Abstract
Patients with rheumatoid arthritis (RA) are twice as likely as their healthy peers to suffer from cardiovascular disease. RA is also a major cause of disability and reduced quality of life. Clinical trials of exercise and physical activity interventions demonstrate positive effects on muscle strength, function, aerobic capacity, mood and disability. While RA management guidelines emphasize the role of exercise and physical activity in the management of RA, the description of physical activity and exercise is vague and patients with RA remain less physically active than their healthy counterparts. This review discusses the benefits of physical activity and current physical activity recommendations in RA, describes measurement techniques to assess physical activity, and synthesizes the data from interventions to promote physical activity and improve health outcomes in adults with RA.
Keywords: function & aerobic capacity, physical activity, rheumatoid arthritis
Rheumatoid arthritis (RA), a chronic inflammatory autoimmune disease, affects approximately 0.5–1% of the population in Northern Europe and North America, with a mean annual incidence of 0.02–0.05%. Global estimates of RA prevalence are slightly lower [1]. RA-associated fatigue, muscle weakness, joint pain and inflammation lead to reduced function, diminished aerobic capacity, disability and early mortality. Mortality rates are attributable to multiple factors. Primary among these factors is an increased prevalence of cardiovascular disease associated with immune-mediated ischemic heart disease and accelerated atherosclerosis. Additionally, patients with RA may have hypertension, obesity, metabolic disturbances and be physically inactive [2]. Physical inactivity is a modifiable and potentially potent risk factor. This review focuses on current literature regarding physical activity in adults with RA and interventions to promote physical activity in this population.
Definitions of physical activity & exercise
Physical activity refers to physical motion produced by skeletal muscles that produces energy expenditure from minimal to maximal intensity. Exercise is commonly referred to as a purposeful, planned behavior that results in energy expenditure. While exercise interventions may or may not be supervised, most physical activity interventions are incorporated into leisure time and lifestyle behaviors and are generally unsupervised.
Evidence for the benefits of exercise
Exercise and physical activity are known to impact the immune system. Participation in exercise leads to the production of pro- and anti-inflammatory cytokines such as TNF-α, IL-1, IL-1ra, IL-6 and IL-10. Changes are also evident in leukocyte subsets such as neutrophils, lymphocytes including T, B and NK cells, and monocytes and plasma concentrations of CRP. The impact of exercise on immune response is proportional to the magnitude of exercise, with the greatest changes apparent following strenuous and eccentric exercise. Endurance training appears to decrease the resting levels of many inflammatory markers. Thus, exercise intensity, duration and mode can lead to differential impacts on the immune system. These data form the basis for exercise recommendations in adults with RA and other chronic illnesses [3].
RA-associated inflammation is associated with accelerated atherosclerosis. While the precise mechanism for this trend is uncertain, researchers postulate that high-grade systemic inflammation leads to vascular and metabolic effects [2]. Physical activity and exercise have been shown to improve myocardial contractility. Additionally, inflammation can lead to rheumatoid cachexia or diminished lean muscle mass, which significantly affects muscle force production and function [4].
Data regarding exercise interventions for persons with RA demonstrate that exercise is both safe and effective in enhancing function, improving cardiovascular capacity, muscle strength and lean muscle mass, and increasing quality of life [2-8]. Exercise also reduces fatigue and symptoms of depression [8]. Conn et al. in a meta-analysis of 28 studies of physical activity interventions for adults with arthritis (including osteoarthritis and RA) published from 1970 to 2005, reported effect sizes of 0.69 for physical activity, 0.49 for pain and small effect sizes for function (0.14; intervention vs control). These data suggest that interventions to promote physical activity provide modest benefit for adults with arthritis. Separate analyses for adults with RA were not conducted [9].
Hurkmans et al. conducted a Cochrane review of eight randomized controlled trials (RCTs) of aerobic and dynamic strengthening exercises specifically designed for adults with RA to determine the effectiveness of exercise on aerobic capacity, pain and function. Data from 575 adults with low-to-moderate RA disease activity were analyzed. The authors reported a large significant effect for short-term aerobic capacity, defined as programs 12 weeks in length (pooled effect size 0.99 [95% CI: 0.29–1.68]), immediately following the intervention and a nonsignificant trend for positive effect on functional ability (pooled effect size of 0.03 [95% CI: −0.46–0.51]) for supervised land-based programs of short duration. Results for programs of longer duration and for short-term aquatic exercise were less impactful [7]. In another meta-analysis of 14 RCTs of aerobic exercise in 1040 patients with stable RA, Baillet and colleagues reported significant improvements in function (standardized mean difference [SMD] = 0.24), pain (SMD = 0.31) and quality of life (SMD = 0.39) with no deleterious effects to joints [6]. Evidence synthesized for the development of a recent clinical practice guideline for RA supports these findings [10]. Of the 382 studies incorporated into the guidelines, data from RCTs of aerobic exercise and dynamic strength training supported the use and positive benefits of exercise to manage symptoms of RA [10].
Recommendations for the management of RA: implications for exercise & physical activity
Physical activity and exercise are known to reduce the risk of cardiovascular disease and are recommended for all people, regardless of health status. The American Heart Association and the American College of Sports Medicine recommends 30 min of moderate intensity exercise five-times per week to prevent heart disease [11]. The US CDC has published physical activity recommendations for persons with arthritis based on a synthesis of data from studies of physical activity and exercise. Patients with RA and other arthritic conditions are encouraged to engage in either 30 min of moderate exercise five-times per week or 30 min of vigorous exercise three-times per week [101]. These recommendations parallel those of the American College of Sports Medicine [11].
Recognizing the benefits of physical activity and exercise on muscle force production, promotion of lean muscle mass, aerobic capacity and reduction of cardiovascular disease in RA, numerous professional organizations include exercise and physical activity in their RA disease management guidelines. However, these guidelines are less prescriptive about the use of physical activity or exercise. For example, the European League Against Rheumatism (EULAR) provides a general statement about the use of nonpharmacologic interventions as adjuncts to medications and promotes the use of exercise and physical activity for the management of RA [12]. The Royal Australian College of General Practitioners and the Australian National Health and Medical Research Council also support the use of exercise. These guidelines do not provide specific parameters for physical activity participation. Instead, these guidelines emphasize consideration of the patients’ needs and preferences regarding exercise and physical activity [13]. The Pan-American League Against Rheumatism states that equal importance should be placed upon nonpharmacologic interventions and medical therapy, and therefore encourages physical therapy and exercise. Explicit parameters for physical activity and exercise prescription are not provided (Table 1) [14]. Despite the role of exercise and physical activity for reducing symptoms and long-term consequences of RA, RA practice guidelines, in general, do not provide parameters to help guide patients and providers to select the best exercise and physical activity prescriptions. In particular, details regarding appropriate intensity, frequency, mode and duration of exercise and physical activity are lacking, in part owing to the state of the research in this area [9].
Table 1.
Published recommendations for physical activity and rheumatoid arthritis management guidelines.
| Professional organization | Recommendations | Ref. |
|---|---|---|
| American College of Rheumatology (ACR) | No mention of physical activity Physical therapy is recommended |
[50] |
| American College of Sports Medicine and American Heart Association |
Physical activity specific. Recommends 30 min of moderate physical activity five-times per week or 20 min of vigorous aerobic physical activity three-times per week |
[11] |
| British Society of Rheumatology (BSR) | Aerobic exercise should be encouraged with the caveat that one needs to be careful about minimizing joint destruction |
[51] |
| Royal Australian College of General Practitioners and the Australian National Health and Medical Research Council |
General physical activity and exercise are encouraged. Individualized exercise that incorporates patients’ needs and preferences are encouraged to reduce the adverse effects of the disease on muscle strength, endurance and aerobic capacity |
[13] |
| Latin American Rheumatology Associations of the Pan-American League of Associations for Rheumatology (PANLAR) and the Grupo Latinoamericano de Estudio de Artritis Reumatoide |
Guidelines emphasize the important role of nonpharmacological interventions – equal in importance to medical management. Recommends physical therapy and exercise. No specific mention of physical activity |
[14] |
| European League Against Rheumatism and Early RA (EULAR) |
Nonpharmaceutical interventions can be used as adjuncts to medical therapy and include dynamic exercises, occupational therapy and hydrotherapy |
[12] |
RA: Rheumatoid arthritis.
Estimates of physical activity participation in persons with RA
Individuals with arthritis are less physically active than their healthy counterparts. Information from the National Health Interview (NHIS), a large US population-based survey, reports the age-adjusted prevalence of sedentary lifestyles as 51.2% among persons with arthritis compared with 27.2% in the general US population [15]. Additionally, approximately 44% of US adults with arthritis report no leisure time physical activity, 8% more than adults without arthritis (36%). This trend is seen globally. In a large cross-sectional study of 10,755 healthy Swedish adults and those with chronic diseases, similar disparities in physical activity participation were reported [16]. Of the 10,755 participants in the study, 1125 were diagnosed with RA, 526 with chronic obstructive pulmonary disease, 2149 with diabetes mellitus and 6960 were healthy adults. Leisure time physical activity was measured using a four-item scale:
-
■
Sedentary (mostly sitting down or low activity 2 h a week)
-
■
Moderate exercise (low activity 2 h a week)
-
■
Moderate regular exercise (high activity 30 min, one- to two-times a week)
-
■
Regular exercise and training (high activity 30 min performed three-times a week)
Responses to this item were then dichotomized into low activity level (responses one and two) and high activity level (responses three and four) [17]. The authors found physical activity levels were lower among persons with RA (25.6 vs 39.8%) compared with healthy individuals. Importantly, individuals with RA also reported greater activity limitations when compared with all patient groups [16]. In The Netherlands, van den Berg conducted an observational study of 400 adults with RA to determine whether or not they were physically active and to identify the type of physical activities that individuals with RA engage in. Of the 252 individuals who responded to the survey, 201 (80%) reported they engaged in some form of physical activity. Supervised exercise performed in groups and unsupervised leisure activities such as bicycling, walking and swimming were ranked the most favorable [18]. Despite the known benefits of physical activity, adults with RA are less active than healthy counterparts and some data suggest that those who engage in modest levels of physical activity prefer leisure time activities.
Self-report & objective measures of physical activity
Measurement of physical activity is obtained using indirect techniques such as self-report questionnaires and through the use of motion acquisition devices such as accelerometers. An accelerometer is a small computerized device worn at the waist or on the arm that digitally records the acceleration of motion of the body during routine activities and exercise. Some accelerometers are uni-axial, capturing movement in a single plane of motion. Tri-axial accelerometers capture motion in all three planes (frontal, saggital and transverse) and tend to provide more precise estimates of movement. Commercial accelerometers categorize data into exercise bouts or counts. These counts are then converted into metabolic equivalents (METs) to provide an estimate of the ratio of work metabolic rate to resting metabolic rate. Resting metabolic rate is defined as one MET or 1 kc/kg/h, whereas walking at a self-determined pace on level ground is estimated to be equivalent to three METs [102]. MET is the standard measurement used in exercise science, physical therapy and cardiovascular medicine for determining energy output.
Accelerometers provide an objective measurement of physical activity and record behaviors such as the frequency and duration and intensity of physical activity along with episodes of sedentary behavior. This is accomplished through a data reduction process in analysis. As such, accelerometry has become the gold standard for physical activity assessment in large epidemiologic studies. However, the use of accelerometers for physical activity assessment in persons with RA presents unique challenges owing to the relatively sedentary life-styles of these patients [19,20]. Seminak et al. in an analysis of 107 persons with RA reported the need to increase the nonwear threshold or periods of time with minimal or ‘0’ activity often corresponding to periods when the device is not being worn or when movement is so minimal it is difficult to detect [19]. In general population studies this ‘nonwear’ time is set at 60 min to allow for episodes when the individual is essentially sedentary but still wearing the device. This threshold is necessary as the accelerometer may not be able to distinguish between sedentary behavior and nonwear. Based on data analysis from these 107 subjects with RA, the authors recommend setting the nonwear threshold to 90 min for persons with RA. Despite its challenges, accelerometers have gained in popularity as they provide more detailed information about physical activity behavior and are relatively easy to use. New mobile versions of accelerometers via cellphone applications and PDAs are reaching the market and provide more opportunities for e pidemiologic research in this area.
Numerous self-report measures of physical activity exist and vary from instruments evaluating attributes of neighborhood environments that promote physical activity such as the Physical Activity Neighborhood Environment scale [21] to questionnaires that quantify physical activity participation such as the International Physical Activity Questionnaire (IPAQ) [22-24] and Behavioral Risk Factor Surveillance System (BRFSS) [25,101]. The IPAQ survey assesses a number of physical activity domains including leisure time and domestic, occupational and transport-related activities, and allows for quantification of these activities as low, moderate or vigorous. The survey also collects information on sedentary behaviors such as sitting time. A total summed score is generated from the responses and expressed in METs. Validation studies comparing IPAQ responses with accelerometer-derived estimates of physical activity report varied results. Hagstromer et al. recruited 980 individuals aged 18–65 years to assess the validity of the IPAQ. All subjects completed the IPAQ and wore an accelerometer for 7 consecutive days. Significant low-to-moderate correlations were found between the two estimates of physical activity (r = 0.07–0.36). The authors noted the IPAQ data tended to record higher values for sitting and vigorous intensity physical activity compared with the accelerometer [26]. Despite some of the limitations of the IPAQ, this survey has been translated and used in large international comparison studies of physical activity and is considered to be a useful measure for epidemiologic studies of physical activity participation [22-24].
The BRFSS survey has been used in large epidemiologic cohort studies of healthy populations and persons with arthritis. The CDC has used the BRFSS for over a decade to assess the prevalence and impact of arthritis on physical activity. The BRFSS appears to be a reliable and valid estimate of physical activity [25,101]. In a 2007 study, 60 individuals completed the BRFSS survey three-times via phone interview over a 22-day period. All subjects wore a pedometer and an accelerometer, and completed a daily activity log. The results indicated that the test–retest reliability varied by activity type (r = 0.35–0.53 for moderate activity; r = 0.80–0.86 for vigorous activity; and r = 0.67–0.84 for recommended activity) [27]. In sum, a variety of well-validated and reliable instruments exist to assess self-reported physical activity. While they have proven to be useful in large epidemiologic studies, a combination of accelerometry and self-report measures may provide the most useful clinical profile of physical activity behaviors in RA.
Factors associated with physical activity participation in persons with RA
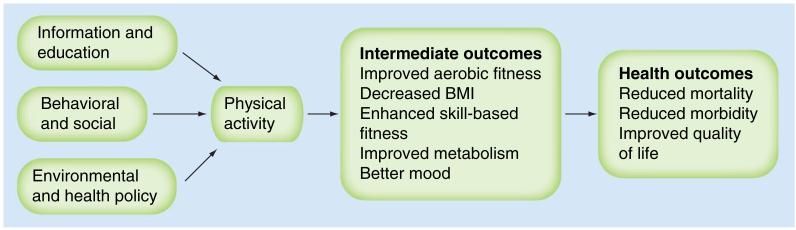
By definition, physical activity requires active personal engagement. Unfortunately, not all individuals are successful at maintaining an active lifestyle, especially those with RA. Physical activity interventions can be categorized as educational, behavioral (counseling) or environmental (e.g., policy) and are designed to target immediate outcomes such as health behaviors and, ultimately, health outcomes (Figure 1). Numerous theories have been proposed to conceptualize the relationship between psychosocial factors and health behaviors. Recent studies have used elements of the self-determination theory to examine factors associated with physical activity participation. The self-determination theory postulates that engagement in and maintenance of physical activity is directly related to whether or not an individual has an autonomous or coerced regulation style and if an individual actively establishes health behavior goals [28]. To assess this theory, Hurkmans and colleagues surveyed 271 patients with RA recruited from three hospital outpatient clinics and found that younger individuals who displayed autonomous behavior regulation by setting their own goals for physical activity were more likely to engage in physical activity [29]. To examine the impact of attitudes and beliefs about physical activity, Erlich-Jones enrolled adults with RA in a cross-sectional study and found patients who were motivated (p = 0.007) or who perceived positive benefits for physical activity (p < 0.05) reported higher levels of physical activity engagement, adjusting for age, sex, BMI, race and disease activity [30]. The data emphasize the importance of addressing beliefs, attitudes and perceptions of physical activity when developing physical activity programs. Patients’ perceptions of diminished joint health due to exercise also influences their exercise/physical activity behaviors. Using a qualitative approach, Law and colleagues recruited 18 subjects (six male) to participate in focus groups to explore perceptions about exercise and RA. The data indicated that patients with RA were cognizant of the benefits of exercise but felt their healthcare providers were uncertain of which exercises they should perform and unclear about the impact of specific exercises on joint integrity. Patients were concerned that exercise may cause harm to their joints and exacerbate their RA. Patients also reported that they often avoided exercise when their joints were painful [31]. While these studies were cross-sectional in nature and exploratory, the data suggest clear and direct information is needed for both patients and healthcare team members about the use of exercise for adults with RA.
Figure 1. Pathway for promoting physical activity in persons with rheumatoid arthritis.
Data taken from [55].
Perceived quality of life is also linked with engagement in physical activity. Kruger et al. in a large observational study examining quality of life and physical activity among 9173 US adults reported that health-related quality of life was lower among less physically active adults after adjusting for other potential confounders [32]. In a large international cross-sectional study of 5235 patients with RA from 21 countries, Sokka et al. reported that physical inactivity was associated with: older age, more comorbidities, less education, female gender, obesity, poor function, fatigue, pain and greater RA disease activity. In their study, only 13.8% of all patients exercised three or more times per week. In 19 of the 21 countries, between 60 and 80% of patients with RA engaged in no regular physical activity [33]. The data from these trials point toward the need to promote physical activity participation in adults with RA and, in doing so, patients may experience improvements in quality of life.
Due to the fluctuating nature of the disease, a major focus of RA management is patient education regarding self-management, including physical activity pacing. Physical activity pacing involves regular rests and titration of activity in response to disease flares. A recent cross-sectional study designed to assess physical activity pacing in adults with RA suggests that this may not be the ideal strategy. Because patients with RA in this study already demonstrated low physical activity levels, the patients were essentially sedentary after self-pacing [34]. However, this is a single study and cannot stand alone. When one combines the epidemiologic data about physical inactivity in adults with RA seen across the globe with the results of this study, the data support the fact that healthcare providers need to be cognizant of current physical activity levels prior to counseling patients about activity pacing (Table 2).
Table 2.
Studies assessing psychosocial factors influencing physical activity participation in adults with rheumatoid arthritis (2007–present).
| Study (year) | Design & sample | Objective | Outcomes | Ref. |
|---|---|---|---|---|
| Brodin et al. (2009) |
Qualitative semi-structured interviews n = 9 persons with RA aged 21–82 years Country: Sweden |
To ascertain how patients perceive exercise |
Four different ways to determine intensity of PA: focus on alterations of bodily features; focus on will power and awareness; focus on type and performance of activity; and focus on consequences of the disease. Discrepancy between health professionals and patients |
[52] |
| Cuperus et al. (2012) |
Cross-sectional n = 30 persons with RA Country: The Netherlands |
To examine the relationship between activity pacing and physical inactivity |
PA levels assessed by self-reported measures were significantly higher than when assessed by an accelerometer-based activity monitor. Activity pacing was associated with lower levels of PA |
[34] |
| Ehrlich-Jones et al. (2011) |
Cross-sectional n = 185 persons with RA Country: USA |
To determine relationship between beliefs, motivation and worries about PA |
Stronger beliefs about the effectiveness of PA for managing arthritis and increased motivation to engage in PA are related to higher levels of PA participation |
[30] |
| Hurkmans et al. (2010) |
Cross-sectional mailed survey n = 271 patients with RA 66% female Country: The Netherlands |
To determine relationship between current PA, preferred regulation style and supportiveness of the patients’ rheumatologists |
Younger age, female sex, higher education level, shorter disease duration, lower disease activity and a more autonomous regulation were associated with more PA. Younger age and a more autonomous regulation were significantly associated with higher levels of PA (p < 0.001 and p < 0.050, respectively). |
[29] |
| Hurkmans et al. (2011) |
Cross-sectional n = 370 healthcare providers including: rheumatologists (n = 126), nurses (n = 132) and physical therapists (n = 112) Country: The Netherlands |
To examine the extent that healthcare providers promote PA in patients with RA and how they perceive their competencies and educational needs |
More than 90% of providers agreed that PA is an important health goal for RA patients and regularly advised their patients to engage in PA |
[53] |
| Law et al. (2010) |
Qualitative focus groups 18 adults with RA (6 male and 12 female) Country: UK |
To explore RA patients’ perceptions of the effects of exercise on joint health |
Patients are aware of the benefits of exercise and impact on joints but they believed healthcare providers were uncertain of the impact of specific exercises on joints |
[31] |
| van den Berg et al. (2007) |
Observational study n = 400 persons with RA Country: The Netherlands |
To determine the proportion of people meeting the Dutch public health recommendation for PA (moderate PA for 30 min on ≥5 days/week) and total minutes of PA per week were calculated. Data compared with representative sample of the general Dutch population |
252 patients responded. The proportion of patients meeting PA recommendations was similar to that of the general population (57% in categories 45–64 years; 59% in categories ≥65 years and 58% in the total groups). Average number of PA minutes per week was significantly lower in RA population among 45–64 year olds (1836 vs 2199, respectively; p = 0.001) |
[18] |
| Vervloesem et al. (2012) |
Observational study n = 154 ambulatory care patients with RA Country: Belgium |
Patients divided into two groups based on willingness to participate in an exercise program completed questionnaires to determine barriers to exercise |
73% indicated they were willing to participate in an exercise program. Positive responders were more often female (p < 0.05), and had a higher education (p < 0.05). In the negative responders, higher scores were found in the general health perception (54.7 vs 47.4) and vitality (61.6 vs 53.7) and they reported lower reassuring thoughts (11.9 vs 12.9) compared with the positive responders (all p < 0.05) |
[54] |
PA: Physical activity; RA: Rheumatoid arthritis.
Clinical studies of interventions to promote physical activity in persons with RA
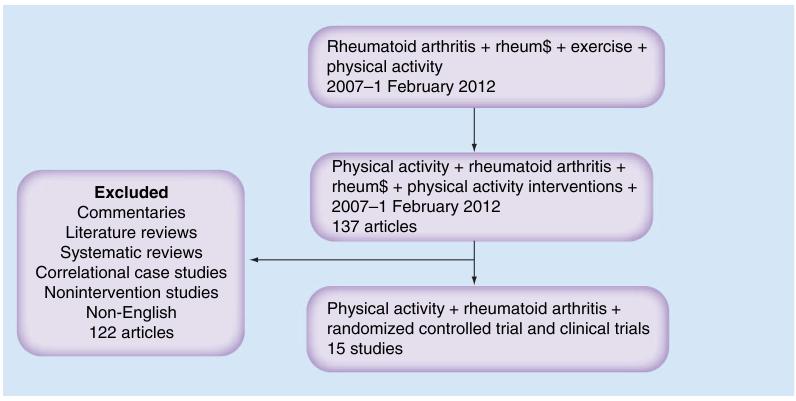
Physical activity is a complex behavior involving psychosocial and environmental factors. Therefore, interventions to promote physical activity in RA may target providers or may target patients’ knowledge, self-management skills, environment or motivation/self-determination. Given that the meta-analyses and reviews published covered articles from 1970 to 2006, a search of interventions currently used to promote physical activity in persons with RA was performed using the following databases (Medline, PubMed, EMBASE, Cumulative Index to Nursing & Allied Health Literature, PsychInfo and the Cochrane Library) for published papers from 2007 to February 2012. The Medical Subjects Headings (MESH) terms used in combination were ‘RA,’ ‘rheum$’, ‘physical activity’, ‘physical activity intervention’ and ‘exercise’. The initial search yielded 1359 papers (Figure 2). The search was then restricted to studies focusing on activity interventions. Full articles were obtained for assessment when the articles met the inclusion criteria. The inclusion criteria were:
-
■
Studies published in English
-
■
Studies of physical activity interventions or whole body exercise or studies that aimed to promote physical activity
-
■
Studies involving RA patients
Figure 2. Results of literature search on randomized and controlled trials of physical activity interventions in rheumatoid arthritis.
Studies of persons with other inflammatory arthritis, connective tissue disease or degenerative arthritis were excluded if adults with RA were not included or if the data was not analyzed separately for this subset. Studies that focused on modalities and other nonpharmacologic interventions plus physical activity were also excluded. If the title and abstract did not provide sufficient information to discern eligibility then the full-text manuscript was examined. Conference proceedings and dissertations were not included. Due to the dearth of randomized controlled studies of interventions to promote physical activity, both randomized and controlled clinical trials of interventions of physical activity are included and discussed. Studies were ranked for quality using the Jadad scale [35].
In total, 15 articles were identified describing physical activity interventions. Of these, ten studies (67%) were RCTs [4,36-44], three were follow-up studies to an RCT [45-47] and the remaining studies were clinical trials of interventions (CTs) [48,49]. Ten studies tested an exercise intervention [4,36,37,40-44,48,49]. Of these studies, one was a multicenter study [42] and one used a team approach [37]. The intervention duration of the ten physical activity and exercise trials varied from 4 weeks to 1 year, with four studies testing interventions lasting 1 year. A fairly equal number of studies selected a strengthening program or aerobic capacity training program as the mode of intervention, although dose, frequency and intensity, and level of supervision of exercise varied across trials. One study combined both aerobic training and dynamic strength training [43]. Among the RCTs of exercise interventions, two studies compared home exercise to supervised exercise programs [40,41], the remaining studies used usual care and one a waitlist control [37]. The majority of the RCTs were of good quality with a Jadad score of three out of five (Table 3) [35].
Table 3.
Randomized controlled trials and clinical controlled trials of interventions to promote physical activity in rheumatoid arthritis.
| Study (year) | Sample & recruitment |
Intervention(s) | Outcomes | Jadad score |
Ref. |
|---|---|---|---|---|---|
| Baillet et al. (2009) |
RCT (matching employed for some demographic features) RA: n = 50 Country: France |
Rx: DEP Control: conventional joint rehabilitation group Duration: 4 weeks |
At 4 weeks, DEP significantly improved function (HAQ by 0.2; p = 0.04) quality of life (p = 0.01) and aerobic fitness (mean difference: 0.2; p = 0.02). No statistical differences in outcomes at 6- and 12-month follow-up |
3 | [36] |
| Breedland et al. (2011) |
RCT RA: n = 34 Intervention (n = 19) Control (n = 15) Country: The Netherlands |
Rx: multidisciplinary group therapy program of education and physical exercise designed to increase aerobic capacity and muscle strength combined with education to increase self-efficacy Control: wait list Duration: 8 weeks |
Intervention group showed significant improvement (12.1%) in VO2, max at week 9 compared with the control group (−1.7%) No between-group changes were found |
3 | [37] |
| Brodin et al. (2008) |
Multicenter RCT n = 228 persons with RA (169 women and 59 men, mean age 55 years) Rx: (n = 134) Control: (n = 94) Country: Sweden |
Rx: coaching program to undertake physical activity of moderate intensity 30 min/day, 4 days/week, Control: standard care Duration: 1 year |
Assessments performed every 3 months. At 1 year, the intervention group reported greater quality of life compared with controls (p = 0.025) and muscle strength (Timed-Stands Test; p < 0.001, Grippit; p = 0.003) |
3 | [38] |
| deJong et al. (2009) |
18-month follow-up to RCT RA: n = 71 Country: The Netherlands |
Rx: continuation of unsupervised high-intensity exercise program Control: usual care Duration: 2 years |
The majority of subjects continued to exercise at 18 months. Compared with nonexercisers, those who exercised maintained their muscle strength without increased disease activity or progression of radiological damage |
3 | [45] |
| Feinglass et al. (2012) |
RCT n = 226 patients with RA and OA (57.5% with RA) Country: USA |
Rx: counseling by PA coaches Control: physician advice to exercise Duration: 6 months |
Accelerometer used to measure PA. Subjects who demonstrated the lowest function at baseline had the largest mean absolute and relative physical improvementat follow-up, regardless of intervention group status |
3 | [39] |
| Hurkmans et al. (2010) |
RCT n = 110 sedentary adults with RA Country: The Netherlands |
Rx1: IT individualized PA program, a bicycle ergometer and group contacts Rx2: GT using web-based materials and email Duration: 1 year |
At 1 year follow-up significant improvements in the percentage of adults with RA meeting the guidelines for moderate intensity physical activity in both groups (19% [IT] and 24% [GT]). A greater percentage of adults in the IT group met the recommendations for vigorous activity at follow-up compared with baseline |
3 | [44] |
| Hsieh et al. (2009) |
RCT RA: n = 30 female Chinese patients Country: Taiwan |
Rx: Physical therapist supervised 30 min aerobic exercise three-times per week Combination of ROM, strength and aerobic exercise at 50–80% VO2 max Control: 30 min home aerobic exercise group three-times per week Duration: 8 weeks |
Significant difference in changed score between pre- and post-exercise data was observed between the supervised aerobic exercise and home aerobic exercise groups regarding aerobic capacity (p < 0.0001). Pre- and post-exercise within- group comparisons showed significant improvement (20%) in aerobic capacity only in the supervised aerobic exercise group. Pre- and post-exercise within-group comparison showed significant improvement in five and three items of disease- related variables in supervised aerobic exercise and home aerobic exercise groups, respectively |
4 | [40] |
| Lemmey et al. (2011) |
RCT n = 28 adults with established RA Country: Wales |
Rx: PRT two-times per week Control: ROM exercises Duration: 24 weeks |
PRT significantly increased lean muscle mass and appendicular lean mass. Trunk fat decreased by 2.5 kg (NS). Strength significantly improved in all performance tests: chair stand test (by 30%), knee extensors by 25%, arm curls by 23%, walk time by 17%. Significant changes reported in immune markers of inflammation. No significant gains in control group found |
4 | [4] |
| Lemmey et al. (2012) |
3-year follow-up to RCT n = 18 patients with RA Country: Wales |
Rx: High-intensity PRT Control: low-intensity range of movement exercise Duration: 24 weeks |
At 3 years, PRT subjects remained significantly leaner than control subjects and retained most of the improvement in walking speed gained from training. Stable RA patients had similar rates of lean mass loss but elevated rates of fat mass accretion relative to age-matched sedentary non-RA controls |
4 | [47] |
| Neuberger et al. (2007) |
RCT RA: n = 220 adults Ages: 40–70 years Country: USA |
Rx 1: class-based low impact aerobic exercise Rx 2: home exercise Control: no Rx, asked to keep prestudy exercise levels constant Duration: 12 weeks |
Symptoms (pain, fatigue and depression) decreased significantly at 12 weeks (p < 0.04) for the class exercise group compared with controls. Rx group demonstrated significant interaction effects of time and group for timed walk and grip strength: Rx group improved more than the controls (p < or = 0.005). No significant increases in measures of disease activity. Fatigue and perceptions of benefits and barriers to exercise influenced amount of exercise |
3 | [41] |
| Sjöquist et al. (2010) |
RCT (multicenter) – ten clinics n = 228 persons with early RA (74% women) disease duration of 1 year Country: Sweden |
Rx: person-based coaching to adopt physical activity (moderate activity, 30 min/day more than 4 days/week) Control: subjects could seek physical therapy or engage in group exercise on their own Duration: 1 year |
Primary outcome was self-reported health. 146 patients included in analysis Clusters with less affected variables had significantly better general health perception at baseline than those with more affected variables. Furthermore, coached individuals in more affected clusters significantly improved self-reported health status compared both to those coached in less affected clusters and to those in more affected clusters in the control group |
3 | [42] |
| Sjöquist et al. (2011) |
Follow-up to RCT (multicenter) n = 158 with early RA Country: Sweden |
Rx: coaching to adopt physical activity (moderate activity, 30 min/day more than 4 days/week) Control: as above (2010) Follow-up 1 year after Rx ended |
A mailed survey assessed PA participation and general health perception and pain. The function (HAQ) and disease activity (DAS 28) were collected at regular obtained from medical visits. No long-term improvement in perceived general health or other outcomes were found at follow-up |
[46] | |
| Strasser et al. (2011) |
RCT n = 40 persons with RA >2 years Ages: 41–73 years Country: Austria |
Rx: supervised muscle strength training (sets of weight bearing exercises for all major muscle groups plus endurance training using cycle ergometer two-times per week) Control: usual care Duration: 6 months |
Subjects in the Rx group demonstrated reductions in disease activity (p = 0.06) and pain (p = 0.05) general health (p = 0.04) and functional ability (p = 0.06) at 6 months. Cardiorespiratory endurance improved by 10% (p < 0.001). Strength increased by 14% in the Rx group. Lean body mass increased, and the percentage of body fat decreased significantly (p < 0.05) |
3 | [43] |
| Uhlig et al. (2010) |
CCT n = 15 persons with RA Country: Norway |
Rx: tai chi group exercise two-times weekly Duration: 12 weeks |
Improved lower-limb muscle function at 12-week follow-up. Patients reported improved physical condition, reduced stress, increased body awareness, confidence in moving, balance and less pain during exercise and in daily life |
NA | [48] |
| van der Giesen et al (2010) |
CCT n = 81 persons with RA Country: The Netherlands |
Rx: intensive group exercise program by 25 physical therapists trained to provide program Duration: 1 year |
Muscle strength improved significantly, whereas aerobic capacity, functional ability, psychological functioning and disease activity did not change. All providers willing to provide the program in the future; future reimbursement by health insurance companies unclear |
NA | [49] |
CCT: Clinical controlled trial; DAS-28: Disease Activity Score; DEP: Dynamic exercise program; GT: General physical activity information; HAQ: Health Assessment Questionnaire; IT: Internet-based; NA: Not applicable; NS: Nonsignificant; OA: Osteoarthritis; PA: Physical activity; PRT: Progressive resistance training; RA: Rheumatoid arthritis; RCT: Randomized control trial; ROM: Range of motion; Rx: Treatment; VOz Oxygen consumption.
Although study outcomes examining the impact of exercise (aerobic or strengthening) on health varied, the majority of studies assessed function, general health and disease activity (Table 4). Measurements of function usually included a combination of validated self-report measures and performance tests. Disease activity was assessed with laboratory markers (CRP, sedimentation rates and cytokines) or a combination of immune markers and joint assessments. Two studies formally assessed joint damage using radioagraphs [36,45]. In each study, the intervention included a dynamic exercise program. deJong et al. evaluated the impact of unsupervised long-term high-intensity exercise versus usual care and found no significant increase in joint progression with exercise [45]. However, if joint damage was evident at the start of the trial some changes in joint integrity were evident. Baillet et al. examined the impact of short-term (4 weeks) dynamic exercise based on the recommendations for exercise published by the American College of Sports Medicine [11] versus a conventional joint exercise program and reported no changes in joint integrity with exercise at the 12-month follow-up and no adverse events [36].
Table 4.
Outcome measures assessed in clinical trials of physical activity interventions for adults with rheumatoid arthritis.
| Study (year) | Disease status |
Pain | Joint damage |
Function disability |
Global health mood |
Self- efficacy |
QoL | Aerobic fitness/ capacity |
Strength dexterity |
Barriers attitudes expectations |
BMI | Physical activity |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baillet et al. (2009) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [36] | |||||
| Breedland et al. (2011) |
✓ | ✓ | ✓ | ✓ | ✓ | [37] | |||||||
| Brodin et al (2008) | ✓ | ✓ | ✓ | ✓ | [38] | ||||||||
| deJong et al. (2009) |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [45] | |||||
| Feinglass et al. (2012) |
✓ | ✓ | ✓ | ✓ | [39] | ||||||||
| Hurkmans et al. (2010) |
✓ | ✓ | ✓ | ✓ | [44] | ||||||||
| Hsieh et al. (2009) | ✓ | ✓ | [40] | ||||||||||
| Lemmey et al. (2009) |
✓ | ✓ | ✓ | ✓ | ✓ | [4] | |||||||
| Lemmey et al. (2012) |
✓ | ✓ | [47] | ||||||||||
| Neuberger et al. (2007) |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [41] | ||||
| Sjoquist et al. (2010) plus (2011) |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [42] | ||||
| Strauser et al. (2011) |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [43] | |||||
| Uhlig et al. (2010) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [48] | |||||
| van der Giesen et al. (2010) |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [49] |
QoL: Quality of life.
No studies reported an increase in disease activity with exercise. Regardless of the intervention type (exercise/physical activity or counseling to promote physical activity), studies demonstrated improvements in improved function, enhanced general health (mood state) or perceived health, muscle strength (including lean muscle mass) and aerobic capacity (improvements in timed walk or aerobic fitness). Studies incorporating dynamic exercise tended to yield greater gains in muscle strength, whereas studies incorporating aerobic activities yielded greater gains, regardless of study duration, in aerobic capacity when the treatment subjects were compared with control subjects. Studies with interventions of longer duration (1 year) tended to report significant changes in measured outcomes. For the majority of studies, benefits were found immediately after the intervention and generally did not persist at followup. Five studies (33%) employed an intervention lasting 3 months or less [36,37,40,41,48]. Of the two follow-up studies of exercise interventions, the exercise intervention lasted 2 years [45] and 24 weeks [47]. Lemmey et al.’s study evaluated the long-term benefits of a high intensity, progressive, resistive exercise program performed twice a week for 24 weeks on muscle mass and function [47]. At the 3-year follow-up, intervention subjects remained more fit (greater lean muscle mass) and demonstrated similar walking speeds as noted following the intervention. Follow-up assessments of the two intervention studies suggest some maintenance of primary outcomes at follow-up [45,47].
Five studies used a counseling intervention or education to promote physical activity [37-39,42,46]. Of these, one combined a conditioning exercise program and education [37]. The other studies focused on counseling or coaching interventions [38,39,42] and one was a follow-up study to the primary counseling intervention [46]. Feinglass compared counseling for physical activity via coaches to physician advice regarding physical activity and assessed subject participation in physical activity using accelerometers [39]. The authors reported significant improvements in physical activity participation after 6 months, especially among subjects who were less active at baseline. Sjöquist et al. used a multicenter approach to assess the impact of a year long person-based coaching intervention versus control. Subjects in the control group could seek physical therapy on their own or engage in group exercises [46]. Of the 228 adults with RA who were recruited, data from 146 were available for the analysis. The data suggested significant improvements in perceived health following the intervention. These results were more dramatic for those who had more severe disease symptoms at baseline. A follow-up study suggested improvements were not maintained long term.
Discussion
Exercise and physical activity are related but different constructs, as exercise is one subset of physical activity. The majority of studies in RA focus on varied modes of exercise to promote health outcomes such as strength, aerobic capacity, pain and perceived health. Despite the heterogeneity of interventions, exercise and physical activity studies do not report adverse events or exacerbation of RA disease. Measurement of physical activity, particularly in persons with RA, is difficult because adults with RA are relatively inactive. However, accelerometers appear to be a promising and more objective means of documenting physical activity. These devices are not commonly used in clinical settings due to cost, but provide a means of documenting physical activity behaviors across a spectrum of classifications (sedentary, lifestyle, moderate and vigorous). Numerous physical activity surveys exist and may be the best method for documenting physical activity participation in large epidemiologic studies. Among those available, the IPAQ appears to be used most internationally and has established reliability and validity in many languages.
Similar to the data on physical activity interventions among healthy adults, physical activity and exercise interventions appear to have short-term benefits for persons with RA with respect to function, perceived health status, aerobic capacity and quality of life. There were no reported deleterious effects on disease activity or joint pain. Joint damage was less frequently evaluated, perhaps owing to associated costs of radiographs. Studies examining psychosocial factors associated with physical activity participation emphasize the need for a multimodal approach to physical activity interventions, combining behavioral strategies with exercise. Interventions to promote physical activity (through counseling or coaching) are scarce. Those that do exist indicate that such strategies may be effective in promoting physical activity participation, although long-term benefits are unclear.
Conclusion
Physical activity, whether supervised exercise or activity incorporated into daily lifestyles is an important component of RA management. The data on exercise has consistently demonstrated, through meta-analyses and systematic reviews, that exercise promotes health without exacerbating disease activity and pain. Management guidelines published by professional societies encourage physical activity and exercise for adults with RA but do not include details regarding physical activity. Thus, many health-care providers are unclear as to the best activity prescriptions to give their patients with RA. Coaching and counseling for physical activity appears to have a positive impact on physical activity participation. More studies to assess long-term impacts and best practices in coaching for physical activity in RA are needed.
Future perspective
Given the advances in technology for monitoring physical activity, patients may soon be able to accurately and objectively track their physical activity patterns and help rheumatologists to identify those patterns with respect to immune response and disease progression. This information combined with pharmacologic advances in RA may drastically reduce RA-associated disability and positively impact cardiovascular risk in RA.
Executive summary.
-
■
Physical activity participation in persons with rheumatoid arthritis is suboptimal.
-
■
Medical organizations recommend the use of physical activity to manage symptoms of rheumatoid arthritis, but details pertaining to the correct dose, frequency and duration are lacking.
-
■
Providers’ lack of clarity and specificity about physical activity guidelines may impact patients’ physical activity behavior.
-
■
Physical activity measurement in rheumatoid arthritis presents challenges due to the low levels of activity participation evident in this population.
-
■
Accelerometry is considered the gold standard for objectively assessing physical activity, although it is costly and requires training to analyze data.
-
■
Interventions to promote physical activity participation include information/education, counseling and behavioral approaches.
-
■
Modes of delivery of physical activity interventions vary from telephonic counseling to structured interventions using peers as well as healthcare providers.
-
■
Data on the effectiveness of physical activity interventions is limited compared with information on structured supervised exercise programs, but provide promising benefits with respect to improved function, mood and quality of life.
Footnotes
Financial & competing interests disclosure
Funding for this article was provided in part by NIH R03 AR057133-02. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■ of considerable interest
- 1.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev. 2005;4(3):130–136. doi: 10.1016/j.autrev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 2 ■■.Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJ, et al. Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheumatology. 2008;47(3):239–248. doi: 10.1093/rheumatology/kem260. Excellent summary of the literature on cardiovascular disease, rheumatoid arthritis and physical activity.
- 3.Flachenecker P. Autoimmune diseases and rehabilitation. Autoimmunity Reviews. 2012;11:219–225. doi: 10.1016/j.autrev.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 4 ■■.Lemmey AB, Marcora SM, Chester K, Wilson S, Casanova F, Maddison PJ. Efficacy of progressive resistance training for patients with rheumatoid arthritis and recommendations regarding its prescription. Int. J. Clin. Rheumatol. 2011;l6(2):189–205. Well-designed randomized controlled trial of examining impact of progressive resistive exercise on body composition and function.
- 5.Brodin N, Eurenius E, Jensen I, et al. Coaching patients with early rheumatoid arthritis to healthy physical activity: a multicenter, randomized, controlled study. Arthritis Rheum. 2008;59(3):325–331. doi: 10.1002/art.23327. [DOI] [PubMed] [Google Scholar]
- 6 ■■.Baillet A, Zeboulon N, Gossec L, et al. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: meta-analysis of randomized controlled trials. Arthritis Care Res. 2010;62(7):984–992. doi: 10.1002/acr.20146. Succinct analysis of aerobic exercise interventions in rheumatoid arthritis.
- 7.Hurkmans E, van derGiesen FJ, VlietVlieland TPM, Schoones J, van den Ende EC. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst. Rev. 2009;(4):CD006853. doi: 10.1002/14651858.CD006853.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns AP, McVeigh JG. A systematic review of the effects of dynamic exercise in rheumatoid arthritis. Rheumatol. Int. 2009;30(2):147–158. doi: 10.1007/s00296-009-1090-5. [DOI] [PubMed] [Google Scholar]
- 9.Conn VS, Hafdahl AR, Minor MA, Nielsen PJ. Physical activity interventions among adults with arthritis: meta-analysis of outcomes. Semin. Arthritis Rheum. 2008;37(5):307–316. doi: 10.1016/j.semarthrit.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Forestier R, André-Vert J, Guillez P, et al. Non-drug treatment (excluding surgery) inrheumatoid arthritis: clinical practice guidelines. Joint Bone Spine. 2009;76(6):691–698. doi: 10.1016/j.jbspin.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health. Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 12.Combe B, Landewé R, Lukas C, et al. EULAR recommendations for the management of early arthritis: Report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann. Rheum. Dis. 2006;66:34–45. doi: 10.1136/ard.2005.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Royal Australian College of General Practitioners and the Australian National Health and Medical Research Council . Recommendations For The Diagnosis And Management Of Early Rheumatoid Arthritis. Royal Australian College of General Practitioners; Melbourne, Australia: 2009. pp. 1–50. [Google Scholar]
- 14.Massardo L, Suárez-Almazor ME, Cardiel MH, Nava A, Levy RA, Laurindo I. Management of patients with rheumatoid arthritis in latin america: a consensus position paper from Pan-American League of Associations of Rheumatology and Grupo Latino Americano De Estudio de Artritis Reumatoide. J. Clin. Rheumatol. 2009;15(4):203–210. doi: 10.1097/RHU.0b013e3181a90cd8. [DOI] [PubMed] [Google Scholar]
- 15.Cheng YJ, Hootman JM, Murphy LB, Langmaid GA, Helmick CG. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation – United States, 2007-2009. MMWR. 2010;59(39):1261–1265. [PubMed] [Google Scholar]
- 16.Arne M, Janson C, Janson S, Boman G, et al. Physical activity and quality of life in subjects with chronic disease: chronic obstructive pulmonary disease compared with rheumatoid arthritis and diabetes mellitus. Scand. J. Prim. Health Care. 2009;27:141–147. doi: 10.1080/02813430902808643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exer. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 18■.Van den Berg MH, de Boer IG, le Cessie S, Breedveld FC, VlietVlieland TPM. Most people with rheumatoid arthritis undertake leisure time physical activity and exercise in the Netherlands: an observational study. Aust. J. Physiother. 2007;53(2):113–118. doi: 10.1016/s0004-9514(07)70044-2. Provides insight into the types of physical activities persons with rheumatoid arthritis undertake.
- 19.Semanik P, Song J, Chang R, Manheim L, Ainsworth B, Dunlop D. Assessing physical activity in persons with rheumatoid arthritis using accelerometry. Med. Sci. Sports Exerc. 2010;42(8):1493–1501. doi: 10.1249/MSS.0b013e3181cfc9da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida GJM, Wasko MCM, Jeong K, Moore CG, Piva SR. Physical activity measured by the sensewear armband in women with rheumatoid arthritis. Phys. Ther. 2011;91(9):1367–1376. doi: 10.2522/ptj.20100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallis J, Kerr J, Carlson J, et al. Evaluating a brief self-report measure of neighborhood environments for physical activity research and surveillance: physical activity neighborhood environment scale (PANES) J. Phys. Act. Health. 2010;7:533–540. doi: 10.1123/jpah.7.4.533. [DOI] [PubMed] [Google Scholar]
- 22.Hallal PC, Victora CG. Reliability and validity of the International Physical Activity Questionnaire (IPAQ) Med. Sci. Sports Exerc. 2004;36(3):556–562. doi: 10.1249/01.mss.0000117161.66394.07. [DOI] [PubMed] [Google Scholar]
- 23.Bauman A, Ainsworth B, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J. Phys. Act. Health. 2009;6(S1):S5–S8. doi: 10.1123/jpah.6.s1.s5. [DOI] [PubMed] [Google Scholar]
- 24.Bauman A, Bull F, Chey T, et al. The International Prevalence Study on Physical Activity: results from 20 countries. Int. J. Behav. Nutrit. Phys. Act. 2009;6(1):21. doi: 10.1186/1479-5868-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James NT, Miller CW, Fos PJ, et al. Health status, physical disability, and obesity among adult Mississippians with chronic joint symptoms or doctor-diagnosed arthritis: findings from the Behavioral Risk Factor Surveillance System, 2003. Prev. Chronic Dis. 2008;5(3) Abstract 85. [PMC free article] [PubMed] [Google Scholar]
- 26.Hagstromer M, Ainsworth B, Oja P, Sjostrom M. Comparison of a subjective and objective measure of physical activity in a population sample. J. Phys. Act. Health. 2010;7(4):541–550. doi: 10.1123/jpah.7.4.541. [DOI] [PubMed] [Google Scholar]
- 27.Yore M, Ham S, Ainsworth B, et al. Reliability and validity of the instrument used in BRFSS to assess physical activity. Med. Sci. Sports Exerc. 2007;39(8):1267–1274. doi: 10.1249/mss.0b013e3180618bbe. [DOI] [PubMed] [Google Scholar]
- 28.Deci EL, Ryan RM. The ‘what’ and ‘why’ of goal pursuits: human needs and the self-determination of behavior. Psychol. Inq. 2000;11:227–268. [Google Scholar]
- 29.Hurkmans EJ, Maes S, de Gucht V, et al. Motivation as a determinant of physical activity in patients with rheumatoid arthritis. Arthritis Care Res. 2010;62(3):371–377. doi: 10.1002/acr.20106. [DOI] [PubMed] [Google Scholar]
- 30.Ehrlich-Jones L, Lee J, Semanik P, Cox C, Dunlop D, Chang RW. Relationship between beliefs, motivation, and worries about physical activity and physical activity participation in persons with rheumatoid arthritis. Arthritis Care Res. 2011;63(12):1700–1705. doi: 10.1002/acr.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31 ■.Law RJ, Breslin A, Oliver EJ, et al. Perceptions of the effects of exercise on joint health in rheumatoid arthritis patients. Rheumatology. 2010;49(12):2444–2251. doi: 10.1093/rheumatology/keq299. Interesting data on patients’ views of healthcare providers knowledge about physical activity and exercise on joint health.
- 32.Kruger J, Bowles HR, Jones DA, et al. Health-related quality of life, BMI and physical activity among US adults (≥ 18 years): National Physical Activity and Weight Loss Survey, 2002. Int. J. Obesity. 2007;31(2):321–327. doi: 10.1038/sj.ijo.0803386. [DOI] [PubMed] [Google Scholar]
- 33 ■■.Sokka T, Hakkinen A, Kautiainen H, et al. for the QUEST-RA Group Physical inactivity in patients with rheumatoid arthritis: data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. 2008;59(1):42–50. doi: 10.1002/art.23255. Provides comprehensive information on physical activity from an international perspective.
- 34.Cuperus N, Hoogeboom TJ, Neijland Y, van den Ende CH, Keijsers N. Are people with rheumatoid arthritis who undertake activity pacing at risk of being too physically inactive? Clin. Rehabil. 2012 doi: 10.1177/0269215512437417. doi:10.1177/0269215512437417. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 36.Baillet A, Payraud E, Niderprim VA, et al. A dynamic exercise programme to improve patients’ disability in rheumatoid arthritis: a prospective randomized controlled trial. Rheumatology. 2009;48(4):410–415. doi: 10.1093/rheumatology/ken511. [DOI] [PubMed] [Google Scholar]
- 37.Breedland I, Scheppingen C, Leijsma M, Verheij-Jansen NP, van Weert E. Effects of a group-based exercise and educational program on physical performance and disease self-management in rheumatoid arthritis: a randomized controlled study. Phys. Ther. 2011;91(6):879–983. doi: 10.2522/ptj.20090010. [DOI] [PubMed] [Google Scholar]
- 38.Brodin N, Eurenius E, Jensen I, Nisell R, Opava CH, the Para Study Group Coaching patients with early rheumatoid arthritis to healthy physical activity: a multicenter, randomized, controlled study. Arthritis Rheum. 2008;59(3):325–331. doi: 10.1002/art.23327. [DOI] [PubMed] [Google Scholar]
- 39.Feinglass J, Song J, Semanik P, et al. Association of functional status with changes in physical activity: insights from a behavioral intervention for participants with arthritis. Arch. Phys. Med. Rehabil. 2012;93(1):172–175. doi: 10.1016/j.apmr.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh LF, Chen SC, Chuang CC, Chai HM, Chen WS, He YC. Supervised aerobic exercise is more effective than home aerobic exercise in female Chinese patients with rheumatoid arthritis. J. Rehabil. Med. 2009;41(5):332–337. doi: 10.2340/16501977-0330. [DOI] [PubMed] [Google Scholar]
- 41.Neuberger GB, Aaronson LS, Gajewski B, et al. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57(6):943–952. doi: 10.1002/art.22903. [DOI] [PubMed] [Google Scholar]
- 42.Sjoquist ES, Almqvist L, Asenlof P, Lampa J, Opaya CH. Physical-activity coaching and health status in rheumatoid arthritis: a person-oriented approach. Disabil. Rehabil. 2010;32(10):816–825. doi: 10.3109/09638280903314069. [DOI] [PubMed] [Google Scholar]
- 43.Strasser B, Leeb G, Strehblow C, Schobersberger W, Haber P, Cauza E. The effects of strength and endurance training in patients with rheumatoid arthritis. Clin. Rheumatol. 2011;30(5):623–632. doi: 10.1007/s10067-010-1584-2. [DOI] [PubMed] [Google Scholar]
- 44.Hurkmans EJ, van den Berg MH, Ronday KH, Peeters AJ, le Cessies S, Vlieland TPM. Maintenance of physical activity after Internet-based physical activity interventions in patients with rheumatoid arthritis. Rheumatology. 2010;49(1):167–172. doi: 10.1093/rheumatology/kep285. [DOI] [PubMed] [Google Scholar]
- 45.deJong Z, Munneke M, Kroon HM, et al. Long-term follow-up of a high-intensity exercise program in patients with rheumatoid arthritis. Clin. Rheumatol. 2009;28(6):663–671. doi: 10.1007/s10067-009-1125-z. [DOI] [PubMed] [Google Scholar]
- 46.Sjöquist ES, Brodin N, Lampa J, et al. Physical activity coaching of patients with rheumatoid arthritis in everyday practice: a long-term follow-up. Musculoskeletal Care. 2011;9(2):75–85. doi: 10.1002/msc.199. [DOI] [PubMed] [Google Scholar]
- 47.Lemmey AB, Williams SL, Marcora SM, Jones J, Maddison PJ. Are the benefits of a high-intensity progressive resistance training program sustained in rheumatoid arthritis patients? A 3-year follow-up study. Arthritis Care Res. 2012;64(1):71–75. doi: 10.1002/acr.20523. [DOI] [PubMed] [Google Scholar]
- 48.Uhlig T, Fongen C, Steen E, et al. Exploring Tai Chi in rheumatoid arthritis: a quantitative and qualitative study. BMC Musculoskelet. Disord. 2010;11:43. doi: 10.1186/1471-2474-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Giesen FJ, van Lankveld W, Hopman-Rock M, et al. Exploring the public health impact of an intensive exercise program for patients with rheumatoid arthritis: a dissemination and implementation study. Arthritis Care Res. 2010;62(6):865–872. doi: 10.1002/acr.20138. [DOI] [PubMed] [Google Scholar]
- 50.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46(2):328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 51.Luqmani R, Hennell S, Estrach C, Basher D, Birrell F, Bosworth A. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years) Rheumatology. 2009;48(4):436–439. doi: 10.1093/rheumatology/ken450a. [DOI] [PubMed] [Google Scholar]
- 52.Brodin N, Swärdh E, Biguet G, Opava CH. Understanding how to determine the intensity of physical activity--an interview study among individuals with rheumatoid arthritis. Disabil. Rehabil. 2009;31(6):458–465. doi: 10.1080/09638280802131853. [DOI] [PubMed] [Google Scholar]
- 53.Hurkmans EJ, de Gucht V, Maes S, Peeters AJ, Ronday HK, VlietVlieland TP. Promoting physical activity in patients with rheumatoid arthritis: rheumatologists’ and health professionals’ practice and educational needs. Clin. Rheumatol. 2011;30(12):1603–1609. doi: 10.1007/s10067-011-1846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vervloesem N, Van Gils N, Ovaere L, Westhovens R, Van Assche D. Are personal characteristics associated with exercise participation in patients with rheumatoid arthritis? a cross-sectional explorative survey. Musculoskeletal Care. 2012;10(2):90–100. doi: 10.1002/msc.1003. [DOI] [PubMed] [Google Scholar]
- 55.Iversen MD. Achieving the HP2010 arthritis objective: counseling to increase physical activity (PA) for persons with arthritis; Presented at: the Annual Scientific Meeting of American College of Rheumatology; San Diego, CA, USA. 2005.Nov 14, [Google Scholar]
Websites
- 101.CDC . Behavioral Risk Factor Surveillance System Survey Data. US Department of Health and Human Services, CDC; Atlanta, GA, USA: [Accessed 15 December 2011]. 2007. www.cdc.gov/brfss/index.htm. [Google Scholar]
- 102.Win Swim Software [Accessed 1 April 2012];The meaning of MET. www.winswim.com/MET>htm.