Abstract
Background
The intrauterine environment strongly influences adult disease susceptibility. We utilized a rat model of third trimester maternal diabetes to test the hypothesis that adult offspring exposed to hyperglycemia in utero display increased blood pressure and alterations in vascular responsiveness.
Methods
Diabetes was induced by streptozotocin injection to pregnant rats on gestation day 13 (term 21 day) and partially controlled with insulin injections. Hemodynamic function was evaluated in 6–12 month old offspring.
Results
Male but not female offspring of diabetic mothers (ODM) had significantly increased blood pressure compared to controls, heart rate was similar. For both sexes, heart rate baroreflex responses were similar as were in vivo hemodynamic responses to angiotensin II, NOS inhibition and ganglionic blockade. Aortic contractility to angiotensin II was similar in both groups. NOS inhibition and the Cu/Zn superoxide dismutase (SOD) inhibitor diethyldithiocarbamate but not the SOD-mimetic Tempol significantly increased contractile responses to angiotensin II in controls but not ODM. NADPH stimulated superoxide production was greater in male ODM than controls (p<0.05).
Conclusions
Exposure to hyperglycemia in utero results in sex specific cardiovascular changes in adult offspring. Impaired NO - reactive oxygen species signaling may play a significant role in the hemodynamic phenotype of ODM.
The number of pregnancies complicated by diabetes mellitus continues to increase throughout the developed world, a figure that will rise with the increasing prevalence of type 2 diabetes among young adult women 1,2. Infants of diabetic mothers represent a group of patients with an increased risk of perinatal mortality and morbidity, including cardiomyopathy, macrosomia, hypoglycemia, hypocalcemia, and respiratory distress syndrome 3. Recently, long-term complications in offspring of diabetic mothers have been recognized, including development of type 2 diabetes, dyslipidemia and obesity independently of the genetic background of the offspring 4–8. While considerable attention is focused on the increased metabolic abnormalities in adult offspring of diabetic mothers, cardiovascular alterations, including hypertension, are also lifelong consequences of in-utero exposure to maternal diabetes. In a Pima Indian population, offspring of mothers who suffered diabetes during pregnancy had higher systolic arterial blood pressure in adolescence compared with offspring of mothers who developed type 2 diabetes mellitus after the index pregnancy, independent of obesity 9. In a separate population enrolled in the Diabetes in Pregnancy follow-up study, 10–14 year old offspring of diabetic mothers had significantly higher systolic and mean arterial blood pressures than offspring of nondiabetic mothers 10.
The etiology of hypertension in offspring of diabetic mothers (ODM) has not been extensively explored. The majority of studies examining cardiovascular dysfunction in ODM have used animals made diabetic either prior to pregnancy or early in pregnancy by injection of streptozotocin, an alkylating agent with selective toxicity to the beta cells of pancreatic islets 11. Studies in rats demonstrate that male but not female offspring of dams made diabetic prior to mating or on the 7th day of pregnancy are hypertensive by 2–3 months of age 12–14. Findings of these studies and others suggest changes in baroreflex sensitivity, vascular responsiveness, activity of the renin-angiotensin and NO systems and arachidonic acid metabolism may contribute to the development of hypertension in ODM 15,16. Because gestational onset diabetes, characterized by maternal hyperglycemia during the last third of gestation, is the most common form of diabetes to affect pregnancy, our laboratory has focused on a rat model whereby maternal hyperglycemia is induced by streptozotocin at the beginning of the third and final week of gestation.
Using this model of maternal diabetes, with hyperglycemia moderated by twice daily insulin, we previously described significantly enhanced aortic contractility and endothelial dysfunction in female but not male offspring 17. We therefore sought to extend studies in this model to determine whether offspring exposed to short-term (7-day) maternal hyperglycemia in utero would display sex-specific increased blood pressure. As studies in animal models suggest abnormalities in autonomic function may precede overt hypertension, we also analyzed baroreflex control of heart rate 18. Finally, we chose to further our previously reported findings of altered ex vivo aortic vascular responsiveness and endothelial dysfunction in offspring of hyperglycemic mothers and explore potential mechanisms contributing to our previous observations. Diabetes during pregnancy is associated with increased markers of oxidative injury in the offspring 19,20. Thus, the isolated vessels studies focused on the contribution of reactive oxygen species to ex vivo aortic vascular dysfunction. Importantly, we cross-fostered all pups to non-diabetic mothers to insure that any identified pathophysiology would be related to the in utero environment and not alterations in postnatal nutritional intake or behavioral patterns associated with maternal diabetes.
RESULTS
In vivo experiments
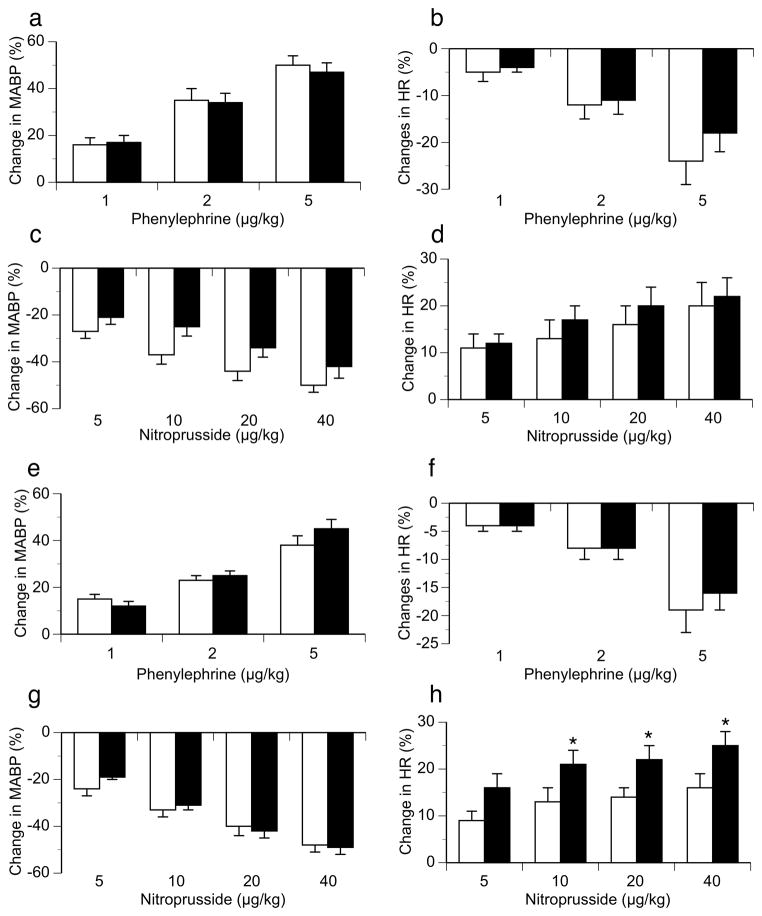
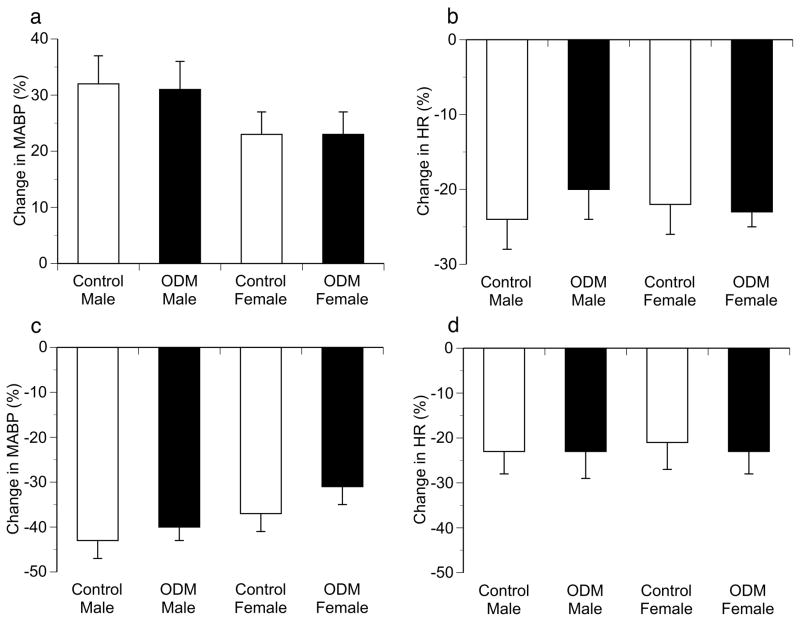
Male ODM displayed significantly greater resting mean arterial pressure (123 ± 5 vs. 102 ± 4 mmHg; P < 0.01) but similar resting heart rate (360 ± 11 vs. 370 ± 7 bpm) compared to controls (Figure 1). In contrast, no differences in MABP or HR were identified between female ODM and control animals. Peak changes in MABP and HR in response to phenylephrine and nitroprusside were similar in male ODM and control animals (Figure 2). Female ODM also had similar increases in MABP and reflex bradycardia compared to controls in response to phenylephrine (Figure 2). However, female ODM demonstrated greater reflex tachycardia in response to sodium nitroprusside (10, 20 and 40 μg/kg) compared to controls, though MABP responses were similar between groups (Figure 2). To evaluate baroreflex sensitivity, slopes of the linear regression line relating percentage change from baseline for mean blood pressure and heart rate in response to phenylephrine and sodium nitroprusside for each animal were calculated. No differences in the MABP-HR relationship were identified between ODM and control animals for either male (−1.1 ± 0.14 vs. −1.01 ± 0.18 %ΔHR/%Δ MABP, respectively) or female animals (−1.56 ± 0.24 vs. −1.2 ± 0.22 %ΔHR/%Δ MABP, respectively; P = 0.0548).
Figure 1.
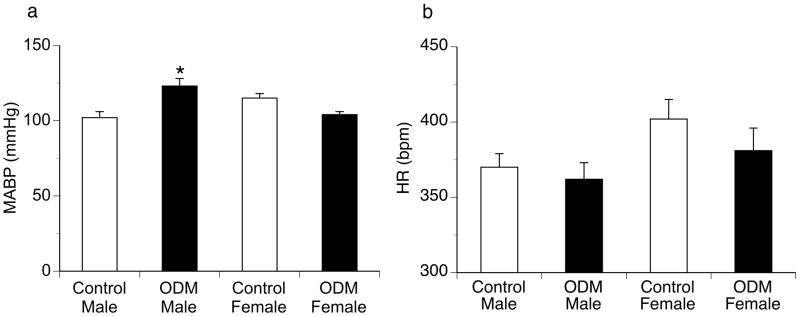
Resting mean arterial blood pressure (MABP) (a) and heart rate (HR) (a) in control and ODM male and female adult rats. Data are reported as mean ± SE. bpm, beats per minute. * p < 0.01 compared to control male.
Figure 2.
Changes in systemic hemodynamics to increasing doses of phenylephrine and nitroprusside in male (top two rows) and female (bottom two rows) control (□) and ODM (■) adult rats. (a) MABP responses to phenylephrine in males. (b) HR responses to phenylephrine in males. (c) MABP responses to nitroprusside in males. (d) HR responses to nitroprusside in males. (e) MABP responses to phenylephrine in females. (f) HR responses to phenylephrine in females. (g) MABP responses to nitroprusside in females. (h) HR responses to nitroprusside in females. Data are reported as mean ± SE. * p < 0.05 compared to control females for similar dose.
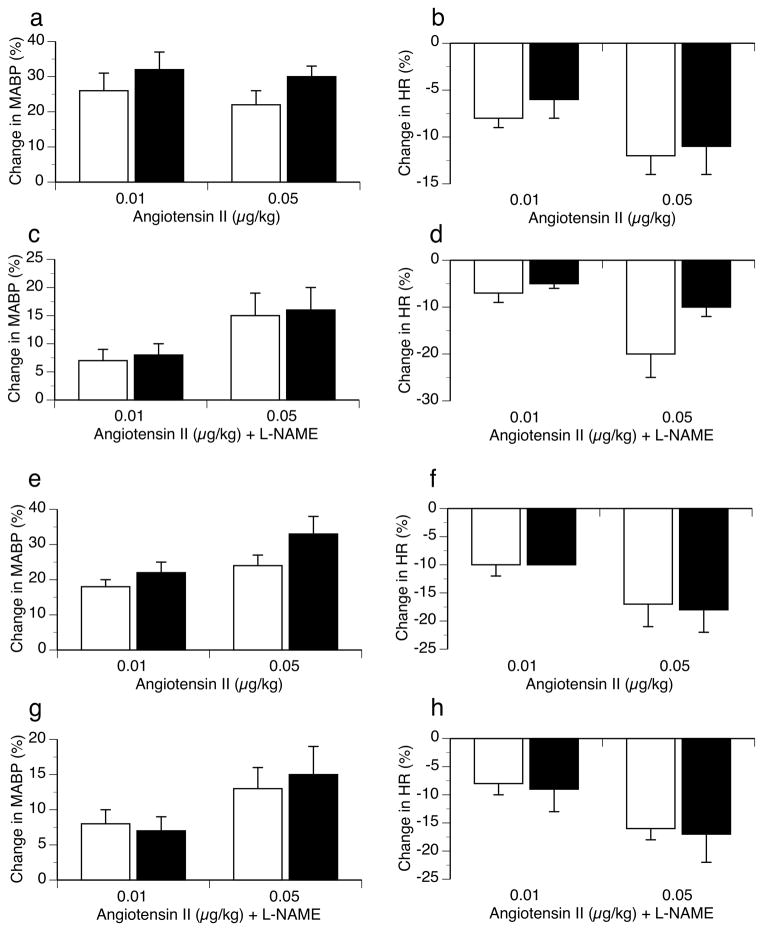
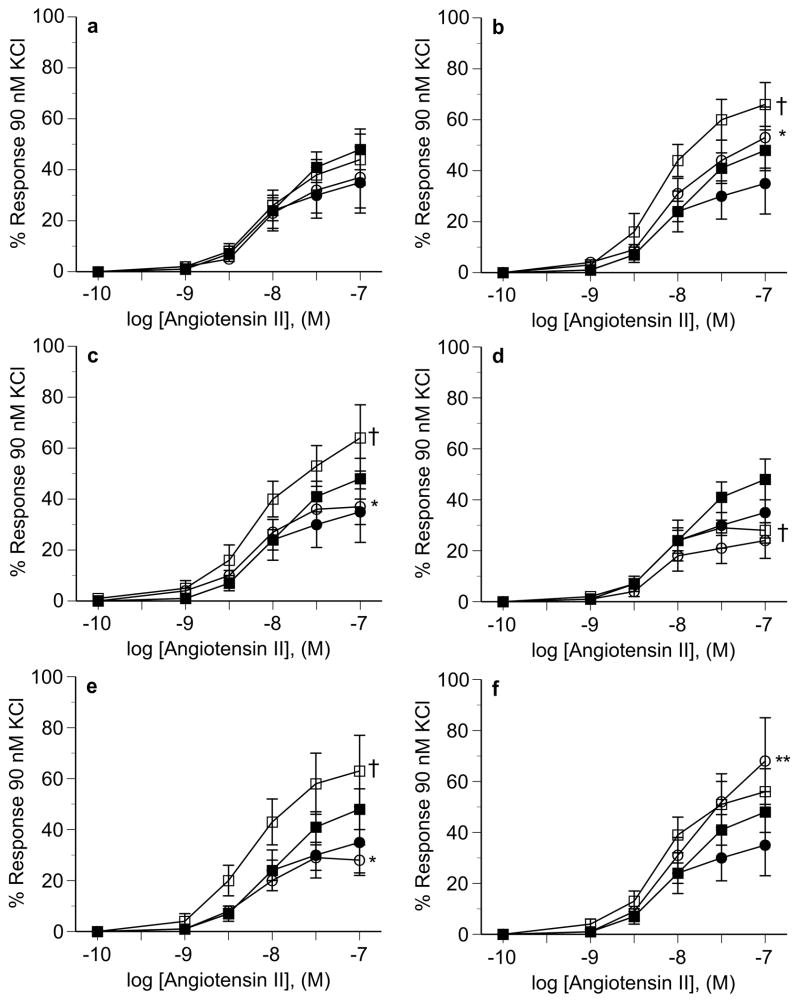
Changes in MABP and HR in response to the NO synthase inhibitor L-NAME and the ganglionic blocking agent chlorisondamine are shown in Figure 3. No differences in hemodynamic responses to either agent were seen between ODM and control animals of either sex. Changes in MABP and HR in response to angiotensin II alone or in the presence of L-NAME were also similar between groups in both sexes (Figure 4).
Figure 3.
Changes in systemic hemodynamics (expressed as percent change from baseline) to L-NAME (top panels) and chlorisondamine (bottom panels) in control and ODM male and female animals. (a) Change in MABP in response to L-NAME. (b) Change in HR in response to L-NAME. (c) Change in MABP in response to chlorisondamine. (d) Change in HR in response to chlorisondamine. Data are reported as mean ± SE.
Figure 4.
Changes in systemic hemodynamics (expressed as percent change from baseline) to increasing doses of angiotensin II before and after L-NAME (10 mg/kg i.v.) in male (top two rows) and female (bottom two rows) control (□) and ODM (■) adult rats. (a) MABP responses to angiotensin II in males. (b) HR responses to angiotensin II in males. (c) MABP responses to angiotensin II + L-NAME in males. (d) HR responses to angiotensin II + L-NAME in males. (e) MABP responses to angiotensin II in females. (f) HR responses to angiotensin II in females. (g) MABP responses to angiotensin II + L-NAME in females. (h) HR responses to angiotensin II + L-NAME in females. Data are reported as mean ± SE.
Ex vivo vascular function in male ODM
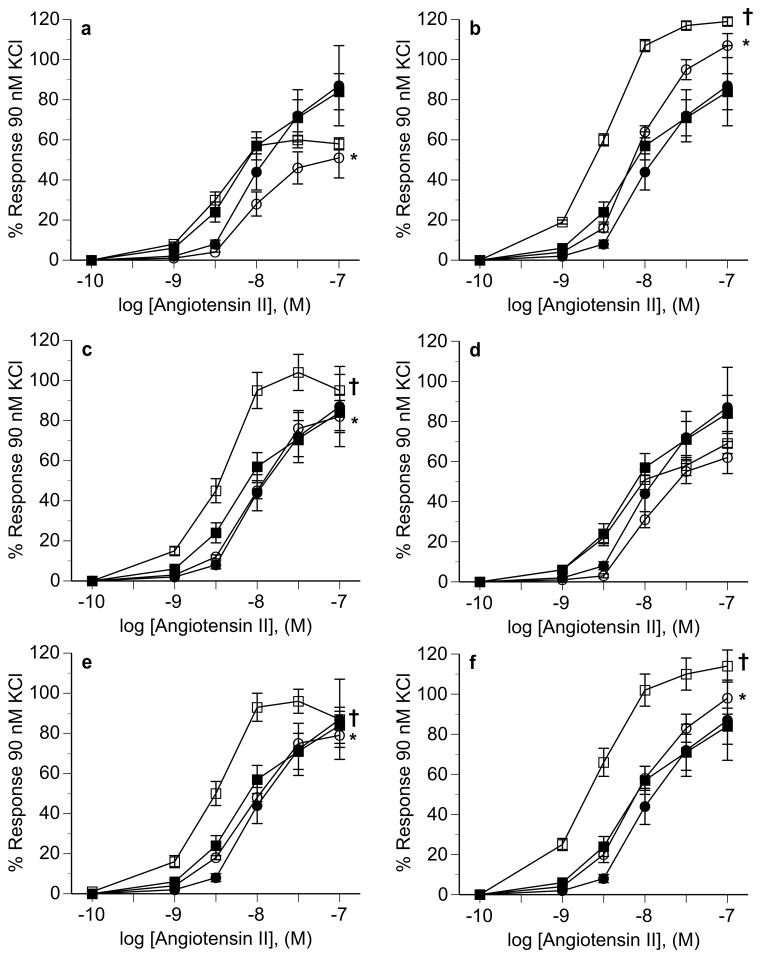
Aortic segments from male ODM and control animals exhibited similar vasoconstrictive responses to angiotensin II (Figure 5). However, in the presence of the angiotensin II type 2 (AT2) receptor antagonist PD123319, the response to angiotensin II was attenuated in ODM compared to control (Figure 5A). Constrictive responses to angiotensin II in the presence of the AT1 receptor antagonist losartan were completed blocked in both groups (data not shown). The NO synthase inhibitor L-NNA and the Cu/Zn SOD inhibitor DETC significantly increased responses to angiotensin II in controls but not ODM whereas the superoxide scavenger Tempol attenuated responses to angiotensin II in controls but not ODM (Figure 5B–5D). The combined effects of L-NNA with DETC resulted in similar changes to either L-NNA or DETC, without any evidence of additive effects (Figure 5E). Similarly, combined L-NNA and Tempol resulted is changes in responses similar to L-NNA alone in both groups (Figure 5F).
Figure 5.
Male aortic responsiveness to angiotensin II in the absence and presence of PD 123319 (a), L-NNA (b), DETC (c), Tempol (d), L-NNA + DETC (e), and L-NNA + Tempol (f). In each panel: angiotensin alone in control (■) and ODM (●); angiotensin in presence of specific agent in control (□) and ODM (○). Data are reported as mean ± SE. * p < 0.05 between control an ODM in presence of specific agent in each panel (treatment effect by ANOVA), † p < 0.05 between control in absence and presence of specific agent (treatment effect by ANOVA). M, molar.
Ex vivo vascular function in female ODM
Similar to that seen in males, aortic segments from female ODM and control animals exhibited similar vasoconstrictive responses to angiotensin II alone (Figure 6A). No effect on constrictive responses was seen in the presence of PD 123319 (Figure 6A). L-NNA and DETC again significantly increased responses to angiotensin II in controls but not ODM, while Tempol had not significant effect on the response (Figure 6B–6D). As seen in males, the combined effects of L-NNA with DETC resulted in similar changes to either L-NNA or DETC, without any evidence of additive effects (Figure 6E). Interestingly, the response to angiotensin II in the presence of Tempol + L-NNA was similar between control and ODM female groups (Figure 6F).
Figure 6.
Female aortic responsiveness to angiotensin II in the absence and presence of PD 123319 (a), L-NNA (b), DETC (c), Tempol (d), L-NNA + DETC (e), and L-NNA + Tempol (f). In each panel: angiotensin alone in control (■) and ODM (●); angiotensin in presence of specific agent in control (□) and ODM (○). Data are reported as mean ± SE. * p < 0.05 between control an ODM in presence of specific agent in each panel (treatment effect by ANOVA), † p < 0.05 between control in absence and presence of specific agent (treatment effect by ANOVA). ** p< 0.05 between ODM in absence and presence of specific agent (treatment effect by ANOVA). M, molar.
Superoxide production
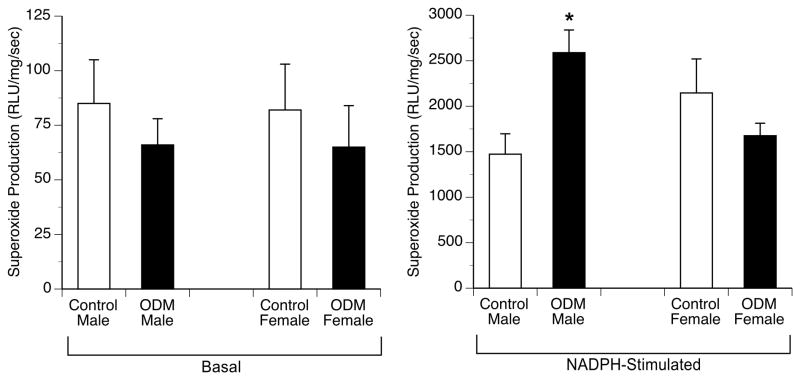
Basal superoxide production, as determined by chemiluminescence was similar between groups in both sexes. NADPH stimulated superoxide production was 1.5–2 fold higher in aorta from male ODM compared to control, whereas in females, NADPH stimulated superoxide production was similar between groups (Figure 7).
Figure 7.
Basal and NADPH-oxidase stimulated superoxide production determined by lucigenin-enhanced chemiluminescence of abdominal aorta from control and ODM animals. Values reported in relative light units (RLU) · mg dry weight of tissue−1 · s−1. Data are reported as mean ± SE. * p < 0.05 compared with control male.
DISCUSSION
It is increasingly being recognized that exposure to hyperglycemia in utero results in long-term consequences, including the development of vascular dysfunction and hypertension 9,13,14. The majority of animal studies evaluating the effects of hyperglycemia during pregnancy have studied offspring of mothers made diabetic before or relatively early in gestation and were reared by these same mothers (i.e. not cross-fostered). As such, a critical window of exposure to maternal diabetes resulting in cardiovascular morbidity in the offspring has not been established and could potentially occur during the late prenatal or postnatal period. We evaluated hemodynamics and vascular responsiveness in offspring exposed to maternal hyperglycemia only in the last third of pregnancy, similar to that occurring with gestational diabetes. Furthermore, all rat pups were cross-fostered to non-diabetic mothers, effectively removing the postnatal period as a contributor to the offspring phenotype. The results of these studies demonstrate that exposure to hyperglycemia late in gestation results in hypertension and increased NADPH stimulated superoxide production in male but not female offspring at 6–12 months of age. However, ODM of both sexes displayed abnormalities in aortic vascular reactivity related to altered NO and reactive oxygen species (ROS) signaling/bioavailability.
In a previous study using this same animal model, we identified that female but not male ODM exhibited vascular and endothelial dysfunction, as evidenced by increased aortic contractile responses to endothelin-1 and noradrenaline, and decreased vasorelaxation to the endothelial dependent vasodilator, acetylcholine 17. Therefore, we were surprised to identify males, but not females, were hypertensive at 6–12 months of age. Interestingly, sex-specific hypertension has been shown in a variety of developmental programming models. Both moderate maternal protein restriction, and uteroplacental insufficiency results in hypertension in male but not female offspring 21–23. The etiology of sex differences in developing hypertension is unclear, though potential mechanisms, including differences in the amounts of sex hormones or fundamental differences at the level of gene expression, have recently been reviewed 24.
We utilized in vivo assessment of systemic hemodynamics and vascular function to identify components of neurohumoral regulation of cardiovascular function that may contribute to hypertension in male ODM. Baroreflex dysfunction has been observed in hypertension both as a primary cause and a secondary effect 25. In a model of maternal diabetes in which pregnant dams were made diabetic by streptozotocin on the 7th day of pregnancy, Wichi et al. found increased blood pressure responses to phenylephrine and impaired baroreflex mediated tachycardia elicited by decreasing MABP with nitroprusside in male offspring 14. In contrast, we found no differences in dose response changes in MABP and HR to phenylephrine and nitroprusside or the slope of the baroreflex response to either increasing or decreasing blood pressure between male ODM and control animals, suggesting baroreflex function remains intact. Reasons for the discrepancy in findings between our study and those of Wichi et al. may relate to differences in age of offspring, duration of maternal diabetes or timing of the studies related to surgical catheter placement. However in females, the heart rate baroreflex response to unloading baroreceptors, elicited by decreasing blood pressure with nitroprusside, tended to be greater in ODM compared to controls. Specifically, nitroprusside injection elicited significantly greater increases in HR (10, 20, 40 μg/kg) in ODM compared to control animals, though decreases in blood pressure were similar. Heart rate baroreflex sensitivity also tended to be different (p = 0.06), though failure to reach significance related to the slight but not significantly different decreases in blood pressure between groups. The finding of increased sensitivity of the baroreflex is opposite that expected in a pre-hypertensive state, where impairment (decreased sensitivity) of the baroreflex is likely to be observed. Without further study, it is not possible to say whether this effect was mediated at the level of the baroreceptor, related to central processing of the afferent signal, or to end-organ response differences.
We previously found evidence for aortic endothelial dysfunction in female ODM, evidenced by decreased vasorelaxation to acetylcholine, and attenuation of the increased vasoconstrictive responses to endothelin-1 and noradrenaline in ODM compared to controls by removal of endothelium or following L-NNA exposure 17. Cavanal et al. also found basal aortic NO production was decreased in ODM compared to controls 26. In contrast to these findings in isolated vessels, we found no differences in the pressor response following L-NAME, suggesting endogenous NO bioavailability and function was similar between groups. To further interrogate the NO buffering system that may mask a predisposition to hypertension, we examined pressor responses to bolus doses of angiotensin II before and after L-NAME. No significant differences in the blood pressure responses between treatment groups in either sex were identified, again suggesting similarly intact NO signaling pathways in control and ODM. The findings demonstrate that abnormalities in endothelial function and NO bioavailability in the aorta may not, in fact, be present throughout the vascular bed, particularly in resistance vessels that contribute to maintenance of blood pressure.
Sympathetic function is essential to blood pressure regulation, and overactivity of sympathetic nerves may have an important role in the development of hypertension and related cardiovascular disorders 25. In many experimental models of hypertensive rats, there appears to be an important contribution from the sympathetic nervous system. A number of models of developmental programming of hypertension also suggest an important role of sympathetic function contributing to the phenotype 27–29. To determine the contribution of the sympathetic drive to the maintenance of blood pressure, we administered the ganglionic blocking agent chlorisondamine. Impairment of the sympathetic drive by blocking ganglionic transmission results in a rapid blood pressure fall, which is related to the degree of sympathetic activity. We hypothesized that male ODM would exhibit a bigger decrease in blood pressure in response to chlorisondamine compared to controls, suggesting increased sympathetic vascular tone in this group. However, the blood pressure decreases associated with chlorisondamine, expressed both as percent change or absolute change in mmHg (data not shown), were not different in ODM compared to control male or female animals. We recognize these findings do not rule out a significant contribution of the sympathetic nervous system in contributing to hypertension in ODM. For example, increased renal sympathetic nerve activity may alter renal excretory function, and thus contribute to altered renal sodium handling and ultimately hypertension. Along these lines, it has previously been shown that offspring of rats with STZ-induced diabetes beginning on day 0 of gestation have reduced nephron number, salt sensitive hypertension and altered renal sodium handling 30. Further studies are needed to see if a similar phenotype is present in our model of maternal diabetes.
Using ex vivo wire myography, we found inhibition of NO synthase by L-NNA resulted in enhanced angiotensin II mediated vasoconstriction in control but not ODM animals, findings consistent with those we previously reported in this model and suggesting impaired NO bioavailability in ODM. Whether this is related to decreased levels of the substrate L-arginine, NO synthase, uncoupling of NO synthase or scavenging by increased levels of superoxide remains to be determined 26. A number of studies have demonstrated that a suboptimal uterine environment, including maternal obesity, diabetes and pre-eclampsia, alters oxidative pathways in the placenta and offspring 31–33. Evidence of oxidative stress and disruption of oxidative signaling remains well into the postnatal period and adulthood. Using lucigenin-enhanced chemiluminescence, we found that aorta from male but not female ODM generated 1.5–2 fold higher amounts of superoxide in response to NADPH than did controls. In addition to smooth muscle contraction and vascular tone, reactive oxygen species mediate a number of vascular physiologic functions, including transcription factor activation, gene expression, cell growth, and apoptosis. Thus, conclusions about the effects of chronic exposure to elevated superoxide levels are difficult, though ROS-mediated cellular injury is thought to contribute to cardiovascular disease. The acute effects of altered ROS species are more easily examined, though remain complex. In endothelial-denuded rat aortic rings, Shen et al. demonstrated that different ROS species induced contraction via separate mechanisms of action 34. Using the superoxide generator pyrogallol, Demirci et al. demonstrated that the effects on endothelium dependent vasodilation were dose dependent, improving Ach-mediated relaxation at low concentration but attenuating responses at higher concentrations 35. A similar dose-dependent effect was seen when phenylephrine-induced contractions were studied, with low doses of pyrogallol diminishing contraction and high doses enhancing contractility. In the present study, we found Tempol had no significant effect on angiotensin II mediated contractions. On the other hand, DETC significantly enhanced contractions in control but not ODM animals. We speculate that in control animals, with intact and functional endothelium, DETC resulted in a small increase in vascular superoxide levels that enhanced contraction. In contrast, in ODM, in which endothelial dysfunction is already present and superoxide levels are elevated, DETC has little effect on contractile responses. Because studies were not performed in endothelial-denuded vessels, we cannot comment on potential direct effects of superoxide or other reactive oxygen species on smooth muscle cells in this model.
There are several limitations of the study. First, offspring were studied over a range of ages (6–12 months), and it is possible that age-related effects exist, but not identified. Certainly, some of the difficulties in reconciling our findings with those of previous studies may relate to the large age range of the rats used in our studies. An age related effect of increased blood pressure (seen in male ODM) on composition and function of the arterial wall may also be more pronounced in older vs. younger adult rats and not accounted for in the experimental design 36. Second, vascular reactivity and superoxide production studies were performed in the aorta, a conduit vessel, and not in resistance vessels, were alterations in vascular function likely have a more physiologic contribution to the maintenance of blood pressure. Finally, because we used streptozotocin to induce maternal diabetes, our model is one of late gestation insulinopenia and hyperglycemia and not gestational diabetes, a condition of insulin resistance.
In summary, we identified that exposure to maternal diabetes during the last third of gestation results in hypertension in adult male but not female offspring. Results of the in vivo studies, examining baroreflex function, the NO contribution to blood pressure and vascular sympathetic tone failed to identify abnormalities in these systems that may directly contribute to hypertension. However, oxidative stress may contribute to the underlying phenotypes in ODM as the maternal diabetic in utero environment results in increased vascular superoxide production in male offspring and endothelial dysfunction and impaired NO-ROS signaling in offspring of both sexes.
METHODS
Animal Preparation
All procedures were performed within the regulations of the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Iowa. Pregnant Sprague-Dawley rats (Charles River Laboratories, Wilmington MA) received an i.p. injection of either streptozotocin (Sigma-Aldrich, St. Louis, MO) (STZ; 50 mg/kg of body weight in 10 mmol/l citrate buffer (pH 3.5) or an equivalent volume of citrate buffer alone on gestational day 13. STZ-injected rats had blood glucose measured twice daily via tail-nicking, using the LifeScan OneTouch Ultra Blood Glucose Monitoring System (LifeScan, Inc., Milpitas, CA). Hyperglycemia was partially controlled and ketosis was avoided by subcutaneous injection of insulin (Humulin; Eli Lilly and Co., Indianapolis) in the morning and insulin-glargine (Lantus; Sanofi-Aventis, Bridgewater, NJ) each evening to maintain blood glucose between 100 and 400 mg/dl. Control rats were injected with saline twice daily. Dams were allowed to delivery spontaneously. All pups were cross-fostered with non-diabetic postpartum dams. Litters were culled to ten pups, selecting to preserve the largest pups. Pups of both sexes were weaned on to standard rat chow on day 21. We have previously described this methodological approach and detail regarding maternal glycemic control and the metabolic status of the offspring 17.
In vivo experimental protocol
At 6–12 months of age, offspring exposed to late gestation hyperglycemia and offspring of control mothers of both sexes were studied (n = 6 – 8 for each sex and each condition). This was a wide range in ages of animals studied, however, we think this is a valid grouping based on: 1) the distribution of ages of the animals was similar in all groups; and 2) several groups have shown that systolic blood pressure, heart rate, nitroprusside and acetylcholine-induced relaxation of aortic rings are unchanged in young adult and old adult WKY rats 37–39. Anesthesia was induced and maintained with inhalational isoflurane mixed with oxygen. The left femoral triangle was opened, a polyethylene catheter (PE 50, Intramedic, Franklin Lakes, NJ) was inserted and secured into the femoral artery, while a PE 10 catheter was inserted and secured into the femoral vein. Catheters were tunneled subcutaneously to a mid-scapular exit and connected to a Covance Infusion Harness (Instech Laboratories, Inc., Plymouth Meeting, PA), which allows the rat freedom of movement. Postoperative analgesia included bupivacaine (International Laboratory Ltd., San Bruno CA) placed topically along suture lines prior to closing the skin. Normal saline with 25 U/ml heparin was continuously infused at 5 μl/min in the arterial catheter to maintain patency while the venous catheter was filled with 0.05 ml of 0.9% NaCl with 500 U/ml heparin. After surgery, animals were allowed free access to food and water. Three days were allowed for recovery from surgery before experiments were performed.
Arterial blood pressure was recorded using Statham P23 Db pressure transducers (Spectramed, Critical Care Division, Oxnard, CA), MacLab hardware and software (ADInstruments, Colorado Springs, CO), and a G4 Powerbook (Apple Computers, Cupertino, CA). Heart rate (HR) was monitored with a cardiotachometer triggered from the arterial pressure pulse wave. On the first study day, baseline blood pressure and heart rate were determined. We then examined the function of the arterial baroreflex by measurement of dose response changes in mean arterial blood pressure (MABP) and HR to phenylephrine (1, 2, and 5 μg/kg, i.v.) and sodium nitroprusside (5, 10, 20, and 40 μg/kg, i.v.). A 20–30 minute recovery period was allowed for MABP and HR to return to baseline before administration of each subsequent dose. All agents were given intravenously as bolus injection, with the volume adjusted to 0.1 ml. On the following day, dose response blood pressure and heart rate changes to angiotensin II (0.05, and 0.1 μg/kg, i.v.), were examined to assess functional response to angiotensin receptor stimulation. After a 1-hr recovery period, baseline blood pressure and heart rate were again determined followed by administration of the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 10 mg/kg, i.v., Sigma-Aldrich). This approach allows assessment of the contribution of NO to maintenance of resting blood pressure. A dose response to angiotensin II (0.01, and 0.05 μg/kg, i.v.) was again performed, allowing assessment of the contribution of NO to buffering the pressor response resulting from angiotensin II. On the final day of study, rats received the ganglionic blocker chlorisondamine (5 mg/kg, i.v.). This approach allows us to assess the contribution of vascular sympathetic tone to the maintenance of baseline blood pressure.
All cardiovascular measurements were performed in conscious, freely moving rats. Baseline blood pressure and heart rate were reported as an average over time (at least 30 min). All drugs were given i.v. as bolus injection, with the volume of agent adjusted to 0.1 ml. The doses used for the various pharmacologic agents administered in vivo were chosen based upon review of the literature, and preliminary studies examining the range of responses to the agents 40–42. Hemodynamic responses to increasing doses of phenylephrine, sodium nitroprusside, and angiotensin II are reported as a maximal percentile change from resting blood pressure and heart rate immediately prior to giving the drugs. Subsequent injections were given only after hemodynamics had returned to basal levels.
Ex vivo vascular reactivity
In a separate group of animals (n = 6 – 7 for each sex and each condition), aortic vascular reactivity was assessed using methods previously described 17. Briefly, offspring were euthanized and descending thoracic aorta segments harvested, cleansed of adherent connective tissue and sectioned into 2-mm-long rings. Aortic rings were mounted in individual 18-ml isolated organ chambers (Radnoti Glass Technology Inc., Monrovia, CA) and connected to an isometric force transducer. Contractile responses were recorded with a MacLab 8E (ADInstruments, Colorado Springs, CO) and stored on a Power Macintosh 8600 computer. Passive stretch was set at 90% of the tension required to obtain peak responses to potassium chloride (KCl) (2.5 g, determined in preliminary studies) and the rings were allowed to equilibrate in bicarbonate-buffered physiological salt solution (PSS) at 37°C for 60 min before the start of experimentation. The composition of the PSS was as follows (in mM): 130NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4·7 H2O, 14.9 NaHCO3, 1.6 CaCl2·H20, 5.5 dextrose and 0.03 CaNa2-EDTA (pH 7.30). PSS was aerated with a mixture of 95% O2–5% CO2.
To evaluate the separate and combined roles of angiotensin type 1 and type 2 receptors, superoxide production and nitric oxide production on the vasoconstrictive effects of angiotensin II, aortic vessel segments were incubated, respectively with the angiotensin II type 1 receptor antagonist losartan (10−6M), the angiotensin II type 2 receptor antagonist PD-123319 (10−7M), the superoxide dismutase mimetic Tempol (1 mM), the Cu/Zn superoxide dismutase inhibitor diethyldithiocarbamate (DETC, 10 mM), the NO synthase inhibitor NG-nitro-L-arginine (L-NNA, 10−4M), Tempol + L-NNA, and DETC + L-NNA. Separate baths were used to assess the cumulative concentration responses to angiotensin II (10−11 to 10−7M) in the absence and presence of the agents described above. All PSS reagents and vasoactive compounds were acquired from Sigma-Aldrich (St. Louis, MO). Doses of pharmacologic agents were determined based upon our previous experience with these agents and literature review 43,44. Angiotensin II was used as the constriction agent as our previous studies demonstrated no differences in constriction between aortas obtained from control and ODM animals. This approach allowed us to be confident that any measured differences in the constrictive response to angiotensin II was related to the presence of the additional pharmacologic agent(s) added to the bath.
Chemiluminescence
To assess the effects of in utero exposure to hyperglycemia on vascular reactive oxygen species, basal superoxide anion production was measured by lucigenin (25μM)-enhanced chemiluminescence after 5 min of dark adaptation as previously described (luminometer model FB12; Zylux) 45. Proximal abdominal aorta excised from animals undergoing ex vivo vascular reactivity studies and sectioned into 3 mm rings was utilized. NADPH oxidase-dependent superoxide production was measured as the diphenylene-iodonium (10–4 mol/l)-inhibitable chemiluminescence measured after the addition of the enzyme substrate NADPH (10–4 mol/l). Superoxide anion production, as determined by chemiluminescent relative light units (RLU), was normalized to weight of the aortic segment.
Data analysis
For each animal, baroreflex sensitivity was determined by calculating the slope of the linear regression relating the percent change in MABP to percent change in HR. Animals of each sex were analyzed separately. Comparisons between groups were performed by Student’s unpaired, two-tailed t-test or ANOVA, factor for treatment group and drug concentration. If ANOVA identified significant differences, defined as P<0.05, pairwise comparisons were made using Tukey test, with P<0.05 considered significant. Variances were compared using the variance ration test (F-test). All results are expressed as means ± SE.
Acknowledgments
Statement of financial support: Ikaria, Inc. (to RK), National Institutes of Health (NIH; grant DK077599 to TDS) and NIH grant R01 DK081548 (to AWN).
Footnotes
Conflict of Interest Statement: None of the authors have any conflicts of interest to report.
References
- 1.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28:579–84. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 2.Shaw J. Epidemiology of childhood type 2 diabetes and obesity. Pediatr Diabetes. 2007;8 (Suppl 9):7–15. doi: 10.1111/j.1399-5448.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- 3.Nold JL, Georgieff MK. Infants of diabetic mothers. Pediatr Clin North Am. 2004;51:619–37. viii. doi: 10.1016/j.pcl.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30 (Suppl 2):S169–74. doi: 10.2337/dc07-s211. [DOI] [PubMed] [Google Scholar]
- 5.Lee H, Jang HC, Park HK, Cho NH. Early manifestation of cardiovascular disease risk factors in offspring of mothers with previous history of gestational diabetes mellitus. Diabetes Res Clin Pract. 2007;78:238–45. doi: 10.1016/j.diabres.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia. 2002;45:991–6. doi: 10.1007/s00125-002-0865-y. [DOI] [PubMed] [Google Scholar]
- 7.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol. 2007;50:972–9. doi: 10.1097/GRF.0b013e31815a61d6. [DOI] [PubMed] [Google Scholar]
- 8.Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21:149–57. doi: 10.1080/14767050801929430. [DOI] [PubMed] [Google Scholar]
- 9.Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab. 2005;90:3225–9. doi: 10.1210/jc.2005-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho NH, Silverman BL, Rizzo TA, Metzger BE. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. J Pediatr. 2000;136:587–92. doi: 10.1067/mpd.2000.105129. [DOI] [PubMed] [Google Scholar]
- 11.Mansford KR, Opie L. Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. Lancet. 1968;1:670–1. doi: 10.1016/s0140-6736(68)92103-x. [DOI] [PubMed] [Google Scholar]
- 12.Holemans K, Gerber RT, Meurrens K, De Clerck F, Poston L, Van Assche FA. Streptozotocin diabetes in the pregnant rat induces cardiovascular dysfunction in adult offspring. Diabetologia. 1999;42:81–9. doi: 10.1007/s001250051117. [DOI] [PubMed] [Google Scholar]
- 13.Rocha SO, Gomes GN, Forti AL, et al. Long-term effects of maternal diabetes on vascular reactivity and renal function in rat male offspring. Pediatr Res. 2005;58:1274–9. doi: 10.1203/01.pdr.0000188698.58021.ff. [DOI] [PubMed] [Google Scholar]
- 14.Wichi RB, Souza SB, Casarini DE, Morris M, Barreto-Chaves ML, Irigoyen MC. Increased blood pressure in the offspring of diabetic mothers. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1129–33. doi: 10.1152/ajpregu.00366.2004. [DOI] [PubMed] [Google Scholar]
- 15.Duong Van Huyen JP, Vessieres E, Perret C, et al. In utero exposure to maternal diabetes impairs vascular expression of prostacyclin receptor in rat offspring. Diabetes. 2010;59:2597–602. doi: 10.2337/db10-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes GN, Gil FZ. Prenatally programmed hypertension: role of maternal diabetes. Braz J Med Biol Res. 2011;44:899–904. doi: 10.1590/s0100-879x2011007500109. [DOI] [PubMed] [Google Scholar]
- 17.Segar EM, Norris AW, Yao JR, et al. Programming of growth, insulin resistance and vascular dysfunction in offspring of late gestation diabetic rats. Clin Sci (Lond) 2009;117:129–38. doi: 10.1042/CS20080550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon FJ, Matsuguchi H, Mark AL. Abnormal baroreflex control of heart rate in prehypertensive and hypertensive Dahl genetically salt-sensitive rats. Hypertension. 1981;3:I135–41. doi: 10.1161/01.hyp.3.3_pt_2.i135. [DOI] [PubMed] [Google Scholar]
- 19.Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006;27:327–32. doi: 10.1016/j.placenta.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Grissa O, Ategbo JM, Yessoufou A, et al. Antioxidant status and circulating lipids are altered in human gestational diabetes and macrosomia. Transl Res. 2007;150:164–71. doi: 10.1016/j.trsl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–62. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 22.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–7. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65:1339–48. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1941–52. doi: 10.1152/ajpregu.90724.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohmeier TE. The sympathetic nervous system and long-term blood pressure regulation. Am J Hypertens. 2001;14:147S–54S. doi: 10.1016/s0895-7061(01)02082-9. [DOI] [PubMed] [Google Scholar]
- 26.de Cavanal MF, Gomes GN, Forti AL, et al. The influence of L-arginine on blood pressure, vascular nitric oxide and renal morphometry in the offspring from diabetic mothers. Pediatr Res. 2007;62:145–50. doi: 10.1203/PDR.0b013e318098722e. [DOI] [PubMed] [Google Scholar]
- 27.MohanKumar SM, King A, Shin AC, Sirivelu MP, MohanKumar PS, Fink GD. Developmental programming of cardiovascular disorders: focus on hypertension. Rev Endocr Metab Disord. 2007;8:115–25. doi: 10.1007/s11154-007-9047-z. [DOI] [PubMed] [Google Scholar]
- 28.Nuyt AM, Alexander BT. Developmental programming and hypertension. 2009;18:144–52. doi: 10.1097/MNH.0b013e328326092c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segar JL, Roghair RD, Segar EM, Bailey MC, Scholz TD, Lamb FS. Early gestation dexamethasone alters baroreflex and vascular responses in newborn lambs before hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;291:R481–8. doi: 10.1152/ajpregu.00677.2005. [DOI] [PubMed] [Google Scholar]
- 30.Nehiri T, Duong Van Huyen JP, Viltard M, et al. Exposure to maternal diabetes induces salt-sensitive hypertension and impairs renal function in adult rat offspring. Diabetes. 2008;57:2167–75. doi: 10.2337/db07-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinalski M, Sledziewski A, Telejko B, Zarzycki W, Kinalska I. Antioxidant therapy and streptozotocin-induced diabetes in pregnant rats. Acta diabetologica. 1999;36:113–7. doi: 10.1007/s005920050153. [DOI] [PubMed] [Google Scholar]
- 32.Raza H, John A. Glutathione metabolism and oxidative stress in neonatal rat tissues from streptozotocin-induced diabetic mothers. Diabetes Metab Res Rev. 2004;20:72–8. doi: 10.1002/dmrr.422. [DOI] [PubMed] [Google Scholar]
- 33.Tarry-Adkins JL, Chen JH, Jones RH, Smith NH, Ozanne SE. Poor maternal nutrition leads to alterations in oxidative stress, antioxidant defense capacity, and markers of fibrosis in rat islets: potential underlying mechanisms for development of the diabetic phenotype in later life. FASEB J. 2010;24:2762–71. doi: 10.1096/fj.10-156075. [DOI] [PubMed] [Google Scholar]
- 34.Shen JZ, Zheng XF, Kwan CY. Differential contractile actions of reactive oxygen species on rat aorta: selective activation of ATP receptor by H2O2. Life Sci. 2000;66:PL291–6. doi: 10.1016/s0024-3205(00)00539-7. [DOI] [PubMed] [Google Scholar]
- 35.Demirci B, McKeown PP, Dvm UB. The bimodal regulation of vascular function by superoxide anion: role of endothelium. BMB Rep. 2008;41:223–9. doi: 10.5483/bmbrep.2008.41.3.223. [DOI] [PubMed] [Google Scholar]
- 36.Chamiot-Clerc P, Renaud JF, Safar ME. Pulse pressure, aortic reactivity, and endothelium dysfunction in old hypertensive rats. Hypertension. 2001;37:313–21. doi: 10.1161/01.hyp.37.2.313. [DOI] [PubMed] [Google Scholar]
- 37.Demirci B, McKeown PP, Bayraktutan U. Blockade of angiotensin II provides additional benefits in hypertension- and ageing-related cardiac and vascular dysfunctions beyond its blood pressure-lowering effects. J Hypertens. 2005;23:2219–27. doi: 10.1097/01.hjh.0000191906.03983.ee. [DOI] [PubMed] [Google Scholar]
- 38.Gomes P, Simao S, Silva E, et al. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxidative medicine and cellular longevity. 2009;2:138–45. doi: 10.4161/oxim.2.3.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvia L, Miriam P, Petr K, Jaroslav K, Josef Z. Effects of aging and hypertension on the participation of endothelium-derived constricting factor (EDCF) in norepinephrine-induced contraction of rat femoral artery. European journal of pharmacology. 2011;667:265–70. doi: 10.1016/j.ejphar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 40.Butler DG, Butt DA, Puskas D, Oudit GY. Angiotensin II-mediated catecholamine release during the pressor response in rats. J Endocrinol. 1994;142:19–28. doi: 10.1677/joe.0.1420019. [DOI] [PubMed] [Google Scholar]
- 41.Gordon FJ, Mark AL. Impaired baroreflex control of vascular resistance in prehypertensive Dahl S rats. Am J Physiol. 1983;245:H210–7. doi: 10.1152/ajpheart.1983.245.2.H210. [DOI] [PubMed] [Google Scholar]
- 42.Hashmi-Hill MP, Graves JE, Sandock K, Bates JN, Robertson TP, Lewis SJ. Hemodynamic responses elicited by systemic injections of flavin adenine dinucleotide in anesthetized rats. J Cardiovasc Pharmacol. 2007;50:94–102. doi: 10.1097/FJC.0b013e31805c162a. [DOI] [PubMed] [Google Scholar]
- 43.Kober T, Konig I, Weber M, Kojda G. Diethyldithiocarbamate inhibits the catalytic activity of xanthine oxidase. FEBS Lett. 2003;551:99–103. doi: 10.1016/s0014-5793(03)00876-7. [DOI] [PubMed] [Google Scholar]
- 44.Shastri S, Gopalakrishnan V, Poduri R, Di Wang H. Tempol selectively attenuates angiotensin II evoked vasoconstrictor responses in spontaneously hypertensive rats. J Hypertens. 2002;20:1381–91. doi: 10.1097/00004872-200207000-00025. [DOI] [PubMed] [Google Scholar]
- 45.Roghair RD, Miller FJ, Scholz TD, Lamb FS, Segar JL. Endothelial superoxide production is altered in sheep programmed by early gestation dexamethasone exposure. Neonatology. 2008;93:19–27. doi: 10.1159/000105521. [DOI] [PubMed] [Google Scholar]