response to revascularization. In line with this postulation, we have shown that endothelial function improved in renovascular hypertensive pigs treated with an MCP-1 inhibitor10. Furthermore, IL-6 and TNF-α levels in RAS patients are elevated shortly after PTRA, but decrease 1 month later42. Importantly, our study showed an inverse relationship between release of pro-inflammatory markers and renal functional recovery, as well as with basal GFR responses to Ach. Therefore, inflammation may impair response to revascularization by ameliorating STK endothelial function and contribute to systemic inflammation by releasing inflammatory markers.
Importantly, both basal levels and responses to Ach of STK-RBF and STK-GFR, as well as STK-net release of e-Selectin, TNF-α, and MCP-1, remained significant predictors of ΔGFR upon Bonferroni adjustment, underscoring their predominant role as chief determinants of response to revascularization in swine ARAS.
Interestingly, CLK release of the inflammatory markers e-Selectin and MCP-1 also inversely correlated with ΔGFR. These findings extend previous studies in experimental43 and clinical44 RAS showing CLK inflammation and fibrosis. Furthermore, unilateral CLK nephrectomy is associated with post-atrophic regeneration of the STK in experimental RAS45, underscoring the contribution of the non-stenotic kidney to the pathophysiology of RAS.
Our study is limited by the short duration of the disease, relatively small group of young animals, and lack of comorbidities like atherosclerosis, essential hypertension, or diabetes that might modify parenchymal injury and revascularization outcomes. Hence, PTRA was more successful in restoring blood pressure control in our pigs than typically achieved in humans. Moreover, the majority of RAS patients are treated with statins and/or blockers of the renin-angiotensin-system, which may modulate both blood pressure and renal response to PTRA. The absence of correlation between urinary albumin and ΔGFR in our study may be related to the relatively early stage of the disease. Also, we cannot rule out the possibility that some aspects of the stenoses that were not fully captured by our measurements contributed to differences between the groups. In addition, BOLD-MRI data were available only in 9 of the revascularized RAS animals. The statistical relationship between basal STK-GFR and its change in response to PTRA (ΔGFR) should be interpreted with caution, because it might reflect mathematical artifacts46. Although these limitations may affect the translational potential of our observations, our study represents an important first step in identification of parameters to predict renal response to PTRA in human RAS. The extent of renal tissue injury and functional decline in our model are similar to human kidneys47, 48. Furthermore, our assessment of single-kidney hemodynamics and function is clinically applicable47, and provides a unique opportunity to evaluate parameters that may help predict response to PTRA in RAS. Future studies need to confirm these results in humans.
In conclusion, lower basal STK-GFR, “tubular hibernation” (decreased STK tubular oxygen consumption) reflected by diminished response to furosemide, and preserved endothelial functional reserve to acetylcholine may predict recovery of GFR after revascularization in RAS. Conversely, elevated release of inflammatory markers from both kidneys is associated with attenuated renal functional recovery after PTRA. These tools may be potentially clinically applicable for identification of patients likely to improve renal function after revascularization.
Bulleted Points.
What is Known?
Renal revascularization often shows little additive benefit over medical therapy for improvement in renal function in patients with renal artery stenosis
Identification of basal parameters to predict which specific patients would respond to revascularization could be invaluable
What this Article Adds?
This study shows that low basal stenotic-kidney glomerular filtration rate with preserved response to acetylcholine may predict benefit from revascularization
We also found that renal inflammation and robust stenotic kidney-R2* responses to furosemide (possibly reflecting avid tubular oxygen consumption) are associated with less favorable outcomes
These tools may be potentially clinically applicable for identification of patients likely to improve renal function after revascularization.
Acknowledgments
Sources of Funding
This study was partly supported by the NIH grant numbers: HL085307, DK73608, HL77131 and C06-RR018898, and by the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: A population-based study. J Vasc Surg. 2002;36:443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 2.Conlon PJ, O'Riordan E, Kalra PA. New insights into the epidemiologic and clinical manifestations of atherosclerotic renovascular disease. Am J Kidney Dis. 2000;35:573–587. doi: 10.1016/s0272-6386(00)70002-3. [DOI] [PubMed] [Google Scholar]
- 3.Rihal CS, Textor SC, Breen JF, McKusick MA, Grill DE, Hallett JW, Holmes DR., Jr Incidental renal artery stenosis among a prospective cohort of hypertensive patients undergoing coronary angiography. Mayo Clin Proc. 2002;77:309–316. doi: 10.4065/77.4.309. [DOI] [PubMed] [Google Scholar]
- 4.Excerpts from united states renal data system 1997 annual data report. Am J Kidney Dis. 1997;30:S1–S213. [PubMed] [Google Scholar]
- 5.Kennedy DJ, Colyer WR, Brewster PS, Ankenbrandt M, Burket MW, Nemeth AS, Khuder SA, Thomas WJ, Shapiro JI, Cooper CJ. Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am J Kidney Dis. 2003;42:926–935. doi: 10.1016/j.ajkd.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: A randomized trial. Ann Intern Med. 2009;150:840–848. W150–W841. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 7.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 8.Harden PN, MacLeod MJ, Rodger RS, Baxter GM, Connell JM, Dominiczak AF, Junor BJ, Briggs JD, Moss JG. Effect of renal-artery stenting on progression of renovascular renal failure. Lancet. 1997;349:1133–1136. doi: 10.1016/s0140-6736(96)10093-3. [DOI] [PubMed] [Google Scholar]
- 9.Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: Implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2001;16:765–770. doi: 10.1093/ndt/16.4.765. [DOI] [PubMed] [Google Scholar]
- 10.Zhu XY, Chade AR, Krier JD, Daghini E, Lavi R, Guglielmotti A, Lerman A, Lerman LO. The chemokine monocyte chemoattractant protein-1 contributes to renal dysfunction in swine renovascular hypertension. J Hypertens. 2009;27:2063–2073. doi: 10.1097/HJH.0b013e3283300192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favreau F, Zhu XY, Krier JD, Lin J, Warner L, Textor SC, Lerman LO. Revascularization of swine renal artery stenosis improves renal function but not the changes in vascular structure. Kidney Int. 2010;78:1110–1118. doi: 10.1038/ki.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebrahimi B, Gloviczki M, Woollard JR, Crane JA, Textor SC, Lerman LO. Compartmental analysis of renal bold mri data: Introduction and validation. Invest Radiol. 2012;47:175–182. doi: 10.1097/RLI.0b013e318234e75b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez SI, Warner L, Haas JA, Bolterman RJ, Textor SC, Lerman LO, Romero JC. Increased hypoxia and reduced renal tubular response to furosemide detected by bold magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009;297:F981–F986. doi: 10.1152/ajprenal.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, Textor SC. Comparison of 1.5 and 3 t bold mr to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol. 2009;44:566–571. doi: 10.1097/RLI.0b013e3181b4c1e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO. Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol. 2011;46:41–47. doi: 10.1097/RLI.0b013e3181f0213f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urbieta-Caceres VHZX, Jordan KL, Tang H, Textor K, Lerman A, Lerman LO. Selective improvement in renal function preserved remote myocardial microvascular integrity and architecture in experimental renovascular disease. Atherosclerosis. 2011;221:350–358. doi: 10.1016/j.atherosclerosis.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eirin A, Gloviczki ML, Tang H, Gossl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs197. Epub July 6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol. 2001;281:F630–F638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 19.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol. 1999;10:1455–1465. doi: 10.1681/ASN.V1071455. [DOI] [PubMed] [Google Scholar]
- 20.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector ct: Comparison with electron-beam ct. Radiology. 2007;243:405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 21.Eirin A, Zhu XY, Urbieta-Caceres VH, Grande JP, Lerman A, Textor SC, Lerman LO. Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol. 2011;300:F1394–F1401. doi: 10.1152/ajprenal.00697.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30:1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chrysochou C, Mendichovszky IA, Buckley DL, Cheung CM, Jackson A, Kalra PA. Bold imaging: A potential predictive biomarker of renal functional outcome following revascularization in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27:1013–1019. doi: 10.1093/ndt/gfr392. [DOI] [PubMed] [Google Scholar]
- 24.Alhadad A, Ahle M, Ivancev K, Gottsater A, Lindblad B. Percutaneous transluminal renal angioplasty (ptra) and surgical revascularisation in renovascular disease--a retrospective comparison of results, complications, and mortality. Eur J Vasc Endovasc Surg. 2004;27:151–156. doi: 10.1016/j.ejvs.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Galaria II, Surowiec SM, Rhodes JM, Illig KA, Shortell CK, Sternbach Y, Green RM, Davies MG. Percutaneous and open renal revascularizations have equivalent long-term functional outcomes. Ann Vasc Surg. 2005;19:218–228. doi: 10.1007/s10016-004-0165-8. [DOI] [PubMed] [Google Scholar]
- 26.Radermacher J, Chavan A, Bleck J, Vitzthum A, Stoess B, Gebel MJ, Galanski M, Koch KM, Haller H. Use of doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344:410–417. doi: 10.1056/NEJM200102083440603. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Zhao L, Shen L, Xu D, Huang B, Wang Q, Lin J, Zou Y, Ge J. Comparison of various niches for endothelial progenitor cell therapy on ischemic myocardial repair: Coexistence of host collateralization and akt-mediated angiogenesis produces a superior microenvironment. Arterioscler Thromb Vasc Biol. 2012;32:910–923. doi: 10.1161/ATVBAHA.111.244970. [DOI] [PubMed] [Google Scholar]
- 28.Soulez G, Therasse E, Qanadli SD, Froment D, Leveille M, Nicolet V, Turpin S, Giroux MF, Guertin MC, Oliva VL. Prediction of clinical response after renal angioplasty: Respective value of renal doppler sonography and scintigraphy. AJR Am J Roentgenol. 2003;181:1029–1035. doi: 10.2214/ajr.181.4.1811029. [DOI] [PubMed] [Google Scholar]
- 29.van Jaarsveld BC, Krijnen P, Pieterman H, Derkx FH, Deinum J, Postma CT, Dees A, Woittiez AJ, Bartelink AK, Man in 't Veld AJ, Schalekamp MA. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch renal artery stenosis intervention cooperative study group. N Engl J Med. 2000;342:1007–1014. doi: 10.1056/NEJM200004063421403. [DOI] [PubMed] [Google Scholar]
- 30.Webster J, Marshall F, Abdalla M, Dominiczak A, Edwards R, Isles CG, Loose H, Main J, Padfield P, Russell IT, Walker B, Watson M, Wilkinson R. Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and newcastle renal artery stenosis collaborative group. J Hum Hypertens. 1998;12:329–335. doi: 10.1038/sj.jhh.1000599. [DOI] [PubMed] [Google Scholar]
- 31.Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: A randomized trial. Essai multicentrique medicaments vs angioplastie (emma) study group. Hypertension. 1998;31:823–829. doi: 10.1161/01.hyp.31.3.823. [DOI] [PubMed] [Google Scholar]
- 32.Ramos F, Kotliar C, Alvarez D, Baglivo H, Rafaelle P, Londero H, Sanchez R, Wilcox CS. Renal function and outcome of ptra and stenting for atherosclerotic renal artery stenosis. Kidney Int. 2003;63:276–282. doi: 10.1046/j.1523-1755.2003.00734.x. [DOI] [PubMed] [Google Scholar]
- 33.Muray S, Martin M, Amoedo ML, Garcia C, Jornet AR, Vera M, Oliveras A, Gomez X, Craver L, Real MI, Garcia L, Botey A, Montanya X, Fernandez E. Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis. 2002;39:60–66. doi: 10.1053/ajkd.2002.29881. [DOI] [PubMed] [Google Scholar]
- 34.Ronden RA, Houben AJ, Kessels AG, Stehouwer CD, de Leeuw PW, Kroon AA. Predictors of clinical outcome after stent placement in atherosclerotic renal artery stenosis: A systematic review and meta-analysis of prospective studies. J Hypertens. 2010;28:2370–2377. doi: 10.1097/HJH.0b013e32833ec392. [DOI] [PubMed] [Google Scholar]
- 35.Thanyasiri P, Kathir K, Celermajer DS, Adams MR. Endothelial dysfunction and restenosis following percutaneous coronary intervention. Int J Cardiol. 2007;119:362–367. doi: 10.1016/j.ijcard.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Lerman LO, Taler SJ, Textor SC, Sheedy PF, 2nd, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996;49:846–854. doi: 10.1038/ki.1996.117. [DOI] [PubMed] [Google Scholar]
- 37.Lerman L, Textor SC. Pathophysiology of ischemic nephropathy. Urol Clin North Am. 2001;28:793–803. doi: 10.1016/s0094-0143(01)80034-3. ix. [DOI] [PubMed] [Google Scholar]
- 38.Cheung CM, Chrysochou C, Shurrab AE, Buckley DL, Cowie A, Kalra PA. Effects of renal volume and single-kidney glomerular filtration rate on renal functional outcome in atherosclerotic renal artery stenosis. Nephrol Dial Transplant. 2010;25:1133–1140. doi: 10.1093/ndt/gfp623. [DOI] [PubMed] [Google Scholar]
- 39.Johns EJ. Inflammation: The underlying foe in renovascular hypertension? J Hypertens. 2009;27:1964–1965. doi: 10.1097/HJH.0b013e328331a881. [DOI] [PubMed] [Google Scholar]
- 40.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 41.Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, Lerman A, Lerman LO. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol. 2004;15:958–966. doi: 10.1097/01.asn.0000117774.83396.e9. [DOI] [PubMed] [Google Scholar]
- 42.Alhadad A, Guron G, Fortuna-Nowakowska E, Saeed A, Mattiasson I, Jensen G, Lindblad B, Gottsater A, Herlitz H. Renal angioplasty causes a rapid transient increase in inflammatory biomarkers, but reduced levels of interleukin-6 and endothelin-1 1 month after intervention. J Hypertens. 2007;25:1907–1914. doi: 10.1097/HJH.0b013e328244e2ca. [DOI] [PubMed] [Google Scholar]
- 43.Mai M, Geiger H, Hilgers KF, Veelken R, Mann JF, Dammrich J, Luft FC. Early interstitial changes in hypertension-induced renal injury. Hypertension. 1993;22:754–765. doi: 10.1161/01.hyp.22.5.754. [DOI] [PubMed] [Google Scholar]
- 44.Tullis MJ, Zierler RE, Caps MT, Bergelin RO, Cantwell-Gab K, Strandness DE., Jr Clinical evidence of contralateral renal parenchymal injury in patients with unilateral atherosclerotic renal artery stenosis. Ann Vasc Surg. 1998;12:122–127. doi: 10.1007/s100169900127. [DOI] [PubMed] [Google Scholar]
- 45.Gobe GC, Axelsen RA, Searle JW. Cellular events in experimental unilateral ischemic renal atrophy and in regeneration after contralateral nephrectomy. Lab Invest. 1990;63:770–779. [PubMed] [Google Scholar]
- 46.Williams GW, Forsythe SB, Textor SC, Tarazi RC. Analysis of relative change and initial value in biological studies. Am J Physiol. 1984;246:R122–R126. doi: 10.1152/ajpregu.1984.246.1.R122. [DOI] [PubMed] [Google Scholar]
- 47.Gloviczki ML, Glockner JF, Crane JA, McKusick MA, Misra S, Grande JP, Lerman LO, Textor SC. Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension. 2011;58:1066–1072. doi: 10.1161/HYPERTENSIONAHA.111.171405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55:961–966. doi: 10.1161/HYPERTENSIONAHA.109.145227. [DOI] [PMC free article] [PubMed] [Google Scholar]