Abstract
Backgrounds & Aims
Clinical esophageal manometry can be technically challenging. We investigated the prevalence and causes of technically imperfect, high-resolution esophageal pressure topography (EPT) studies at a tertiary referral hospital.
Methods
We reviewed 2,000 consecutive clinical EPT studies that had been performed with consistent technique and protocol. A study was considered technically imperfect if there was a problem with pressure signal acquisition, if the catheter did not pass through the esophagogastric junction (EGJ), or if there were less than 7 evaluable swallows (without double-swallowing, etc). Data from the technically imperfect studies were interpreted blindly to determine a diagnosis; this diagnosis was compared with that based on chart review.
Results
We identified 414 technically imperfect studies (21% of the series). These were attributed to fewer than 7 evaluable swallows (58%), inability to traverse the EGJ (29%), sensor or thermal compensation malfunction (7%), and miscellaneous artifacts (6%). The most frequent causes of failure to traverse the EGJ were a large hiatal hernia (50%) and achalasia (24%). The condition most frequently associated with an incomplete swallow protocol was achalasia (33%). Despite the limitations, the diagnosis of achalasia was correctly achieved by blinded interpretation in 77% of cases and non-blinded interpretation in 94% of cases.
Conclusion
Technically imperfect EPT studies are common in a tertiary care center; large hiatal hernia and achalasia were the most frequent causes. However, despite the technical limitations, the data could still be interpreted, especially in the context of associated endoscopic and radiographic data.
Keywords: esophageal motor function, non-obstructive dysphagia, gastroesophageal reflux disease, High-resolution manometry, diagnostic yield
Introduction
Esophageal manometry is used in clinical practice to define esophageal motor function and guide treatment based on motor abnormalities. The main clinical indication is the evaluation of non obstructive dysphagia. However, other potential indications are the evaluation of chest pain and gastroesophageal reflux disease 1. Esophageal manometry is especially important in assessing dysphagia when achalasia is in the differential diagnosis.
Guidelines have been published to standardize the performance and interpretation of esophageal manometry 2. Recommendations involve the equipment, patient preparation, performance of the study, and analysis of the data. However these guidelines were devised for conventional manometry. High-resolution manometry (HRM) combines closely spaced pressure sensors and data presentation in the form of esophageal pressure topography plots (EPT). This technique offers several advantages compared to conventional manometry 3; most importantly, it increases the diagnostic yield in cases of dysphagia 4–5. Additionally, the analysis of EPT studies is more easily learned 6 and EPT may facilitate a better understanding of esophageal motor defects associated with poor bolus transit and symptoms. Thus, HRM is rapidly replacing conventional manometry in both research applications and clinical practice.
As EPT becomes increasingly utilized in clinical practice, there is an increasing need for guidelines both with respect to interpretation and to the technical aspects of the study. Guidelines developed for conventional manometry may not be universally applicable to HRM. Moreover, additional guidelines may be appropriate based on specific equipment and system characteristics. Although HRM has inherent advantages, its performance is still associated with both technical challenges and the more generic probe placement issues encountered with any manometric study. However, the format of EPT makes it easier to recognize these technical limitations and, potentially, to interpret studies cognizant of these limitations. Given these issues, a systematic analysis of limitations in EPT studies encountered in clinical practice may be helpful to establish guidelines of how to manage them. Consequently, the aims of this study were to determine the prevalence and causes of technically imperfect EPT studies experienced at a tertiary referral center and to ascertain how frequently an accurate diagnosis could still be obtained despite the limitations.
Methods
Subjects
A series of 2,000 clinical EPT studies performed from January 2007 to May 2010 done using a consistent technique (Manoscan ™ (Given Imaging, Los Angeles, CA)) were systematically reviewed. Studies were uniformly done without using protective sheaths on the HRM probes. Patients presented with diverse conditions consistent with an esophageal referral practice. The study protocol was approved by the Northwestern University Institutional Review Board.
Study protocol
HRM studies were done in a supine position after at least a 6-hr fast. The HRM catheter was a 4.2 mm outer diameter solid-state assemblies with 36 circumferential sensors at 1-cm intervals (Given Imaging, Los Angeles, CA). Transducers were calibrated at 0 and 300 mmHg using externally applied pressure. The manometry assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with about 3 intra-gastric sensors. The assembly was fixed in place by taping it to the nose. The study protocol included at least 30-s baseline recording and ten 5-ml swallows separated by at least 20 s. Patients were coached to swallow only once and on command to the degree that this was possible. The probe was removed before stopping the recording for post-study thermal compensation.
EPT analysis
EPT data were analyzed using ManoView ™ analysis software (Given Imaging, Los Angeles, CA). The data were corrected for the thermal sensitivity of the pressure sensors using the thermal compensation function. The criteria for categorizing a study as technically imperfect were: 1) problems with pressure signal acquisition or calibration (pressure sensor dysfunction, absence of post-study thermal compensation), 2) non-esophageal (vascular or cardiac) pressure artifact in the EPT plot obscuring the esophageal recording, 3) probe placement that failed to include the upper esophageal sphincter in the recording, 4) probe placement that failed to traverse the esophago-gastric junction (EGJ) or diaphragm, or 5) if there were less than 7 evaluable test swallows either because of an abbreviated study or because of consistent double-swallowing, belching, gagging, etc (Figure 1). These criteria were devised post hoc from review of the American Neurogastroenterology and Motility Society guidelines on components of a clinical manometry evaluation 2, adapting them as necessary for HRM. Given that the Chicago Classification defines esophageal motility disorders in EPT based on 10 evaluable swallows 7 a minimum of 7 swallows available for analysis seemed to be reasonable, especially to identify diagnoses which required at least 20% of abnormal swallows.
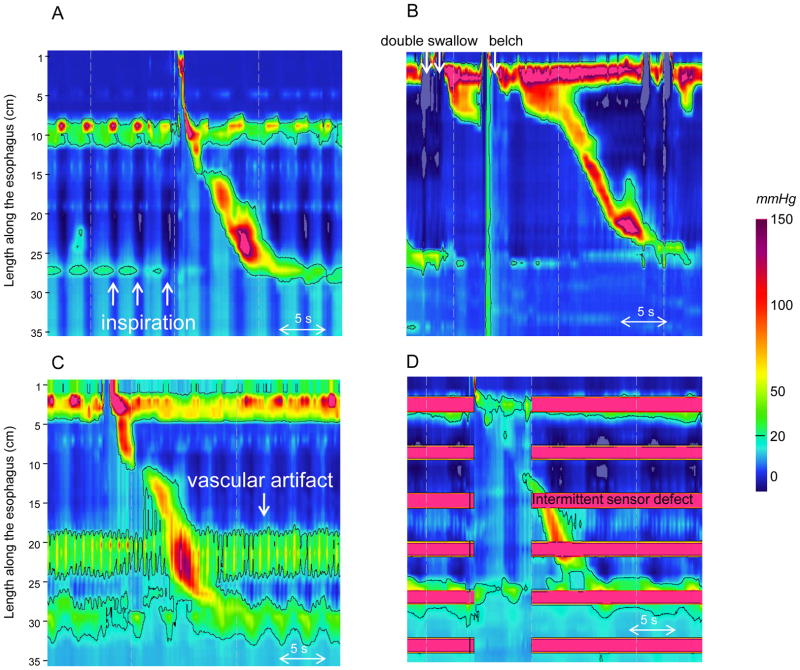
Figure 1.
Examples of technically imperfect studies. In Panel A the catheter did not pass through the EGJ because of a large hiatal hernia. During inspiration (white arrows), the pressure decreased in all the recording sensors indicating that all pressure sensors were in the chest. In Panel B, it was not possible to obtain 7 evaluable swallows because of double swallows and belches. In Panel C, a vascular artifact was observed in the distal esophagus. Panel D illustrates a technically imperfect study with multiple malfunctioning pressure sensors.
Technically imperfect studies were then blindly reviewed by 2 observers (LB/SR) to determine an EPT diagnosis despite the limitations. EPT diagnosis was based on established metrics measured on as many individual swallows as possible (integrated relaxation pressure (IRP), peristaltic integrity at 20-mmHg isobaric contour, contractile front velocity, distal contractile integral and bolus pressurization pattern). The final diagnosis was given in accordance with the latest published Chicago Classification 7. In order to gauge the impact of technical limitations on clinical management, the final EPT diagnosis arrived at by the managing physician, who was aware of the entire clinical context of the study, was also determined.
Clinical Data
Clinical data were explored on the subjects with technically imperfect studies. Clinical diagnosis was achieved by the managing physician (JEP/PJK). This diagnosis was based on symptoms, esophagogastroduodenoscopy (EGD), barium swallow and clinical outcome determined from chart review. The managing physicians utilized the technically imperfect EPT study in determining management according to their best judgment.
Statistical analysis
Qualitative data and frequency were described in percentage. EPT diagnosis was compared to clinical diagnosis. Sensitivity, specificity, positive and negative predictive values to diagnose achalasia despite technically imperfect studies were calculated.
Results
Frequency and causes of technically imperfect EPT studies
We identified 414 technically imperfect studies (21% of the series) in 386 patients. The distribution of causes for this designation is illustrated in Figure 2. The most frequently encountered technical limitation was of an abbreviated study with fewer than 7 evaluable swallows (12% of the series). This was most commonly attributable to the patient consistently double-swallowing (n=105, 44%), belching (n=88, 36%), or being intolerant of the procedure (n=48, 20%). Achalasia was the most frequently condition associated with an incomplete swallow protocol (n=78, 33% of all patients with incomplete studies). The second most common condition was a previous history of foregut surgery in 22 patients (9% of patients with incomplete protocol swallow). There was no other dominant explanation for the remaining 58% of incomplete studies.
Figure 2.
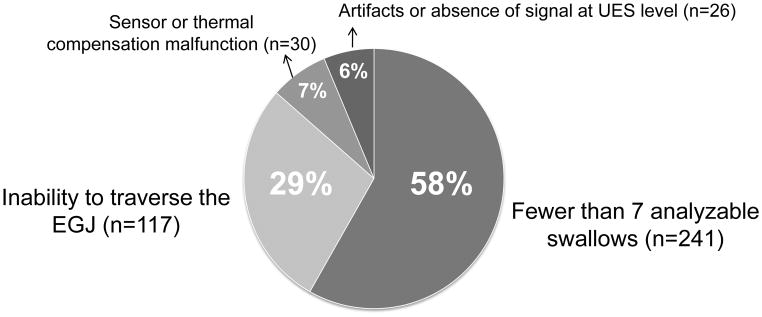
Causes of technically imperfect EPT studies. Fewer than 7 analyzable swallows and an inability to traverse the EGJ or diaphragm were the most common causes of technically imperfect studies.
Inability to traverse the EGJ or diaphragm was the second most common technical limitation encountered (6% of the series of 2,000 consecutive EPT studies and 28% of the imperfect studies). The most frequent causes of the difficulty were large hiatal hernias such that no sensors were below the diaphragm (n=58, 50%) and achalasia (n=28, 24%). Another common condition leading to an inability to place the manometry catheter across the EGJ was previous foregut surgery noted in 20 cases (17%): fundoplication, n=7; gastric bypass, n=5; gastrectomy, n=3; esophagectomy, n=3; gastric stapling, n=2. Other miscellaneous causes were: small hiatal hernia (n=1), extreme angulation at the EGJ (n=2: one anatomic variant and one previous left lower lung lobe resection), distal stricture in a context of suspected eosinophilic esophagitis (n=1), narrowing esophagus in a context of eosinophilic esophagitis (n=1), scleroderma (n=1), neoplasm involving the tongue (n=1) and dilated esophagus on chest X-ray (n=1). In 2 cases clinical data were not available.
The recording abnormalities leading to technically imperfect studies were equally distributed between technical malfunction (sensor problems, absence of thermal compensation) and recording artifacts (vascular artifacts, absence of signal at the level of the upper esophageal sphincter).
Impact of technically imperfect EPT studies on clinical management
Based on the initial EPT reports prepared by the managing clinician, technically limited studies were judged non-diagnostic such that further workup was required in only 27 cases (6.5% of all technically imperfect studies). To differentiate absent peristalsis from achalasia, an esophagram was recommended in eleven cases (catheter not traversing EGJ in eight and incomplete swallow protocol in three) and endoscopy-assisted catheter placement in six cases (catheter not traversing EGJ). No specific recommendation was made in the remaining ten cases (six of weak peristalsis in a context of incomplete protocol; four cases of the catheter not traversing the EGJ because of anatomical or post-surgical conditions).
Based on EGD, barium swallows and clinical outcome, the diagnosis of achalasia was established in 125 instances of technically imperfect studies (30%). Table 1 summarizes the blinded re-interpretation of the EPT studies of these patients. The blinded interpretation correctly diagnosed achalasia in 96 cases (77%). The 4 cases of rapid propagation/spasm on EPT corresponded to treated achalasia (one botulinum toxin injection, one Heller myotomy, two pneumatic dilation). Examples of technically imperfect EPT studies in patients with achalasia are given in Figure 3.
Table 1.
Esophageal pressure topography (EPT) review diagnosis of technically imperfect studies in patients with an ultimate clinical diagnosis of achalasia. The non-blinded diagnosis was made by the initial interpreter of the EPT study who was aware of the complete clinical context of the case.
| <7 evaluable swallows | Catheter not traversing EGJ | Technical malfunction | Recording artifacts | |
|---|---|---|---|---|
|
| ||||
| Clinical achalasia (n) | 78 | 28 | 4 | 14 |
|
| ||||
| EPT review diagnosis (n, %) | ||||
| Achalasia | 62 (79%) | 18 (64%) | 4 (100%) | 12 (86%) |
| Absent peristalsis | 5 (6%) | 6 (21%) | 0 | 0 |
| Frequent failed/Hypotensive | 6 (8%) | 1 (4%) | 0 | 2 (14%) |
| Rapid propagation/Spasm | 4 (5%) | 0 | 0 | 0 |
| Undetermined | 2 (2%) | 3 (11%) | 0 | 0 |
|
| ||||
| Non-blinded diagnosis (n,%) | ||||
| Achalasia | 74 (95%) | 25 (89%) | 4 (100%) | 13 (93%) |
| Absent peristalsis | 0 | 2 (7%) | 0 | 0 |
| Frequent failed/Hypotensive | 3 (4%) | 1 (4%) | 0 | 1 (7%) |
| Rapid propagation/Spasm | 1 (1%) | 0 | 0 | 0 |
Figure 3.
Examples of technically imperfect studies in patients with achalasia. In Panel A, the EPT study was considered imperfect as each swallow was followed by a belch. The mean integrated relaxation pressure (IRP) was 10 mmHg. Esophageal residual contractions were observed with a borderline value of distal latency (4.8 s). This EPT was classified as rapid propagation by the blinded reviewers. The chart review revealed that the patient had a previous Heller myotomy and the managing physician’s diagnosis was treated type III achalasia. In Panel B, the catheter did not pass through the EGJ. The EPT diagnosis of achalasia was based on the absence of peristalsis, pan-esophageal pressurization and consistent EGD findings. In Panel C, each swallow was consistently followed by a belch. The EPT diagnosis of achalasia was based on the absence of EGJ relaxation (IRP= 32 mmHg) and absent peristalsis. In Panel D, dysfunction of several pressure sensors occurred intermittently. The EPT diagnosis of achalasia was based on the absence of EGJ relaxation (IRP= 22 mmHg), the absence of peristalsis and the occurrence of pan-esophageal pressurization.
In seven instances the diagnosis of achalasia was suspected on EPT but not confirmed with clinical data. For all of these studies the catheter did not pass through the EGJ. In six cases, achalasia was then suspected based on absent peristalsis and pan-esophageal pressurization: two patients had a giant paraesophageal hernia (in both cases dysphagia resolved after surgical reduction of the hernia without myotomy or EGJ dilation); one patient had a prior gastrectomy for cancer and presented with a recurrence manifest as stenosis at the esophago-jejunal anatomosis; one patient had an esophagectomy for cancer; the other two studies were performed in the same patient with history of gastric bypass surgery and a stenotic gastro-jejunal anastomosis; the diagnosis of pseudo-achalasia secondary to prior surgery was established clinically in this patient. In the last case, achalasia was suspected because of premature contractions associated with distal pressurization. The patient had a large paraesophageal hernia and patient’s dysphagia resolved after surgical reduction of hernia.
As detailed above, using a blinded EPT review the overall sensitivity of technically imperfect studies to diagnose achalasia was 77% with a specificity of 98%, a positive predictive value of 93% and a negative predictive value of 91%. However, in the context of actual clinical care, when the entire clinical context of the case was known to the reviewing clinician, achalasia was correctly diagnosed despite a technically imperfect EPT in 94% of instances (Table 1). For purposes of comparison, achalasia was diagnosed in 166 of the 1586 ‘perfect’ EPT studies analyzed in this series. Clinical data confirmed achalasia in 153 cases (92%). Among the remaining 13 patients, seven had a pseudo-achalasia pattern after surgery (five Nissen fundoplication, two gastric bypass), one had an esophageal tumor, one had a Schatzki ring, two had eosinophilic esophagitis, one was unresolved and clinical data was not available in one patient.
Cases of large hiatal hernias
Among the series of 2,000 EPT studies, we identified 111 patients with a large hiatal hernia (at least 5 cm on EGD examination). EPT studies were considered as imperfect in 63 cases of large hiatal hernia (57%). Traversing the crural diaphragm to place the tip of the catheter in the abdominal cavity was most challenging in these patients as it was not achieved in 59 (53% of patients with large hiatal hernia). An assisted endoscopic placement was attempted in 11 cases: it was successful in only 4 cases. To compare, over the same period, an endoscopic placement was attempted in 50 cases of achalasia and facilitated traversing the EGJ in 90%. Finally even if the tip of the catheter was not in the abdominal cavity, esophageal peristalsis was still evaluable in patients with large hiatal hernia. As mentioned, it is important to note that a false positive diagnosis of achalasia (Figure 4) was achieved in 3 imperfect EPT studies in patients with large hiatal hernia whereas this diagnosis was never made in 44 large hiatal hernia patients with a complete EPT study. The abnormal catheter position within the hernia and pressurization above the diaphragm may have led to a false positive IRP in these patients.
Figure 4.
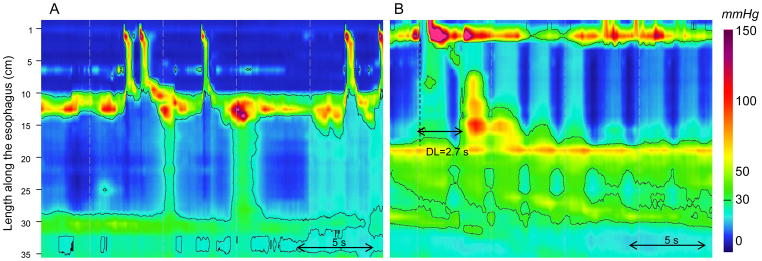
False positive diagnosis of achalasia in patients with large hiatal hernias. In the two EPT studies the tip of the catheter was not in the abdominal cavity. In Panel A, absence of lower esophageal sphincter (LES) relaxation during swallowing was associated with absent peristalsis and pan-esophageal pressurization. In Panel B, absence of LES relaxation was associated with a premature contraction characterized by a distal latency (DL) < 4.5 s. The abnormal IRP is a manifestation of the abnormal EGJ position related to the large hernia and not an intrinsic defect in inhibition.
Discussion
This systematic review of clinical data revealed that technically imperfect EPT studies were experienced in approximately 20% of the cases in a tertiary care center. Achalasia and large hiatal hernia were the most frequent causes and may suggest referral center bias. However, despite the technical limitations, blinded interpretation of these studies still correctly diagnosed achalasia in 77% of the cases with excellent specificity. Non-blinded interpretation, done by the managing clinician aware of the entire clinical context of the case achieved the achalasia diagnosis in 94% of instances, a value comparable to that achieved with technically ‘perfect’ studies (92%).
An incomplete swallow protocol was the main limitation experienced in EPT studies. This limitation is a consequence of a poor tolerability with belches and double swallows secondary to discomfort. The minimal number of swallows required to consider an esophageal high-resolution manometry study accurate for motility disorders evaluation is unclear, however, it is reasonable to utilize guidelines for conventional manometry 8–9. Since, the Chicago Classification is also based on the analysis of 10 swallows in the supine position we decided to consider a study as limited when fewer than 7 swallows were analyzable. However, this criterion might be too stringent for EPT and a smaller number of analyzable swallow may be sufficient to diagnose achalasia as normal peristalsis is absent in these patients. However, normal peristalsis may co-exist with abnormalities in other motility disorders and a sufficient number of swallows would be required to diagnose those. For example, only 20% of abnormal swallows are required for the diagnosis of distal esophageal spasm.
Traversing the EGJ with the manometry catheter can be challenging and was not achieved 12% of the time in our experience. Careful examination is mandatory to ascertain optimal placement and a deep inspiration maneuver may help to localize the diaphragm. Fluoroscopy might also be helpful to control the placement of the catheter. In cases of achalasia with anatomic deformity, tight EGJ or large para-esophageal hernia, an endoscopy-assisted placement should be considered. In our experience this placement was more often successful in patients with achalasia than with large hiatal hernias.
It was not surprising that achalasia was the main cause of technically imperfect studies in our series as achalasia can be associated with hypercontractility at the EGJ, dilated esophagus and saliva and/or food retention. Impaired EGJ opening and dilation represent a technical challenge to traverse the EGJ as the catheter will often coil in the distal esophagus. Additionally, the presence of esophageal retention may also reduce tolerability and lead to an incomplete swallow protocol. However, esophageal manometry is essential in the diagnosis and the management of patients with achalasia and thus, it is important to obtain valid recordings. Of note, the Chicago Classification is not intended for patients previously treated for achalasia 7 and some of the missed diagnoses may be attributable to the normalization of the IRP after treatment. Despite technical limitations, achalasia was correctly diagnosed using EPT in 77% of cases by a blinded observer. The absence of esophageal peristalsis associated with pan-esophageal pressurization is characteristic of type II achalasia and this subtype is the most easily recognized based on the pressurization pattern 10. Furthermore, when the interpreter was aware of the entire clinical context of the case, particularly endoscopic and radiographic findings, achalasia was correctly diagnosed in 94% of cases. Therefore, achalasia was often diagnosed with an excellent level of confidence despite a technically imperfect study.
The clinical significance of technical limitations in EPT studies depends on the indication for the manometry. The evaluation of EGJ relaxation is essential to differentiate achalasia from absent peristalsis. However, in a patient referred for a pre-operative evaluation for large hiatal hernia, traversing the EGJ is less critical providing that peristalsis is present. An EPT study should therefore be interpreted in the clinical context of the presenting complaint and the adjunct information available.
This systematic review of technical imperfections might be useful to provide some guidelines for clinical practice. A careful check of the catheter prior to the procedure should be done to make sure that all sensors are working. Correct placement through the EGJ should be systematically confirmed using a deep breath maneuver to differentiate intra-abdominal and intra-thoracic pressures. When traversing the EGJ is challenging, endoscopic placement may be attempted. In our experience, it was successful in 90% of patients with achalasia but in only 53% in patients with large hiatal hernias. Finally, patient education and reassurance are essential to minimize double swallowing, belching and gagging.
This study has some important limitations in terms of generalizability. We reported the experience of a single tertiary center with expertise in the management of esophageal disorders. This is the reason for the high rate of achalasia and large hiatal hernia in this series and it is possible that other causes for technically imperfect studies may be more prevalent in a more general clinical practice. However, esophageal manometry is a specific technique and this study highlights the issues that will be encountered in clinical manometry and practitioners should be aware of these particular technical issues. Moreover, we did not use protective sheaths which might generate specific artifacts. These potential artifacts should be taken into account in a general practice.
In summary, technically imperfect EPT studies are not uncommon in clinical practice and recognition of these technical issues is important in determining diagnostic accuracy. Most of the time, these technical limitations are the consequence of the patient’s condition (achalasia) or issues related to anatomy (hernia, post-surgical). Despite these limitations, the diagnosis of achalasia is still achieved with good sensitivity and excellent specificity.
Acknowledgments
Financial support:
This work was supported by R01 DK079902 (JEP) from the Public Health Service
Abbreviations
- EPT
esophageal pressure topography
- EGJ
esophago-gastric junction
- HRM
high resolution manometry
- IRP
integrated relaxation pressure
Footnotes
Author’s contributions:
SR: Analysis and interpretation of data, Drafting of manuscript
PJK: Study concept and design, Analysis and interpretation of data, Drafting of manuscript
LB: Analysis and interpretation of data
KB: Analysis and interpretation of data
DL: Analysis and interpretation of data
JEP: Study concept and design, Analysis and interpretation of data
Drafting of manuscript
Potential Competing Interests:
Sabine Roman and John E. Pandolfino have served as consultant for Given Imaging.
References
- 1.Pandolfino JE, Kahrilas PJ American Gastroenterological A. American Gastroenterological Association medical position statement: Clinical use of esophageal manometry. Gastroenterology. 2005;128:207–208. doi: 10.1053/j.gastro.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Murray JA, Clouse RE, Conklin JL. Components of the standard oesophageal manometry. Neurogastroenterol Motil. 2003;15:591–606. doi: 10.1046/j.1365-2982.2003.00446.x. [DOI] [PubMed] [Google Scholar]
- 3.Kahrilas PJ. Esophageal motor disorders in terms of high-resolution esophageal pressure topography: what has changed? Am J Gastroenterol. 2010;105:981–987. doi: 10.1038/ajg.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clouse RE, Staiano A, Alrakawi A, et al. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95:2720–2730. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 5.Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16:533–542. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 6.Grubel C, Hiscock R, Hebbard G. Value of spatiotemporal representation of manometric data. Clin Gastroenterol Hepatol. 2008;6:525–530. doi: 10.1016/j.cgh.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Pandolfino JE, Fox MR, Bredenoord AJ, et al. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209–224. doi: 10.1053/j.gastro.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145–151. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: A New Clinically Relevant Classification by High-Resolution Manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]