Abstract
Factor H (fH) is an endogenous negative regulator of the alternative pathway (AP) that binds polyanions as well as complement activation fragments C3b and C3d. The AP is both necessary and sufficient to develop collagen Ab–induced arthritis (CAIA) in mice; the mechanisms whereby normal control of the AP is overcome and injury develops are unknown. Although primarily a soluble circulating protein, fH can also bind to tissues in a manner dependent on the carboxyl-terminal domain containing short consensus repeats 19 and 20. We examined the role of fH in CAIA by blocking its binding to tissues through administration of a recombinant negative inhibitor containing short consensus repeats 19 and 20 (rfH19-20), which impairs fH function and amplifies surface AP activation in vitro. Administration of rfH19-20, but not control rfH3-5, significantly worsened clinical disease activity, histopathologic injury, and C3 deposition in the synovium and cartilage in wild-type and fH+/− mice. In vitro studies demonstrated that rfH19-20 increased complement activation on cartilage extracts and injured fibroblast-like synoviocytes, two major targets of complement deposition in the joint. We conclude that endogenous fH makes a significant contribution to inhibition of the AP in CAIA through binding to sites of immune complex formation and complement activation.
Introduction
Complement, a major component of innate immunity, is thought to play an important role in the pathogenesis of human rheumatoid arthritis (RA) (1). In this setting, uncontrolled complement system activation causes injury of cartilage and synovium in joints through redirection of its powerful effector mechanisms of inflammation onto self tissues.
Activation of the complement system is not normally allowed on the surface of host cells and tissues but is rapidly promoted on pathogens. The mechanisms that normally provide this regulation are complex and finely balanced. However, dysregulation of these mechanisms can lead to excessive complement activation, inflammation, and injury to self tissues. For example, deficiencies and hypofunctional polymorphic variants or mutations in the negative regulators of the complement system are associated with the development of atypical hemolytic uremic syndrome (2, 3), membranoproliferative glomerulonephritis type II (also called dense deposit disease) (3), age-related macular degeneration (4, 5), and systemic lupus erythematosus (6). Additionally, excessive complement activation plays a role in ischemia-reperfusion injury (7) and multiple sclerosis (8).
Complement activation on the surface of host cells and tissues or on the surface of pathogens is initiated through three distinct pathways designated the classical, alternative (AP), and lectin pathways. Complement activation by each of these pathways is greatly increased through the amplification loop, which utilizes the same proteins as the alternative pathway. Each of these three pathways utilizes unique molecules and mechanisms for their initiation; however, the pathways all converge on C3 and C5 to generate the same effector molecules, including C3a, C5a, the membrane attack complex (MAC), and fragments of C3 (C3b, iC3b, C3dg/C3d) that interact with complement receptors.
We and others have shown that the AP is uniquely both necessary and sufficient for mice to develop passive transfer collagen Ab–induced arthritis (CAIA). The classical and lectin pathways initiate complement activation but are not required for this disease model. CAIA is an immune complex–induced model of the effector phase of human RA (1, 9–12) that is dependent on complement activation and exhibits disease-related contributions from C3a, C5a, and the MAC (13). The AP consists of four proteins designated factor B (fB), factor D (fD), properdin, and C3. The AP does not absolutely depend on a recognition protein for its initiation, but rather is slowly and continuously activated by a mechanism called “tickover” where C3 is spontaneously hydrolyzed and changes conformation, forming C3(H2O) that is a C3b-like molecule. Recent studies have also suggested that properdin can serve a recognition function on some target surfaces and initiate C3b binding and AP engagement (14). Properdin-deficient mice develop less CAIA; therefore, properdin plays a role in AP-mediated arthritis (15).
In tickover, C3(H2O) associates with fB, then allowing fD to cleave fB into fragment Ba, which is released, and fragment Bb, which remains associated to form the C3 convertase (activating) enzyme C3(H2O)Bb. Additionally, the AP is engaged as the amplification loop when fB binds to surface-bound C3b that is generated by any of the three pathways. This association results in fD cleavage of fB and further generation of the C3 convertase C3bBb, followed by formation of the C3bBbC3b complex, which serves as the C5 convertase.
How the normal AP regulatory mechanisms that limit the effects of tickover and the amplification loop are overcome in CAIA is unknown, especially on the acellular cartilage that lacks cell membrane–associated complement regulatory proteins. Factor H (fH), a 155-kDa protein, is a circulating endogenous negative regulator of the AP of the complement system. fH is constitutively expressed in the liver and distributed systematically in body fluids (16). fH controls the AP in the fluid phase of blood as well as on the solid phase (cell surfaces) using three different mechanisms: 1) preventing the binding of fB to C3(H2O) and C3b, 2) promoting the dissociation of the C3(H2O)Bb and C3bBb complexes, and 3) acting as an obligate cofactor for serum factor I to cleave C3b into the hemolytically inactive iC3b form (17–19). Using these mechanisms, fH can prevent the formation of AP C3 and C5 convertases.
Both fH and the alternatively transcribed fH-like 1 protein have been shown to be expressed and secreted by synovial fibroblasts and are present in the synovial fluid derived from RA patients (20). fH is composed of 20 repeating domains designated short consensus repeats (SCRs); subdomains of fH contain multiple SCRs and exhibit different C3b binding specificities and functions. For example, SCRs 1–4 bind to C3b and regulate the AP by decay acceleration and cofactor activity. SCRs 5–7 contain a polyanion binding site (21, 22). SCRs 19 and 20 are particularly important in fH function as they bind effectively to the combination of polyanions and C3b and C3d deposited on the surface (23, 24). Once fH is anchored to the cell surface using SCRs 5–7 and 19 and 20, it can then act on surface-bound C3b through SCRs 1–4. Polymorphisms and mutations in these C3b and polyanion binding SCRs, as well as the generation of anti-fH auto-Abs against these domains, lead to the impaired regulation of the AP and to multiple diseases (25, 26). SCRs 19 and 20 are particularly relevant to the surface control of AP activation because they greatly increase fH binding to the surface (27).
Although fH regulates AP initiation through tickover and the C3b-catalyzed amplification loop, its role in the modulation of immune complex injury initiated by high-affinity pathogenic autoantibodies is poorly understood (28). In this study, we have used the CAIA model as well as ex vivo methods to show that endogenous fH can play a key role in immune complex–induced joint injury by limiting the surrounding cartilage and cell-bound amplification that follows initial complement activation.
Materials and Methods
Animals
Ten-week-old C57BL/6 male wild-type (WT) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice in different treatment groups were matched for age and sex. C57BL/6 fH−/− mice were maintained in our own colony and were backcrossed for 10 generations onto the C57BL/6 strain prior to use in in vivo studies, and they were confirmed for genetic purity (29, 30). A total of 132 WT and gene-targeted homozygous or heterozygous fH-deficient mice were used for in vivo studies. Mice were maintained in filter top cages with four to five mice in each cage in a barrier animal facility with a climate-controlled environment having 12 h light/dark cycles. All mice were fed breeder’s chow provided by the Center for Laboratory Animal Care, University of Colorado Denver. WT, C3−/−, and C4−/− sera were obtained from genetically deficient mice bred onto the C57BL/6 background. All experimental procedures were approved by the Animal Review Committee of the University of Colorado Denver.
Genotyping of fH+/− mice by PCR
To obtain fH heterozygous-deficient (fH+/−) mice, fH−/− C57BL/6 mice were bred with WT C57BL/6 mice for >20 generations. The identity of fH+/− mice was confirmed by tail DNA–based PCR using three primers, 5′-GTA AAGGTCCTCCTCCAAGAG-3′, 3′-GGT ATA AAC CTT TGC ACC-5′, and neo primer 5′-GGG GAT CGG CAA TAA AAA GAC-3′, at a ratio of 1:2:0.5 and 55°C annealing temperature (30). A 2% agarose gel was used to characterize the presence of 576- and 400-bp fragments originating from WT and fH−/− genotypes, respectively. The presence of both bands, that is, of 576 bp and of 400 bp, confirmed the identity of fH+/− mice. The genotypes of fH−/−, fH+/−, and fH+/+ experimental mice were confirmed before and after the development of disease by genomic DNA, and the phenotypes were confirmed by Western blot analysis of fH levels in sera.
Recombinant fH19-20 and fH3-5 protein purification
A recombinant protein containing SCR domains 19 and 20 of mouse fH (rfH19-20) was purified as previously described for human rfH19-20 (31). This domain of fH is necessary for fH interactions with surface-bound C3b/C3d as well as cell surface glycosaminoglycans. rfH19-20 efficiently competes with full-length fH in vitro for binding to these cell ligands, without affecting fluid phase complement regulation (31). We have not determined the in vivo half-life of mouse rfH19-20. Another recombinant protein containing the SCR domains 3–5 of human fH (rfH3-5), which has no known tissue binding characteristics, was used as a negative control for in vivo and in vitro studies. We have not used murine rfH3-5 because it was not available; however, there is a very high degree of similarity between murine and human fH (32). This protein was also produced in Pichia pastoris and purified in a similar fashion (33). The immunogenicity of human fH (rfH3-5) in mice is not known.
Induction of CAIA and establishment of treatment protocol
CAIA was induced using a mixture of four mAbs (three IgG2a and one IgG2b) (Arthrogen) reactive to bovine, human, and mouse type II collagen (CII) (Chondrex) (10). All four mAbs recognize the conserved epitopes (CB11) shared by CII across species. All mice received i.p. injections of anti-CII mAb on day 0, and 50 μg/mouse LPS from Escherichia coli strain 0111B4 was injected i.p. on day 3. LPS was injected to synchronize the development of arthritis in mice. Preliminary experiments were performed with WT mice using five different doses of anti-CII mAb, 0.5, 2, 4, 6, and 8 mg/mouse, to establish the dose-dependent level of clinical disease activity. Preliminary experiments were also carried out to ascertain the dose of rfH19-20 required to give maximal enhancement of disease. Intraperitoneal injections of 50 (2.3 μM) or 150 μg (6.9 μM) rfH19-20 were carried out 15 min after the injection of mAb to CII on day 0 and again 15 min after LPS on day 3 (total of 100 and 300 μg/mouse). The estimated molar ratio of rfH19-20 to fH would be 2.3:3.3 and 6.9:3.3 (or 2.3:1.1) for 50 or 150 μg/mouse rfH19-20, respectively, assuming a total of 1.5 ml serum and 500 μg/ml fH present in mouse. The results of these preliminary studies indicated that the use of 2 mg/mouse anti-CII mAb, or lower, resulted in disease scores sufficiently low to allow enhancement to be ascertained, and that 100–300 μg rfH19-20 was required to increase disease activity. Control experiments were performed with 300 μg rfH3-5, a region of fH structure containing SCRs 3–5 that is not involved in binding to substrates. CAIA was also examined in fH+/− mice treated with and without rfH19-20 or rfH3-5 using a submaximal dose of 0.5 mg/mouse anti-CII mAb. CAIA was also examined in fH+/− mice using a dose of 8 mg/mouse anti-CII mAb. All mice treated with PBS or rfH19-20 or rfH3-5 started to develop arthritis on day 4 and were sacrificed on day 10. Blood was drawn intraorbitally from all mice at day 0 prior to injection of mAb to CII, at day 3 prior to LPS injection, and at day 10 prior to sacrifice.
Examination for clinical disease activity
The severity of clinical disease activity in all mice was determined daily according to our published criteria (34) by two trained laboratory personnel acting independently and blinded to the treatment. Clinical disease activity scores (DAS) were evaluated on a 3-point scale per paw: 0, normal joint; 1, slight inflammation and redness; 2, severe erythema and swelling affecting the entire paw with inhibition of use; and 3, deformed paw or joint with ankylosis, joint rigidity, and loss of function. The total score for DAS was based on all four paws with a maximum score of 12 for each mouse.
Histopathology of knee joints
Both forepaws and the entire right hind limb, including the paw, ankle, and knee, were surgically excised at day 10 from all mice and fixed immediately in 10% buffered formalin (Biochemical Sciences). The preparation of tissue samples and histological analysis from mice treated with PBS, rfH19-20, or rfH3-5 was performed as previously described (10). All sections were read by a trained observer who was also blinded to the treatment and to the clinical DAS of each mouse. The joint sections were scored on a scale of 0–5 for inflammation, pannus formation, cartilage damage, and bone damage. The overall score was calculated as the total of the four individual parameters with each parameter represented as the mean value for five joints per mouse.
Immunohistochemistry for C3 and fH deposition over time
An experiment evaluating the serial deposition of C3 and fH on the surface of the cartilage and in the synovium during early phases of CAIA was performed. Mice were sacrificed at 0.5, 1, 2, 4, 8, 24, 72, 96, 120, and 144 h after injection of mAb to CII, and knees were excised and fixed in formalin. A total of 30 WT mice were used for this time-course study (n = 3 for each time point). In the experiments with rfH19-20 and rfH3-5, mice were sacrificed on day 10 with excision of both forepaws and right hind limb (knee joint, ankle, and paw) and fixation in formalin. C3 deposition in the joints (synovium and cartilage) was identified using polyclonal goat anti-mouse C3 antisera (ICN Pharmaceuticals), and joints were scored as previously described (9). fH localization was examined with a polyclonal goat antiserum specific for human fH that is cross-reactive with mouse fH (Quidel), as previously described (10). Knee joints from fH−/− with no disease were used a negative controls to establish the baseline.
Alternative pathway hemolytic assay
Lysis of sheep erythrocytes (ES) was measured by mixing, on ice, gelatin veronal buffer (5 mM veronal, 145 mM NaCl, 0.02% NaN3 [pH 7.3], 0.1% gelatin), normal mouse serum (40% final; Rockland Immunochemicals), and 0.1 M MgEGTA (5 mM final concentration) in the presence of buffer alone, 0–259 μg/ml murine rfH19-20 (0–18 μM), or 0–389 μg/ml human rfH3-5 (0–18 μM). Cells (5 × 106) were added and the mixture (24 μl total) was immediately transferred to a 37°C water bath and incubated for 20 min. To determine the extent of hemolysis, 200 μl cold gelatin veronal buffer containing 10 mM EDTA was added, the samples were centrifuged, and the OD of 200 μl supernatant was determined at 414 nm. The percentage lysis was determined by subtracting the A414 in the absence of serum, and dividing by the maximum possible A414 determined by water lysis of the erythrocytes.
In vitro effect of rfH19-20 on the level of C3 deposition on cartilage microparticles
Articular cartilage from 16-wk-old C57BL/6 WT mice (n = 5) and C3−/− (n = 3) mice without CAIA was obtained by surgically removing the knee joints. Skin, muscle, fat, tendon, extra tissue, and synovium were carefully removed. Articular cartilage located on the surface of tibia and tarsus bone was scrapped using a sterile sharp surgical razor blade. Cartilage fragments from each mouse (right and left knee joints) were pooled. Cartilage pieces were washed four times in 1× PBS (Ca2+/Mg2+ deficient and containing 0.05 M EDTA), dried at room temperature, and stored at −70°C prior to use. Following thawing, cartilage fragments were homogenized thoroughly into microparticles using a Polytron homogenizer. Cartilage microparticles were resuspended in 300 μl 1× PBS. These cartilage microparticles did not completely dissolve in solution and made a turbid suspension because of their size.
To examine competitive inhibition of fH by rfH19-20 on C3 deposition on the surface of cartilage microparticles by ELISA, a 50-μl suspension was used. Sera from fH−/− (n = 5), fH+/− (n = 5), and fH+/+ (n = 5) were used as a source for C3 and fH. Sera (50 μl) from fH−/−, fH+/−, and fH+/+ mice were incubated for 30 min at 4°C with 450 μg/ml rfH19-20 or 450 μg/ml rfH3-5. After incubation, pretreated sera were mixed with cartilage microparticles for 1 h at 37°C, followed by washing four times with buffer (1× PBS, 0.5% Tween 20). To measure C3 deposition, cartilage microparticles were incubated in Eppendorf tubes for 1 h at 37°C with HRP-conjugated goat anti-C3 antiserum (Cappel) diluted 1:4000 in freshly prepared PBS/0.05% Tween 20. After washing five times with wash buffer, cartilage microparticles were resuspended in 150 μl tetramethylbenzidine substrate solution A (BD Biosciences), followed by transferring 50 μl into preblocked (1× PBS, 1% BSA, 0.05% Tween 20) 96-well Costar ELISA plates. Fifty microliters tetramethylbenzidine substrate solution B (BD Biosciences) was added for 3 min to each well to develop the color reaction. The reaction was stopped using 50 μl 2 N H2SO4. Absorbance was determined as mentioned previously (35). Data were expressed using the following formula: mean OD with cartilage microparticles treated with rf19-20/serum from WT mice minus OD without rfH19-20/without serum. Cartilage microparticles, prepared identically in parallel from C3−/− mice and treated with autologous sera from C3−/− mice, were also used as additional negative controls. Minimal background (<0.4 OD units) was observed with the control cartilage microparticles alone from WT or C3−/− mice incubated with Ca2+/Mg2+-deficient buffer in the absence of sera. This background was subtracted from experimental OD values obtained with sera. The data are expressed as mean OD ± SEM.
Effect of rfH19-20 on C3 deposition assessed on UV light–exposed fibroblast-like synoviocytes
To evaluate the competitive inhibition of fH by rfH19-20 or rfH3-5 on C3 deposition on the surface of stressed and injured cells, fibroblast-like synoviocytes (FLS) were used. The FLS line used in this study was derived and established from the synovium of mice with collagen-induced arthritis (CIA) (a gift from Dr. Gary S. Firestein, University of California San Diego). FLS were cultured in DMEM media containing high glucose (Sigma-Aldrich), 10% FBS (Atlas Biologicals), 1% penicillin-streptomycin (Life Technologies), 0.5% gentamicin (Life Technologies), and 1% glutamine (Life Technologies). To induce injury, FLS were cultured in a serum-free media for 24 h, which was followed by an exposure to UV light (312 nm) (Fotodyne) for 18 h (36, 37). After washing twice with 1× PBS, cells (∼2 × 105 cells/tube) were mixed and incubated for 1 h on ice with sera as a source of C3 and fH from fH−/− (n = 5), fH+/− (n = 5), and fH+/+ (n = 5) mice. Sera were pretreated with and without 450 μg/ml rfH19-20 or rfH3-5 as described above. C3 deposition on UV-exposed FLS was examined by using flow cytometry. The cells were washed twice and stained for 30 min on ice with FITC-conjugated anti-mouse C3 mAb (IgG2) (Cedarlane Laboratories). A matched isotype rat IgG2a FITC-conjugated (Cedarlane Laboratories) and FITC-conjugated anti-mouse C3 Abs were used without treatment with rfH19-20 or rfH3-5 to determine levels of nonspecific binding. Following three washes, FLS were analyzed for bound C3 deposition by flow cytometry (Beckman Coulter). Dead cells and cell debris were electronically gated out. Five different experiments were performed, and data shown are expressed as mean channel fluorescence of 10,000 cells, where increased fluorescence indicates increased C3 deposited on the surface of injured FLS.
Effect of rfH19-20 on immune complex–mediated C3b deposition and C5 activation in vitro
The effect of rfH19-20 on C3b deposition and C5a generation induced by adherent mAb to CII in vitro was examined by ELISA using sera from WT, C4−/−, and C3−/− mice. Preparation of the culture plates with adherent anti-CII mAb was carried out as previously described (9). Serum samples were serially diluted in a Ca2+-deficient buffer so that only the AP would be active. Ca2+-deficient buffer contains 10 mM EGTA and 5 mM MgCl2. EGTA at 10 mM inhibits classical pathway activation with no effect on the AP. Sera with or without rfH19-20 (0, 5, 10, or 20 μg/10 μl serum) were preincubated for 30 min at 4°C and then added to the wells for 1 h at 37°C. Following washes in PBS/0.5% Tween 20, HRP-conjugated goat anti-mouse C3 antiserum (MP Biomedicals) diluted 1:4000 in freshly prepared PBS/0.05% Tween 20 was added to the wells. Plates were incubated at room temperature for another 1 h. After six more washes, the color reaction and absorbance were determined as previously described (9). Data were expressed using the following formula: mean OD with adherent mAb to CII with serum minus OD without serum. Minimal background was observed with the controls of anti-CII mAb alone incubated with Ca2+-deficient buffer in the absence of sera. The levels of C5a in the supernatants from the above experiments, as well as from in vivo studies with sera obtained at days 0, 3, and 10, were measured using a specific C5a ELISA (BD Biosciences) (13).
Serum levels of C3 and fH
The absolute serum levels of C3 in fH−/− mice were measured by ELISA, as previously described (9). In these studies, a serum from WT mice was used as a positive control and a serum from C3−/− mice was used as a negative control. The absolute serum levels of fH in fH+/− mice were also measured using a commercial kit designated TSZ ELISA (Biotang, Framingham, MA) with minor modifications to minimize background. Sera from WT and fH−/− mice were used as positive and negative controls, respectively, in ELISA and in Western blot analyses.
Statistical analyses
All mice were included in the final analysis of arthritis incidence, disease severity, and histologic scoring. An unpaired two-sample t test was used to analyze these data. All data were expressed as the mean ± SEM, with p < 0.05 considered significant.
Results
C3 deposition on the cartilage increases in early CAIA
We have previously demonstrated the presence of high levels of fixed C3 fragments and fH in the joint at the 10 d end point in the CAIA model (10). To evaluate the early levels of locally deposited C3 and the major AP inhibitor fH early in the evolution of CAIA, a histologic examination of the synovium and cartilage was performed through 144 h after injection of anti-CII mAb. The results showed low baseline fH levels at all time points through 96 h, with slight but nonsignificant late increases in synovium and cartilage at 120 and 144 h, compared with 1 h (Fig. 1). In contrast, C3 deposition on the cartilage showed a large increase over baseline levels beginning at 8 h after injection of the mAb to CII (p < 0.001 at 72, 96, 120, and 144 h versus 1 h) (Fig. 1).
FIGURE 1.
Scoring for inflammation and cartilage damage, as well as staining for C3 deposition and fH in knee joints of mice with CAIA. Histopathologic scoring for inflammation (black filled circle) and cartilage damage (black open circle) from the knee joints (right and left) was performed following tissue processing and toluidine blue staining of sections. C3 deposition in knee joints in the synovium (red filled circle) and on the surface of cartilage (red open circle) is illustrated, as is fH deposition in the synovium (blue filled circle) and on the surface of cartilage (blue open circle). The data are expressed as mean of disease/baseline ± SEM (n = 3/time point). Baseline shows background levels of inflammation, cartilage damage, and C3 and fH deposition in the knee joints of WT mice without treatment with mAb to CII (n = 3).
These results suggested an early imbalance between the levels of complement activation as assessed by C3 fragment deposition, which increased greatly, compared with deposition of the regulatory protein fH, which lagged substantially behind and did not achieve a similar early increase. We have previously shown that there is an increase in deposition of fH locally in the knee joints of WT mice at day 10 with CAIA, 7 d after the LPS injection (10). This finding is especially relevant because there are no endogenous complement AP inhibitors normally present on the cartilage, other than fluid phase–derived fH that could bind to that site. Because of these observations, we sought to further define the role of endogenous fH in the evolution of CAIA.
Inhibition of endogenous fH by rfH19-20 enhances CAIA
To examine the effects of blocking fH function in mice injected with anti-CII mAb, we used the murine rfH19-20 protein that, similar to its human counterpart (31), is able to block the surface binding of mouse fH and thus increase AP-dependent activity (Supplemental Fig. 1). As expected, incubation of ES in the presence of 40% normal mouse serum and MgEGTA resulted in no lysis of ES due to the inhibitory effects of endogenous fH. However, in the presence of increasing amounts of added murine or human rfH19-20, lysis of ES was observed due to blocking binding of endogenous fH to the ES. Lysis of ES seen with murine rH19-20 showed an IC50 of 6.2 μM (89 μg/ml). The control protein (rfH3-5) from a region of fH demonstrating no known binding interactions exhibited no permissive effect on lysis of the ES at any of the concentrations tested.
To examine the potential effects of fH blockade in vivo in CAIA, we first had to determine a dose of anti-CII mAb that would provide less than maximal injury. After evaluating dose-dependent injury, as outlined in Materials and Methods, we found that a dose of 0.5 mg anti-CII mAb/mouse injected i.p. was sufficient to induce measurable injury but still allow the detection of increases in disease severity with blockade of endogenous fH.
We observed that mice injected with 0.5 mg/mouse anti-CII mAb exhibited significant increases in DAS after injection of rfH19-20 on day 1 after the mAb and on day 3 after LPS. Injection of 300 μg rfH-19-20 led to significant increases in DAS on days 5–10 after injection of the mAb to CII as compared with mice treated with PBS alone or 300 μg control protein rfH3-5 (Fig. 2A). The average DAS on day 10 were 3.6 ± 0.40, 3.2 ± 0.37, and 9.4 ± 1.4 in mice treated with PBS only, rfH3-5, and rfH19-20, respectively. Mice treated with 300 μg rfH19-20 showed 2.6- and 2.9-fold increases in the DAS on day 10 in comparison with the mice treated with PBS only or with rfH3-5 (p < 0.004 and p < 0.0026, respectively). At day 10, the prevalence was 100% in all three treatment groups (Fig. 2B). Furthermore, no significant differences in the increased DAS were observed between groups treated with a total of 100 or 300 μg rfH19-20, suggesting that we had achieved the maximal effect of endogenous fH blockade at these two doses (data not shown).
FIGURE 2.
Amplifying effect of rfH19-20 on arthritis induced by anti-CII mAb. The data shown are derived from the indicated days after the mAb injection. (A) Clinical DAS over the duration of the experiment. (B) Prevalence of arthritis (%) over the duration of the experiment. The data represent the mean ± SEM based on n = 5 for each group. *p < 0.05 in comparison with PBS treatment or rfH3-5 treatment.
Blockade of endogenous fH by rfH19-20 enhances histological changes and C3 deposition in the joint in CAIA
Histopathologic analyses on joints from mice treated with PBS or with 300 μg rfH19-20 or rfH3-5 showed changes consistent with the DAS. A significant increase (p < 0.05) was seen in the total histopathology score as well as in the individual scores for inflammation, pannus, cartilage damage, and bone damage in mice treated with a 300 μg rfH19-20 in comparison with mice treated with PBS or rfH3-5 (Fig. 3A). The levels of C3 deposition in the synovium as well as in the cartilage were also significantly increased in the mice treated with rfH19-20 in comparison with treatment with PBS or rfH3-5 (p < 0.05) (Fig. 3B). Representative tissue sections of histology and C3 deposition in knee joints are shown in Fig. 4.
FIGURE 3.
Increased histopathologic injury scores and elevated C3 deposition in the joints of WT mice injected i.p. with 0.5 mg/mouse anti-CII mAb on day 0 followed by LPS on day 3 and treated with either PBS, 300 μg rfH19-20, or 300 μg rfH3-5. At day 10 following anti-CII mAb injection, mice were sacrificed. Histopathologic scoring for inflammation, pannus formation, and cartilage and bone damage from five joints, including forelimbs (right and left) and one hind limb (right knee, ankle, and paw), was performed following tissue processing and toluidine blue staining of sections. (A) Individual component histopathologic scores from WT mice treated with PBS, 300 μg/mouse rfH19-20, or 300 μg/mouse rfH3-5. (B) C3 deposition in knee joints in the synovium, on the surface of cartilage, and total scores (synovium plus cartilage) from the same mice. All data are represented as scores (mean ± SEM) based on n = 5 in each group. *p < 0.05 in comparison with WT mice for individual histopathologic injury and C3 deposition scores.
FIGURE 4.
Representative histopathology and C3 deposition images from the knee joints of WT mice injected i.p. with 0.5 mg/mouse anti-CII mAb and treated with PBS, 300 μg/mouse rfH19-20, or 300 μg/mouse rfH3-5. The top three panels from left to right (A–C) show staining with toluidine blue (blue color) from the knee joints of WT mice treated with PBS (left panel), 300 μg rfH19-20/mouse (center panel), and 300 μg rfH3-5/mouse (right panel). Areas of synovium (S; black arrow), cartilage (C; yellow arrow), and meniscus (M; yellow arrow) are identified. The bottom three panels from left to right (D–F) show staining with anti-C3 Ab (brown color) from the knee joints of WT mice treated with PBS (left panel), 300 μg rfH19-20/mouse (center panel), and 300 μg rfH3-5/mouse (right panel). Areas of synovium (S), cartilage (C), and meniscus (M) are identified with black arrows. Original magnification for all images shown is ×20. Scale bars, 0.1 mm (100 μm).
The absolute serum levels of C5a were also measured before (day 0), during (day 3), and after the induction of CAIA (day 10) in mice using 0.5 mg anti-CII mAb and treated with PBS, 300 μg rfH19-20, or 300 μg rfH3-5. No differences were found between all three groups, suggesting that the disease-enhancing effects of rfH19-20 were manifest locally within the joint and, as expected by the mechanism of action, rfH19-20 did not lead to systemic complement activation (data not shown).
We also studied the enhancing effects of local fH inhibition using a higher dose (2 mg/mouse) of anti-CII mAb to induce CAIA. This approach showed an increase in DAS only after administration of 300 μg/mouse but not 100 μg/mouse rfH19-20 (Supplemental Fig. 2). Histopathology and C3 deposition scores were not changed by treatment with either dose of rfH19-20 (Supplemental Fig. 3). This result confirmed the dose-dependency of the in vivo effects of manipulating AP activation and also illustrated that one can increase the levels of local anti-CII mAb deposition and complement activation, which then exceeds the threshold under which endogenous fH can be readily demonstrated to play an enhancing role.
CAIA is markedly decreased in fH−/− mice
We also assessed the role of fH in CAIA by using mice that had been gene targeted to eliminate expression of this protein (29). Despite the nearly complete absence of circulating C3 in the baseline state in fH−/− mice, previous studies had demonstrated an increase in renal injury and dysfunction following treatment with Abs to glomerular basement membrane; this result was interpreted as indicative of an effect of local C3 production on injury in the kidneys (29). In contrast to those results, we observed that CAIA was markedly reduced in fH−/− mice with a significant decrease in the DAS between WT and fH−/− mice at days 5–10 (Fig. 5A). The DAS in WT and fH−/− and mice at day 10 were 10.6 ± 0.93 and 2.2 ± 0.49, respectively (mean ± SEM). The prevalence of disease in both WT and fH−/− mice was 100% at day 10 (Fig. 5B). The histopathologic injury scores and C3 deposition scores were also markedly decreased in the fH−/−mice in comparison with WT mice (Fig. 6).
FIGURE 5.
DAS and prevalence in WT and fH−/− mice with CAIA induced by 8 mg/mouse anti-CII mAb injected i.p. on day 0 followed by an i.p. injection of LPS on day 3. Mice were evaluated daily by an observer blinded to the genotype of each mouse. The x-axis illustrates the day after mAb injection. (A) Clinical DAS of WT and fH−/− mice. (B) Prevalence of disease in WT and fH−/− mice. Data represent the mean ± SEM based on n = 5 in each group. *p < 0.05 for DAS in fH−/− in comparison with WT mice from days 5–10.
FIGURE 6.
Histopathologic injury scores and C3 deposition in the joints of WT and fH−/− mice injected i.p. with 8 mg/mouse anti-CII mAb. At day 10 following injection, mice were sacrificed and histopathologic scoring for inflammation, pannus formation, and cartilage and bone damage was performed. (A) Individual component histopathology scores from WT (n = 5) and fH−/− (n = 4) mice. (B) C3 deposition in knee joints in the synovium, on the surface of cartilage, and total scores (synovium plus cartilage) from WT and fH−/− mice. All data are represented as scores (mean ± SEM). *p < 0.05 in comparison with WT mice of individual histopathology and C3 deposition scores.
To further explore the mechanism underlying the lack of development of CAIA in fH−/− mice, levels of serum C3 were determined in WT and fH−/− mice at days 0, 3, and 10 (before, during, and after CAIA, respectively). C3 was nearly absent in sera from fH−/− mice in comparison with sera from WT mice at all time points; the reduction in the level of C3 in the sera of fH−/− mice versus WT mice was 92, 95, and 94% at days 0, 3 and 10, respectively (data not shown). These data confirm that fH−/− mice do not maintain a sustained level of C3 in their circulation, which likely explains the marked reduction rather than enhancement in CAIA.
CAIA is not decreased in fH+/− mice but is enhanced by rfH19-20
To evaluate the effect of decreased but not absent circulating fH levels on injury induction, CAIA was examined in fH+/− mice. To first confirm the expected levels of C3 and fH levels in heterozygous deficient mice, we examined serum levels of these proteins. There was no significant difference in the absolute serum level of C3 between WT (n = 5) and fH+/− (n = 6) mice, with OD values of 2.16 ± 0.04 and 1.94 ± 0.11 U (mean ± SEM) (p = 0.106), respectively. In contrast, the absolute serum levels of fH were 30% reduced in the sera from 8-wk-old fH+/− mice compared with WT mice (data not shown). Western blot analyses confirmed the presence of a 155-kDa band of fH in the serum from fH+/− mice identical in size to the band of fH present in the serum from WT mice; this band was absent in the serum from fH−/− mice (data not shown).
CAIA performed with a submaximal dose of anti-CII mAb (0.5 mg/mouse) showed no significant differences in DAS between fH+/− and WT mice at all time points (Fig. 7A). The DAS in fH+/− and WT mice at day 10 were 2.0 ± 0 (n = 4) and 1.5 ± 0.133 (n = 4), respectively (mean ± SEM). The prevalence of disease in all mice was 100% at day 10 (Fig. 7B). CAIA was also examined in fH+/− and WT mice by using a high dose of anti-CII mAb (8 mg/mouse anti-CII mAb). There were again no significant differences in DAS between fH+/− (n = 5) and WT (n = 5) mice at all time points (Fig. 7C). The prevalence of disease in both fH+/− and WT mice was 100% at day 10 (Fig. 7D). Thus, in contrast to fH−/− mice, fH+/− mice were not protected from CAIA even using a high dose of anti-CII mAb.
FIGURE 7.
CAIA in fH+/− mice treated with rfH19-20. CAIA was induced in WT and fH+/− mice with two doses of anti-CII mAb, 0.5 or 8 mg/mouse injected i.p. on day 0 followed by an i.p. injection of LPS on day 3. The data are expressed versus days after mAb injection. (A) DAS scores with 0.5 mg/mouse anti-CII mAb (mean ± SEM with n = 4 for each group). (B) Prevalence of disease (%) with 0.5 mg/mouse. (C) DAS scores with treatment with 8 mg/mouse anti-CII mAb (mean ± SEM with n = 5 for each group). (D) Prevalence of disease (%) with 8 mg/mouse anti-CII mAb. (E) DAS scores with CAIA with 0.5 mg/mouse anti-CII mAb. fH+/− mice were treated with PBS or rfH19-20 (total of 300 μg/mouse) at day 0 and at day 3 (mean ± SEM with n = 4 for each group). (F) Prevalence of disease (%) in fH+/− mice treated with PBS or rfH19-20 (300 μg/mouse).
These results suggested that the levels of circulating fH were not the primary determinants of the severity of CAIA that develops. To further evaluate the effects of local blockade of fH function in the setting of decreased circulating levels, we examined the effect of rfH19-20 (300 μg/mouse) on CAIA in fH+/− mice using a submaximal dose of anti-CII mAb (0.5 mg/mouse). There was a significant increase at all time points in DAS in fH+/− mice treated with rfH19-20 (Fig. 7E), as was also seen with WT mice (Fig. 2A). The DAS in fH+/− mice treated with PBS and fH+/− mice treated with rfH19-20 mice at day 10 were 1.75 ± 0.25 and 5.5 ± 0.645 (mean ± SEM), respectively. The prevalence of disease in both fH+/− mice treated with PBS and fH+/− mice treated with rfH19-20 was 100% at day 10 (Fig. 7F). There were no significant differences in serum C3 levels before (day 0), during (day 3), and after (day 10) the development of CAIA in WT and fH+/− mice (data not shown). In summary, fH+/− mice, despite demonstrating no inherent differences in the development of CAIA, became more susceptible to CAIA when treated with rfH19-20 as an inhibitor of local target surface fH regulatory effects.
rfH19-20 enhances C3 deposition on cartilage microparticles in vitro
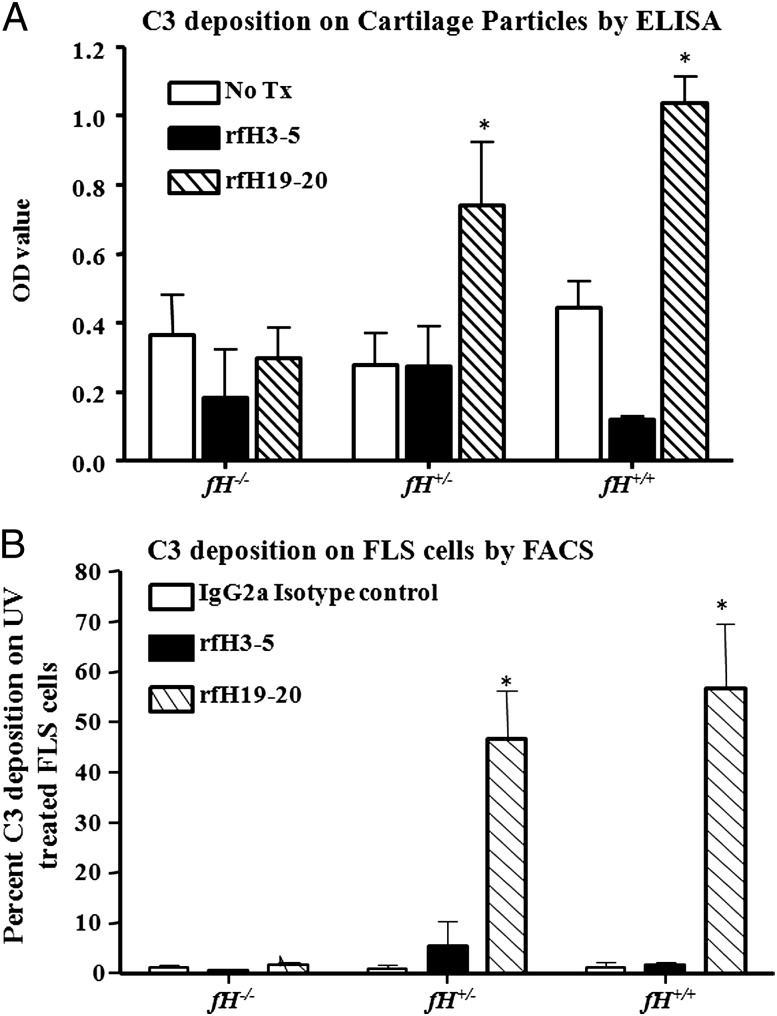
As an in vitro model of the joint in CAIA, we examined the effects of rfH19-20 on complement deposition on cartilage. A significant increase in C3 deposition was observed on cartilage microparticles from WT mice pretreated with rfH19-20, using sera from either fH+/− or fH+/+ mice as sources of fH and C3 (Fig. 8A). Consistent with the in vivo phenotypes, the C3 deposition OD values were 0.30 ± 0.087, 0.87 ± 0.16, and 1.03 ± 0.074 on cartilage microparticles treated with sera from fH−/−, fH+/−, and fH+/+ mice, respectively, containing rfH19-20. This increase in C3 deposition was ∼3-fold and was significant (p < 0.05) on the cartilage microparticles treated with sera from fH+/− and fH+/+ mice containing rfH19-20 as compared with cartilage microparticles treated with sera from fH−/− mice containing rfH19-20 (Fig. 8A). No significant increase in C3 deposition was seen on the surface of cartilage microparticles treated with sera from fH−/−, fH+/−, and fH+/+containing the control rfH3-5. A minimum level of C3 deposition was observed on the surface of cartilage microparticles from C3−/− mice treated with autologous sera (data not shown). This background was equivalent to C3 deposition on WT cartilage microparticles without any treatment. In summary, these data demonstrate that exogenous rfH19-20 can compete with endogenous serum fH for in vitro binding to the cartilage surface.
FIGURE 8.
Competitive inhibition of endogenous fH with rfH19-20 increased C3 deposition on the surface of cartilage microparticles from WT mice and also on FLS derived from mice with CIA. (A) No treatment of cartilage microparticles (empty square), cartilage microparticles treated with rfH3-5 plus sera from fH−/−, fH+/−, and fH+/+ (solid filled square), and cartilage microparticles treated with rfH19-20 plus sera from fH−/−, fH+/−, and fH+/+ mice (hatched line square). Cartilage microparticles from C3−/− mice treated with C3−/− sera were used as a negative control (data not shown). All data related to the C3 deposition on cartilage microparticle from WT mice are represented as OD value (mean ± SEM) based on n = 7 knee joints from five WT mice. *p < 0.05 for cartilage microparticles treated with fH+/− sera plus rfH19-20 versus cartilage microparticles treated with fH+/− sera only, and also for cartilage microparticles treated with fH+/+ sera plus rfH19-20 versus cartilage microparticles treated with fH+/+ sera only. (B) C3 deposition on the UV-exposed FLS was examined by flow cytometry. Rat IgG2a isotype was used as a control for nonspecific binding (open square), FLS treated with the rfH3-5, a nonspecific control protein (black filled square), and FLS treated with rfH19-20 (hatched line square). Five independent flow cytometry experiments were performed, and the data are shown as percentage (mean ± SEM) C3 deposition. *p < 0.05 for FLS treated with fH+/− sera plus rfH19-20 versus FLS cells treated with fH+/− sera plus rfH3-5, and also for FLS treated with fH+/+ sera plus rfH19-20 versus FLS treated with fH+/+ sera plus rfH3-5.
rfH19-20 enhances C3 deposition on stressed FLS in vitro
To examine the effect of rfH19-20 on C3 deposition to an injured cell surface, FLS were used as an ex vivo surrogate of synovial cells. Little early C3 deposition on the synovium in CAIA was observed in Fig. 1 as fibroblasts express complement inhibitory proteins on the cell surface (35). Thus, to model later time points we used serum-starved and UV-treated FLS. Cell cycle analysis using propidium iodide 18 h after serum starvation and UV treatment of FLS confirmed that the cells were stressed, as there was a 20% decrease in S phase and a 11% decrease in G2M phase compared with nontreated FLS. Damaged FLS pretreated with sera from either WT or fH+/− mice (as sources of fH and C3) exhibited 34- (WT) and 28-fold (fH+/−) increased levels of fixed C3 on their surface in the presence of rfH19-20 in comparison with FLS treated with either serum containing rfH3-5 (Fig. 8B). There was also little C3 deposition on damaged FLS pretreated with sera from fH−/− mice in the presence of rfH19-20 or rfH3-5. Furthermore, normal untreated FLS failed to exhibit any C3b deposition in the presence of sera from WT, fH−/−, or fH+/− mice (data not shown). These results indicate that exogenous rfH19-20 competes with endogenous fH for binding to the surface of injured FLS in vitro and promotes complement deposition in a similar fashion as observed on the synovium in vivo.
rfH19-20 does not increase C3 deposition or C5a generation in vitro induced by adherent anti-CII mAb
To directly assess whether rfH19-20 was able to control the level of complement activation induced by anti-CII mAb, the in vitro effect of rfH19-20 on C3 deposition and C5a generation induced by anti-CII mAb adherent to wells in ELISA plates was determined using sera from WT, C4−/− (AP only), and C3−/− mice without CAIA. rfH19-20 (5 μg/10 μl serum) or CR2-fH (2 μg/10 μl serum) was used as inhibitors of AP activation (34), with serial dilutions of sera (1:10 through 1:320) in Ca2+-sufficient buffer (SB) or Ca2+-deficient buffer (DB). The levels of C3 deposition and C5a generation measured with SB reflect all three complement activation pathways, whereas the levels with DB reflect activation only of the AP. The same levels of C3 deposition were observed with all dilutions of sera from WT and C4−/− mice in SB or DB in the absence or presence of rfH19-20 (Supplemental Fig. 4A–C). Similarly, there were no differences in C5a generation measured in the supernatants using sera diluted in SB or DB in the absence or presence of rfH19-20 (Supplemental Fig. 4D–F). As expected, sera from C3−/− mice did not induce any detectable C3 fragment deposition or C5a generation. CR2-fH was used as a positive control for inhibition of the AP and, as expected, demonstrated a nearly complete specific inhibition. These results indicate that rfH19-20 does not enhance complement activation induced by immune complexes in vitro probably because fH does not bind to IgG and C3 fragments alone but requires binding with polyanions, which are absent on the surface of ELISA plate wells.
Discussion
The major observation of these studies is that endogenous fH is capable of inhibiting activation of the AP of complement on cartilage and synovium in joints in vivo exposed to a submaximal level of anti-CII mAb. This conclusion was derived from experiments in CAIA, a locally initiated immune complex model of joint disease, treated with rfH19-20 to prevent engagement of full-length endogenous fH. Thus, we show that endogenous fH provides an important level of protection from joint damage in immune complex arthritis. A second observation is that this form of immune complex–induced arthritis may begin in the cartilage with initial Ab deposition and complement activation, then secondarily involve the synovium after injury to synoviocytes.
In this study we report that competitive blockade by murine rfH19-20 of the binding of endogenous fluid phase fH to the cartilage and injured FLS surface significantly increased CAIA in WT mice. Further support for the conclusion that fH plays a key role in regulating AP-induced complement deposition on cartilage and cell surfaces in the joint is derived from studies with fH+/− heterozygous-deficient mice. These mice exhibit lower circulating levels of fH but are not more susceptible to CAIA unless they are treated with rfH19-20 to disrupt tissue binding of endogenous fH. rfH19-20 impairs only surface control of the AP by fH and does not influence the systemic activation of the complement system as indicated by unchanged serum levels of C5a. Thus, fH controls AP activation on cartilage and injured FLS in vivo in a manner dependent on the fH SCR19-20 domain, indicating that the AP can be regulated on these joint surfaces.
These findings are very likely to be relevant to the initiation of arthritis in humans (38). In man, circulating autoantibodies, including anti-CII Abs, are present for several years prior to the onset of clinically apparent arthritis (39). Substantial evidence suggests that in RA joint-based inflammation is initiated through Ag/Ab complexes that are present on the cartilage surface (40). The observation that only injured FLS, but not normal FLS expressing complement regulatory proteins, could exhibit C3 binding suggests that cartilage damage may precede injury to the synovium. Initial complement activation by solid phase immune complexes in the cartilage may lead to secondary damage to the FLS and thus to subsequent development of synovitis.
The in vitro models that we have used allow us to evaluate three stages in the evolution of inflammation in CAIA. The first stage is complement activation by solid phase immune complexes represented by anti-CII mAb bound to a surface (10). However, rfH19-20 exhibits no effect in this assay probably secondary to the absence of polyanions on the IgG mAb or surface of the well for engagement of fH from serum. The second stage is amplification of complement activation on cartilage. This stage is controlled to a high degree by fH as demonstrated using cartilage microparticles in vitro where the addition of rfH19-20 greatly increases complement activation as measured by C3 deposition. This conclusion is also consistent with the in vivo observation of initial C3 deposition on the cartilage, after injection of anti-collagen mAb, in the absence of fH deposition (Fig. 1). The third stage is FLS injury where complement activation is likely to be most relevant to the latter stages of injury in CAIA. Synovial cells are now stressed and susceptible to complement-induced injury, further increasing the requirement for fH control of AP activation. Notably, this same role for fH in regulation of inflammation is observed in ischemic injury in renal tubular epithelial cells (41) and in oxidative stress in retinal pigmented epithelial cells (42)
fH is increasingly recognized as a centrally important protein in the regulation and control of AP activation on multiple tissue sites. The normal concentration of fH in plasma ranges from 233 to 269 μg/ml in young and old individuals, respectively (43), although other studies suggest that the concentration of fH in plasma is 2-fold higher (44, 45). The primary synthesis of fH occurs in the liver (16); however, other cell types can produce fH, including monocytes (46), endothelial cells (47), keratinocytes (48), and synovial cells (49). We also found that mice completely deficient in fH developed no arthritis and that these mice were resistant to CAIA, which is in contrast to the membranoproliferative glomerulonephritis type II in fH−/− mice and accelerated injury following the injection of subnephritogenic doses of antisera (29). It is not clear why this apparent paradox exists. The serum levels of C3, however, are markedly low in these deficient mice, and it is known that the primary source of pathogenic C3 activation for inflammatory joint disease is from the circulation (50). Our results are consistent with that conclusion.
The recombinant form of fH SCR 19 and 20 that we have used, rfH19-20, is a very useful tool with which to evaluate the role of surface-bound fH (27, 31). This molecule exhibits no effect on fluid phase regulation of C3 (31), but only acts where fH is dependent on surface-bound C3b/C3d fragments and tissue polyanions to exert its local regulatory effect. This protein has been used previously to block fH function both in vitro (23, 31) and in vivo (33, 41). Importantly, recent structure/function studies have provided us with a substantial understanding of the mechanistic underpinnings of this domain both in the context of its interactions with ligands (21, 22) as well as its effects on full-length fH binding (27). Our data show that rfH19-20 can enhance C3 deposition to cartilage particles and to UV-treated FLS in vitro. However, whether rfH19-20 can bind to inflamed tissues in mice in vivo, such as synovium, is not known. There are other sites on fH besides SCR 19 and 20 that can bind to glycosaminoglycan (GAG)-specific tissues. It has been determined that mouse rH19-20 is readily found in ischemic-injured renal tubules, but not in normal tubules, of mice treated with 50 μg of the protein (41).
There are some potential limitations to the interpretation of our results. First, we were able to overcome the blocking effect of rfH19-20 by increasing the dose of anti-CII mAb to a level that resulted in greater clinical disease activity. As the AP amplification loop is characterized by a threshold effect, it appears that higher doses of anti-CII mAb exceeded that threshold where endogenous fH could exert its inhibitory capacity. Second, we have not directly evaluated the role of the membrane complement regulatory proteins Crry, DAF, and CD59 either in vivo or in the control of AP activation on FLS in vitro. Recent studies demonstrated an increase in inflammation and injury in the Ag-induced arthritis model in the absence of CD59, consistent with a proinflammatory effect of MAC formation (51). Additional studies will be necessary to understand the specific roles of the membrane-bound regulatory proteins in CAIA. Third, CAIA is considered a model of effector function in RA. To fully understand the roles of fH in the evolution of autoimmunity and AP activation, evaluation of the CIA active immunization model will be necessary. Fourth, in our studies we were not able to examine the relative targeting to and levels of rfH19-20 within the joint as there are no immunochemical reagents yet available that discriminate between rfH19-20 and endogenous fH. Fifth, we cannot rule out the possibility that other GAG-binding regions of fH, such as 5–7, which have not been evaluated in this study, may serve as inhibitors of full-length fH function on synovium or cartilage based on the specific nature of the GAGs in these tissues. However, because rfH19-20 has been used successfully as an inhibitor in other models, and is the only region of fH that effectively binds to both C3b/d and GAGs, it is likely that it is the most efficient inhibitor of fH (33, 41). Furthermore, it has been shown that rfH19-20 competes very efficiently with full-length fH for binding to C3b/d and GAGs on cell surfaces. When domains 19 and 20 are not present in the full-length protein (or are functionally mutated), fH requires between 5- and 50-fold more protein to bind to C3b-coated cells such as human and sheep erythrocytes, and it requires ∼12-fold more protein to carry out cell surface–related functions (such as decay acceleration) (52). Therefore, although fH can still bind to cell surfaces without having the C-terminal domains, it is very inefficient and no longer protects effectively. Such is the case of individuals that have mutations in the C terminus of fH who lose the ability to bind to C3b/d and/or GAGs through their C terminus and develop inflammatory diseases. Finally, fH is one member of a larger family of fH-related proteins (CFHRs), whose in vivo roles are not well understood. However, CFHRs bind to C3b/C3d molecules on surfaces and may modulate fH functions in vivo (5, 53). Further evaluation of the tissue-specific roles of CFHR proteins both at the mRNA and protein levels is warranted to more fully understand the role of fH and other soluble AP regulatory proteins in CAIA and other diseases.
In conclusion, using a combination of in vivo models and informative in vitro analyses, we demonstrate that endogenous fH is essential to the control of complement activation and AP amplification in joints. Our studies also suggest that immune complex–induced joint disease begins in the cartilage and secondarily involves the synovium. An essential role for fH in human RA is consistent with previous studies demonstrating markedly elevated levels of AP activation fragments in the joints of patients with RA, even in the absence of similar increases in the circulation (54–56). Prior studies have demonstrated that targeting of fH regulatory function to the joint at sites of C3 fixation using a recombinant protein-based approach markedly ameliorates inflammation and damage in CAIA (34). Our results demonstrate that endogenous fH is a key regulator of inflammation in the joint and point toward the importance of continued efforts to therapeutically regulate AP activation at this site.
Acknowledgments
We thank Stephanie Hyatt and Brandt Levitt for assisting in various complement ELISA assays and for determining clinical disease scores in experimental mice.
This work was supported by National Institutes of Health Grant AR051749 (to V.M.H.).
The online version of this article contains supplemental material.
- AP
- alternative pathway
- CAIA
- collagen Ab-induced arthritis
- CIA
- collagen-induced arthritis
- CII
- type II collagen
- DAS
- disease activity score
- DB
- Ca2+-deficient buffer
- ES
- sheep erythrocyte
- fB
- factor B
- fD
- factor D
- fH
- factor H
- FLS
- fibroblast-like synoviocyte
- GAG
- glycosaminoglycan
- MAC
- membrane attack complex
- RA
- rheumatoid arthritis
- rfH19-20
- recombinant factor H SCR domains 19 and 20
- rfHSCR3-5
- recombinant factor H SCR domains 3–5
- SB
- Ca2+-sufficient buffer
- SCR
- short consensus repeat
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ji H., Ohmura K., Mahmood U., Lee D. M., Hofhuis F. M., Boackle S. A., Takahashi K., Holers V. M., Walport M., Gerard C., et al. 2002. Arthritis critically dependent on innate immune system players. Immunity 16: 157–168 [DOI] [PubMed] [Google Scholar]
- 2.Richards A., Kathryn Liszewski M., Kavanagh D., Fang C. J., Moulton E., Fremeaux-Bacchi V., Remuzzi G., Noris M., Goodship T. H., Atkinson J. P. 2007. Implications of the initial mutations in membrane cofactor protein (MCP; CD46) leading to atypical hemolytic uremic syndrome. Mol. Immunol. 44: 111–122 [DOI] [PubMed] [Google Scholar]
- 3.Zipfel P. F., Misselwitz J., Licht C., Skerka C. 2006. The role of defective complement control in hemolytic uremic syndrome. Semin. Thromb. Hemost. 32: 146–154 [DOI] [PubMed] [Google Scholar]
- 4.Schaumberg D. A., Hankinson S. E., Guo Q., Rimm E., Hunter D. J. 2007. A prospective study of 2 major age-related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Arch. Ophthalmol. 125: 55–62 [DOI] [PubMed] [Google Scholar]
- 5.Zipfel P. F., Heinen S., Józsi M., Skerka C. 2006. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol. Immunol. 43: 97–106 [DOI] [PubMed] [Google Scholar]
- 6.Valentijn R. M., van Overhagen H., Hazevoet H. M., Hermans J., Cats A., Daha M. R., van ES L. A. 1985. The value of complement and immune complex determinations in monitoring disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 28: 904–913 [DOI] [PubMed] [Google Scholar]
- 7.Chan R. K., Ibrahim S. I., Takahashi K., Kwon E., McCormack M., Ezekowitz A., Carroll M. C., Moore F. D., Jr., Austen W. G., Jr. 2006. The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia-reperfusion. J. Immunol. 177: 8080–8085 [DOI] [PubMed] [Google Scholar]
- 8.Rus H., Cudrici C., Niculescu F. 2005. C5b-9 complement complex in autoimmune demyelination and multiple sclerosis: dual role in neuroinflammation and neuroprotection. Ann. Med. 37: 97–104 [DOI] [PubMed] [Google Scholar]
- 9.Banda N. K., Takahashi K., Wood A. K., Holers V. M., Arend W. P. 2007. Pathogenic complement activation in collagen antibody-induced arthritis in mice requires amplification by the alternative pathway. J. Immunol. 179: 4101–4109 [DOI] [PubMed] [Google Scholar]
- 10.Banda N. K., Thurman J. M., Kraus D., Wood A., Carroll M. C., Arend W. P., Holers V. M. 2006. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J. Immunol. 177: 1904–1912 [DOI] [PubMed] [Google Scholar]
- 11.Hietala M. A., Jonsson I. M., Tarkowski A., Kleinau S., Pekna M. 2002. Complement deficiency ameliorates collagen-induced arthritis in mice. J. Immunol. 169: 454–459 [DOI] [PubMed] [Google Scholar]
- 12.Hietala M. A., Nandakumar K. S., Persson L., Fahlén S., Holmdahl R., Pekna M. 2004. Complement activation by both classical and alternative pathways is critical for the effector phase of arthritis. Eur. J. Immunol. 34: 1208–1216 [DOI] [PubMed] [Google Scholar]
- 13.Banda N. K., Hyatt S., Antonioli A. H., White J. T., Glogowska M., Takahashi K., Merkel T. J., Stahl G. L., Mueller-Ortiz S., Wetsel R., et al. 2012. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J. Immunol. 188: 1469–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hourcade D. E. 2006. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J. Biol. Chem. 281: 2128–2132 [DOI] [PubMed] [Google Scholar]
- 15.Dimitrova P., Ivanovska N., Belenska L., Milanova V., Schwaeble W., Stover C. 2012. Abrogated RANKL expression in properdin-deficient mice is associated with better outcome from collagen-antibody-induced arthritis. Arthritis Res. Ther. 14: R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopp A., Hebecker M., Svobodova E., Jozsi M. 2012. Factor H: a complement regulator in health and disease and a mediator of cellular interactions. Biomolecules 2: 46–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. 1977. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein β1H for cleavage of C3b and C4b in solution. J. Exp. Med. 146: 257–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. 1976. Control of the amplification convertase of complement by the plasma protein β1H. Proc. Natl. Acad. Sci. USA 73: 3268–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whaley K., Ruddy S. 1976. Modulation of the alternative complement pathways by β1H globulin. J. Exp. Med. 144: 1147–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friese M. A., Hellwage J., Jokiranta T. S., Meri S., Müller-Quernheim H. J., Peter H. H., Eibel H., Zipfel P. F. 2000. Different regulation of factor H and FHL-1/reconectin by inflammatory mediators and expression of the two proteins in rheumatoid arthritis (RA). Clin. Exp. Immunol. 121: 406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajander T., Lehtinen M. J., Hyvärinen S., Bhattacharjee A., Leung E., Isenman D. E., Meri S., Goldman A., Jokiranta T. S. 2011. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc. Natl. Acad. Sci. USA 108: 2897–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan H. P., Schmidt C. Q., Guariento M., Blaum B. S., Gillespie D., Herbert A. P., Kavanagh D., Mertens H. D., Svergun D. I., Johansson C. M., et al. 2011. Structural basis for engagement by complement factor H of C3b on a self surface. Nat. Struct. Mol. Biol. 18: 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira V. P., Herbert A. P., Cortés C., McKee K. A., Blaum B. S., Esswein S. T., Uhrín D., Barlow P. N., Pangburn M. K., Kavanagh D. 2009. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J. Immunol. 182: 7009–7018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehtinen M. J., Rops A. L., Isenman D. E., van der Vlag J., Jokiranta T. S. 2009. Mutations of factor H impair regulation of surface-bound C3b by three mechanisms in atypical hemolytic uremic syndrome. J. Biol. Chem. 284: 15650–15658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meri S. 2007. Loss of self-control in the complement system and innate autoreactivity. Ann. N. Y. Acad. Sci. 1109: 93–105 [DOI] [PubMed] [Google Scholar]
- 26.Ricklin D., Hajishengallis G., Yang K., Lambris J. D. 2010. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11: 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pangburn M. K. 2002. Cutting edge: localization of the host recognition functions of complement factor H at the carboxyl-terminal: implications for hemolytic uremic syndrome. J. Immunol. 169: 4702–4706 [DOI] [PubMed] [Google Scholar]
- 28.Ratnoff W. D., Fearon D. T., Austen K. F. 1983. The role of antibody in the activation of the alternative complement pathway. Springer Semin. Immunopathol. 6: 361–371 [DOI] [PubMed] [Google Scholar]
- 29.Pickering M. C., Cook H. T., Warren J., Bygrave A. E., Moss J., Walport M. J., Botto M. 2002. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 31: 424–428 [DOI] [PubMed] [Google Scholar]
- 30.Alexander J. J., Wang Y., Chang A., Jacob A., Minto A. W., Karmegam M., Haas M., Quigg R. J. 2007. Mouse podocyte complement factor H: the functional analog to human complement receptor 1. J. Am. Soc. Nephrol. 18: 1157–1166 [DOI] [PubMed] [Google Scholar]
- 31.Ferreira V. P., Herbert A. P., Hocking H. G., Barlow P. N., Pangburn M. K. 2006. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J. Immunol. 177: 6308–6316 [DOI] [PubMed] [Google Scholar]
- 32.Cheng Z. Z., Hellwage J., Seeberger H., Zipfel P. F., Meri S., Jokiranta T. S. 2006. Comparison of surface recognition and C3b binding properties of mouse and human complement factor H. Mol. Immunol. 43: 972–979 [DOI] [PubMed] [Google Scholar]
- 33.Takeda K., Thurman J. M., Tomlinson S., Okamoto M., Shiraishi Y., Ferreira V. P., Cortes C., Pangburn M. K., Holers V. M., Gelfand E. W. 2012. The critical role of complement alternative pathway regulator factor H in allergen-induced airway hyperresponsiveness and inflammation. J. Immunol. 188: 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banda N. K., Levitt B., Glogowska M. J., Thurman J. M., Takahashi K., Stahl G. L., Tomlinson S., Arend W. P., Holers V. M. 2009. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J. Immunol. 183: 5928–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guc D., Gulati P., Lemercier C., Lappin D., Birnie G. D., Whaley K. 1993. Expression of the components and regulatory proteins of the alternative complement pathway and the membrane attack complex in normal and diseased synovium. Rheumatol. Int. 13: 139–146 [DOI] [PubMed] [Google Scholar]
- 36.Clydesdale G. J., Dandie G. W., Muller H. K. 2001. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol. Cell Biol. 79: 547–568 [DOI] [PubMed] [Google Scholar]
- 37.Pickering M. C., Fischer S., Lewis M. R., Walport M. J., Botto M., Cook H. T. 2001. Ultraviolet-radiation-induced keratinocyte apoptosis in C1q-deficient mice. J. Invest. Dermatol. 117: 52–58 [DOI] [PubMed] [Google Scholar]
- 38.Arend W. P., Firestein G. S. 2012. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol 8: 573–586 [DOI] [PubMed] [Google Scholar]
- 39.Mullazehi M., Wick M. C., Klareskog L., van Vollenhoven R., Rönnelid J. 2012. Anti-type II collagen antibodies are associated with early radiographic destruction in rheumatoid arthritis. Arthritis Res. Ther. 14: R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vetto A. A., Mannik M., Zatarain-Rios E., Wener M. H. 1990. Immune deposits in articular cartilage of patients with rheumatoid arthritis have a granular pattern not seen in osteoarthritis. Rheumatol. Int. 10: 13–19 [DOI] [PubMed] [Google Scholar]
- 41.Renner B., Ferreira V. P., Cortes C., Goldberg R., Ljubanovic D., Pangburn M. K., Pickering M. C., Tomlinson S., Holland-Neidermyer A., Strassheim D., et al. 2011. Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. Kidney Int. 80: 165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z., Lauer T. W., Sick A., Hackett S. F., Campochiaro P. A. 2007. Oxidative stress modulates complement factor H expression in retinal pigmented epithelial cells by acetylation of FOXO3. J. Biol. Chem. 282: 22414–22425 [DOI] [PubMed] [Google Scholar]
- 43.Hakobyan S., Harris C. L., Tortajada A., Goicochea de Jorge E., García-Layana A., Fernández-Robredo P., Rodríguez de Córdoba S., Morgan B. P. 2008. Measurement of factor H variants in plasma using variant-specific monoclonal antibodies: application to assessing risk of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 49: 1983–1990 [DOI] [PubMed] [Google Scholar]
- 44.de Paula P. F., Barbosa J. E., Junior P. R., Ferriani V. P., Latorre M. R., Nudelman V., Isaac L. 2003. Ontogeny of complement regulatory proteins: concentrations of factor h, factor I, c4b-binding protein, properdin and vitronectin in healthy children of different ages and in adults. Scand. J. Immunol. 58: 572–577 [DOI] [PubMed] [Google Scholar]
- 45.Esparza-Gordillo J., Soria J. M., Buil A., Almasy L., Blangero J., Fontcuberta J., Rodríguez de Córdoba S. 2004. Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics 56: 77–82 [DOI] [PubMed] [Google Scholar]
- 46.Whaley K. 1980. Biosynthesis of the complement components and the regulatory proteins of the alternative complement pathway by human peripheral blood monocytes. J. Exp. Med. 151: 501–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooimans R. A., van der Ark A. A., Buurman W. A., van Es L. A., Daha M. R. 1990. Differential regulation of complement factor H and C3 production in human umbilical vein endothelial cells by IFN-γ and IL-1. J. Immunol. 144: 3835–3840 [PubMed] [Google Scholar]
- 48.Timár K. K., Pasch M. C., van den Bosch N. H., Jarva H., Junnikkala S., Meri S., Bos J. D., Asghar S. S. 2006. Human keratinocytes produce the complement inhibitor factor H: synthesis is regulated by interferon-γ. Mol. Immunol. 43: 317–325 [DOI] [PubMed] [Google Scholar]
- 49.Friese M. A., Manuelian T., Junnikkala S., Hellwage J., Meri S., Peter H. H., Gordon D. L., Eibel H., Zipfel P. F. 2003. Release of endogenous anti-inflammatory complement regulators FHL-1 and factor H protects synovial fibroblasts during rheumatoid arthritis. Clin. Exp. Immunol. 132: 485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monach P. A., Verschoor A., Jacobs J. P., Carroll M. C., Wagers A. J., Benoist C., Mathis D. 2007. Circulating C3 is necessary and sufficient for induction of autoantibody-mediated arthritis in a mouse model. Arthritis Rheum. 56: 2968–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams A. S., Mizuno M., Richards P. J., Holt D. S., Morgan B. P. 2004. Deletion of the gene encoding CD59a in mice increases disease severity in a murine model of rheumatoid arthritis. Arthritis Rheum. 50: 3035–3044 [DOI] [PubMed] [Google Scholar]
- 52.Pangburn M. K., Pangburn K. L., Koistinen V., Meri S., Sharma A. K. 2000. Molecular mechanisms of target recognition in an innate immune system: interactions among factor H, C3b, and target in the alternative pathway of human complement. J. Immunol. 164: 4742–4751 [DOI] [PubMed] [Google Scholar]
- 53.Hebecker M., Józsi M. 2012. Factor H-related protein 4 activates complement by serving as a platform for the assembly of alternative pathway C3 convertase via its interaction with C3b protein. J. Biol. Chem. 287: 19528–19536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambert P. H., Nydegger U. E., Perrin L. H., McCormic J., Fehr K., Miescher P. A. 1975. Complement activation in seropositive and seronegative rheumatoid arthritis: 125I-C1q binding capacity and complement breakdown products in serum and synovial fluid. Rheumatology 6: 52–59 [PubMed] [Google Scholar]
- 55.Nydegger U. E., Zubler R. H., Gabay R., Joliat G., Karagevrekis C. H., Lambert P. H., Miescher P. A. 1977. Circulating complement breakdown products in patients with rheumatoid arthritis: correlation between plasma C3d, circulating immune complexes, and clinical activity. J. Clin. Invest. 59: 862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schur P. H., Austen K. F. 1968. Complement in human disease. Annu. Rev. Med. 19: 1–24 [DOI] [PubMed] [Google Scholar]