Abstract
Aging is associated with many changes in sleep, with one of the most prominent being a reduction in slow wave sleep. Traditional measures of this phenomenon rely on spontaneous activity and typically confound the incidence and amplitude of delta waves. The measurement of evoked K-complexes during sleep, enable separate assessment of incidence and amplitude taken from the averaged K-complex waveform. The present study describes data from 70 normal healthy men and women aged between 19 and 78 years. K-complexes were evoked using short auditory tones and recorded from a midline array of scalp sites. Significant reductions with age were seen in the amplitude of the N550 component of the averaged waveform, which represents the amplitude of the K-complex, with linear regression analysis indicating approximately 50% of the variance was due to age. Smaller, yet still significant reductions were seen in the ability to elicit K-complexes. The data highlight the utility of evoked K-complexes as a sensitive marker of brain aging in men and women.
1. Introduction
Sleep is a ubiquitous behavior in humans that is associated with a characteristic pattern of changes in brain electrical activity across the night. Two different sleep states are definable using EEG and other physiological measures: Rapid eye movement (REM) sleep with wake-like EEG activity, but muscle paralysis and non-rapid eye movement (NREM) sleep with theta (4–7 Hz) and delta (0–4Hz) frequency EEG and unique EEG features such as sleep spindles and K-complexes (KCs). NREM sleep can be further subdivided based on EEG features into stage 1, with the onset of theta activity; stage 2, with theta, spindles and KCs; and slow wave sleep (SWS) with at least 20% delta activity (Rechtschaffen and Kales, 1968).
KCs were first described by Loomis et al. (Loomis et al., 1938) and represent an unusual class of EEG features in that they can occur spontaneously or be evoked by a variety of external stimuli (Colrain, 2005). Their spontaneous occurrence can define the onset of stage 2 NREM sleep (Rechtschaffen and Kales, 1968). The accepted definition for KCs is “EEG waveforms having a well delineated negative sharp wave which is immediately followed by a positive component. The total duration of the complex should exceed 0.5 sec.” (Rechtschaffen and Kales, 1968). They are thus by definition, delta frequency waveforms, as a period of greater than 0.5 seconds corresponds to a frequency of less than 2Hz. Spontaneous and evoked KCs have an extremely variable morphology (Paiva and Rosa, 1991) due to them being embedded in the ongoing EEG, and their summation with background EEG activity. It is for this reason that Bastien and Campbell (1992) proposed the use of averaging to extract the signal (pure KC) from the noise of the unrelated random EEG. Such averaging of KCs in NREM sleep produces an averaged evoked delta frequency waveform with a characteristic pattern of peaks starting and ending with positive components (P2 and P900). The large negative N550 component in between these positive peaks represents the averaged amplitude of the “pure” KC.
KCs share a common generation mechanism with the continuous delta activity seen in SWS. Delta EEG reflects EEG synchronization produced either by thalamocortical cells operating in a burst-firing pattern following hyperpolarization (Steriade et al., 1991) or by cortical cells operating in this manner independent of the thalamus (Steriade et al., 1993a). Aging has been shown to impact delta generation during sleep with a decrease in the incidence of spontaneous KCs (Crowley et al., 2002a; Kubicki et al., 1989) and incidence and amplitude (N550) of evoked KCs (Crowley et al., 2002b; Crowley et al., 2004). However, these studies only tested groups of young (mean age of around 23 years in both studies) and old (mean age of 66 years in one study and 76 in the other) adults. The progression of change in the KC across the adult lifespan is, therefore, unknown.
Aging also reliably leads to a decrease in the amount of SWS (Bliwise, 1993; Blois et al., 1983; Ehlers and Kupfer, 1989; Feinberg et al., 1967; Larsen et al., 1995; Smith et al., 1977). However as highlighted in a recent meta analysis of studies about sleep across the lifespan (Ohayon et al., 2004) many of the studies did not contain middle aged subjects. Of the 47 studies used to describe the impact of age on adult sleep only seven compared the sleep of young, middle aged and elderly adults whereas seventeen studies compared young and elderly subjects directly. A recent study (Bonnet and Arand, 2007) addressed this limitation by studying the sleep of 76 subjects aged 18 to 70 years and reported that SWS declined with age with an overall correlation of r = −0.62 (p <.01).
The reduction in SWS with aging is thought to primarily reflect a decrease in the amplitude of delta activity, rather than an absence of slow frequency activity (Bliwise, 1993), as the scoring of SWS requires not only the presence of at least 20% delta activity per epoch, but that the delta activity have an amplitude of at least 75 μV (Rechtschaffen and Kales, 1968). However, aging has also been reliably associated with decreases in NREM sleep delta power which reflects both amplitude and incidence of delta waves (Blois et al., 1983; Ehlers and Kupfer, 1989; Feinberg and Campbell, 2003; Feinberg et al., 1981; Landolt et al., 1996; Mourtazaev et al., 1995). The use of evoked KCs as an index of delta production enables the separation of incidence and amplitude variables that are confounded in more traditional SWS and delta power measures.
The aim of the present experiment was to test the hypothesis that evoked delta frequency responses, as indexed by the amplitude of the N550 evoked potential component, would show a linear decline with increasing age over the adult lifespan. Confirmation of the hypothesis would indicate that evoked delta responses might provide a functional index of age-related brain macrostructural and microstructural changes.
2. Methods
2.1 Subjects
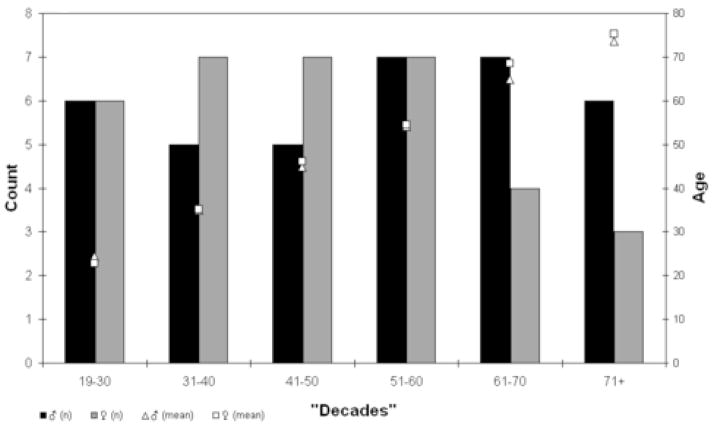
Data were collected from 70 normal healthy adults (34 women) ranging in age from 19 to 78 years. The age distributions for each sex are presented in Figure 1. The men had a mean (± SD) age of 50.6 ± 17.3 years, which did not differ significantly from that of the women (46.7 ± 16.8 years) (t(68)=0.94, p=0.4).
Figure 1.
Age distribution of the sample. Histograms reflect the number of men (black) and women (gray) in each decade, with the count displayed on the left Y axis. The unfilled triangles reflect the mean age per decade of men and unfilled squares the mean age per decade of women, with the age values plotted on the right hand Y axis.
Data were drawn from a number of studies from our laboratory, all using the same equipment, with identical stimulus and similar recording parameters. Eight control subjects were drawn from each of the Nicholas et al. (2002) and Afifi et al. (2003) studies, seventeen subjects from the Crowley et al. (2002b) study and thirty seven subjects from ongoing studies of normal aging and alcoholism. All subjects were in general good health and free from sleep disorders. All subjects reported normal hearing with no need for hearing aids. For the subjects over the age of 65 years hearing was verified using an audiometer to be within 15dB ISO at 1000Hz. Bastien and Campbell(1992) have reported that a 20 dB decrease in stimulus intensity had no impact on N550 amplitude. All subjects were tested during wakefulness to ensure that they could hear the tones that would be presented to them during sleep.
2.2 Stimuli
Auditory stimuli consisted of 1000Hz pure tones presented for 50ms (2ms rise time) at 80 dB(A). These stimuli have previously been shown to be optimal for KC elicitation (Bastien and Campbell, 1992). The tones were presented binaurally via E-A-RTONE 3A insert earphones, with a random inter stimulus interval of between 15 and 30 seconds. A minimum of 200 stimuli were presented in stable stage 2 sleep during each night. When subjects entered SWS or REM sleep or the EEG showed signs of arousal or motor artifact, stimulus presentation was halted, until stage 2 sleep had been re-entered. Data were not collected during REM due to the absence of K-complexes form this sleep stage. SWS was avoided due to the potential for the age-related decline in SWS to confound the results.
2.3 EEG data collection
EEG and evoked potential data were recorded from 5 midline scalp sites adapted from the international 10/20 system (Fz, FCz, Cz, CPz, Pz), using Grass gold-plated 10mm electrodes (with the exception of the subjects from (Afifi et al., 2003) where the data were collected using a Neuroscan Quick Cap electrode array). Two channels of electro-oculogram (EOG) and a submental electromyogram (EMG) were also recorded. All EEG channels were referenced to linked earlobes. Raw data were acquired using Neuroscan Synamps® amplifiers and stored for offline analysis using Neuroscan Scan™ software. Signals were continuously displayed in real time and filtered optimally for visual recognition of sleep stages (Rechtschaffen and Kales, 1968) (30sec per page, EEG & EOG: band pass filter, 0.3 – 30Hz; EMG: band pass filter, 10 – 100Hz.). For analysis: raw EEG data were epoched time-locked to tone stimuli, and were filtered (“zero phase shift” - 0.3 to 30Hz) offline. Individual epochs were corrected for baseline differences across the pre-stimulus period.
Evoked KCs were defined using a modification of the Rechtschaffen and Kales (1968) criteria, using data from Cz and Fz, with the additional parameter that the negative peak of the KC had to occur between 400 and 900 ms after the tone. No amplitude criterion was used. Epochs containing movement artifacts observed in EOG or EMG channels were discarded. All KC responses within stage 2 sleep were then averaged for each subject to produce an averaged KC evoked potential at all sites. The proportion of stimuli producing a KC was recorded as the KC incidence value for each subject.
Component identification was achieved using Scan™ software. The P2 component was defined as the most positive peak between 100 and 300ms post stimulus. The N550 was defined as the most negative value between 400 and 900ms post stimulus and the P900 as the most positive peak between 800 and 1200ms post stimulus. Amplitudes were then determined relative to the average of the pre-stimulus baseline. All peaks were determined within all electrode sites.
2.4 Statistical Analysis
KC incidence and the amplitude and latency values for each peak, at each electrode site were subjected to linear regression analysis to determine the relationship of each variable to age. Univariate ANOVAs were used to investigate changes in KC incidence according to age and sex. Amplitude and latency of each peak component was subjected to a mixed model ANOVA, with electrode site (Fz, FCz, Cz, CPz and Pz) as a repeated measures factor and sex as a between groups factor. Age was entered as a covariate. The age × site interaction term in this model provided a test of differences of the age regression slopes between different electrode sites. The site and age × site effects were tested for sphericity and when found, degrees of freedom were adjusted using the Greenhouse-Geisser correction but original degrees of freedom are reported.
3 Results
3.1 K-complex incidence
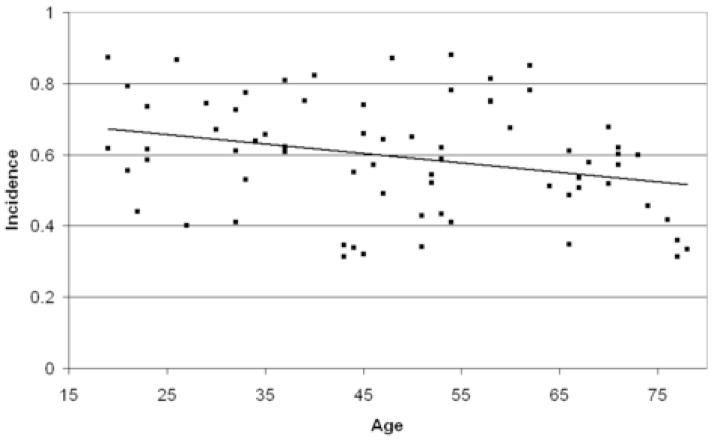
KC incidence is displayed in Figure 2. Univariate ANOVA indicated a significant effect of age (F (1,67) = 5.90, p = 0.018) but no effect of sex (F (1,67) = 0.114, p = 0.737). There was a significant linear decrease in KC incidence with age (R2 = .079, F (1,68)=5.87, p = 0.018).
Figure 2.
KC incidence (proportion of stimuli producing a K-complex) as a function of age. Data represent one point per subject, with the solid line indicating the linear regression function fitted to the data.
3.2 Evoked potential components from averaged KC waveforms
Averaged evoked KC waveforms at each electrode site are presented in Figure 3 broken down by “decade” as per the age distributions in Figure 1.
Figure 3.
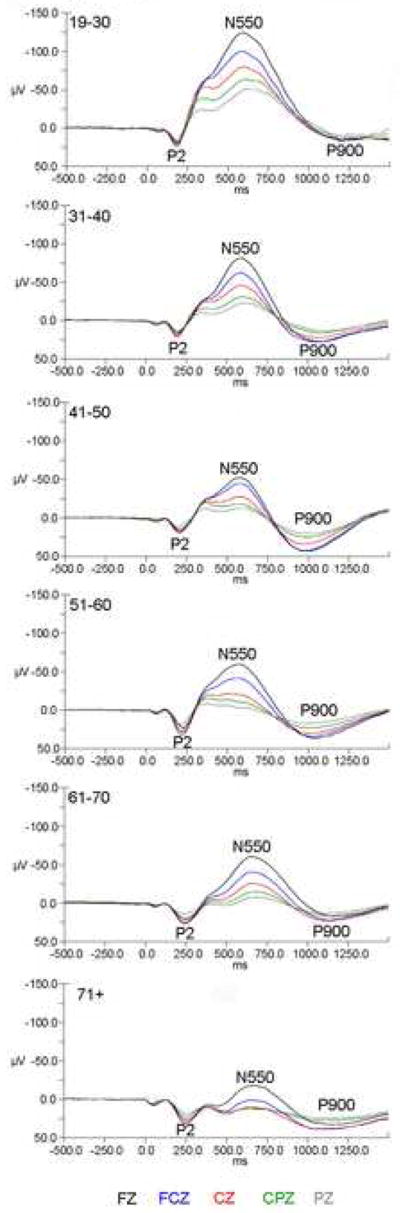
Grand mean waveforms for each decade. In each set of waveforms data are plotted with negative voltages up the Y axis.
3.2.1 N550
N550 amplitude data for each electrode site are presented in Figure 4. ANOVA of N550 amplitude revealed a significant decrease with age (F (1,65) = 94.22, p < 001) but no significant effect of sex (F (1,65) = 0.35, p = 0.6). There was a significant effect of site (F (4, 260) =35.78, p < 0.001) and a significant interaction between age and site (F (4, 260) = 4.41, p = 0.024), indicating a significant difference in the slopes of the linear regression equations between sites. As can be seen in the bottom panel of Figure 4 and Table 1, the slopes are steepest at Fz and flatten progressively in an anterior to posterior progression. All linear regression equations were significant (p < 0.001 in all cases), with R2 values indicating linear relationships explaining between 49% and 59% of the age variance. The regression equations predict a 15.24 μV per decade decrease in amplitude at Fz, and decreases of 14.58, 13.28, 14.44 and 9.87 μV per decade at FCz, CZ, CPZ and PZ respectively.
Figure 4.
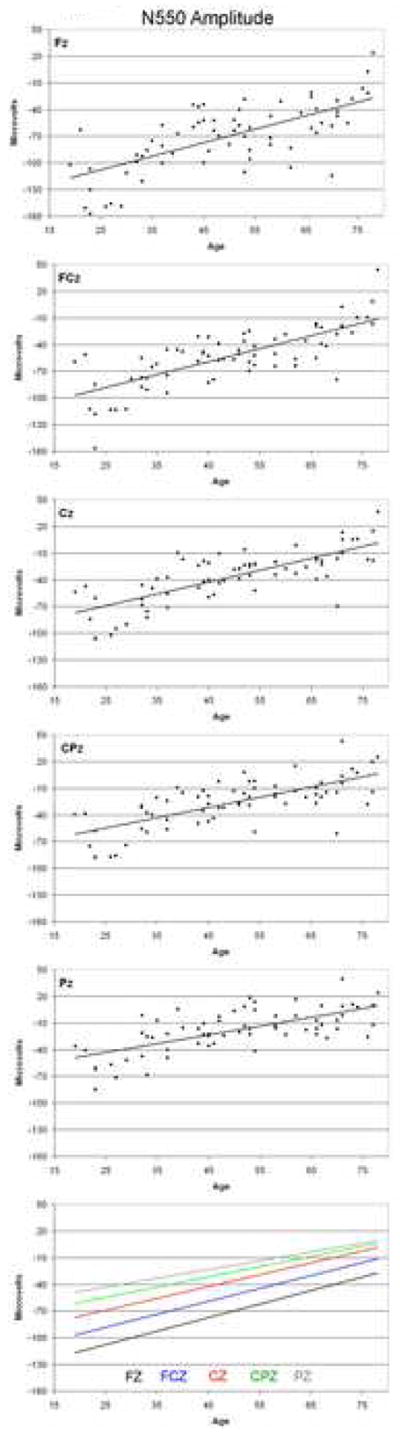
N550 amplitude as a function of age at each electrode site. Data represent one point per subject, with the solid line indicating the linear regression function fitted to the data.
Table 1.
Linear regression analysis for age with N550, P2, and P900 amplitude and latency at each electrode site.
| N550 | P2 | P900 | |||||
|---|---|---|---|---|---|---|---|
| R2 | Slope | R2 | Slope | R2 | Slope | ||
| Amplitude | Fz | 0.490*** | 1.524 | 0.115** | 0.172 | 0.050 | 0.307 |
| FCz | 0.573*** | 1.458 | 0.133** | 0.208 | 0.062* | 0.294 | |
| Cz | 0.587*** | 1.328 | 0.089* | 0.190 | 0.066* | 0.259 | |
| CPz | 0.519*** | 1.444 | 0.027 | 0.090 | 0.053 | 0.055 | |
| Pz | 0.485*** | 0.987 | 0.010 | 0.050 | 0.004 | −0.072 | |
| Latency | Fz | 0.068* | 1.049 | 0.262*** | 1.196 | 0.001 | −0.246 |
| FCz | 0.048 | 0.978 | 0.282*** | 1.302 | 0.006 | −0.633 | |
| Cz | 0.060* | 0.1.150 | 0.263*** | 1.305 | 0.003 | −0.633 | |
| CPz | 0.046 | 1.076 | 0.263*** | 1.305 | 0.008 | −0.633 | |
| Pz | 0.012 | 0.639 | 0.218*** | 1.243 | 0.004 | −0.682 | |
p <0.05,
p <0.01,
p <0.001.
ANOVA of N550 latency revealed a modest increase with age (F (1,65) = 4.22, p = 0.044). There was no significant effect of site (F (1,65) = 1.65, p = 0.2), no significant effect of sex (F (1,65) = 0.17, p = 0.7), and no significant interaction between age and site (F (4,260) = 1.42, p = 0.2). As indicated in Table 1, linear regression equations were significant at Fz (p = 0.029) and Cz (p = 0.042) only, with R2 values indicating that only 6% to 7% of the age variance was explained at these sites.
3.2.2 P2
P2 amplitude data for each electrode site are presented in Figure 5. ANOVA of P2 amplitude revealed a significant increase with age (F (1,65) = 6.82, p = 0.011), but no significant effect of site (F (4,260) =1.43, p = 0.243), and no significant effect of sex (F (1,65) = 0.001, p = 0.970). There was a significant interaction between age and site (F (4,260) = 3.62, p = 0.032), indicating a significant difference in the slopes of the linear regression equations between sites. As can be seen in the bottom panel of Figure 5 and in Table 1, the slopes at FCz, Cz and Fz were steeper than those at the more posterior sites and the linear regression equations for these sites were all significant, whereas those for CPz and Pz were not. The R2 values indicate that the strongest relationship, at FCz, explained only about 13% of the variance of of P2 amplitude with age.
Figure 5.
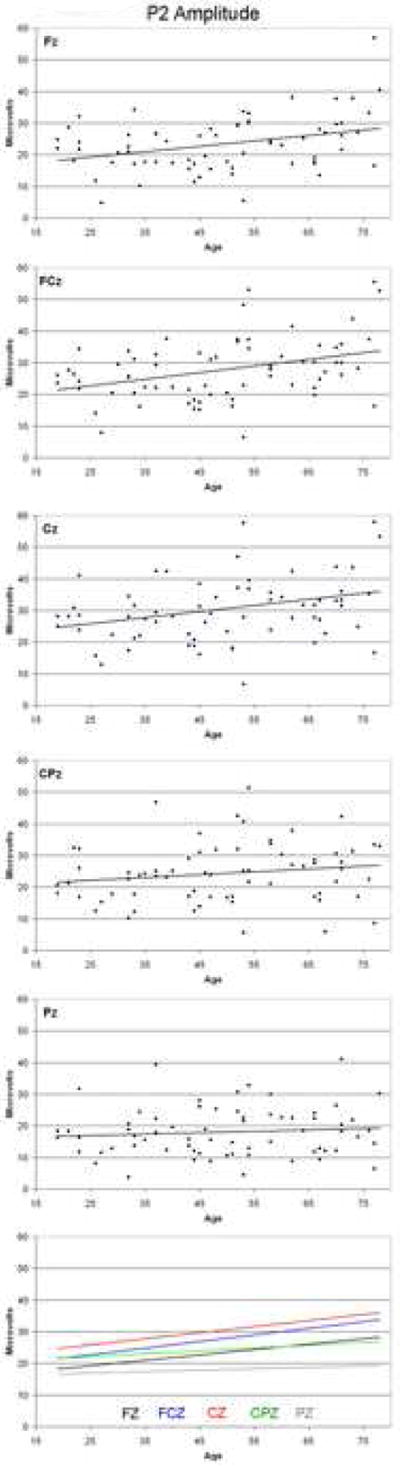
P2 amplitude as a function of age at each electrode site. Data represent one point per subject, with the solid line indicating the linear regression function fitted to the data.
P2 latency data for each electrode site are presented in Figure 6. ANOVA of P2 latency also revealed a significant increase with age (F (1,65) = 20.72, p < 0.001), but no significant effect of site (F (4,260) = 1.45, p = 0.236), no significant effect of sex (F (1,65) = 0.21, p = 0.652), and no significant interaction between age and site (F (4,260) = 0.59, p = 0.594) (see bottom panel of Figure 6). As indicated in Table 1, linear regression equations were significant for all sites, with the R2 values indicating that this variable accounts for between 22 and 28% of the age variance.
Figure 6.
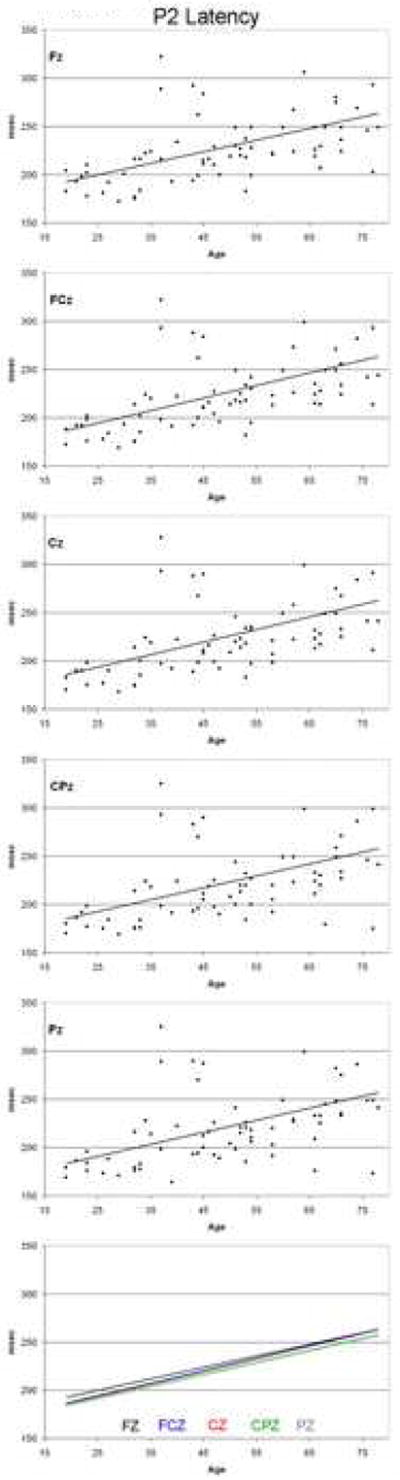
P2 latency as a function of age at each electrode site. Data represent one point per subject, with the solid line indicating the linear regression function fitted to the data.
3.2.3 P900
ANOVA of P900 amplitude revealed no significant effect of age (F (1,66) = 2.68, p = 0.106), and no significant effect of site (F (4,264) =0.5, p = 0.479). There was a significant effect of sex (F (1,66) = 4.81, p = 0.032), and a significant interaction between age and site (F (4,264) = 4.67, p = 0.022), indicating a significant difference in the slopes of the linear regression equations between sites. As can be seen in Table 1, the linear regression equations were significant only at FCz and Cz. However, the R2 values indicate that these relationships explain only approximately 6% of the variance of age.
ANOVA of P900 latency revealed no significant effects of age (F (1,66)=0.4, p = 0.5), sex (F (1,66)=0.158, p = 0.7), or site (F (4,264)=0.27, p = 0.9) and no interaction between age and site (F (4,264)=0.30, p = 0.9). The linear regression relationships with age were non-significant at all sites (see Table 1).
4. Discussion
The hypothesis of a linear decline in N550 amplitude was supported by the data, with R2 values indicating that greater than 50% of the variance in the measure is attributable to aging. While the age relationship was significant at all measured electrode sites, the slope of the age related decline was steepest at Fz and showed a progressive anterior-posterior flattening. Significant increases in P2 amplitude with age were also seen at anterior sites, and there was an overall increase in P2 latency. Evoked KC incidence decreased significantly in a linear fashion with increasing age, however the R2 values were modest, indicating that less than 10% of the variance in the measure is attributable to age.
KCs are single instances of delta frequency EEG waveforms. Delta EEG during sleep can be generated in two ways: within cortical cells operating in a burst-firing pattern following hyperpolarization and activation of low threshold calcium channels (Steriade et al., 1993b); or by thalamocortical cells (Steriade et al., 1991) operating in a similar manner. As sleep state becomes deeper, and GABAergic cells in the thalamic reticular nucleus are less impacted by ascending brainstem input, thalamocortical cells are able to generate either delta or transmit spindle activity, depending on their level of hyperpolarization. As this hyperpolarization becomes greater and the spindles give way to delta activity
There is now converging evidence from multiple sources that KCs are a purely cortical form of delta EEG rather than a thalamocortical phenomenon. Amzica and Steriade (Amzica and Steriade, 1997, 1998b) have argued that the spontaneous KC generation is due to the activity of a cortically generated slow (<1Hz) oscillation, that has been recorded from cortical neurons in anesthetized animals (Steriade et al., 1993b) during natural slow wave sleep in cats (Steriade et al., 1996) and naturally sleeping humans (Achermann and Borbely, 1997; Amzica and Steriade, 1997). The cortical nature of the slow oscillation was demonstrated by its survival in athalamic preparations (Steriade et al., 1993a), its absence in the thalamus of decorticated cats (Timofeev and Steriade, 1996), and the disruption of its long-range synchronization after disconnection of intracortical synaptic linkages (Amzica and Steriade, 1995). Multi-site depth recordings in both animals (Amzica and Steriade, 1998a) and humans (Wennberg and Lozano, 2003) show polarity inversion which necessitates a supra-thalamic, i.e. cortical generator. Rossetti et al. (2005) recently reported a case study of a patient with thalamic vasogenic edema. During the period of the edema, spindles were absent from the sleep EEG, but spontaneous KCs were unaffected. Consistent with these finding, neither KC incidence nor KC N550 amplitude are different in responses to stimuli presented during a sleep spindle compared to those presented in the absence of a spindle (Crowley et al., 2004), and the scalp distribution of evoked KCs is the same for auditory and respiratory stimuli (Colrain et al., 1999) despite the thalamocortical relay projecting to very different areas of cortex. Finally, Marini et al. were able to show normal spontaneous KC activity in the rat (Marini et al., 2004), and that these were unaffected by destruction of the thalamic reticular nucleus with ibotenic acid (Marini, 2008). All of the above data would seem to indicate that the processes for generating spindles and KCs are independent, with sleep spindles produced within the thalamus (Steriade et al., 1987) and KCs reflecting cortical delta generation. However, spindles are also altered by aging (Crowley et al., 2002b), and it remains possible that some characteristics of spindles (e.g., duration, frequency, density or amplitude) and KC amplitude may well be correlated in terms of how they change across the lifespan.
The formal definition of K-complexes erroneously states that it “… is generally maximal over vertex regions” (Rechtschaffen and Kales, 1968). There is now a substantial literature indicating that the N550 measured in KC averages in adults, has a topographic distribution indicating frontal or fronto-central maxima, bilateral symmetry, and a gradual fall off in voltage from the midline frontal/fronto-central area to the posterior and lateral scalp regions (Colrain et al., 1999; Cote et al., 1999; Niiyama et al., 1995). In adult humans, KCs thus represent a highly synchronized cortical phenomenon that has a substantial frontal predominance. The present data support these earlier studies showing that the amplitude of the N550 peak is maximal at frontal electrode sites. In addition, they show for the first time that the frontal dominance of the N550 persists across the adult lifespan.
Changes in amplitude presumably reflect changes in the “capacity” to generate high amplitude delta waveforms, once triggered. The frontal scalp distribution and probable extrathalamic generation of the KC indicate that it may be indexing a similar regional process to that reported by Dang-Vu and colleagues (Dang-Vu et al., 2005). H215O PET was measured during sleep as determined by concurrent C3-A2 and C4-A1 EEG recordings. Delta power across stage2 and SWS was negatively correlated with regional cerebral blood flow (rCBF) in regions previously grouped together by Damasio (1994) as ventromedial prefrontal cortex (VMPFC). Specifically, negative correlations were found with rCBF in medial frontal cortex, orbitofrontal cortex, anterior cingulate gyrus, basal forebrain, anterior hypothalamus, putamen, anterior part of the insula and the precuneus. Importantly, there was no correlation with activation in the thalamus. The authors interpreted their data as relating to the generation of an extra-thalamic delta activity as distinct from the clock-like delta produced by thalamo-cortical loops. If N550 amplitude in evoked KCs is indeed indexing the same process, the present data would indicate its potential utility as a functional measure of the impact of aging on VMPFC.
The N550 amplitude data from the present study extend the literature showing decreased delta amplitudes with aging. Most studies have concentrated on comparing groups of young and older subjects whereas a strength of this study is the investigation of changes in an evoked delta waveform across the adult lifespan. It has been known for over thirty years that the greatest change in slow wave sleep delta activity occurs in adolescence and early adulthood (Feinberg, 1974) and it can be argued that in some of the studies reporting a decrease in delta power with increasing age, a substantial proportion of the impact occurs early in adult aging. For example, Ehlers and Kupfer (1989) reported that 31–40 year old men had less power in 0.5–2Hz and 1–2Hz activity than 21–30 year old men, but that there was no further decrease seen in 51–70 year olds. Mourtazaev et al. (1995) reported that their finding of decreased slow wave power with aging was mainly based on the difference between the youngest group (26–35 years) and the other age groups (61–60, 66–75 and 85–101 years), with less difference being observed between the older groups. The present finding of a linear decrease in delta amplitude is more consistent with the linear reduction in SWS reported in the recent Bonnet and Arand (2007) study, although with a more of the age variance explained (R2 of 0.50 vs. 0.380). It is also consistent with the brain changes seen with aging, for example Pfefferbaum et al. (1994) and highlights the sensitivity of the averaged KC as a measure of delta production. Other measures, such as SWS and delta power that show a reduced rate of change in older subjects, have features that may mask the linear decline revealed in theN550 measure. Thus SWS, is confounded by scoring rules that require delta frequency EEG, be at least 75 μV in amplitude (Rechtschaffen and Kales, 1968), and delta power is a composite measure of amplitude and incidence of waveforms. N550 amplitude is a “pure” delta amplitude measure.
In the present study the slope of the linear relationship between KC N550 amplitude and age is steepest at frontal sites and then becomes progressively flatter with more caudal electrode sites. Steeper rates of decline in frontal delta are consistent with the data presented by Münch et al. (2004) where older subjects (57–74 years) displayed a substantially reduced increase in delta power at frontal sites following sleep deprivation when compared with the younger subjects.
The present data show significant decreases in amplitude and incidence of evoked delta waveforms with aging, but indicate that the incidence measure is less impacted by age than is amplitude. The oldest subject was still able to produce KCs to 33.3% of the stimuli and the average KC incidence rate for the 71–78 year-olds was 0.48 ± 0.13, representing a 27% decrease from the 0.65 ± 0.18 seen in the 19–30 year-olds. In comparison, the incidence of spontaneous KCs has previously been reported as reduced by about 20% in older compared to younger subjects (Crowley et al., 2002a). By way of comparison the N550 amplitude at Fz of the oldest subjects showed an 80.3% decrease when compared to the 19–30 year-olds. The finding of a relatively conserved ability to produce KCs in normal elderly is consistent with our previous report comparing delta production in normal elderly to that in Alzheimer’s Disease (AD) patients (Crowley et al., 2005).
The present data are also consistent with other studies showing that delta incidence is less impacted by aging than delta amplitude. For example, Feinberg (1974) used a 50 μV criterion for SWS in a study of 105 normal subjects from ages 4 to 96 years. There were dramatic reductions in total SWS when comparing the adult groups to young children (4–10 year olds) and adolescents (11–16 year olds), however, the only SWS effect seen among the adults subjects was that the 17–23 year-olds had more stage 4 than the 67–96 year olds. The reduced amplitude is unlikely to have negatively affected SWS scoring given that SWS has been shown to be scored reliably with no amplitude criterion (Webb and Dreblow, 1982). Indeed, Smith Karacan & Yang (1977) found no age related changes in incidence when all waves greater than 5 μV were assessed.
The decrease in KC incidence presumably indicates that the cortex is less often in the hyperpolarized state conducive to KC production. The underlying cause of this change is not known, but may relate to altered cortical dynamics with a disruption of the slow frequency oscillation. Recent work has highlighted the role of glial cells in producing the slow frequency oscillation thought to be necessary for K-complex and delta generation (Amzica and Massimini, 2002). Age related changes in glial function may thus be partly responsible for the finding.
In vivo studies (Manns et al., 2000, 2003) have demonstrated that the cholinergic neurons of the nucleus basalis of Meynert (NB) discharge in a rhythmic bursting mode, which is subtended by calcium conductances when cells are hyperpolarized (Alonso et al., 1996; Khateb et al., 1992). The dramatic reduction of KC incidence (as low as 5%), in AD patients in the Crowley et al. (2005) study, were thus interpreted as potentially due to the pathology of the NB seen in AD patients. The less dramatic change in KC incidence seen with normal aging in the present data set could nonetheless reflect a more subtle change in this mechanism.
The finding of an increase in P2 amplitude with age highlights the fact that the reduction in N550 is not merely due to physical changes in the body with age that might produce an overall damping of EEG amplitude. As with KC incidence, regression equations for P2 amplitude and latency explained substantially less of the age variance than was the case for N550 amplitude. P2 is a difficult component to interpret (Crowley and Colrain, 2004), but presumably is some reflection of the state of the cortex at the start of the KC, and thus the present data highlight altered cortical dynamics with aging. Pfefferbaum et al. (1980) have previously P2 amplitude as increasing with aging in evoked potential recorded during wakefulness, despite most “awake” auditory evoked potential components showing reduced amplitudes with increased age (Kok, 2000). Data collection during other sleep stages (REM and SWS) might further inform the understanding of P2 and indeed other components, but this will require an additional study given that only stage 2 data are available for analysis in the present experiment.
The present data do not show a significant main effect of sex for any variable other than P900 amplitude which was larger in women than men. The lack of a sex difference in either KC incidence or KC N550 amplitude is interesting in the context of other studies previously reporting sex differences in other delta EEG measures. Studies have typically reported that women have increased SWS or delta power relative to men, with the difference persisting into old age (Carrier et al., 2001; Mourtazaev et al., 1995). However, the incidence of spontaneous KCs is not higher in young or old women relative to age-matched men (Crowley et al., 2002a). Studies which have evaluated aging effects on delta activity have typically not reported significant age × sex interactions (Carrier et al., 2001; Mourtazaev et al., 1995), concluding that the process of aging has similar impacts on delta EEG in men and women, as is the case in the present data.
5. Conclusions
The present data point to N550 amplitude in the average of evoked KCs as being a highly sensitive marker of aging in both men and women. Evoked KCs appear to reflect a functional measure of the ability of the aging brain to produce large amplitude synchronized waveforms, and thus act as an indirect measure of how aging impacts the functional integrity of brain networks, particularly those in frontal regions. Given that these measures are derived from sleep EEG, they arguably provide measures that are free from potentially confounding factors present in recordings made during wakefulness in older subjects, such as the ability to understand, follow or remember instructions. Sleep can be viewed as an optimal baseline state in which to assess functional properties of the CNS. Given recent experimental and theoretical developments relating to the role of delta EEG in reflecting a compensatory state for synaptic potentiation during wakefulness (Tononi and Cirelli, 2003), and the role of delta sleep in supporting daytime learning and memory (Goder et al., 2006) and neuropsychological function (Anderson and Horne, 2003), the ability to measure delta production in a way that sensitively indexes normal aging may prove important in aiding the understanding of how changes in sleep EEG support changes in brain function in older adults.
Acknowledgments
The work was funded by NIH (NIAAA) grant AA14211.
Footnotes
6.1 Disclosure statement: None of the authors have any potential or actual conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achermann P, Borbely AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81(1):213–222. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiol Neurobiol. 2003;136(2–3):221–234. doi: 10.1016/s1569-9048(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Alonso A, Khateb A, Fort P, Jones BE, Muhlethaler M. Differential oscillatory properties of cholinergic and noncholinergic nucleus basalis neurons in guinea pig brain slice. Eur J Neurosci. 1996;8(1):169–182. doi: 10.1111/j.1460-9568.1996.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Amzica F, Massimini M. Glial and neuronal interactions during slow wave and paroxysmal activities in the neocortex. Cereb Cortex. 2002;12(10):1101–1113. doi: 10.1093/cercor/12.10.1101. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Disconnection of intracortical synaptic linkages disrupts synchronization of a slow oscillation. Journal of Neuroscience. 1995;15(6):4658–4677. doi: 10.1523/JNEUROSCI.15-06-04658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F, Steriade M. The K-complex: its slow (<1-Hz) rhythmicity and relation to delta waves. Neurology. 1997;49(4):952–959. doi: 10.1212/wnl.49.4.952. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998a;82(3):671–686. doi: 10.1016/s0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998b;107(2):69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Anderson C, Horne JA. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40(3):349–357. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- Bastien C, Campbell K. The evoked K-complex: All-or-none phenomenon? Sleep. 1992;15(3):236–245. doi: 10.1093/sleep/15.3.236. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Blois R, Feinberg I, Gaillard JM, Kupfer DJ, Webb WB. Sleep in normal and pathological aging. Experientia. 1983;39(6):551–558. doi: 10.1007/BF01971096. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. EEG arousal norms by age. J Clin Sleep Med. 2007;3(3):271–274. [PMC free article] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old) Psychophysiology. 2001;38(2):232–242. [PubMed] [Google Scholar]
- Colrain IM. The K-complex: A seven-decade history. Sleep. 2005;28(2):255–273. doi: 10.1093/sleep/28.2.255. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response? J Sleep Res. 1999;8(4):273–280. doi: 10.1046/j.1365-2869.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Cote KA, de Lugt DR, Langley SD, Campbell KB. Scalp topography of the auditory evoked K-complex in stage 2 and slow wave sleep. J Sleep Res. 1999;8(4):263–272. doi: 10.1046/j.1365-2869.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- Crowley K, Sullivan EV, Adalsteinsson E, Pfefferbaum A, Colrain IM. Differentiating pathologic delta from healthy physiologic delta in patients with Alzheimer disease. Sleep. 2005;28(7):865–870. doi: 10.1093/sleep/28.7.865. [DOI] [PubMed] [Google Scholar]
- Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM. The effects of normal aging on sleep spindle and K-complex production. Clin Neurophysiol. 2002a;113(10):1615–1622. doi: 10.1016/s1388-2457(02)00237-7. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol. 2004;115(4):732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Trinder J, Colrain IM. An examination of evoked K-complex amplitude and frequency of occurrence in the elderly. J Sleep Res. 2002b;11(2):129–140. doi: 10.1046/j.1365-2869.2002.00293.x. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Trinder J, Colrain IM. Evoked K-Complex Generation: The Impact of Sleep Spindles and Age. Clinical Neurophysiology. 2004;115(2):471–476. doi: 10.1016/j.clinph.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Damasio A. Emotion Reason and the Human Brain. Grosset/Putnam; New York: 1994. Descartes’s Error. [Google Scholar]
- Dang-Vu TT, Desseilles M, Laureys S, Degueldre C, Perrin F, Phillips C, Maquet P, Peigneux P. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage. 2005;28(1):14–21. doi: 10.1016/j.neuroimage.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Ehlers C, Kupfer D. Effects of age on delta and REM sleep parameters. Electroencephalography and Clinical Neurophysiology. 1989;72:118–125. doi: 10.1016/0013-4694(89)90172-7. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10(3–4):283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Campbell IG. Kinetics of non-rapid eye movement delta production across sleep and waking in young and elderly normal subjects: theoretical implications. Sleep. 2003;26(2):192–200. doi: 10.1093/sleep/26.2.192. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Fein G, Floyd TC, Aminoff M. Sleep EEG waveform profiles in young and elderly normal subjects. In: Perris C, Struwe G, Jansson B, editors. Biological Psychiatry. Elsevier/North Holland Biomedical Press; Amsterdam: 1981. pp. 294–297. [Google Scholar]
- Feinberg I, Koresko RL, Heller N. EEG sleep patterns as a function of normal and pathological aging in man. J Psychiatr Res. 1967;5(2):107–144. doi: 10.1016/0022-3956(67)90027-1. [DOI] [PubMed] [Google Scholar]
- Goder R, Aldenhoff JB, Boigs M, Braun S, Koch J, Fritzer G. Delta power in sleep in relation to neuropsychological performance in healthy subjects and schizophrenia patients. J Neuropsychiatry Clin Neurosci. 2006;18(4):529–535. doi: 10.1176/jnp.2006.18.4.529. [DOI] [PubMed] [Google Scholar]
- Khateb A, Muhlethaler M, Alonso A, Serafin M, Mainville L, Jones BE. Cholinergic nucleus basalis neurons display the capacity for rhythmic bursting activity mediated by low-threshold calcium spikes. Neuroscience. 1992;51(3):489–494. doi: 10.1016/0306-4522(92)90289-e. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biol Psychol. 2000;54(1–3):107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kubicki S, Scheuler W, Jobert M, Pastelak-Price C. The effect of age on sleep spindle and K complex density. EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb. 1989;20(1):59–63. [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738(2):205–212. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- Larsen LH, Moe KE, Vitiello MV, Prinz PN. Age trends in the sleep EEG of healthy older men and women. J Sleep Res. 1995;4(3):160–172. doi: 10.1111/j.1365-2869.1995.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Loomis A, Harvey E, Hobart G. Distribution of disturbance-patterns in the human electroencephalogram, with special reference to sleep. Journal of Neurophysiology. 1938;1:413–430. [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000;20(4):1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003;89(2):1057–1066. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- Marini G. Personal Communication. 2008.
- Marini G, Ceccarelli P, Mancia M. Spontaneous K-complexes in behaving rats. Arch Ital Biol. 2004;142(1):59–67. [PubMed] [Google Scholar]
- Mourtazaev MS, Kemp B, Zwinderman AH, Kamphuisen HA. Age and gender affect different characteristics of slow waves in the sleep EEG. Sleep. 1995;18(7):557–564. doi: 10.1093/sleep/18.7.557. [DOI] [PubMed] [Google Scholar]
- Münch M, Knoblauch V, Blatter K, Schroder C, Schnitzler C, Krauchi K, Wirz-Justice A, Cajochen C. The frontal predominance in human EEG delta activity after sleep loss decreases with age. Eur J Neurosci. 2004;20(5):1402–1410. doi: 10.1111/j.1460-9568.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- Nicholas CL, Sullivan EV, Pfefferbaum A, Trinder J, Colrain IM. The effects of alcoholism on auditory evoked potentials during sleep. J Sleep Res. 2002;11(3):247–253. doi: 10.1046/j.1365-2869.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- Niiyama Y, Fushimi M, Sekine A, Hishikawa Y. K-complex evoked in NREM sleep is accompanied by a slow negative potential related to cognitive process. Electroencephalogr Clin Neurophysiol. 1995;95(1):27–33. doi: 10.1016/0013-4694(95)00021-p. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Paiva T, Rosa A. The K-Complex variability in normal subjects. In: Terzano MG, Halasz P, Declerck AC, editors. Phasic Events and Dynamic Organization of Sleep. Raven; New York: 1991. pp. 167–184. [Google Scholar]
- Pfefferbaum A, Ford JM, Roth WT, Kopell BS. Age-related changes in auditory event-related potentials. Electroencephalogr Clin Neurophysiol. 1980;49(3–4):266–276. doi: 10.1016/0013-4694(80)90221-7. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardised Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. U.S. Government Printing Office; Washington D.C: 1968. [Google Scholar]
- Rossetti AO, Maeder-Ingvar M, Reichhart MD, Despland PA, Bogousslavsky J. Transitory sleep spindles impairment in deep cerebral venous thrombosis. Neurophysiol Clin. 2005;35(1):19–23. doi: 10.1016/j.neucli.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Smith JR, Karacan I, Yang M. Ontogeny of delta activity during human sleep. Electroencephalogr Clin Neurophysiol. 1977;43(2):229–237. doi: 10.1016/0013-4694(77)90130-4. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40Hz) spontaneous cortical rhythms during brain activation. Journal of Neuroscience. 1996;16(1):392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57(1):260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Nunez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991;11(10):3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993a;13(8):3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. Journal of Neuroscience. 1993b;13(8):3852–3865. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. Journal of Neurophysiology. 1996;76(6):4152–4168. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Webb WB, Dreblow LM. A modified method for scoring slow wave sleep of older subjects. Sleep. 1982;5(2):195–199. doi: 10.1093/sleep/5.2.195. [DOI] [PubMed] [Google Scholar]
- Wennberg RA, Lozano AM. Intracranial volume conduction of cortical spikes and sleep potentials recorded with deep brain stimulating electrodes. Clin Neurophysiol. 2003;114(8):1403–1418. doi: 10.1016/s1388-2457(03)00152-4. [DOI] [PubMed] [Google Scholar]