Abstract
Background
Preventing expansion and dyskinetic movement of a myocardial infarction (MI) can limit left ventricular (LV) remodeling. Using a device designed to produce variable alteration of infarct stiffness and geometry, we sought to understand how these parameters affect LV function and remodeling early after MI.
Methods
Ten pigs had posterolateral infarctions. An unexpanded device was placed in 5 animals at the time of infarction, and 5 animals served as untreated controls. One week after MI animals underwent MRI to assess LV size and regional function. In the treatment group, after initial imaging, the device was expanded with 2ml, 4ml, 6ml, 8ml and 10ml of saline. The optimal degree of inflation was defined as that which maximized stroke volume (SV). The device was left optimally inflated in the treatment animals for three additional weeks.
Results
One week after MI, device inflation to ≥6ml significantly (p<0.05) decreased endsystolic volume (ESV) (0ml:59.9ml±3.8, 6ml:54.0ml≥±3.1, 8ml:50.5ml±4.8, 10ml:46.1ml±2.2) and increased ejection fraction (EF) (0ml:34.6%±1.6, 6ml:39.7%±0.9, 8ml:43.1%±2.7, 10ml:44.1%±0.9). SV significantly (p<0.05) improved for the 6ml and 8ml volumes (0ml: 31.2ml±2.6, 6ml: 35.7ml±2.0, 8ml: 37.5ml±1.9) but trended downward for 10ml (36.6ml±2.8). At four-weeks after MI, end-diastolic volume and ESV were unchanged from one-week values in the treatment group while the control group continued to dilate. SV (38.2±4.4ml vs. 34.0.1±4.8ml, p=0.08) and EF (36.0±2.6% vs. 27.6±1.4%, p=0.04) were also better in the treatment animals.
Conclusions
Optimized isolated infarct restraint can limit adverse LV remodeling after MI. The tested device affords the potential for a patient-specific therapy to preserve cardiac function after MI.
Keywords: Device, Heart Failure, MRI, Myocardial Mechanics, Myocardial Remodeling
Introduction
Every year approximately 1.3 million Americans suffer a myocardial infarction (MI).[1] Two-thirds of the heart failure cases occur in patients who have had a MI. [2] Although it is tempting to attribute the association between MI and heart failure simply to a reduction in the amount of healthy myocardium contributing to ejection, the majority of patients who suffer a MI are initially well-compensated hemodynamically and only develop symptomatic heart failure over time as the LV remodels in response to the infarct. In addition to loss of contractile myocardium, the degree of LV remodeling and systolic impairment are related to the size, compliance and geometry of the infarct region.[3,4,5]
Immediately after MI the infarct stops contracting, resulting in the development of a systolic dyskinetic wall motion abnormality. Additionally, a precipitous increase in the systolic compliance of the affected region occurs, and is associated with myocardial thinning and stretching. Systolic infarct compliance remains elevated for weeks to months.[3,6] The persistent stretching and dyskinetic movement of the infarct region leads to adverse LV remodeling and compromises systolic and diastolic LV performance.[3,4,5,7]
A growing body of theoretical [4,5] and experimental [8–13] evidence supports the prevention of infarct expansion early after MI as a viable therapeutic option for limiting adverse LV remodeling and subsequent symptomatic heart failure. Previous experimental studies have employed several techniques to limit infarct expansion and associated LV remodeling, including: complete and partial heart wraps;[8] patches over the infarct;[9,10] and injected polymers.[11,12,13] Results have been encouraging with most studies demonstrating mild to moderate amelioration but not cessation of the remodeling process. The optimal degree of infarct modification and the best means for safely accomplishing has not been determined. In this experiment the effect of a novel expandable device designed to variably alter infarct stiffness and geometry was studied in a porcine model of myocardial infarction (MI).
Material and Methods
Infarct Model and Device Implantation
Pigs weighing 40–50kgs were used in this study. General anesthesia was maintained with a mixture of inhaled isoflurane and oxygen. A transmural posterolateral myocardial infarction of approximately 20% of the LV was produced by ligating the left circumflex artery distal to the first obtuse marginal artery branch in atrioventricular groove. The distal branches off the first obtuse marginal artery and the anterior originating diagonal arteries were also ligated when necessary to achieve a uniform infarction size.
Five animals underwent wound closure and recovery; the other five animals underwent device implantation followed by closure and recovery (Figure 1). The device was composed of a polypropylene mesh shaped to match the area of infarction. The mesh was precisely placed over the infarct and sutured to the demarcation line between viable and nonviable myocardium. A two finger-breath pocket was created in between the mesh and the underlying infarcted tissue. A commercially available balloon catheter (Millar International Inc., Erlanger, KY) was placed beneath the mesh and anchored with sutures. The proximal end of the catheter was tunneled into the subcutaneous tissue to allow for easy access at the one-week study period.
Figure 1. Restraint Device.
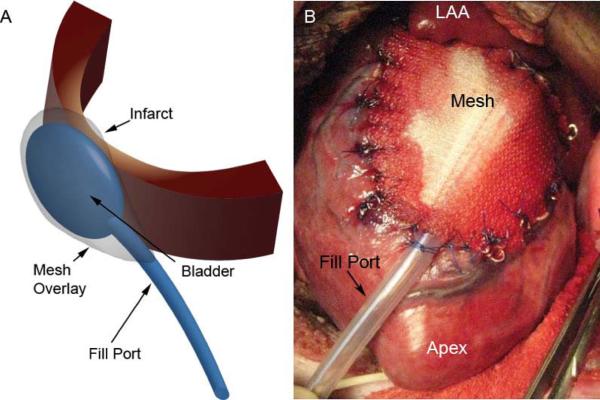
Panel A depicts a schematic drawing of the restraint device used in this study. A bladder type catheter is placed over the infarct between the epicardium and a mesh sutured to the heart surface. External filling is performed via an exteriorized fill port. Panel B is an intraoperative photograph of the implanted device sutured to the LV epicardium directly over the infarct region. MRI compatible markers are sutured to the edge of the mesh and epicardium to delineate the infarct on MR imaging.
All animal experiments were conducted under an experimental protocol approved by the University of Pennsylvania's Institutional Animal Care and Use Committee and were in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication no 85-23, revised 1996).
Magnetic Resonance Imaging
MRI studies were performed using a 3T TIM Trio Scanner (Siemens Inc., Malvern, PA) before infarction as well as at one and four weeks after infarction. Prior to imaging a high fidelity pressure transduction catheter (Millar Instruments, Inc., Houston TX) was advanced into the LV under fluoroscopic guidance to allow for hemodynamic assessment and gating. One week after MI all animals again underwent MRI. In the treatment group after initial imaging, the device was incrementally expanded with 2ml, 4ml, 6ml, 8ml and 10ml of saline to achieve an increasing degree of infarct stiffening and geometric alteration. Regional and global LV geometry and function were serial assessed at each level of device expansion. The optimal infarct restraint was defined as the level of device filling that yielded the largest stroke volume. The device was then left optimally filled in the 5 treatment animals for three additional weeks. MRI four weeks after MI assessed global and regional LV function and the degree of LV remodeling. All images were cardiac and respiratory gated to ensure consistent spatial positioning of the heart during each acquisition using a custom designed system which gates the MRI by monitoring LV pressure and ventilator pressure.
The imaging consisted of localizers followed by a 2D CINE TrueFISP cardiac acquisition using the following parameters: field of view 300 × 244, acq. matrix 192 × 156, TR/TE 3.11/1.53 ms, BW 1184 Hz/pixel, slice thickness 4 mm, 2 signal averages and 6 to 8 k-space lines acquired per cardiac frame. The images were archived and stored for off-line analysis.
A 3D SPAMM tag sequence was used to measure regional LV strain at the 1 week time point with the following parameters: field of view 260 × 260, acq. matrix 256 × 128, TR/TE 7/2.6 ms, BW 330 Hz/pixel, slice thickness 2 mm, 4 signal averages and 4 to 6 k-space lines acquired per cardiac frame. [14]
Data Analysis
The recorded pressure data were analyzed to generate LV hemodynamics for each device volume at one-week after MI and at the four-week after MI time point. The maximum (dP/dtmax) and minimum (dP/dtmin) pressure change were calculated using a linear fit of the pressure versus time data. End-diastolic pressure (EDP) was defined as the LV pressure value prior to the rise in dP/dt. Peak pressure was the maximum LV pressure and the heart rate was averaged over 10 cardiac cycles.
LV volume data was obtained from the CINE MR images. The short axis slices were reformatted to nine long axis slices using open access software (ImageJ, NIH). LV contours were drawn for each slice in all the phases. The long-axis contours where then imported into a custom program in MATLAB (MathWorks, Natick MA) to calculate LV end diastolic volume (EDV), LV end systolic volume (ESV), stroke volume (SV), and ejection fraction (EF) from rotational slices. Regional LV volumes and regional ejection fraction function were assessed by contouring the MRI short-axis slices at equally spaced LV locations from base to apex. The LV was divided into five equal sections and the volume of the short axis slices for each section was measured for both end-systole and end-diastole.
Systolic LV regional strain at one-week post infarct was measure from the SPAMM tag images using an optical flow method (OFM).[14] Endocardial and epicardial contours of the LV were manually drawn on the end-diastolic phase for all slices in ImageJ (NIH). OFM was used to produce the x, y, and z displacement flow fields from the stack of tagged images. Maximum principal strain was then calculated from the displacements.
Statistics
Data are presented as mean ± SEM. One-week post MI data data were assessed using one-way repeated measures ANOVA with Tukey post hoc evaluation to account for differences between device volumes. A two-way repeated measures ANOVA with Tukey multiple comparisons was used to analyze the four-week post MI arm of the study to measure differences between control and treated groups and time points and for regional volume and ejection fraction between 0ml and 8ml. Four week hemodynamic data were analyzed using a t-test. P < 0.05 was considered statistically significant for all comparisons.
Results
One- Week Post MI Testing and Optimization
Figure 2 demonstrates representative MRI images at end-diastole and end-systole for the baseline (pre-infarction) time point and for different device inflation volumes at one-week post-MI. From baseline to one-week post-MI there is a significant change in the LV geometry with an increase in LV volume and a dyskinetic bulging of the infarct region. Hemodynamic data are presented in Table 1. The effect of increasing infarct restraint on LV size and function were determined by measuring the changes in EDV, ESV, SV and EF for different device volumes at 1 week. Device volume had no significant effect on end-diastolic volume (EDV) (Table 1). In contrast, device volume had a significant effect on ESV. At device volumes of 4ml and lower, there was no significant change in ESV but at device inflation volumes of 6ml and greater there was a significant decrease in ESV (Table 1). Stroke volume showed relatively little change at the lower device inflation volumes with a significant improvement occurring at device volumes of 6ml and 8ml (Table 1). In all animals, SV was greatest at a device inflation volume of 8ml (Table 1).
Figure 2. The Effect of Device Filling on LV Geometry.
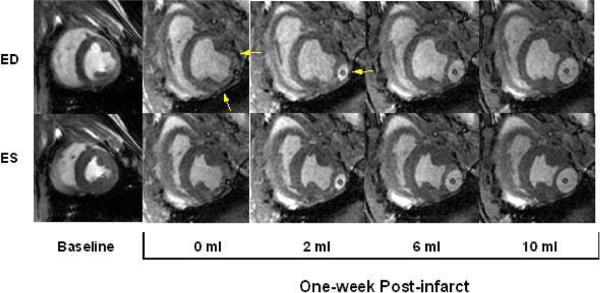
Mid-ventricular end-diastolic (ED) and end-systolic (ES) images at baseline and one week after infarct. From baseline to one-week post infarct the end diastolic volume (EDV) and end systolic volume (ESV) have increased significantly. Progressively increasing device volume from 0 ml to 10 ml decreases the infarct bulging and alters the infarct and LV geometry. Arrows in 0ml image denote the infarct region and in 2ml image point to the device.
Table 1.
Left Ventricular Function and Hemodynamic Parameters 1 Week after Infarction at Varied Device Inflation Levels (Mean± SEM)
| Device Inflation Level | ||||||
|---|---|---|---|---|---|---|
| Parameter | 0 ml | 2 ml | 4 ml | 6 ml | 8 ml | 10 ml |
| End-Diastolic Volume (ml) | 90.0±5.6 | 89.8±5.9 | 94.8±7.1 | 89.7±4.7 | 88.2±4.6 | 82.8±4.9 |
| End-Systolic Volume (ml) | 59.9±3.8 | 59.1±3.9 | 60.3±5.5 | 54.0±3.1* | 50.5±4.8* | 46.1±2.2* |
| Ejection Fraction (%) | 34.6±1.6 | 33.8±3.1 | 36.5±2.8 | 39.7±0.9* | 43.1±2.7* | 44.1±0.9* |
| Stroke Volume (ml) | 31.2±2.6 | 30.7±4.0 | 34.5±3.7 | 35.7±2.0* | 37.5±1.9* | 36.6±2.8 |
| Heart Rate (bpm) | 108.3±8.3 | 108.0±7.5 | 108.3±8.3 | 109.5±5.6 | 109.8±7.4 | 106.7±6.8 |
| LV End Diastolic Pressure (mmHg) | 14.1±3.6 | 12.2±2.3 | 11.7±1.9 | 11.8±1.9 | 11.8±1.9 | 10.4±1.8 |
| Peak LV Pressure (mmHg) | 70.8±5.5 | 65.9±2.4 | 65.8±2.1 | 69.1±4.5 | 67.2±3.2 | 64.6±1.3 |
| dp/dtmax (mmHg/s) | 907±104 | 861±58 | 817±74 | 839±48 | 850±65 | 786±85 |
| dp/dtmin (mmHg/s) | −806±45 | −731±39 | −744±44 | −815±106 | −834±47 | −791±56 |
p<0.05, versus 0ml, On-way ANOVA with repeated measures and Tukey multiple comparison
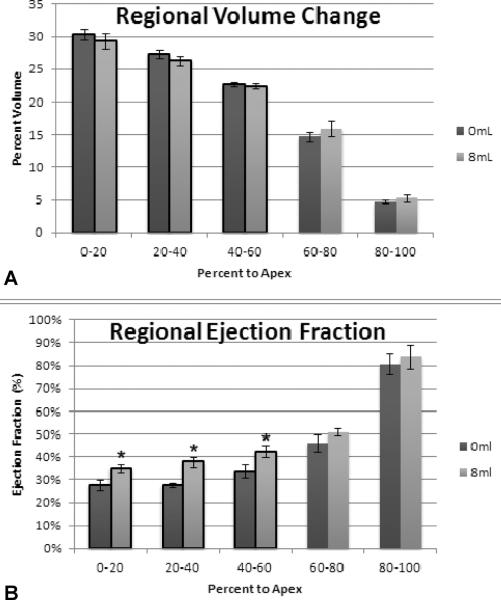
At 1 week, regional changes in EF were also detected at device inflation volumes of 6ml or greater. When the heart was divided into 5 transverse segments from base to apex the greatest improvements in regional EF were observed in the most basilar three segments. These segments included the majority of the infarct and were the regions of the heart in closest approximation to the device. This phenomenon is depicted for a device filling volume of 8ml in Figure 3B. A regional shift in EDV from the basilar segments toward the most apical segments was also observed with increasing device volume (Figure 3A).
Figure 3. Regional Effect of Device Filling on LV Function.
(A) Device filling shifts the end-diastolic volume from the basal region where the device was positioned (0–40%) to the apical region (60–100%). (B) Basal and apical regional ejection fraction increased with device filling. (* p<0.05 versus 0ml)
Regional LV maximum principal strain (Emax) was calculated for the 0ml and 8ml device inflation volumes at 1 week. Strain was measured in the infarct region, the basal remote region at the level to the device and in the apical remote region distant from the device position. Emax is essentially equivalent to radial wall thickening in uninfarcted myocardial regions; for transmural infarction regions increasing Emax is indicative of dyskinetic stretching.
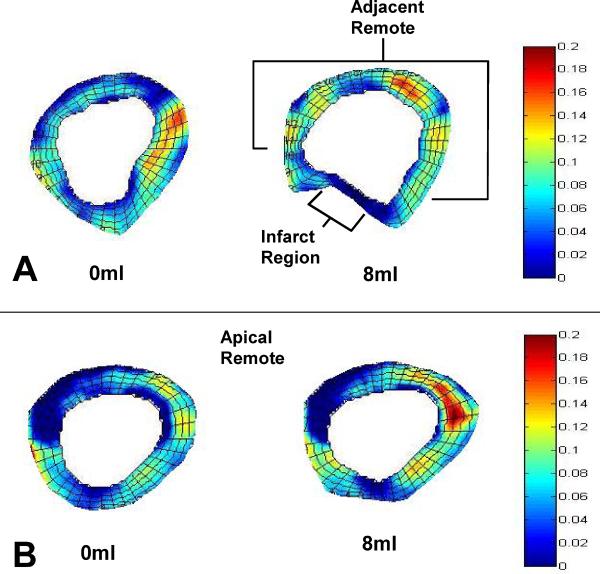
Representative color maps of the regional maximum principal strain are shown in Figure 4. Strain patterns were altered in all regions by device inflation to 8ml. The composite strain data shows device filling significantly increased Emax in both the adjacent remote (0.09±0.0034 vs. 0.08±0.0031, p=0.002) and apical remote regions (0.081±0.0031 vs. 0.072±0.0031, p<0.001), as shown in Figure 5. Systolic stretching of the infarct region was significantly reduced with device inflation as indicated by the decline in infarct Emax (0.046±0.0048 vs.0.022±0.0034, p<0.001).
Figure 4. Color Map of Regional Maximum Principal Strain Pre and Post Device Filling.
(A) Device filling (8ml) altered both the geometry and strain in the basal region where the device was positioned. The septum and posterior wall had increased strain while the infarct region strain diminished. (B) The apical remote geometry was less affected by device filling while strain was significantly altered.
Figure 5. The Effect of Device Filling on Regional Maximum Principal Strain.
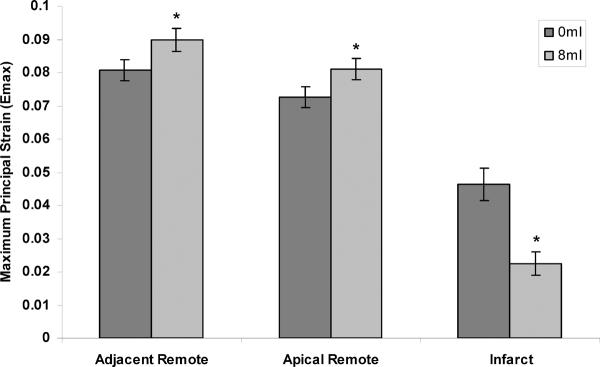
Device filling significantly improved the adjacent remote function by shifting volume from the infarct region to the normal remote region increasing loading. Similar results occur in the apical region where device filling increased apical remote strain. Filling the device significantly decreased infarct strain indicating a stiffer infarct and diminished systolic stretch. (* p<0.05 versus 0ml)
Four- Week Post MI Testing
In the control group EDV continued to increase significantly between the one-week and four-week time points from 86.9±6.3ml to 121.3±6.1ml (p=0.001); ESV in the control group also increased during this time frame from 54.8±2.7ml to 87.0±3.5ml (p=0.001) (Table 2). However, EDV in the treatment group remained stable between one and four-weeks after MI as did ESV. SV and EF were similar in both groups 1 week after MI; however, SV (38.2±4.5ml vs. 32.1±4.8ml, p=0.08) and EF (36.0±2.7% vs. 27.6±1.7%, p=0.02) were both greater in the treatment group by four-weeks after MI (Table 2).
Table 2.
Ventricular function and Hemodynamic Parameters for Control and Treated groups.
| Control | Treated | |||||
|---|---|---|---|---|---|---|
| Baseline | 1-week | 4-week | Baseline | 1-week | 4-week | |
| End-Diastolic Volume (ml) | 61.3±1.2 | 86.9±5.5* | 121.3±5.4† | 64.3±1.3 | 97.2±6.3* | 106.0±7.6‡ |
| End-Systolic Volume (ml) | 36.5±0.8 | 54.8±2.3* | 86.9±3.1† | 38.8±1.0 | 65.1±4.5*• | 67.8±4.9‡ |
| Ejection Fraction (%) | 41.5±0.3 | 36.3±2.6* | 27.6±1.4† | 41.0±0.2 | 32.7±3.7* | 36.0±2.6‡ |
| Stroke Volume (ml) | 33.3±0.3 | 32.0±4.1 | 34.1±3.1 | 29.9±1.7 | 32.0±4.8 | 38.2±4.4 |
| Heart Rate (bpm) | – | – | 102.5±2.9 | – | – | 104.5±7.6 |
| LV End Diastolic Pressure (mmHg) | – | – | 12.6±1.7 | – | – | 6.3±1.0* |
| Peak LV Pressure (mmHg) | – | – | 69.9±2.1 | – | – | 75.7±6.7 |
| dp/dtmax (mmHg/s) | – | – | 636±58 | – | – | 873±177 |
| dp/dtmin (mmHg/s) | – | – | −742±38 | – | – | −952±125 |
p<0.05 Baseline vs. 1-week,
p<0.05 1-week vs 4-week,
p<0.05 Control versus Treated at 4-weeks,
p<0.05 Control versus Treated at 1-week,
Ventricular Function; Two way ANOVA with repeated measures and Tukey multiple comparison. Hemodynamics; t-test.
Hemodynamic data comparing treatment and control groups are presented in Table 2. The only significance between-group difference was observed for LV end diastolic pressure (EDP) which was less in the treatment group (6.8±1.0mmHg) than in the control group (12.6±1.8mmHg), p=0.02.
Comment
Increased compliance (reduced stiffness) of the infarct region results in infarct expansion after MI. Infarct expansion compounds the loss of contractile myocardium. Immediately after MI the compliant infarct becomes an energy sink which reduces systolic pump function: work performed by the uninfarcted myocardium is consumed in stretching the infarct region. The expanding infarct region also alters LV cavity geometry resulting in a right-shift in the pressure-volume relationship and increased LV wall stress. Increased wall stress initiates and sustains the LV remodeling that ultimately leads to symptomatic heart failure and death. [15,16,17]
There is increasing interest in ameliorating adverse LV remodeling by limiting infarct expansion in the early post-MI time period. Therapeutic strategies include complete heart wrapping,[8] partial wrapping,[10] infarct reinforcement with surgical mesh[9] and infarct modification with biomaterials.[11–13] In addition, some studies have suggested that reperfusion therapy and stem cell therapy elicit positive effects on post-MI remodeling by favorably altering infarct material properties.[18, 19] While these approaches to mechanical infarct restraint have demonstrated some efficacy with regard to altering remodeling, definitive improvement in LV contractile function as assessed by ejection fraction and stroke volume have been more difficult to consistently demonstrate. The development of restrictive cardiac physiology and the potential for significant patient-to-patient variability in therapeutic response remain areas of concern.[20,21]
In this experiment we used a device that allowed progressive increase in infarct restraint. LV geometry and function were assessed with cardiac MRI. One week after infarction, device filling resulted in progressive increases in both SV and EF. Interestingly, device filling had virtually no effect on EDV while causing significant decreases ESV at device volumes above 4 ml. Increasing device filling also did not increase LV EDP; increased levels of restraint actually resulted in a trend toward lower EDP.
The precise degree of infarct stiffness was not measured; however, the reduction in infarct Emax with device filling indicated that infarct stretching was greatly reduced during systole (Figure 5). For a similar large animal infarct model, Dang and colleagues [22] employing a finite element model of the LV to estimate that converting a dyskinetic infarct into an akinetic infarct required a 250-fold increase in infarct stiffness. In large animal models of transmural infarcts, this degree of infarct stiffening has only been accomplished by infarct reperfusion within one hour of the onset of ischemia.[18]
The persistent application of optimized infarct restraint improved both LV geometry and function at four-weeks after MI. In the control group, EDV and ESV increased progressively from baseline to four-weeks after MI, while EF progressively decreased during this time. However, in the treatment animals further LV remodeling between the one-week and the four-week post MI time points was prevented. EF and SV were also higher in the treatment group at four-weeks after MI. In addition to reduced LV dilation and improved systolic function the treatment animals also had better diastolic function; LV EDP was 50% less in the treatment group relative to control.
The mechanism by which aggressive localized infarct restraint improves global LV remodeling is likely complex and was not fully elucidated in the experiments reported here. There are at least three elements that contribute to the salutary effects of this type of post-MI infarct restraint. First, transforming a large dyskinetic myocardial segment into an akinetic segment reduces the systolic pump energy losses consumed in stretching the infarct region. Second, infarct restraint reduces LV wall stress, which improves borderzone contractile function and reduces the stimulus for global LV remodeling. Third, aggressive infarct restraint appears - from the results of this study – to effectively improve near and longer-term diastolic function by shifting diastolic volume from the compromised areas of the LV towards more remote regions, thereby allowing healthy myocardium to improve regional function by the Starling mechanism.
A device capable of variable localized restraint was previously described by Hung et al. and used to treat ischemic mitral regurgitation (IMR) in sheep. The focus of that study was fundamentally different from ours. These investigators were interested in how their device affected papillary muscle position and degree of IMR in both acute and chronic IMR models. While these authors did demonstrate a trend toward increased end systolic elastance with device inflation, they did not explore how the device affected LV remodeling and function over time.[23]
In summary, this report adds to a growing body of pre-clinical research that supports the efficacy of early infarct restraint as a means of limiting post-MI LV remodeling. The therapeutic approach used in this study is unique in that variable levels of mechanical restraint were imposed directly on the infarct region. The potential for variable restraint levels and, therefore, patient-specific therapy was demonstrated.
Acknowledgements
This work was supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda, MD (HL63954, HL73021, HL103723 and HL108330). R. C. Gorman and J. H. Gorman III are supported by individual Established Investigator Awards from the American Heart Association, Dallas, TX. W. R.T. Witschey is supported by K99 HL108157 and C. Xu was supported by a National Research Service Award both from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Heart Association Writing Group Heart Disease and Stroke Statistics—2012 Update: A Report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 3.Holmes JW, Borg TK, Covell JW. Structure and mechanics of Healing Myocardial Infarcts. Annual Review Biomedical Engineering. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 4.Pilla JJ, Gorman JH, III, Gorman RC. Theoretical Impact of Infarct Compliance on Left Ventricular Function. Annals of Thoracic Surgery. 2009;87:803–811. doi: 10.1016/j.athoracsur.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogen DK, Rabinowitz SA, Needleman A, McMahon TA, Abelmann WH. An Analysis of the Mechanical Disadvantage of Myocardial Infarction in the Canine Left Ventricle. Circulation Research. 1980;47:728–741. doi: 10.1161/01.res.47.5.728. [DOI] [PubMed] [Google Scholar]
- 6.Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LH, Bogen DK. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 1994;89:2315–2326. doi: 10.1161/01.cir.89.5.2315. [DOI] [PubMed] [Google Scholar]
- 7.Jackson BM, Gorman JH, III, Moainie S, Guy TS, Narula N, Narula J, St John Sutton M, Edmunds LH, Jr, Gorman RC. Extension of Borderzone Myocardium in Postinfarction Dilated Cardiomyopathy. Journal of American College of Cardiology. 2002;40:1160–1167. doi: 10.1016/s0735-1097(02)02121-6. [DOI] [PubMed] [Google Scholar]
- 8.Pilla JJ, Blom AS, Brockman DJ, Bowen F, Yuan Q, Giammarco J, Ferrari VA, Gorman JH, III, Gorman RC, Acker MA. Ventricular Constraint Using the Acorn Cardiac Support Device Reduces Myocardial Akinetic Area in an Ovine Model of Acute Infarction. Circulation. 2002;106:I207–I211. [PubMed] [Google Scholar]
- 9.Kelley ST, Malekan R, Jackson BM, Gorman JH, III, Plappert T, Gorman RC, St John Sutton MG, Edmunds LH., Jr Restraining Infarct Expansion Preserves Left Ventricular Geometry and Function After Acute Anteroapical Infarction. Circulation. 1999;99:135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- 10.Enomoto Y, Gorman JH, III, Moainie SL, Jackson BM, Parish LM, Plappert T, Zeeshan A, St. John-Sutton MG, Gorman RC. Early Ventricular Restraint after Myocardial Infarction: Extent of the Wrap Determines the Outcome of Remodeling. Annals of Thoracic Surgery. 2005;79:881–887. doi: 10.1016/j.athoracsur.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 11.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, Petnehazy Landa N, Feinberg MS, Goitein O, Tsur-Gang O, Shaul M, Klapper L, Cohen S. Intracoronary Injection of In Situ Forming Alginate Hydrogel Reverses Left Ventricular Remodeling After Myocardial Infarction in Swine. Journal of the American College of Cardiology. 2009;54:1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Ryan LP, Matsuzaki K, Noma M, Jackson BM, Eperjesi TJ, Plappert T, St. John Sutton MG, Gorman JH, III, Gorman RC. Dermal Filler Injection: A Novel Approach to Limiting Infarct Expansion. Annals of Thoracic Surgery. 2009;87:148–156. doi: 10.1016/j.athoracsur.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, III, Gorman RC, Burdick JA. Injectable Hydrogel Properties Influence Extent of Post-Infarction Left Ventricular Remodeling in an Ovine Model. Proceedings of the National Academy of Science. 2010;107:11507–11512. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu C, Pilla JJ, Isaac G, Gorman JH, III, Blom AS, Gorman RC, Ling Z, Dougherty L. Deformation Analysis of 3D Tagged Cardiac Images Using an Optical Flow Method. Journal of Cardiovascular Magnetic Resonance. 2010;12:19. doi: 10.1186/1532-429X-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohde LE, Aikawa M, Cheng GC, Sukhova G, Solomon SD, Libby P, Pfeffer J, Pfeffer MA, Lee RT. Echocardiography-Derived Left Ventricular End-Systolic Regional Wall Stress and Matrix Remodeling After Experimental Myocardial Infarction. Journal of the American College of Cardiology. 1999;33:835–842. doi: 10.1016/s0735-1097(98)00602-0. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa Y, Luis Rohde L, Plehn J, Greaves SC, Menapace F, Arnold JMO, Rouleau JL, Pfeffer MA, MD, Lee RT, Solomon SD. Regional Wall Stress Predicts Ventricular Remodeling after Anteroseptal Myocardial Infarction in the Healing and Early Afterload Reducing Trial (HEART): An Echocardiography Based Structural Analysis. American Heart Journal. 2001;141:234–242. doi: 10.1067/mhj.2001.112237. [DOI] [PubMed] [Google Scholar]
- 17.Feygin J, Hu Q, Swingen C, Zhang J. Relationships Between Regional Myocardial Wall Stress and Bioenergetics in Hearts with Left Ventricular Hypertrophy. American Journal of Physiology. 2008;294(5):H2313–H2321. doi: 10.1152/ajpheart.01288.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamoto H, Parish L, Hamamoto H, Ryan LP, Eperjesi TJ, Plappert T, Jackson BM, St John-Sutton MG, Gorman JH, III, Gorman RC. The Effect of Reperfusion on Left Ventricular Regional Remodeling Strains after Myocardial Infarction. Annals of Thoracic Surgery. 2007;84:1528–1537. doi: 10.1016/j.athoracsur.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 19.Hamamoto H, Gorman JH, III, Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, St. John-Sutton MG, Itescu S, Gorman RC. Allogeneic Mesenchymal Precursor Cell Therapy to Limit Post-MI Remodeling: The Effect of Cell Dosage. Annals of Thoracic Surgery. 2009;87:794–802. doi: 10.1016/j.athoracsur.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghanta RK, Rangaraj A, Umakanthan R, Lee L, Laurence RG, Fox JA, Bolman RM, III, Cohn LH, Chen FY. Adjustable Physiological Ventricular Restraint Improves Left Ventricular Mechanics and Reduces Dilatation in an Ovine Model of Chronic Heart Failure. Circulation. 2007;115:1201–1210. doi: 10.1161/CIRCULATIONAHA.106.671370. [DOI] [PubMed] [Google Scholar]
- 21.Jhun CS, Wenk JF, Zhang Z, Wall ST, Sun K, Sabbah HN, Ratcliffe MB, Guccione JM. Effect of Adjustable Passive Constraint on the Falling Left Ventricle: A Finite-Element Model Study. The Annals of Thoracic Surgery. 2010;89:132–137. doi: 10.1016/j.athoracsur.2009.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang ABC, Guccione JM, Mishell JM, Zhang P, Wallace AW, Gorman RC, Gorman JH, III, Ratcliffe MB. Akinetic Myocardial Infarcts Must Contain Contracting Myocytes: Finite Element Model Study. American Journal of Physiology. 2005;288:H1844–H1850. doi: 10.1152/ajpheart.00961.2003. [DOI] [PubMed] [Google Scholar]
- 23.Hung J, Guerrero JL, Handschumacher MD, Supple G, Sullivan S, Levine RA. Reverse Ventricular Remodeling Reduces Ischemic Mitral Regurgitation: Echo-Guided Device Application in the Beating Heart. Circulation. 2002;106:2594–2600. doi: 10.1161/01.cir.0000038363.83133.6d. [DOI] [PubMed] [Google Scholar]