Abstract
No previous study addressed whether in the general population estimated glomerular filtration rate (eGFR [Chronic Kidney Disease Epidemiology Collaboration formula]) adds to the prediction of cardiovascular outcome over and beyond ambulatory blood pressure. We recorded health outcomes in 5322 subjects (median age, 51.8 years; 43.1% women) randomly recruited from 11 populations, who had baseline measurements of 24-hour ambulatory blood pressure (ABP24) and eGFR. We computed hazard ratios using multivariable-adjusted Cox regression. Median follow-up was 9.3 years. In fully adjusted models, which included both ABP24 and eGFR, ABP24 predicted (P≤0.008) both total (513 deaths) and cardiovascular (206) mortality; eGFR only predicted cardiovascular mortality (P=0.012). Furthermore, ABP24 predicted (P≤0.0056) fatal combined with nonfatal events as a result of all cardiovascular causes (555 events), cardiac disease (335 events), or stroke (218 events), whereas eGFR only predicted the composite cardiovascular end point and stroke (P≤0.035). The interaction terms between ABP24 and eGFR were all nonsignificant (P≥0.082). For cardiovascular mortality, the composite cardiovascular end point, and stroke, ABP24 added 0.35%, 1.17%, and 1.00% to the risk already explained by cohort, sex, age, body mass index, smoking and drinking, previous cardiovascular disease, diabetes mellitus, and antihypertensive drug treatment. Adding eGFR explained an additional 0.13%, 0.09%, and 0.14%, respectively. Sensitivity analyses stratified for ethnicity, sex, and the presence of hypertension or chronic kidney disease (eGFR <60 mL/min per 1.73 m2) were confirmatory. In conclusion, in the general population, eGFR predicts fewer end points than ABP24. Relative to ABP24, eGFR is as an additive, not a multiplicative, risk factor and refines risk stratification 2- to 14-fold less.
Keywords: ambulatory blood pressure, population science, renal function, cardiovascular risk factors, epidemiology
Hypertension and chronic kidney disease affect a large proportion of the world’s adult population. Both entities substantially increase the risk of death, cardiovascular disorders, and progression to renal failure, even after accounting for other risk factors.1 Hypertension and renal disease behave as aggregate risk factors, high blood pressure causing renal impairment, and vice versa.1 Several population studies proved that chronic kidney disease at entry predicted cardiovascular mortality and morbidity2-10 and also suggested that people with lower glomerular filtration rate (GFR) within the normal range did not have a significantly higher cardiovascular risk.9
Worldwide, blood pressure is the predominant risk factor without threshold above which the event rate suddenly rises. Ambulatory blood pressure (ABP) monitoring substantially refines the risk stratification in patients with hypertension11 or end-stage renal disease,12 and in population samples.13-16 To our knowledge, no previous study addressed whether in the general population the estimated GFR (eGFR) from the serum creatinine concentration adds to the prediction of cardiovascular outcome over and beyond the 24-hour ambulatory blood pressure (ABP24). We addressed this question in 5322 participants randomly recruited from 11 populations and enrolled in the International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO).
Methods
Study Population
At the time of writing this report, the IDACO17 included 11 randomly recruited population cohorts and 11 785 participants with available data on the conventional and ABP. We excluded 2361 participants, because they were younger than 18 years (n=252), because their conventional blood pressure was not on the database (n=219), or because they had <10 daytime or 5 nighttime blood pressure readings (n=1890). We additionally disregarded 4017 subjects because serum creatinine had not been measured at enrollment and 85 subjects because their serum creatinine concentration was >3 SDs higher than the center- and sex-specific group mean. Thus, the total number of subjects included in the present analysis totaled 5322 (for details, see the expanded Methods in the online-only Data Supplement).
Blood Pressure Measurement
Methods used for conventional and ABP measurement are described in detail in the expanded Methods (online-only Data Supplement). Hypertension was a conventional blood pressure of ≥140 mm Hg systolic or 90 mm Hg diastolic or the use of antihypertensive drugs. Because of the prognostic superiority of ABP over conventional blood pressure, we based our analysis on the former.15 We focused on systolic blood pressure, because in middle-aged and older adults this is the predominant risk factor, both on conventional18 and ambulatory measurement.16
Assessment of Renal Function
To measure the serum creatinine concentration, all laboratories applied Jaffe’s method19 with modifications described elsewhere20,21 to overcome interferences and limitations. The samples were run on automated analyzers in certified laboratories that participated in external quality control programs. We used the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI)22 to estimate the GFR from sex, age, and the serum creatinine concentration. In a subsample of 2962 participants (55.7%), we checked the presence of proteinuria by means of a semiquantitative dipstick method (n=1287)23-26 or by measurement of albumin in a 24-hour urine collection (n=1675).23,27 Proteinuria was a positive dipstick test (any degree) or a 24-hour urinary albumin excretion of ≥30 mg.28
Other Measurements
We used the questionnaires originally administered in each cohort to obtain information on each participant’s medical history and smoking and drinking habits. Body mass index was body weight in kilograms divided by height in meters squared. We measured serum cholesterol and blood glucose by automated enzymatic methods. Diabetes mellitus was the use of antidiabetic drugs, a fasting blood glucose concentration of ≥7.0 mmol/L,14,23-26,29 a random blood glucose concentration of ≥11.1 mmol/L,23,26,30 a self-reported diagnosis,23,30 or diabetes mellitus documented in practice or hospital records.
Ascertainment of Events
We ascertained vital status and the incidence of fatal and nonfatal diseases from the appropriate sources in each country, as described in previous publications.31-33 Fatal and nonfatal stroke did not include transient ischemic attacks. Coronary events encompassed death from ischemic heart disease, sudden death, nonfatal myocardial infarction, and coronary revascularization. Cardiac events comprised coronary end points and fatal and nonfatal heart failure. The composite cardiovascular end point included all aforementioned end points plus cardiovascular mortality. In all outcome analyses, we only considered the first event within each category.
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.1.3 (SAS Institute, Cary, NC). For comparison of means and proportions, we applied the large sample z test and the χ2 statistic, respectively. In exploratory analyses, we plotted incidence rates by center- and sex-specific quartiles of the distributions of the 24-hour systolic blood pressure and eGFR, while standardizing by the direct method for center, sex, and age (≤40, 40–60, and ≥60 years). We used Kaplan-Meier survival function estimates, and the log-rank test to compare incidence rates across center- and sex-specific quartiles of the 24-hour systolic blood pressure or eGFR. We applied Cox regression to compute standardized hazard ratios (HRs), which express the risk for a 1-SD increase in the independent variables. We checked the proportional hazards assumption by the Kolmogorov-type supremum test and by testing the interaction terms between follow-up duration and the risk variables. The HRs were adjusted for center, sex, age, body mass index, smoking and drinking, serum cholesterol, history of cardiovascular disease, diabetes mellitus, and treatment with antihypertensive drugs. To adjust for center, we pooled participants recruited in the framework of the European Project on Genes in Hypertension (Kraków, Novosibirsk, Padova, and Pilsen). Additionally, in fully adjusted models, when computing the HRs for the ABP24, we accounted for eGFR and vice versa. We tested heterogeneity in the HRs across subgroups by introducing the appropriate interaction term in the Cox model. We plotted the 10-year risk of all-cause mortality and cardiovascular events in relation to the 24-hour systolic blood pressure and eGFR. Finally, we applied the generalized R2 statistic (see Expanded Methods in the online-only Data Supplement)34 to assess the refinement in risk prediction by adding the 24-hour blood pressure or eGFR to Cox models on top of other covariables. Statistical significance was an α-level of <0.05 on 2-sided tests.
Results
Baseline Characteristics
The study population consisted of 3709 Europeans (69.7%), 531 Asians (10.0%), and 1082 South Americans (20.3%). The 5322 participants included 2293 women (43.1%). Hypertension was present in 2239, of whom 1035 (46.3%) were taking blood pressure–lowering drugs. Mean (±SD) age was 51.8±16.8 years. At enrollment, 1322 participants (24.8%) were smokers and 2380 (44.7%) reported intake of alcohol. In the whole study population, conventional blood pressure averaged (±SD) 131.1±21.0 mm Hg systolic and 79.9±11.4 mm Hg diastolic. The ABPs24 were 122.7±14.2 and 73.8±8.3 mm Hg, respectively. Mean serum creatinine was 0.99±0.17 mg/dL and mean eGFR 79.4±16.7 mL/min per 1.73 m2. eGFR was 45 to 60 mL/min per 1.73 m2 in 552 participants (10.4%) and 30 to 45 mL/min per 1.73 m2 in 58 (1.1%). Among the 2962 participants, who underwent testing for proteinuria, 67 had a positive dipstick test (any degree of proteinuria) and 179 had a 24-hour urinary albumin excretion of ≥30 mg; the number of participants with severe proteinuria on dipstick testing or having a 24-hour albuminuria in excess of 300 mg amounted only to 77 and 2, respectively.
The center- and sex-specific quantiles of serum creatinine and eGFR are listed in Tables S1 and S2 (available in the online-only Data Supplement). Across center- and sex-specific quartiles of eGFR, baseline characteristics were significantly different (P≤0.05), with the exception of the percentage of participants drinking alcohol (Table 1).
Table 1. Baseline Characteristics of Participants.
| Quartiles of Estimated Glomerular Filtration Rate |
||||
|---|---|---|---|---|
| Characteristic | Low | Medium-Low | Medium-High | High |
| Number (%) with characteristic | ||||
| All subjects in category | 1330 | 1331 | 1330 | 1331 |
| eGFR, mL/min per 1.73 m2 | 61.2 (9.4) | 73.8 (8.1) | 84.1 (8.5) | 98.4 (12.2) |
| European | 926 (69.6) | 928 (69.7) | 926 (69.6) | 929 (69.8) |
| Asian | 134 (10.1) | 132 (9.9) | 133 (10.0) | 132 (9.9) |
| South American | 270 (20.3) | 271 (20.4) | 271 (20.4) | 270 (20.3) |
| Women | 571 (42.9.) | 575 (43.2) | 573 (43.1) | 574 (43.1) |
| Antihypertensive treatment | 425 (32.0) | 276 (20.7) | 199 (15.0) | 135 (10.1) |
| Smokers | 262 (19.7) | 306 (23.0) | 365 (27.4) | 389 (29.2) |
| Using alcohol | 586 (44.1) | 602 (45.2) | 615 (46.2) | 577 (43.4) |
| Diabetes mellitus | 97 (7.3) | 63 (4.7) | 76 (5.7) | 69 (5.2) |
| Cardiovascular disorder | 216 (16.2) | 130 (10.0) | 103 (7.7) | 67 (5.0) |
| Mean±SD of characteristic | ||||
| Age, y | 60.6±12.8 | 53.6±14.9 | 49.4±16.2 | 43.3±17.6 |
| Body mass index, kg/m2 | 26.6±4.4 | 26.0±4.1 | 25.5±4.2 | 24.7±4.3 |
| Conventional pressure, mm Hg | ||||
| Systolic | 138.3±20.8 | 132.0±20.8 | 128.8±20.1 | 125.2±20.0 |
| Diastolic | 82.5±11.1 | 81.0±11.4 | 79.2±11.0 | 77.1±11.4 |
| Ambulatory pressure, mm Hg | ||||
| 24-h systolic | 126.4±14.5 | 123.3±14.7 | 121.5±13.7 | 119.8±13.3 |
| 24-h diastolic | 75.4±8.5 | 74.3±8.5 | 73.3±8.0 | 72.0±7.9 |
| Nighttime systolic | 115.3±15.9 | 112.0±15.8 | 110±14.4 | 108.6±13.6 |
| Nighttime diastolic | 66.5±9.7 | 65.1±9.6 | 63.7±8.7 | 62.7±8.9 |
| Daytime systolic | 132.1±15.4 | 129.5±15.3 | 127.9±14.7 | 126.3±14.5 |
| Daytime diastolic | 80.2±9.2 | 79.7±9.2 | 78.8±8.8 | 77.4±8.6 |
| Blood glucose, mmol/L | 5.5±1.7 | 5.3±1.3 | 5.3±1.5 | 5.1±1.3 |
| Serum cholesterol, mmol/L | 5.8±1.1 | 5.7±1.1 | 5.5±1.2 | 5.3±1.1 |
Baseline characteristics of participants by center- and sex-specific quartiles of eGFR. All between-quartile differences were significant (P≤0.05) with the exception of drinking (P=0.40). Because quartiles were center- and sex-specific, the proportion of Europeans, Asians, and South Americans and women and men was similar across quartiles. To convert cholesterol and glucose from mmol/L to mg/dL, divide by 0.0259 and 0.0555, respectively. eGFR indicates estimated glomerular filtration rate.
Incidence of Events
In the overall study population, the median follow-up was 9.3 years (fifth to 95th percentile interval, 2.5–17.4 years). Across centers, median follow-up ranged from 2.5 years (fifth to 95th percentile interval, 2.3–2.6 years) in JingNing to 17.8 years (16.6–18.2 years) in Dublin. During 50 587 person-years of follow-up, 513 participants died (10.1 per 1000 person-years) and 555 experienced a fatal or nonfatal cardiovascular complication (11.0 per 1000 person-years). Mortality included 206 cardiovascular and 275 noncardiovascular deaths, 27 deaths from unknown cause, and 5 deaths because of renal failure. Considering cause-specific first cardiovascular events, the incidence of fatal and nonfatal stroke amounted to 27 and 170, respectively. Cardiac events consisted of 30 fatal and 129 nonfatal cases of acute myocardial infarction, 21 deaths from ischemic heart diseases, 5 sudden deaths, 12 fatal and 75 nonfatal cases of heart failure, and 48 cases of surgical or percutaneous coronary revascularization. The composite cardiovascular end point also includes 20 fatal cases of peripheral arterial disease and 18 unspecified cardiovascular deaths.
Exploratory Analyses
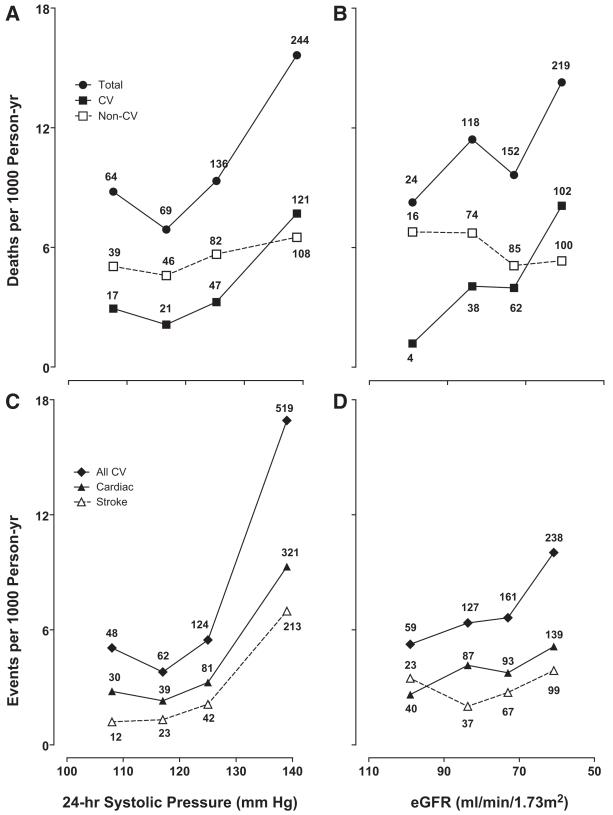
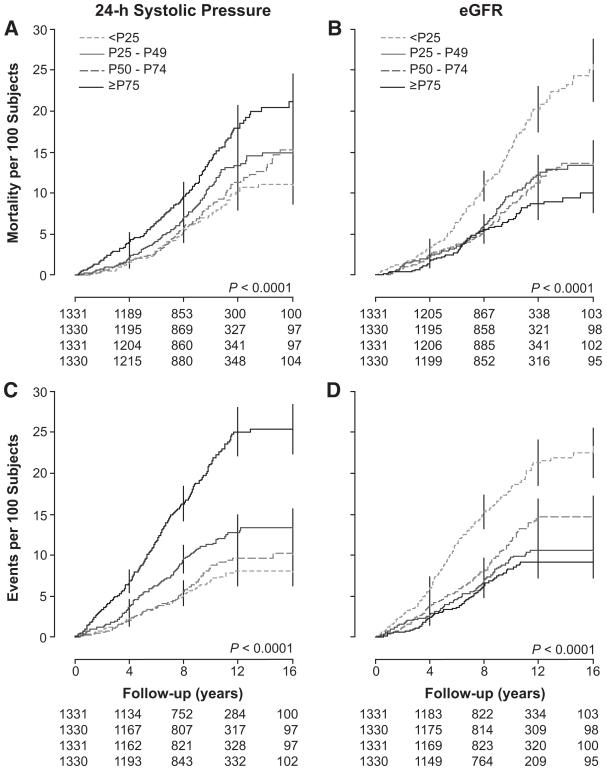
In exploratory analyses, we plotted death and event rates standardized for center, sex, and age across quartiles of the 24-hour systolic blood pressure and eGFR (Figure 1). Rates consistently increased with higher blood pressure and lower eGFR. The P values for linear trend were significant (P≥0.039) with the exception of those for noncardiovascular mortality (P≥0.71). We obtained similar findings by plotting Kaplan-Meier survival function estimates (Figure 2) for cardiovascular mortality and the composite cardiovascular end points by center- and sex-specific quartiles of the 24-hour systolic blood pressure and eGFR. P values for the corresponding log-rank tests were significant (P<0.0001).
Figure 1.
Incidence of total mortality (A and B) and cardiovascular (CV) events (C and D) across quartiles of the 24-hour systolic blood pressure (A and C) and estimated glomerular filtration rate (eGFR) (B and D). The scale on the horizontal axis is ascending for blood pressure and descending for eGFR. Incidence rates were standardized for center, sex, and age groups (<40, 40-60, and ≥60 years) by the direct method. The number of events contributing to the rates is presented. All P values for trend were significant.
Figure 2.
Kaplan-Meier survival function estimates for total mortality (A and B) and for all cardiovascular events (C and D) by center- and sex-specific quartiles of the 24-hour systolic blood pressure (A and C) and eGFR (B and D). All P values for the log-rank test were significant. eGFR indicates estimated glomerular filtration rate.
Mortality
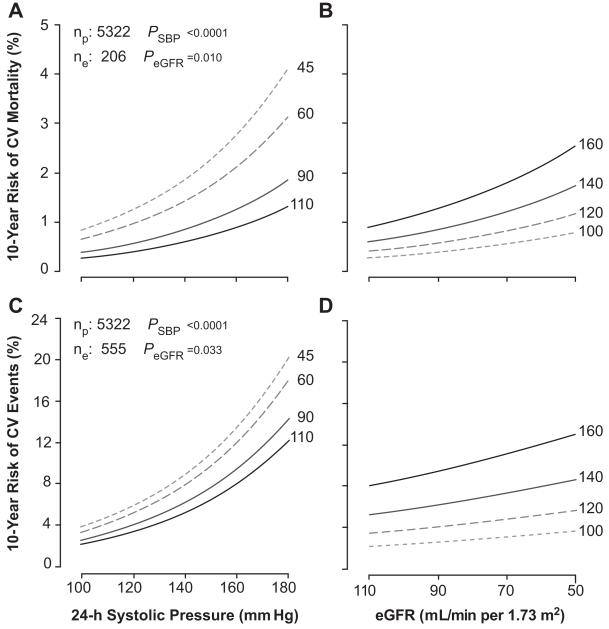
In multivariable Cox models, we adjusted for center, sex, age, body mass index, smoking and drinking, serum cholesterol, history of cardiovascular disease, diabetes mellitus, and antihypertensive drug treatment. In adjusted models, not including eGFR (Table 2), the 24-hour systolic blood pressure predicted both total and cardiovascular mortality (P≤0.008), but not noncardiovascular mortality (P=0.46). In adjusted models, not including the 24-hour systolic blood pressure, eGFR predicted cardiovascular mortality (P=0.012), but not all-cause and non-cardiovascular mortality (P≥0.23). In fully adjusted models, which included both 24-hour systolic blood pressure and eGFR, these findings were consistent. The R2 statistics for adding eGFR as a predictor of cardiovascular mortality over and beyond the 24-hour systolic blood pressure was 0.13% (Table 3), which was 2.6 times less than for adding the 24-hour systolic blood pressure. The interaction terms between 24-hour systolic blood pressure and eGFR in relation to the mortality outcomes were nonsignificant (0.082≤P≤0.89). Figure 3 shows the 10-year absolute risk of cardiovascular mortality associated with the 24-hour systolic blood pressure and eGFR in the whole study population.
Table 2. Standardized Hazard Ratios in Relation to 24-h Systolic Blood Pressure and Estimated Glomerular Filtration Rate in 5322 Participants.
| 24-h Systolic Pressure |
1/HR eGFR |
||||
|---|---|---|---|---|---|
| End Point (No.) | Model | Hazard Ratio | P Value | Hazard Ratio | P Value |
| Mortality | |||||
| All causes (513) | A | 1.12 (1.03–1.22) | 0.0081 | 1.05 (0.91–1.20) | 0.50 |
| FA | 1.12 (1.03–1.22) | 0.0080 | 1.05 (0.91–1.21) | 0.49 | |
| Cardiovascular (206) | A | 1.33 (1.18–1.50) | <0.0001 | 1.34 (1.07–1.67) | 0.012 |
| FA | 1.33 (1.18–1.50) | <0.0001 | 1.35 (1.07–1.69) | 0.010 | |
| Noncardiovascular (275) | A | 0.96 (0.85–1.08) | 0.46 | 0.89 (0.74–1.08) | 0.23 |
| FA | 0.95 (0.84–1.08) | 0.46 | 0.89 (0.74–1.08) | 0.23 | |
| Fatal plus nonfatal events | |||||
| All cardiovascular (555) | A | 1.37 (1.27–1.47) | <0.0001 | 1.15 (1.01–1.31) | 0.035 |
| FA | 1.37 (1.27–1.47) | <0.0001 | 1.15 (1.01–1.32) | 0.033 | |
| Cardiac (335) | A | 1.28 (1.16–1.41) | <0.0001 | 1.08 (0.91–1.27) | 0.40 |
| FA | 1.28 (1.16–1.41) | <0.0001 | 1.07 (0.91–1.27) | 0.40 | |
| Coronary (257) | A | 1.18 (1.05–1.32) | 0.0056 | 1.07 (0.89–1.30) | 0.46 |
| FA | 1.18 (1.05–1.32) | 0.0055 | 1.08 (0.89–1.30) | 0.45 | |
| Stroke (218) | A | 1.57 (1.40–1.76) | <0.0001 | 1.33 (1.08–1.64) | 0.009 |
| FA | 1.57 (1.40–1.76) | <0.0001 | 1.34 (1.08–1.66) | 0.007 | |
eGFR is the glomerular filtration rate estimated from the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI), as given in reference 22. Hazard ratios, presented with 95% CI, express the risk associated with a 1-SD increase in 24-h systolic blood pressure or a 1-SD decrease in eGFR. For eGFR, the inverse of the hazard ratio is presented, so that higher values, associated with lower eGFR, reflect higher risk. All models were adjusted for center, sex, age, body mass index, smoking and drinking, serum cholesterol, diabetes mellitus, history of cardiovascular disease, and antihypertensive treatment. Adjusted models (A) include either the 24-h systolic blood pressure or eGFR, whereas fully adjusted models (FA) include both 24-h systolic blood pressure and eGFR in addition to the aforementioned covariables. eGFR indicates estimated glomerular filtration rate.
Table 3. Predictive Value of the Cox Regression Models.
| Cardiovascular Mortality |
Cardiovascular Events |
Stroke |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Models | Likelihood Ratio | P Value | R2 (%) | Likelihood Ratio | P Value | R2 (%) | Likelihood Ratio | P Value | R2 (%) |
| Basic model | 445.0 | … | 8.03 | 809.7 | … | 14.1 | 353.6 | … | 6.40 |
| 24-h systolic pressure added to basic model |
19.3 | <0.0001 | 0.35 | 62.7 | <0.0001 | 1.17 | 53.3 | <0.0001 | 1.00 |
| eGFR added to basic model | 6.8 | 0.012 | 0.12 | 4.4 | 0.035 | 0.08 | 7.0 | 0.009 | 0.13 |
| eGFR added to basic model also including 24-h systolic pressure |
6.7 | 0.010 | 0.13 | 4.6 | 0.033 | 0.09 | 7.2 | 0.007 | 0.14 |
The basic model included cohort, sex, age, body mass index, smoking and drinking, serum cholesterol, history of cardiovascular disease, diabetes mellitus, and treatment with antihypertensive drugs. P values are for the improvement of the fit across nested models. Values are likelihood ratios and associated P values and generalized R2 statistics for adding 24-h systolic blood pressure or eGFR to the reference model. eGFR indicates estimated glomerular filtration rate.
Figure 3.
Ten-year absolute risk of cardiovascular (CV) mortality (A and B) and all cardiovascular end points (C and D) associated with the 24-hour systolic blood pressure (A and C) and estimated glomerular filtration rate (eGFR; B and D). Risk function estimates were standardized to the mean distribution in the whole study population of cohort, sex, age, body mass index, smoking and drinking, serum cholesterol, history of cardiovascular disease, diabetes mellitus, and treatment with antihypertensive drugs. The 24-hour blood pressure is represented by 4 risk functions corresponding with levels of 100, 120, 140, and 160 mm Hg and eGFR by 4 risk functions corresponding with levels of 45, 60, 90, and 110 mL/min per meter squared Plotted values of the eGFR and 24-hour systolic blood pressure span the fifth to 95th percentile interval. P values are for the independent effect of the estimated glomerular filtration rate (PeGFR) and 24-hour blood pressure (PSBP). np and ne indicate the number of participants at risk and the number of events.
Fatal and Nonfatal Cardiovascular Events
In adjusted analyses, not including eGFR, the 24-hour systolic blood pressure predicted all of the fatal combined with nonfatal outcomes (P≤0.0056). In adjusted models, not including the 24-hour systolic blood pressure, eGFR only predicted the composite cardiovascular end point and fatal plus nonfatal stroke (P≤0.035). In fully adjusted models, which included both 24-hour systolic blood pressure and eGFR, these findings were consistent. The R2 statistics for adding eGFR as predictor of the composite cardiovascular end point or stroke on top of 24-hour systolic blood pressure were 0.09% or 0.14%, respectively. The refinement in risk prediction by eGFR was 7 to 13 times less than for the 24-hour systolic blood pressure. The interaction terms between 24-hour systolic blood pressure and eGFR in relation to the combined fatal plus nonfatal cardiovascular outcomes were nonsignificant (0.31≤P≤0.78). Figure 3 shows the 10-year absolute risk of the combined cardiovascular end point associated with the 24-hour systolic blood pressure and eGFR in the whole study population.
Sensitivity Analyses
Using 24-hour diastolic instead of 24-hour systolic blood pressure produced results similar to those in Tables 2 and 3 (see Tables S3 and S4). Sensitivity analyses of cardiovascular mortality (Table S5) and the composite cardiovascular end point (Table S6), in which we excluded 1 center at a time, produced results similar to those in Table 2.
Furthermore, we did sensitivity analyses for cardiovascular mortality and the composite cardiovascular end point, while stratifying for sex, presence versus absence of hypertension on conventional blood pressure measurement, eGFR <60 versus ≥60 mL/min per 1.73 m2, and European, Asian, or South American ethnicity. In all strata, the 24-hour systolic blood pressure remained a significant predictor of cardiovascular mortality and the composite cardiovascular end point. In models including 24-hour systolic blood pressure and all other covariables, eGFR remained a significant predictor of cardiovascular mortality in 610 patients with eGFR <60 mL/min per 1.73 m2 (58 deaths; 1/HR, 4.32; 95% CI, 1.80–10.3; P=0.001) and in 2239 hypertensive patients (160 deaths; 1/HR, 1.35; CI, 1.04–1.75; P=0.026). eGFR also remained a significant predictor of the composite cardiovascular end point in 3029 men (444 events; 1/HR, 1.19; CI, 1.02–1.38; P=0.032) and 3709 Europeans (443 events; 1/HR, 1.25; 95% CI, 1.07–1.46; P=0.04). Results in participants with and without proteinuria are given in Table S7. The interaction terms of proteinuria (0.1) with the 24-hour systolic blood pressure or 1/eGFR were all nonsignificant (P≥0.06).
Discussion
Our current meta-analysis of individual data included >5322 people randomly recruited from 11 populations and covered on average 9.3 years of follow-up, during which 513 people died and 555 experienced a major cardiovascular complication. The key finding was that, while accounting for the 24-hour systolic blood pressure and other covariables, eGFR was a significant and independent predictor of cardiovascular mortality, all of the cardiovascular events combined and fatal and nonfatal stroke. However, the proportion of the risk explained by eGFR is low.
Several population studies confirmed that eGFR is a predictor of risk, especially at levels <60 mL/min per 1.73 m2.35 From January 2001 until December 2002, Quinn et al8 collected 1 967 827 serum creatinine measurements in 533 798 North Irish patients. Over 4.8 years, 59 980 deaths occurred. Using an eGFR of ≥60 mL/min per 1.73 m2 as reference and with adjustments applied for sex and age, the risk of death increased once eGFR fell to <45 mL/min per 1.73 m2. Findings for cardiovascular mortality (n=22 169) in this North Irish cohort were similar.8 The Nord-Trøndelag Health Study (HUNT II) included 9709 participants followed up for 8.3 years.2 With adjustments applied for sex, age, and the urinary albumin:creatinine ratio, cardiovascular mortality gradually increased, if eGFR was <75 mL/min per 1.73 m2. The Kaiser Permanente Renal Registry included 1 120 295 adults followed up for 2.83 years.10 In multivariable-adjusted analyses, the risk of death increased as eGFR decreased <60 mL/min per 1.73 m2; the HRs were 1.2 for eGFR 45 to 59 mL/min per 1.73 m2, 1.8 for eGFR 30 to 44 mL/min per 1.73 m2, 3.2 for eGFR 15 to 29 mL/min per 1.73 m2, and 5.9 for eGFR <15 mL/min per 1.73 m2. The adjusted HR for cardiovascular events also increased inversely with eGFR; the estimates were 1.4, 2.0, 2.8, and 3.4, respectively.10
The current study extends these previous findings in various ways. First, in addition to total and cardiovascular mortality, our analyses included all fatal combined with nonfatal cardiovascular end points and fatal and nonfatal cardiac events and stroke. Lower eGFR predicted the composite cardiovascular end point and stroke. Konishi et al36 followed 1809 Japanese patients, who underwent a complete coronary revascularization. Over 11.4 years, 127 strokes occurred. In multivariable-adjusted Cox regression, an eGFR of <60 mL/min per 1.73 m2 (321 patients) was associated with a 66% higher risk of stroke.36 Second, to our knowledge, our current study is the first to consider the relative contributions of the ABP24 and renal function as estimated from serum creatinine in risk stratification. Blood pressure was the overriding risk factor. However, our study population included <10% of participants with an eGFR of <60 mL/min per 1.73 m2 (Table S2). Previous studies demonstrated that the risk of all-cause and cardiovascular mortality exponentially increases below this threshold across the stages of renal dysfunction. Third, in contrast to other reports, we did not categorize eGFR according to proposed classification of renal dysfunction, but we analyzed eGFR as a continuous variable.
Equations to estimate the GFR based on the serum creatinine concentration are routinely used to assess renal function. The most commonly used equation is the Modification of Diet in Renal Disease (MDRD) Study equation.37 The MDRD equation was developed in patients with kidney disease and tends to underestimate the measured GFR at levels of 60 mL/min per 1.73 m2, and thus overestimate the prevalence of renal dysfunction. In the present study, we applied the newer CKD-EPI formula,22 which now has been tested in various populations and provides a more accurate estimate of the GFR.38 Recruitment of the population cohorts included in IDACO already started 30 years ago. To measure serum creatinine, from which eGFR is extrapolated, all centers applied Jaffe’s method,19 with modifications.20,21 However, it is unlikely that the serum creatinine levels are comparable across centers. Moreover, serum creatinine varies across ethnicities and is lower in women than in men. To overcome this limitation, we based part of our analyses on center- and sex-specific quartiles of eGFR. In analyses using eGFR as a continuous variable, we adjusted for center and sex. Finally, our results remained consistent, when we excluded one cohort at a time or when stratified for sex or ethnicity.
The strong points of our current report are the use of ambulatory monitoring to assess blood pressure; the relatively large sample size representing populations from Europe, Asia, and South America; and the large number of events, which occurred over a median follow-up of >10 years. Nevertheless, our study also has limitations. First, ≈4000 participants did not have a measurement of serum creatinine at baseline. Participants having a serum creatinine measurement were not specifically selected. Second, several investigators demonstrated that eGFR and albuminuria had additive value in profiling the risk of adverse outcomes in patients39,40 as well as populations.2,6 We did not systematically collect information on proteinuria in the IDACO cohorts. Nevertheless, we did a sensitivity analysis in 2962 participants, in whom information on proteinuria had been collected, albeit with different methods. The nonsignificant interaction terms suggested that in our cohorts, proteinuria did not modify the risk prediction provided by the 24-hour blood pressure or eGFR. However, the subgroup with proteinuria was small and experienced too few events to run a robust analysis. Third, our results suggest that 24-hour systolic blood pressure and eGFR act as additive risk factors for selected outcomes and that they do not potentiate one another. However, the power to demonstrate a significant interaction in Cox regression is generally low. Fourth, the range of systolic blood pressure in our current analysis was wide and included 42.1% patients with hypertension. In contrast, eGFR was reduced to 45 to 60 mL/min per 1.73 m2 (chronic kidney disease stage 3A28) in only 552 participants (10.4%) and to 30 to 45 mL/min per 1.73 m2 (stage 3B28) in only 58 (1.1%). This might explain why blood pressure and eGFR did not behave as synergistic risk factors. Our current results can therefore also not be extrapolated to patients with reduced renal function. Fifth, the R2 statistic is not a perfect measure of the variation explained by Cox models. R2 values can be compared within, but not across studies because of the dependence on censoring. Nevertheless, a measure of explained variance is crucial for the correct interpretation of the prognostic value of a risk factor. P values of HRs do not suffice to compare indicators of risk. Finally, our analysis rested on 11 population-based cohorts with an overrepresentation of European subjects and might not be representative for other ethnic groups, in particular blacks, who might be more susceptible to renal dysfunction.
Perspectives
In the general population, at levels predominantly >60 mL/min per 1.73 m2, eGFR is a weaker predictor of outcome than the 24-hour systolic blood pressure. This does not mean that clinicians should lose interest in measuring glomerular filtration as an index of renal dysfunction or to refine risk stratification. Serum creatinine and creatinine clearance, although most widely used, might lack sensitivity, in particular in the presence of obesity or advanced age. Cystatin C and β-trace protein might be more reliable biomarkers not only to screen for renal dysfunction but also to stratify for cardiovascular risk and to predict the long-term outcome of a variety of patients.41-43 Finally, we are currently addressing the role of the self-measured blood pressure at home in refining risk prediction over and beyond classic risk factors.44 We will investigate whether we can confirm our current findings, if home rather than ABP measurement is used in conjunction with eGFR.
Supplementary Material
Novelty and Significance.
What Is New?
No previous study addressed whether in the general population eGFR predicts cardiovascular outcome over and beyond the ABP24. Population studies on eGFR report only on total and cardiovascular mortality, but not on fatal combined with nonfatal outcomes.
What Is Relevant?
Fully adjusted Cox models included both ABP24 and eGFR. ABP24 predicted (P≤0.008) both total (513 deaths) and cardiovascular (206) mortality; eGFR only predicted cardiovascular mortality (P=0.012). Furthermore, ABP24 predicted (P≤0.0056) fatal combined with nonfatal events as a result of all cardiovascular causes (555 events), cardiac disease (335), or stroke (218), whereas eGFR only predicted (P≤0.035) the composite cardiovascular end point and stroke. The interaction terms between ABP24 and eGFR were nonsignificant (P≥0.082).
Summary
Relative to ABP24, eGFR is as an additive risk factor but refines risk stratification less than ABP24.
Acknowledgments
We gratefully acknowledge the expert assistance of Sandra Covens and Sonja Zuba (Studies Coordinating Center, Leuven, Belgium). The IDACO investigators are listed in reference 17 and in the online-only Data Supplement.
Sources of Funding
This study was supported by the European Union (grants IC15-CT98-0329-EPOGH, LSHM-CT-2006-037093 InGenious HyperCare, HEALTH-F4-2007-201550 HyperGenes and HEALTH-F7-2011-278249 EU-MASCARA, and the European Research Council Advanced Researcher grant 294713 EPLORE) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (grant G.0734.09). The European Union (grants LSHM-CT-2006-037093 and HEALTH-F4-2007-201550) also supported the research groups in Shanghai, Kraków, Padova, and Novosibirsk. The Danish Heart Foundation (grant 01-2-9-9A-22914) and the Lundbeck Fonden (grant R32-A2740) supported the studies in Copenhagen. The Ohasama study received support via Grant-in-Aid for Scientific Research (22590767, 22790556, 23249036, 23390171, and 23790242) from the Ministry of Education, Culture, Sports, Science and Technology, Japan; Health Labor Sciences Research Grant (H23-Junkankitou [Seishuu]-Ippan-005) from the Ministry of Health, Labor, and Welfare, Japan; Japan Arteriosclerosis Prevention Fund; and a grant from the Central Miso Research Institute, Tokyo, Japan. The National Natural Science Foundation of China (grants 30871360 and 30871081), Beijing, China, and the Shanghai Commissions of Science and Technology (grant 07JC14047 and the Rising Star program 06QA14043) and Education (grant 07ZZ32 and the Dawn project) supported the JingNing study in China. The Comisión Sectorial de Investigación Científica de la Universidad de la República (grant I+D GEFA-HT-UY) and the Agencia Nacional de Innovación e Investigación supported research in Uruguay.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.112.197376/-/DC1.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med. 2007;167:2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama M, Metoki H, Terawaki H, Ohkubo T, Kikuya M, Sato T, Nakayama K, Asayama K, Inoue R, Hashimoto J, Totsune K, Hoshi H, Ito S, Imai Y. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population—the Ohasama study. Nephrol Dial Transplant. 2007;22:1910–1915. doi: 10.1093/ndt/gfm051. [DOI] [PubMed] [Google Scholar]
- 4.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 5.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–2182. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 6.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M, Alberta Kidney Disease Network Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 7.Choi SW, Kim HY, Lee YH, Ryu SY, Kweon SS, Rhee JA, Choi JS, Shin MH. eGFR is associated with subclinical atherosclerosis independent of albuminuria: the Dong-gu Study. Atherosclerosis. 2010;212:661–667. doi: 10.1016/j.atherosclerosis.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 8.Quinn MP, Cardwell CR, Kee F, Maxwell AP, Savage G, McCarron P, Fogarty DG. The finding of reduced estimated glomerular filtration rate is associated with increased mortality in a large UK population. Nephrol Dial Transplant. 2011;26:875–880. doi: 10.1093/ndt/gfq505. [DOI] [PubMed] [Google Scholar]
- 9.Di Angelantonio E, Chowdhury R, Sarwar N, Aspelund T, Danesh J, Gudnason V. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ. 2010;341:c4986. doi: 10.1136/bmj.c4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 11.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Pede S, Porcellati C. Ambulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertension. Hypertension. 1998;32:983–988. doi: 10.1161/01.hyp.32.6.983. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55:762–768. doi: 10.1161/HYPERTENSIONAHA.109.144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkubo T, Hozawa A, Nagai K, Kikuya M, Tsuji I, Ito S, Satoh H, Hisamichi S, Imai Y. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama study. J Hypertens. 2000;18:847–854. doi: 10.1097/00004872-200018070-00005. [DOI] [PubMed] [Google Scholar]
- 14.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006;19:243–250. doi: 10.1016/j.amjhyper.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Hansen TW, Kikuya M, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Jeppesen J, Ibsen H, Imai Y, Staessen JA, IDACO Investigators Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25:1554–1564. doi: 10.1097/HJH.0b013e3281c49da5. [DOI] [PubMed] [Google Scholar]
- 16.Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation. 2007;115:2145–2152. doi: 10.1161/CIRCULATIONAHA.106.662254. [DOI] [PubMed] [Google Scholar]
- 17.Thijs L, Hansen TW, Kikuya M, et al. IDACO Investigators. The International Database of Ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12:255–262. doi: 10.1097/mbp.0b013e3280f813bc. [DOI] [PubMed] [Google Scholar]
- 18.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe M. Über den Niederschlag, welchen Pikrinsäure in normalen Harn erzeugt und über eine neue Reaction des Kreatinins. Z Physiol Chem. 1886;10:391–400. [Google Scholar]
- 20.Bowers LD, Wong ET. Kinetic serum creatinine assays. II. A critical evaluation and review. Clin Chem. 1980;26:555–561. [PubMed] [Google Scholar]
- 21.Peake M, Whiting M. Measurement of serum creatinine—current status and future goals. Clin Biochem Rev. 2006;27:173–184. [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staessen JA, Bieniaszewski L, O’Brien ET, Imai Y, Fagard R. An epidemiological approach to ambulatory blood pressure monitoring: the Belgian Population Study. Blood Press Monit. 1996;1:13–26. [PubMed] [Google Scholar]
- 24.Kuznetsova T, Malyutina S, Pello E, Thijs L, Nikitin Y, Staessen JA. Ambulatory blood pressure of adults in Novosibirsk, Russia: interim report on a population study. Blood Press Monit. 2000;5:291–296. doi: 10.1097/00126097-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Kuznetsova T, Staessen JA, Kawecka-Jaszcz K, Babeanu S, Casiglia E, Filipovsky J, Nachev C, Nikitin Y, Peleskã J, O’Brien E. Quality control of the blood pressure phenotype in the European Project on Genes in Hypertension. Blood Press Monit. 2002;7:215–224. doi: 10.1097/00126097-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Schettini C, Bianchi M, Nieto F, Sandoya E, Senra H, Hypertension Working Group Ambulatory blood pressure: normality and comparison with other measurements. Hypertension. 1999;34(4 pt 2):818–825. doi: 10.1161/01.hyp.34.4.818. [DOI] [PubMed] [Google Scholar]
- 28.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 29.Ingelsson E, Björklund-Bodegård K, Lind L, Arnlöv J, Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Wang JG, Gao P, Guo H, Nawrot T, Wang G, Qian Y, Staessen JA, Zhu D. Are published characteristics of the ambulatory blood pressure generalizable to rural Chinese? The JingNing population study. Blood Press Monit. 2005;10:125–134. doi: 10.1097/00126097-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Thijs L, Hansen TW, et al. International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55:1040–1048. doi: 10.1161/HYPERTENSIONAHA.109.137273. [DOI] [PubMed] [Google Scholar]
- 32.Hansen TW, Thijs L, Li Y, et al. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. doi: 10.1161/HYPERTENSIONAHA.109.140798. [DOI] [PubMed] [Google Scholar]
- 33.Boggia J, Thijs L, Hansen TW, et al. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators Ambulatory blood pressure monitoring in 9357 subjects from 11 populations highlights missed opportunities for cardiovascular prevention in women. Hypertension. 2011;57:397–405. doi: 10.1161/HYPERTENSIONAHA.110.156828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillespie BW. [Accessed August 15, 2012];Use of generalized R-squared in Cox regression. APHA Scientific Session and Event Listing 2006. http://apha.confex.com/apha/134am/techprogram/paper_135906.htm.
- 35.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konishi H, Kasai T, Miyauchi K, Kajimoto K, Kubota N, Dohi T, Amano A, Daida H. Association of low glomerular filtration rate with the incidence of stroke in patients following complete coronary revascularization. Circ J. 2011;75:2372–2378. doi: 10.1253/circj.cj-10-1102. [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 38.Carter JL, Stevens PE, Irving JE, Lamb EJ. Estimating glomerular filtration rate: comparison of the CKD-EPI and MDRD equations in a large UK cohort with particular emphasis on the effect of age. QJM. 2011;104:839–847. doi: 10.1093/qjmed/hcr077. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal R, Light RP. GFR, proteinuria and circadian blood pressure. Nephrol Dial Transplant. 2009;24:2400–2406. doi: 10.1093/ndt/gfp074. [DOI] [PubMed] [Google Scholar]
- 40.Rahman M, Ford CE, Cutler JA, et al. ALLHAT Collaborative Research Group Long-term renal and cardiovascular outcomes in Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants by baseline estimated GFR. Clin J Am Soc Nephrol. 2012;7:989–1002. doi: 10.2215/CJN.07800811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jernberg T, Lindahl B, James S, Larsson A, Hansson LO, Wallentin L, Cystatin C. a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation. 2004;110:2342–2348. doi: 10.1161/01.CIR.0000145166.44942.E0. [DOI] [PubMed] [Google Scholar]
- 42.Akerblom Å , Wallentin L, Siegbahn A, Becker RC, Budaj A, Buck K, Giannitsis E, Horrow J, Husted S, Katus HA, Steg PG, Storey RF, Åsenblad N, James SK. Cystatin C and estimated glomerular filtration rate as predictors for adverse outcome in patients with ST-elevation and non-ST-elevation acute coronary syndromes: results from the Platelet Inhibition and Patient Outcomes study. Clin Chem. 2012;58:190–199. doi: 10.1373/clinchem.2011.171520. [DOI] [PubMed] [Google Scholar]
- 43.Bhavsar NA, Appel LJ, Kusek JW, Contreras G, Bakris G, Coresh J, Astor BC, AASK Study Group Comparison of measured GFR, serum creatinine, cystatin C, and β-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis. 2011;58:886–893. doi: 10.1053/j.ajkd.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niiranen TJ, Thijs L, Asayama K, Johansson JK, Ohkubo T, Kikuya M, Boggia J, Hozawa A, Sandoya E, Stergiou GS, Tsuji I, Jula AM, Imai Y, Staessen JA. The International Database of HOme blood pressure in relation to Cardiovascular Outcome (IDHOCO): moving from baseline characteristics to research perspectives. Hypertens Res. 2012 doi: 10.1038/hr.2012.97. Epub ahead of print doi: 10.1038/hr.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.