Abstract
Melioidosis is infection caused by the flagellated saprophyte Burkholderia pseudomallei. TLR5 is a pathogen recognition receptor activated by bacterial flagellin. We studied a genetic variant that encodes a defective TLR5 protein, TLR51174C>T, to elucidate the role of TLR5 in melioidosis. We measured NF-κB activation induced by B. pseudomallei in human embryonic kidney–293 cells transfected with TLR5 and found that B. pseudomallei induced TLR51174C- but not TLR51174T-dependent activation of NF-κB. We tested the association of TLR51174C>T with outcome in 600 Thai subjects with melioidosis. In a dominant model, TLR51174C>T was associated with protection against in-hospital death (adjusted odds ratio: 0.20; 95% confidence interval: 0.08–0.50; p = 0.001) and organ failure (adjusted odds ratio: 0.37; 95% confidence interval: 0.19–0.71; p = 0.003). We analyzed blood cytokine production induced by flagellin or heat-killed B. pseudomallei by TLR51174C>T genotype in healthy subjects. Flagellin induced lower monocyte-normalized levels of IL-6, IL-8, TNF-α, IL-10, MCP-1, IL-1ra, G-CSF, and IL-1β in carriers of TLR51174T compared with carriers of TLR51174C. B. pseudomallei induced lower monocyte-normalized levels of IL-10 in carriers of TLR51174T. We conclude that the hypofunctional genetic variant TLR51174C>T is associated with reduced organ failure and improved survival in melioidosis. This conclusion suggests a deleterious immunoregulatory effect of TLR5 that may be mediated by IL-10 and identifies this receptor as a potential therapeutic target in melioidosis.
Introduction
Melioidosis is infection caused by the Gram-negative, flagellated soil saprophyte and putative bioweapon Burkholderia pseudomallei, which is endemic in parts of southeast Asia and northern Australia (1). Sepsis, the clinical manifestation of the host inflammatory response to infection, is a common clinical presentation of disease, and the lung is the organ most commonly involved (1). In northeast Thailand, where B. pseudomallei is the most common bloodstream isolate, the overall melioidosis mortality rate exceeds 40% and pneumonia confers >two-fold increase in risk of death (2).
Outcome from bacterial infection is dependent on host innate immunity. We and others have previously shown that TLR2 and TLR4—transmembrane pathogen recognition receptors—regulate host responses in B. pseudomallei infection in vitro and in vivo (3, 4). TLR5, a sensor of bacterial flagellin, regulates host defense to a number of lung infections caused by flagellated bacteria (5–9). The importance of TLR5 in regulating the host response to human melioidosis has not been determined.
Host genetic composition determines susceptibility to infection (10, 11). Analysis of genetic variation offers the opportunity to assess the role of specific elements of host innate immunity in human disease (11, 12). A single nucleotide polymorphism in TLR5, TLR51174C>T, is present with a minor allele frequency (MAF) of 5–8% in white and Asian populations. TLR51174T prematurely truncates the TLR5 receptor in the extracellular domain, eliminating the transmembrane and cytoplasmic signaling domains of the receptor (6). This action results in reduced flagellin-dependent activation of innate immunity and host inflammatory responses, possibly in a dominant fashion (6). TLR51174T has been associated with susceptibility to Legionnaire’s disease in a cohort of Dutch subjects exposed to contaminated water (6) but has not been associated with outcome from infection. We proposed testing the role of TLR5 in melioidosis by analyzing the association of TLR51174C>T with outcome in Thais with melioidosis.
Materials and Methods
Bacteria
B. pseudomallei BP-1, B. pseudomallei 1026b, or B. pseudomallei K96243 organisms were grown in Luria–Bertani broth for 6 h (log phase) or 19 h (stationary phase) and heat killed as previously described (3).
Human subjects
The melioidosis case–control study cohort has been previously described (13). Clinical data and blood were obtained prospectively from patients with culture-proven melioidosis admitted to Sappasithiprasong Hospital, Ubon Ratchathani, Thailand, from 1999 to 2005. Consent for enrollment into clinical studies of melioidosis was obtained from subjects or their representatives at the time of recruitment.
For studies using whole blood, fasting blood samples were obtained from healthy white participants in a Harborview Medical Center inflammatory response research study. Enrollment criteria and blood processing have been previously described (14). Thai subjects donating blood at the blood donation center at Sappasithiprasong Hospital in 2010 were recruited for participation in a similar study. Subjects were included if they were between the ages of 18 and 60 and did not report any history of immunodeficiency or inflammatory conditions, chronic diseases, pregnancy in the past 6 mo, anti-inflammatory medication use in the past week, antibiotic use in the past month, vaccination in the past 6 mo, heavy exercise or alcohol consumption in the past 24 h, or smoking in the past month. Those who met enrollment criteria gave written informed consent to participate and provided a postdonation blood sample.
These studies were approved by the University of Washington Human Subjects Division Institutional Review Board; the Ethical Review Committee for Research in Human Subjects, Ministry of Public Health, Thailand; the Ethical Review Committee for Research in Human Subjects, Sappasithiprasong Hospital, Ubon Ratchathani, Thailand; and the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Assays
NF-κB activation was determined with a luciferase reporter assay in Chinese hamster ovary (CHO) cells stably expressing huTLR5 and in human embryonic kidney–293 cells (HEK-293) transiently transfected with huTLR51174C or huTLR51174T, as previously described (6, 15, 16).
For immunoassay studies, 380 μl fresh whole blood in citrate mixed 1:1 with RPMI 1640 media was added to preprepared plates containing 20 μl stimulants. For the Harborview Medical Center study, the stimulant was log phase heat-killed B. pseudomallei 1026b 2.5 × 106 CFU/ml. For the Sappasithiprasong Hospital study, the stimulants were E. coli 0111:B4 LPS 10 ng/ml, S. typhimurium flagellin 500 ng/ml, log phase heat-killed B. pseudomallei 1026b 2.5 × 106 CFU/ml, or heat-killed B. pseudomallei K96243 2.5 × 106 CFU/ml. Plates were incubated at 37°C on a shaking incubator under 5% CO2 for 6 h before being spun down and the plasma was removed and frozen. For the Harborview Medical Center study, plasma IFN-γ, IL-10, IL-12p70, IL-1β, IL-6, IL-8, and TNF-α were subsequently assayed in duplicate, using an electrochemiluminescence imager (Mesoscale Discovery, Gaithersburg, MD). For the Sappasithiprasong Hospital study, IL-6, IL-8, TNF-α, IL-10, MCP-1, IL-1ra, G-CSF, and IL-1β were later assayed in duplicate on a multiplex bead system (Luminex, Austin, TX) using reagents from R&D Systems (Minneapolis, MN) or by ELISA (IL-1β for B. pseudomallei only) (14, 15). For each subject, a complete blood count with differential was performed in the clinical laboratory at the time of phlebotomy.
Genetic methods
DNA was extracted from whole blood using Nucleon BACC3 (GE Healthcare, Buckinghamshire, U.K.) or QIAamp DNA Blood Midi (Qiagen, Hilden, Germany) kits (13). Genotyping was performed using an allele-specific primer extension method (Sequenom, San Diego, CA) with reads by a MALDI-TOF mass spectrometer (13) or was performed using ABI TaqMan assays on an ABI Prism 7900 (Applied Biosystems, Carlsbad, CA).
Statistics
Continuous clinical data are reported as mean values. The association between genotype and outcome was evaluated using the Fisher exact test or by logistic regression. Survival analyses were performed with the log-rank test. For tests of population stratification, allele counts between cases and controls were compared with the χ2 test. Continuous in vitro data expected to follow a normal distribution are reported as mean ± SD. Comparisons between two groups were made using the t test, and comparisons between three or more groups were made using ANOVA and the Bonferroni posttest. Data not normally distributed are reported as median ± interquartile range. Plasma cytokine levels were analyzed in raw form or following normalization to monocyte counts. Given their mostly nonnormal distributions, cytokine values were log10 transformed before analysis by linear regression, adjusting for sex in the Thai cohort (14). Statistics were performed with Stata 11.1 (College Station, TX), incorporating the function genhw. A two-sided p ≤ 0.05 was considered significant.
Results
TLR5 mediates activation of NF-κB by B. pseudomallei
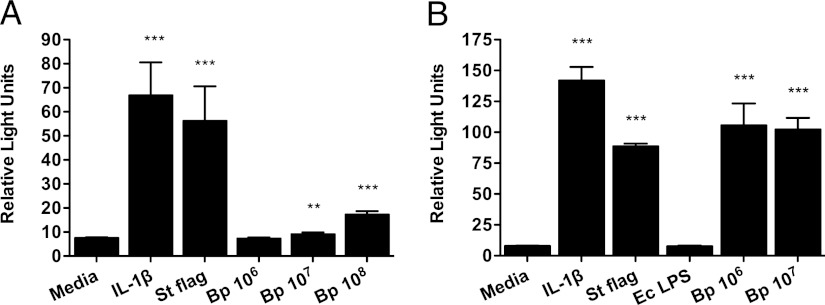
We first tested whether B. pseudomallei activates NF-κB, a transcription factor in the TLR pathway, in a TLR5-dependent manner. As wild-type CHO cells do not respond to flagellin (5), we stimulated CHO cells stably transfected with huTLR5 and an NF-κB luciferase reporter with stationary and log phase killed B. pseudomallei BP-1 (Fig. 1) (16). We examined bacteria from different growth phases because of lost or reduced expression of bacterial flagellin by B. pseudomallei in the stationary phase (S. Peacock, unpublished observations). Although stimulation with only the higher concentrations of stationary phase bacteria resulted in NF-κB activation, we detected robust NF-κB activation upon stimulation with lower concentrations of log phase bacteria. These findings indicated TLR5-mediated innate immune activation by B. pseudomallei.
FIGURE 1.
B. pseudomallei induces TLR5-dependent NF-κB activation. CHO cells stably transfected with TLR5, NF-κB–dependent firefly ELAM luciferase, and control thymidine kinase–driven Renilla luciferase were stimulated with media alone, IL-1β 20 ng/ml, (B) E. coli 0111:B4 LPS 10 ng/ml, S. typhimurium flagellin 100 ng/ml, (A) stationary or (B) log phase heat-killed B. pseudomallei BP-1 at various concentrations in colony-forming units per milliliter. NF-κB activation was determined by the ratio of ELAM to Renilla light emission after stimulation overnight. Data plotted are means ± SDs of triplicate or quadruplicate conditions that represent one of seven experiments in total performed independently. **p ≤ 0.01, ***p ≤ 0.001 by t test for comparisons to cells in media alone.
TLR51174T is associated with protection against death in human melioidosis
To explore the importance of TLR5 signaling in human melioidosis, we selected a common hypofunctional human polymorphism, TLR51174C>T (rs5744168), for study. In a case–control study, we analyzed the association of TLR51174C>T with outcome in a cohort of Thai subjects with culture-proven melioidosis admitted to Sappasithiprasong Hospital. Of 614 subjects with blood available for genotyping who passed quality control checks, survival at hospital discharge was known for 600. Mortality was 23.8%. The characteristics of the melioidosis subjects are listed in Table I. We successfully genotyped TLR51174C>T in 592 individuals (98.7% call rate); 514 (86.8%) were genotype C/C, 76 (12.8%) C/T, and 2 (0.3%) T/T (MAF 6.8%). After confirming Hardy–Weinberg equilibrium in the survivors (p = 0.8), we tested the association of the variant with in-hospital death. We found that 15.9% of survivors were heterozygotes or minor homozygotes, compared with 4.3% of nonsurvivors (contingency table p = 0.0004) (Table II). In a dominant genetic model (grouping carriers of the rare allele—TLR51174CT and TLR51174TT—together), we observed a very strong association of the TLR51174C>T variant with protection against death [odds ratio (OR): 0.24; 95% confidence interval (CI): 0.10–0.56; p = 0.001].
Table I. Characteristics of subjects with melioidosis.
| Alla (n = 600) | Nonsurvivors (n = 143) | Survivors (n = 457) | |
|---|---|---|---|
| Baseline characteristic | |||
| Mean age | 48.6 | 51.7 | 47.7 |
| Female sex | 46 | 43 | 47 |
| Pre-existing conditionb | |||
| Diabetes | 52 | 44 | 55 |
| Chronic liver disease | 2 | 2 | 1 |
| Renal insufficiency | 7 | 13 | 5 |
| Steroid use | 6 | 5 | 6 |
| Clinical presentation | |||
| Bacteremia | 50 | 73 | 43 |
| Pneumonia | 38 | 57 | 33 |
| Urinary tract infection | 12 | 19 | 10 |
| Clinical course | |||
| Shock | 26 | 61 | 15 |
| Respiratory failure | 18 | 68 | 2 |
| Organ failurec | 32 | 81 | 17 |
Numbers are a percentage except for mean age.
Missing data for 4 subjects (bacteremia), 4 subjects (pneumonia), 4 subjects (urinary tract infection), 39 subjects (shock), 29 subjects (respiratory failure), 40 subjects (organ failure).
Unknown pre-existing conditions are considered negative.
Organ failure is defined as presence of shock or respiratory failure.
Table II. Crude association of TLR51174C>T genotype with outcome in subjects with melioidosis.
| General Genetic Model |
Dominant Model |
|||||
|---|---|---|---|---|---|---|
| In-Hospital Death |
||||||
| No | Yes | p Valuea | OR | 95% CI | p Valueb | |
| C/C | 380 | 134 | ||||
| C/T | 70 | 6 | ||||
| T/T | 2 | 0 | 0.0004 | 0.24 | 0.10–0.56 | 0.001 |
| Organ Failure |
||||||
| No |
Yes |
|||||
| C/C | 314 | 163 | ||||
| C/T | 59 | 14 | ||||
| T/T | 2 | 0 | 0.014 | 0.44 | 0.24–0.81 | 0.009 |
General genetic model p value calculated by Fisher exact test.
Dominant model p value calculated by logistic regression.
We next performed an adjusted association of the TLR51174C>T variant with in-hospital death. To identify possible confounders to include in the model, we tested whether TLR51174C>T was associated with any pre-existing condition (diabetes, chronic liver disease, renal insufficiency, and steroid use). We found no association of the variant with any of these factors. However, we considered in the model factors that were independently associated with death: age, diabetes, renal insufficiency, pneumonia, bacteremia, and urinary tract infection. The appropriateness of the antimicrobial regimen did not differ between survivors and nonsurvivors. As pathogen recognition is characterized by cross talk and redundancy (17, 18), we also considered potential confounding effects of other TLR pathway genetic variants. We have previously observed that TLR4 and adapter molecule MyD88 modulate innate immune activation by B. pseudomallei (3). TIRAP is an adapter molecule that cooperates with MyD88. We chose two coding variants, one in TLR4 and one in TIRAP, to analyze in this population: TLR4896A>G (rs4986790) and TIRAP558C>T (rs7932766). Both variants have been previously associated with infection, with functional effects demonstrated by some authors (19–24). We have shown that the TLR4896A>G variant is rare in Thais (MAF < 1%) (13). In melioidosis patients, we identified a trend toward the independent association of TLR4896G with death (OR of death: 4.97; 95% CI: 0.82–30.0; p = 0.08 for a dominant model). TIRAP558C>T has a MAF ∼5% (13) but in melioidosis patients, TIRAP558T was not significantly associated with death (OR of death: 0.83; 95% CI: 0.44–1.58; p = 0.58 for a dominant model). In the model of the association of TLR51174T with death, neither TLR4896G nor TIRAP558T was significant (p = 0.06 or p = 0.49, respectively) or changed the main effect of TLR51174T. In the final model of the association of TLR51174T with death, adjusting for age, diabetes, renal insufficiency, pneumonia, and bacteremia, the protective effect remained very strong (OR: 0.20; 95% CI: 0.08–0.50; p = 0.001).
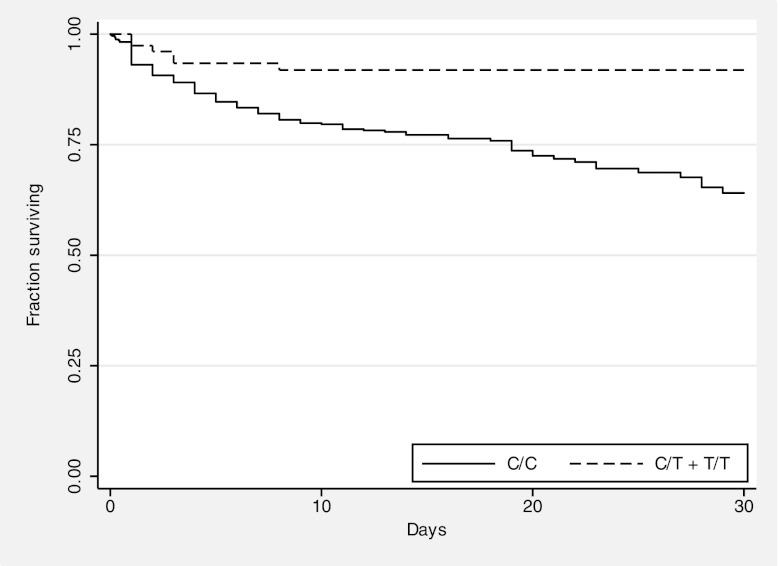
We plotted Kaplan–Meier in-hospital survival curves by TLR51174C>T genotype for all melioidosis cases (Fig. 2). A significant difference was noted in the risk of death between 0 and 30 d for individuals having TLR51174CC genotypes compared with those having TLR51174CT or TLR51174TT genotypes by the log-rank test (p = 0.001).
FIGURE 2.
Survival from melioidosis is enhanced for carriers of TLR51174T. Kaplan–Meier in-hospital survival curves are plotted for melioidosis subjects, grouped by genotype. Curves are significantly different by the log rank test (p = 0.001).
To test whether systemic ancestry differences (population stratification) between cases and controls contributed substantially to our findings, we examined the frequency of 25 unrelated single nucleotide polymorphisms from across the genome (13, 25). We compared the allele frequency of these variants in HapMap European ancestry and Han Chinese Beijing populations and selected the 17 variants with a χ2 > 1.5 (mean χ2 = 22.3). These variants were considered maximally variable between the two populations and might therefore serve as markers of population variation in our cohort. We genotyped these variants in the subset of 434 melioidosis subjects whose DNA was assayed on the Sequenom platform and compared allele frequencies in nonsurvivors with those in survivors (Supplemental Table I). The mean χ2 of all 17 variants was 1.14, suggesting little population stratification (26).
TLR51174T is associated with protection against organ failure in human melioidosis
To determine whether the TLR51174C>T variant was associated with an intermediate phenotype in melioidosis, we identified subjects in our cohort whose hospitalizations were complicated by organ failure, defined as shock or respiratory failure. Of genotyped subjects, 32% (177 of 552) developed organ failure during their hospitalization. Of the subjects with organ failure, 7.9% were genotype TLR51174CT or TLR51174TT, compared with 16.3% of subjects who did not develop organ failure (Table II). In an unadjusted dominant model of the association of the TLR51174C>T with organ failure, the OR was 0.44 (95% CI: 0.24–0.81; p = 0.009). Age, diabetes, pneumonia, bacteremia, and urinary tract infection were independently associated with organ failure, and so were considered in the adjusted model. In the final model incorporating diabetes, pneumonia, and bacteremia, the OR for the association of TLR51174C>T with organ failure was 0.37 (95% CI: 0.19-0.71; p = 0.003).
TLR51174T does not mediate activation of NF-κB by B. pseudomallei
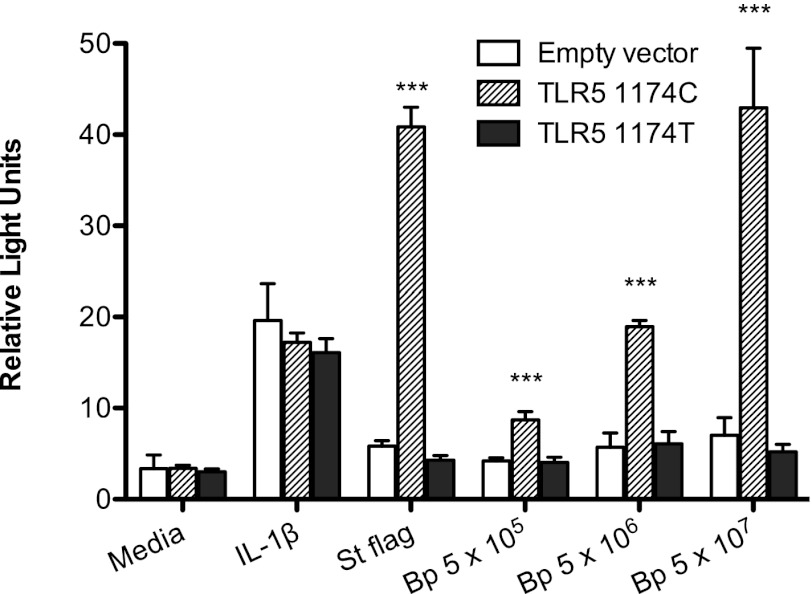
We next assessed how TLR51174c>T modulates innate immune activation by B. pseudomallei in isolation in vitro. As HEK-293 cells do not endogenously express TLRs (27), we transiently transfected HEK-293 cells with either TLR51174C or TLR51174T and an NF-κB luciferase reporter before stimulating the cells with heat-killed log phase B. pseudomallei (Fig. 3). In contrast to TLR51174C, TLR51174T was unable to mediate B. pseudomallei–induced NF-κB activation.
FIGURE 3.
NF-κB activation induced by B. pseudomallei is abrogated by TLR51174T. HEK-293 cells transiently transfected with human TLR51174C or TLR51174T, NF-κB–dependent firefly ELAM luciferase, and control thymidine kinase–driven Renilla luciferase were stimulated with media alone, IL-1β 20 ng/ml, S. typhimurium flagellin 100 ng/ml, or log phase heat-killed B. pseudomallei 1026b at various concentrations in colony-forming units per milliliter. NF-κB activation was determined by the ratio of ELAM to Renilla light emission after 24 h. Data plotted are means ± SDs of triplicate or quadruplicate conditions that represent one of two similar experiments performed independently. ***p ≤ 0.001 by ANOVA with the Bonferroni posttest for comparisons between similarly stimulated cells transfected with empty vector TLR5_1174C or TLR51174T.
B. pseudomallei induces reduced blood cytokine responses ex vivo in carriers of TLR51174T
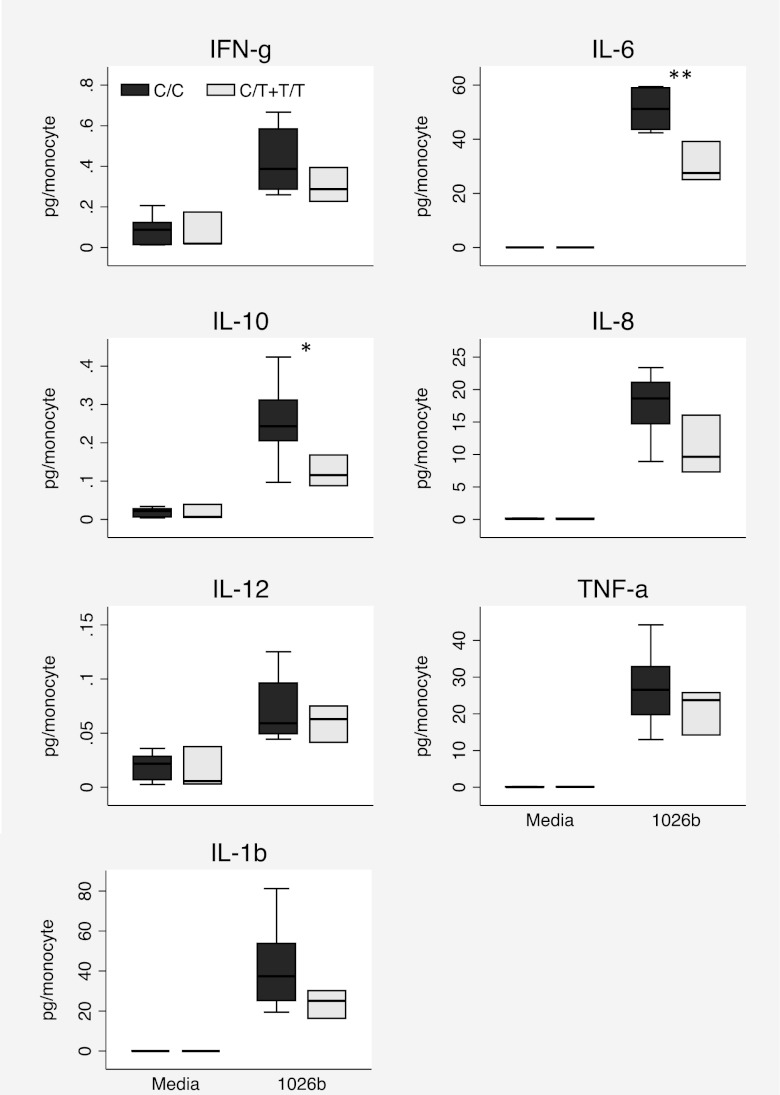
Together, these findings suggested that carriers of TLR51174T may generate impaired inflammatory responses during melioidosis infection that result in reduced organ failure and lower mortality. To establish whether TLR51174C>T is associated with altered cytokine production in response to B. pseudomallei in humans, we obtained blood from 11 healthy white subjects of known TLR51174C>T genotype. We stimulated fresh whole blood ex vivo with heat-killed B. pseudomallei 1026b. We measured IFN-γ, IL-10, IL12p70, IL-1β, IL-6, IL-8, and TNF-α in plasma. We found no TLR51174C>T genotype–dependent differences in raw cytokine production induced by B. pseudomallei. We have found that cytokine levels induced by B. pseudomallei are dependent on leukocyte count (N. Chantratita, submitted for publication). The TLR51174C>T genotype was not associated with total leukocyte count (p = 0.92), but heterozygous or homozygous carriers of TLR51174T had significantly higher monocyte counts than did wild-type subjects (643 ± 177 versus 369 ± 24 cells per microliter, p = 0.029). As monocytes are central drivers of the innate immune response, we normalized cytokine values to monocyte count and compared responses by genotype (Fig. 4). We found that IL-10 and IL-6 levels were significantly lower in carriers of TLR51174T (p = 0.04 and p = 0.009, respectively). A trend toward lower IL-8 levels (p = 0.059) was noted. Although the sample size was small, these data supported a functional effect of TLR51174C>T in innate immune responses to B. pseudomallei.
FIGURE 4.
B. pseudomallei induces lower IL-10 and IL-6 levels in white carriers of TLR51174T. Whole blood was stimulated with media or heat-killed B. pseudomallei 1026b 2.5 × 106 CFU/ml for 6 h. Inflammatory mediators were measured in plasma by electrochemiluminescence assay, normalized to monocyte count, and log10 transformed for statistical analysis by linear regression. n = 8 (TLR51174CC), n = 3 (TLR51174CT+TT). Boxes show the median and interquartile range; whiskers show upper and lower adjacent values; outside values are not shown for clarity. *p ≤ 0.05, **p ≤ 0.01.
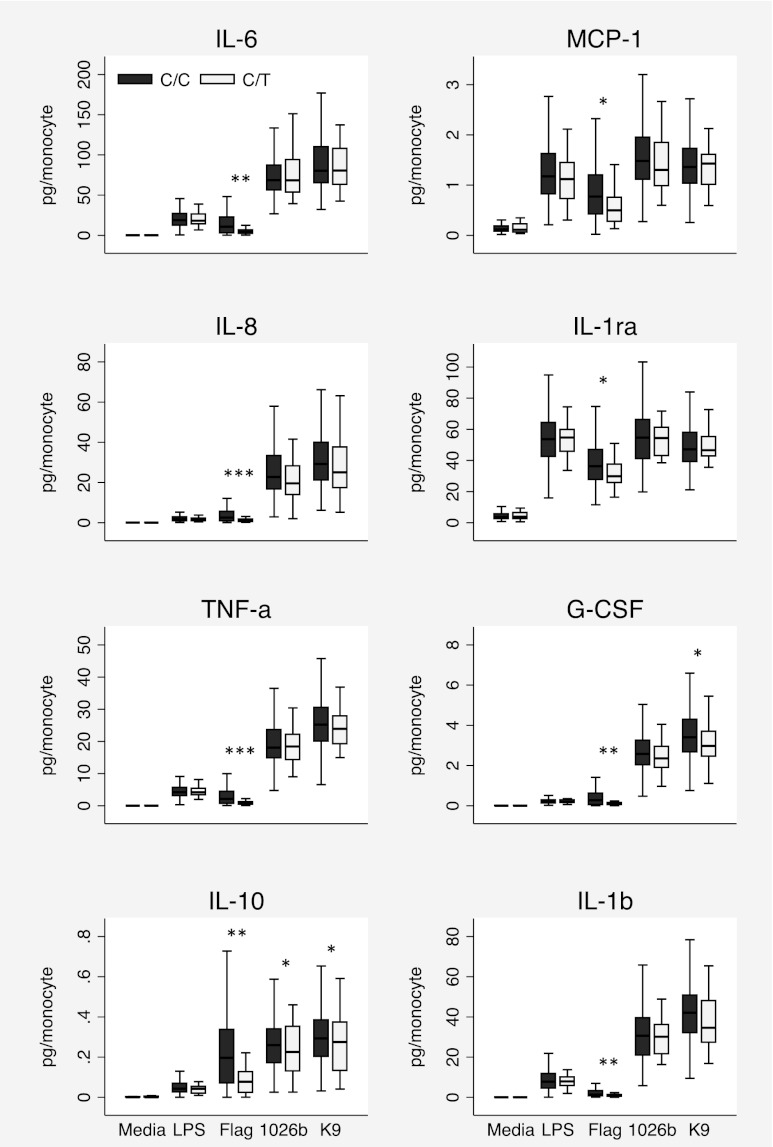
To confirm this effect in a larger cohort and to determine whether this effect was also apparent in a Thai population susceptible to melioidosis, we obtained blood from 300 healthy blood donors at Sappasithiprasong Hospital. We stimulated fresh whole blood with LPS, flagellin, or heat-killed B. pseudomallei 1026b or K96243. We quantified IL-6, IL-8, TNF-α, IL-10, MCP-1, IL-1ra, G-CSF, and IL-1β levels in plasma. We genotyped the subjects at TLR51174C>T and found that 269 (90%) subjects were major homozygotes, 31 (10%) were heterozygotes, and 0 (0%) were minor homozygotes (MAF 5.2%). In this cohort, we did not observe any TLR51174C>T-dependent differences in total leukocyte count (p = 0.22) or monocyte count (p = 0.54). Flagellin stimulation of blood from subjects with the TLR51174CT genotype, compared with TLR51174CC subjects, induced significantly lower levels of all raw cytokine responses except IL-1ra (e.g., median TNF-α concentrations were 260.6 pg/ml compared with 632.7 pg/ml, p < 0.001) and significantly lower levels of all monocyte-normalized cytokine responses (Fig. 5). Stimulation with LPS, a TLR4 agonist, induced no TLR51174C>T-dependent difference in raw or normalized cytokine levels. Stimulation with both strains of B. pseudomallei induced no significant differences in raw cytokine responses, but both strains induced significantly less monocyte-normalized IL-10, and a trend toward lower monocyte-normalized IL-8 levels was observed (p = 0.057 and p = 0.084) in TLR51174CT subjects. K96243 also induced significantly less G-CSF in TLR51174CT subjects after normalization for monocyte count.
FIGURE 5.
B. pseudomallei induces lower IL-10 levels in Thai carriers of TLR51174T. Whole blood was stimulated with media, E. coli 0111:B4 LPS 10 ng/ml, S. typhimurium flagellin 500 ng/ml, heat-killed B. pseudomallei 1026b 2.5 × 106 CFU/ml, or heat-killed B. pseudomallei K96243 2.5 × 106 CFU/ml (K9) for 6 h. Inflammatory mediators were measured in plasma by multiplex bead assay or ELISA (IL-1β for B. pseudomallei only), normalized to monocyte count, and log10 transformed for statistical analysis by linear regression. Boxes show the median and interquartile range; whiskers show upper and lower adjacent values; outside values are not shown for clarity. n = 269 (TLR51174CC), n = 31 (TLR51174CT). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Discussion
The results of this study provide compelling evidence of the importance of TLR5 as a regulator of the host response in melioidosis. In the largest investigation to date of genetic variation as a determinant of outcome from melioidosis, we show that B. pseudomallei induces a TLR5-dependent innate immune response and that a TLR5 genetic variant that encodes a defective TLR5 protein is associated with marked protection against death or organ failure in Thais. We further demonstrate a functional effect of this variant in the blood cytokine response to flagellin and to killed B. pseudomallei.
Although TLR51174C>T is associated with susceptibility to Legionnaires’ disease (Legionella pneumophila pneumonia) and recurrent urinary tract infections (6, 28), our study is the first, to our knowledge, to identify an association of the variant with outcome from infection. TLR51174T has previously been shown to blunt flagellin-dependent IL-6 and IL-8 release in whole blood (6, 14). In addition, we show that levels of IL-10, TNF-α, MCP-1, G-CSF, IL-1ra, and IL-1β induced by flagellin are also reduced in carriers of the variant. It is therefore tempting to postulate that the hypofunctional TLR5 variant broadly impairs the inflammatory response to flagellated infection, resulting in reduced sepsis-induced organ failure and death. This idea is consistent with other studies showing that carriage of the variant is protective in inflammatory conditions, including systemic lupus erythematosus, cystic fibrosis (the variant is associated with a higher body mass index), and Crohn’s disease (29–31). However, IL-10 is the sole cytokine that is consistently reduced in carriers of TLR51174T from two different populations upon stimulation of blood with whole B. pseudomallei, suggesting an effect of the variant that is restricted to IL-10 production when innate immunity is simultaneously activated by multiple ligands. IL-10 is an anti-inflammatory cytokine clearly implicated in sepsis, although its role in modulating the host response during this complex and dynamic process remains poorly understood (32, 33). In a clinical trial of antibiotic therapy in melioidosis, baseline IL-10 levels were increased in nonsurvivors of melioidosis, compared with those in survivors, and the IL-10 level was an independent predictor of mortality (34). A recent study of inhalation melioidosis in African green monkeys found that increased IL-10 levels correlated with reduced survival time on day four following infection (35). These data are concordant with our findings. Conceivably, impaired IL-10 release in carriers of TLR51174T may, in fact, enhance inflammation and control of the invading pathogen in the early stages of infection, resulting in improved organ function and survival. Further investigation is required to elucidate the TLR5-driven and IL-10–dependent mechanisms at play during melioidosis.
We have previously examined the association of TLR genetic variants with susceptibility to melioidosis (13) but did not detect any relationship between TLR51174C>T and disease. These contrasting observations underscore the very different roles the innate immune system plays in modulating host susceptibility to infection versus governing outcome once infection is established. It is often difficult to ascertain the specific route of infection—percutaneous versus inhaled—in clinical cases of melioidosis. However, the differential associations of TLR51174C>T with melioidosis compared with Legionella pneumonia further highlight the importance of additional immune modulators in governing clinical responses to airborne infection caused by flagellated organisms. The protective effect of TLR51174T on outcome from melioidosis and in susceptibility to various inflammatory conditions may explain the relatively high frequency of the hypofunctional allele in a variety of populations.
Our genetic association study has several limitations. Despite our best efforts to select cases and controls appropriately and test for population stratification, unmeasured population stratification may bias our analyses. A second limitation is that the observed mortality in this cohort was lower than in previous descriptions of outcome from melioidosis at this hospital (36). This observation is most likely due to a bias in this study against subjects who died extremely rapidly after admission and did not have blood collected for genotyping by the study team. In addition, our genetic association will require replication in a second, independent population. However, as Sappasithiprasong Hospital is one of the few sites with sufficient infrastructure to perform such a genetic study in melioidosis, we designed as large a clinical study as possible at this single site and performed functional experiments in a similar population.
In summary, a common TLR5 genetic variant that prematurely truncates TLR5 and abrogates TLR5-dependent signaling is strongly associated with improved outcome in human melioidosis. Ex vivo blood stimulation studies indicate that IL-10 is implicated in this process. To our knowledge, this is the first study to suggest an essential role for TLR5 in regulation of the host immune response in melioidosis. Our findings prompt further evaluation of this receptor as a potential target for therapeutic intervention in this severe infection.
Acknowledgments
We thank the staff and patients at Sappasithiprasong Hospital for support; Premjit Amornchai, Aunchalee Thanwisai, and Malinee Oyuchua for DNA extraction; and Malinka Jansson and Sarunporn Tandhavanant for technical assistance.
This work was supported by National Institutes of Health Grants HL094759 and AI057141, the Puget Sound Partners for Global Health, the Parker B. Francis Foundation, the University of Washington Royalty Research Fund, the Wellcome Trust, and the National Institute for Health Research Cambridge Biomedical Research Center.
Portions of this work were presented at the European Melioidosis Network Meeting, September 11, 2009, London, U.K., and at the American Society of Tropical Medicine and Hygiene Annual Meeting, December 4–8, 2011, Philadelphia, PA.
The online version of this article contains supplemental material.
- CHO
- Chinese hamster ovary
- CI
- confidence interval
- HEK-293
- human embryonic kidney–293 cell
- MAF
- minor allele frequency
- OR
- odds ratio.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Wiersinga W. J., van der Poll T., White N. J., Day N. P., Peacock S. J. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 4: 272–282 [DOI] [PubMed] [Google Scholar]
- 2.Limmathurotsakul D., Wuthiekanun V., Chierakul W., Cheng A. C., Maharjan B., Chaowagul W., White N. J., Day N. P., Peacock S. J. 2005. Role and significance of quantitative urine cultures in diagnosis of melioidosis. J. Clin. Microbiol. 43: 2274–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West T. E., Ernst R. K., Jansson-Hutson M. J., Skerrett S. J. 2008. Activation of Toll-like receptors by Burkholderia pseudomallei. BMC Immunol. 9: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga W. J., Wieland C. W., Dessing M. C., Chantratita N., Cheng A. C., Limmathurotsakul D., Chierakul W., Leendertse M., Florquin S., de Vos A. F., et al. 2007. Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (melioidosis). PLoS Med. 4: e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi F., Smith K. D., Ozinsky A., Hawn T. R., Yi E. C., Goodlett D. R., Eng J. K., Akira S., Underhill D. M., Aderem A. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103 [DOI] [PubMed] [Google Scholar]
- 6.Hawn T. R., Verbon A., Lettinga K. D., Zhao L. P., Li S. S., Laws R. J., Skerrett S. J., Beutler B., Schroeder L., Nachman A., et al. 2003. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires’ disease. J. Exp. Med. 198: 1563–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawn T. R., Berrington W. R., Smith I. A., Uematsu S., Akira S., Aderem A., Smith K. D., Skerrett S. J. 2007. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J. Immunol. 179: 6981–6987 [DOI] [PubMed] [Google Scholar]
- 8.Morris A. E., Liggitt H. D., Hawn T. R., Skerrett S. J. 2009. Role of Toll-like receptor 5 in the innate immune response to acute P. aeruginosa pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 297: L1112–L1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de C Ventura G. M., Le Goffic R., Balloy V., Plotkowski M. C., Chignard M., Si-Tahar M., de 2008. TLR 5, but neither TLR2 nor TLR4, is involved in lung epithelial cell response to Burkholderia cenocepacia. FEMS Immunol. Med. Microbiol. 54: 37–44 [DOI] [PubMed] [Google Scholar]
- 10.Sørensen T. I., Nielsen G. G., Andersen P. K., Teasdale T. W. 1988. Genetic and environmental influences on premature death in adult adoptees. N. Engl. J. Med. 318: 727–732 [DOI] [PubMed] [Google Scholar]
- 11.Hill A. V. 2006. Aspects of genetic susceptibility to human infectious diseases. Annu. Rev. Genet. 40: 469–486 [DOI] [PubMed] [Google Scholar]
- 12.Wurfel M. M. 2008. Genetic insights into sepsis: what have we learned and how will it help? Curr. Pharm. Des. 14: 1900–1911 [DOI] [PubMed] [Google Scholar]
- 13.West T. E., Chierakul W., Chantratita N., Limmathurotsakul D., Wuthiekanun V., Emond M. J., Hawn T. R., Peacock S. J., Skerrett S. J. 2012. Toll-like receptor 4 region genetic variants are associated with susceptibility to melioidosis. Genes Immun. 13: 38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wurfel M. M., Gordon A. C., Holden T. D., Radella F., Strout J., Kajikawa O., Ruzinski J. T., Rona G., Black R. A., Stratton S., et al. 2008. Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am. J. Respir. Crit. Care Med. 178: 710–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West T. E., Hawn T. R., Skerrett S. J. 2009. Toll-like receptor signaling in airborne Burkholderia thailandensis infection. Infect. Immun. 77: 5612–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith K. D., Andersen-Nissen E., Hayashi F., Strobe K., Bergman M. A., Barrett S. L., Cookson B. T., Aderem A. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4: 1247–1253 [DOI] [PubMed] [Google Scholar]
- 17.Lee M. S., Kim Y. J. 2007. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 76: 447–480 [DOI] [PubMed] [Google Scholar]
- 18.Netea M. G., van der Graaf C., Van der Meer J. W., Kullberg B. J. 2004. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J. Leukoc. Biol. 75: 749–755 [DOI] [PubMed] [Google Scholar]
- 19.Lorenz E., Mira J. P., Frees K. L., Schwartz D. A. 2002. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch. Intern. Med. 162: 1028–1032 [DOI] [PubMed] [Google Scholar]
- 20.Hawn T. R., Verbon A., Janer M., Zhao L. P., Beutler B., Aderem A. 2005. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires’ disease. Proc. Natl. Acad. Sci. USA 102: 2487–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mockenhaupt F. P., Cramer J. P., Hamann L., Stegemann M. S., Eckert J., Oh N. R., Otchwemah R. N., Dietz E., Ehrhardt S., Schröder N. W., et al. 2006. Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. Proc. Natl. Acad. Sci. USA 103: 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber R. C., Aragaki C. C., Rivera-Chavez F. A., Purdue G. F., Hunt J. L., Horton J. W. 2004. TLR4 and TNF-alpha polymorphisms are associated with an increased risk for severe sepsis following burn injury. J. Med. Genet. 41: 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferwerda B., McCall M. B., Alonso S., Giamarellos-Bourboulis E. J., Mouktaroudi M., Izagirre N., Syafruddin D., Kibiki G., Cristea T., Hijmans A., et al. 2007. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc. Natl. Acad. Sci. USA 104: 16645–16650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawn T. R., Dunstan S. J., Thwaites G. E., Simmons C. P., Thuong N. T., Lan N. T., Quy H. T., Chau T. T., Hieu N. T., Rodrigues S., et al. 2006. A polymorphism in Toll-interleukin 1 receptor domain containing adaptor protein is associated with susceptibility to meningeal tuberculosis. J. Infect. Dis. 194: 1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barreiro L. B., Neyrolles O., Babb C. L., Tailleux L., Quach H., McElreavey K., Helden P. D., Hoal E. G., Gicquel B., Quintana-Murci L. 2006. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med. 3: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reich D. E., Goldstein D. B. 2001. Detecting association in a case-control study while correcting for population stratification. Genet. Epidemiol. 20: 4–16 [DOI] [PubMed] [Google Scholar]
- 27.McNeilly T. N., Mitchell M. C., Nisbet A. J., McAteer S., Erridge C., Inglis N. F., Smith D. G., Low J. C., Gally D. L., Huntley J. F., Mahajan A. 2010. IgA and IgG antibody responses following systemic immunization of cattle with native H7 flagellin differ in epitope recognition and capacity to neutralise TLR5 signalling. Vaccine 28: 1412–1421 [DOI] [PubMed] [Google Scholar]
- 28.Hawn T. R., Scholes D., Li S. S., Wang H., Yang Y., Roberts P. L., Stapleton A. E., Janer M., Aderem A., Stamm W. E., et al. 2009. Toll-like receptor polymorphisms and susceptibility to urinary tract infections in adult women. PLoS ONE 4: e5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawn T. R., Wu H., Grossman J. M., Hahn B. H., Tsao B. P., Aderem A. 2005. A stop codon polymorphism of Toll-like receptor 5 is associated with resistance to systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 102: 10593–10597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blohmke C. J., Park J., Hirschfeld A. F., Victor R. E., Schneiderman J., Stefanowicz D., Chilvers M. A., Durie P. R., Corey M., Zielenski J., et al. 2010. TLR5 as an anti-inflammatory target and modifier gene in cystic fibrosis. J. Immunol. 185: 7731–7738 [DOI] [PubMed] [Google Scholar]
- 31.Gewirtz A. T., Vijay-Kumar M., Brant S. R., Duerr R. H., Nicolae D. L., Cho J. H. 2006. Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G1157–G1163 [DOI] [PubMed] [Google Scholar]
- 32.Bazzoni F., Tamassia N., Rossato M., Cassatella M. A. 2010. Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: lessons from neutrophils. Eur. J. Immunol. 40: 2360–2368 [DOI] [PubMed] [Google Scholar]
- 33.Stanilova S. A. 2010. Functional relevance of IL-10 promoter polymorphisms for sepsis development. Crit. Care 14: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson A. J., Smith M. D., Weverling G. J., Suputtamongkol Y., Angus B. J., Chaowagul W., White N. J., van Deventer S. J., Prins J. M. 2000. Prognostic value of cytokine concentrations (tumor necrosis factor-alpha, interleukin-6, and interleukin-10) and clinical parameters in severe melioidosis. J. Infect. Dis. 181: 621–625 [DOI] [PubMed] [Google Scholar]
- 35.Yeager J. J., Facemire P., Dabisch P. A., Robinson C. G., Nyakiti D., Beck K., Baker R., Pitt M. L. 2012. Natural history of inhalation melioidosis in rhesus macaques (Macaca mulatta) and African green monkeys (Chlorocebus aethiops). Infect. Immun. 80: 3332–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limmathurotsakul D., Wongratanacheewin S., Teerawattanasook N., Wongsuvan G., Chaisuksant S., Chetchotisakd P., Chaowagul W., Day N. P., Peacock S. J. 2010. Increasing incidence of human melioidosis in Northeast Thailand. Am. J. Trop. Med. Hyg. 82: 1113–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]