Abstract
Thymocyte-expressed molecule involved in selection (THEMIS) is a recently identified regulator of thymocyte positive selection. THEMIS’s mechanism of action is unknown, and whether it has a role in TCR-proximal signaling is controversial. In this article, we show that THEMIS and the adapter molecule growth factor receptor–bound protein 2 (GRB2) associate constitutively through binding of a conserved PxRPxK motif within the proline-rich region 1 of THEMIS to the C-terminal SH3-domain of GRB2. This association is indispensable for THEMIS recruitment to the immunological synapse via the transmembrane adapter linker for activation of T cells (LAT) and for THEMIS phosphorylation by Lck and ZAP-70. Two major sites of tyrosine phosphorylation were mapped to a YY-motif close to proline-rich region 1. The YY-motif was crucial for GRB2 binding, suggesting that this region of THEMIS might control local phosphorylation-dependent conformational changes important for THEMIS function. Finally, THEMIS binding to GRB2 was required for thymocyte development. Our data firmly assign THEMIS to the TCR-proximal signaling cascade as a participant in the LAT signalosome and suggest that the THEMIS–GRB2 complex might be involved in shaping the nature of Ras signaling, thereby governing thymic selection.
Introduction
Thymocyte-expressed molecule involved in selection (THEMIS) has recently been identified as a new T cell lineage-specific gene (1–4). Themis−/− mice show impaired positive selection during thymocyte development and severe reduction of mature thymocytes and peripheral T cells. THEMIS expression is high in double-positive (DP) thymocytes, with a marked downregulation after positive selection and in peripheral T cells. Whereas knockout mice did not show hallmarks of autoimmunity (3, 5), the recently described BNm rat strain has linked a frameshift mutation in the Themis gene to inflammatory bowel disease caused by defective regulatory T cell function (6). THEMIS is a highly conserved, 73-kDa cytoplasmic protein without any obvious catalytic or protein–protein interaction domains. Bioinformatics analysis predicts a tandem repeat of a novel, cysteine-containing globular domain (cysteine-containing all β in THEMIS [CABIT]) found in a number of metazoan proteins (2). A putative bipartite nuclear localization sequence is present within the CABIT-2 domain of THEMIS, but no nuclear translocation was detected upon TCR ligation (3, 5). The C-terminal end of THEMIS, predicted to contain little or no secondary structure, harbors two proline-rich regions (PRRs).
In previous work, we identified THEMIS as an early target of tyrosine phosphorylation downstream of TCR (7). TCR-induced tyrosine phosphorylation of THEMIS depended on the adapters linker for activation of T cells (LAT) and Src homology (SH) 2 domain-containing leukocyte protein of 76 kDa (SLP76), and THEMIS appeared to associate to LAT upon TCR stimulation. We and others showed that THEMIS is constitutively associated to the adapter protein growth factor receptor–bound protein 2 (GRB2) (1, 2, 5, 7), suggesting a potential mechanism of THEMIS recruitment onto LAT to regulate the TCR signaling cascade. However, this mechanism and its consequences for THEMIS function in vivo remain to be demonstrated. Initial studies reported only subtle alterations in TCR-proximal signaling in Themis−/− DP thymocytes (1), and THEMIS implication in the TCR signaling machinery has been disputed (2, 5). Thus, precise delineation of when, where, and how THEMIS relocates and undergoes posttranslational modifications during TCR triggering can help clarify its molecular function and role in T cell development.
In this article, we demonstrate that THEMIS PRR1, an atypical binding motif for the C-terminal SH3 domain (SH3C) of GRB2, mediates the constitutive association of THEMIS to GRB2 and THEMIS recruitment via LAT to the immunological synapse (IS) after Ag stimulation. The Lck and ZAP70 kinases control phosphorylation of two tyrosines located shortly upstream of PRR1, and we show that these tyrosines are required for GRB2 binding, which, in turn, boosts THEMIS phosphorylation, revealing an unusual proximal interplay between these two events. Finally, we show that THEMIS mutants defective in GRB2 association do not rescue positive selection in Themis−/− mice. These data definitively support a model of THEMIS regulating key TCR signaling events, and suggest that in DP thymocytes, THEMIS–GRB2 may compete with son of sevenless (SOS)-GRB2 for LAT binding, thus favoring positive selection.
Materials and Methods
Plasmids and Abs
Full-length cDNA encoding human THEMIS was obtained from Open Biosystems (NM_001010923.2; giving rise to a 641-aa protein: Uniprot Q8N1K5-1) and used as the PCR template to generate THEMIS-Strep, carrying a C-terminal One-STrEP-Tag (IBA BioTAGnology, Göttingen, Germany). THEMIS-Strep was cloned into the lentiviral expression vector pHR-SIN-BX-IRES-Emerald (kindly provided by Dr. V. Cerundolo, Weatherall Institute of Molecular Medicine, Oxford, U.K.) to give rise to pHR-THEMIS-Strep. All mutants described were based on pHR-THEMIS-Strep and derived by site-directed mutagenesis (QuickChange II Kit; Agilent Technologies). The lentiviral helper plasmids psPAX2 (Addgene 10703) and pMD2.G (Addgene 12259) were provided by Dr. Didier Trono (Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland) via Addgene. Myc-tagged human GRB2-49L, GRB2-203R, and GRB2-49L/203R constructs (kind gift of Dr. R.A. Weinberg, Massachusetts Institute of Technology Ludwig Center for Cancer Research, Cambridge, MA) were cloned into pHR-SIN-BX-IRES-Emerald by PCR. Plasmids pCEFL-LAT-wild-type (wt)-Myc and pCEFL-LAT-3YF-Myc were kindly provided by Dr. Lawrence E. Samelson (National Institutes of Health, Bethesda, MD) and used as PCR templates to generate LAT-Strep and LAT-3YF-Strep in pHR-SIN-BX-IRES-Emerald. For BM reconstitution experiments, murine THEMIS was cloned into the retroviral expression vector pCMV-IRES-GFP to give rise to pCMV-muTHEMIS-IRES-GFP. Mutants of murine Themis described in this study were derived by site-directed mutagenesis. For expression in 5C.C7 T cells, GFP-tagged versions of the above constructs were expressed from the retroviral pGC vector. All constructs were verified by sequencing. Human wt Lck and ZAP70 were cloned into pEF-BOS expression vector.
Mouse mAbs used included anti-phosphotyrosine (clone 4G10; Millipore); anti-LCK (clone 3A5; Santa Cruz); anti-ZAP70 (2F3.2), anti-LAT (2E9); anti–One-STrEP-Tag mAb (StrepMAB Classic; IBA BioTAGnology); anti-human CD3ε (clone UCHT-1; BioLegend), anti-SLP76 (clone SLP76/03; AbD Serotec), and anti-Myc (clone 4A6; Millipore). Rabbit polyclonal Abs used were anti–phospholipase C γ 1 (anti-PLCγ1; 1249; Santa Cruz); anti-GRB2 (C-23; Santa Cruz); anti–phospho-ZAP70 Y493 (Cell Signaling Technology); anti-actin (Sigma), and anti-THEMIS (Sigma). Goat anti-murine Themis is from Abcam PLC.
Cells, transfections, and lentiviral transductions
CD4+ Jurkat subclone 20 and LAT-deficient Jurkat cells (J.CaM2.5) were maintained in RPMI 1640 (PAA Laboratories) medium supplemented with 10% FBS (Perbio). Human embryonic kidney epithelial cells (HEK293) were maintained in DMEM, 10% FBS. HEK293 cells were transfected by standard calcium phosphate precipitation. Lentiviral particles were produced in HEK293 cells by cotransfection of the pHR-SIN-BX-IRES-Emerald vector with the packaging plasmids psPAX2 and pMD2.G. Forty-eight hours after transfection, viral supernatants were harvested, filtered, and used for the transduction of cells in the presence of 5 μg/ml Polybrene. Puromycin selection was applied 48 h after transduction where appropriate at 1 μg/ml.
Immunoprecipitations and pull-down assays
Resting or anti-CD3–activated wt Jurkat cells or Jurkat cells expressing THEMIS-OST were lysed in ice-cold lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 0.5% dodecyl-β-D-maltoside [Calbiochem], 1 mM Na3VO4, protease inhibitor mixture [Roche]). Lysates were cleared by centrifugation at 14,000 × g for 10 min. THEMIS-Strep pull-downs were carried out on cleared lysates for 15–20 min at 4°C with Streptactin-Sepharose beads (IBA BioTAGnology). After pull down, beads were washed three times with lysis buffer, and bound proteins were eluted with 5 mM biotin. For GFP immunoprecipitations, anti–GFP-agarose (rat monoclonal, clone RQ2; MBL International) was used.
Production of recombinant Themis
HEK293 cells were transduced with the lentiviral expression construct pHR-THEMIS-Strep. GFP expression from the IRES-Emerald cassette was used to sort for highly expressing cells by FACS. Cells were harvested with PBS/EDTA and lysed in standard lysis buffer (see above). Lysates were cleared by centrifugation at 14,000 × g for 10 min and loaded onto a gravity-flow Streptactin-Sepharose column (IBA BioTAGnology, Göttingen, Germany). After washing with 10-column volumes of lysis buffer, bound protein was eluted with elution buffer (100 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 2.5 mM desthiobiotin).
In vitro phosphorylation assay for mass spectrometry
A total of 500 ng recombinant Themis was incubated with 50 ng recombinant Lck (Millipore, Billerica, MA) in kinase buffer (20 mM Tris-HCl pH 7.5, 10 mM MgCl2, 10 mM MnCl2, 1 mM ATP) for 30 min at 30°C. Reactions were stopped by adding reducing SDS NuPAGE sample buffer (Invitrogen) and incubation for 5 min at 95°C, followed by alkylation with 55 mM iodoactamide (Sigma). Proteins were separated on 4–12% gradient Bis-Tris NuPAGE gels (Invitrogen), gels were washed in distilled water, lightly stained with Colloidal Blue (Invitrogen), and subjected to GeLC-mass spectrometry (MS)/MS as described previously (7).
MS data analysis
Samples were analyzed on a Q Exactive (Thermo Scientific) coupled to an Ultimate 3000RSLCnano system (Dionex). Samples were resolved on a 25-cm-long by 75-μm internal diameter home-packed Picotip emitter (New Objective) at a flow rate of 300 nl min−1 using a 120-min gradient. The mass spectrometer was operated in a “top 10” data-dependent mode in which the 10 strongest precursors were selected for fragmentation by HCD. 1+ charged ions were excluded from isolation. Data were converted to .mzXML format using MSconvert (Proteowizard) and uploaded into the central proteomics facility pipeline (CPFP) (8) for analysis. Enzyme was set to trypsin allowing for up to two missed cleavages. Carbamidomethyl cysteine was set as a fixed modification and oxidation (methionine), deamidation (NQ), acetylation (Protein-N), and phosphotyrosine as variable modifications. Mass tolerances for MS and MS/MS peak identifications were 20 ppm and 0.1 Da, respectively. Assignment of a tyrosine phosphorylation site required identification by searches in the CPFP at 1% false discovery rate. InterProphet probability is derived by the combination of results from multiple search engines within CPFP, and improves coverage and confidence over use of a single search engine. Modification localization scoring was performed using the ModLS algorithm (9), a method similar to the AScore algorithm (10). ModLS expands the AScore method to incorporate automatic specificity expansion. For each variable modification chosen for the database search, all amino acid specificities defined in the Unimod database are considered during localization.
Image acquisition and image analysis
Image acquisition and analysis were performed as described in detail elsewhere (11). In brief, T cell–APC interactions were imaged at 37°C. Every 20 s, 1 differential interference contrast and 21 fluorescence images that spanned 20 μm in the z-plane at 1-μm intervals were acquired. The acquisition and analysis software was MetaMorph (Molecular Devices). The formation of a tight cell couple, time 0 in our analysis, was defined as either the first time point with a fully spread T cell–APC interface or 40 s after first membrane contact, whichever occurred first. A region of sensor accumulation was defined by an average fluorescence intensity of >135% of the background cellular fluorescence. To classify spatial accumulation features, we used six mutually exclusive interface patterns: central, invagination, diffuse, lamellal, asymmetric, and peripheral, as defined by strict geometrical constraints (for details see Supplemental Fig. 2). Distal accumulation was scored independently. A T cell was scored to have a uropod as long as an inversion of curvature of the plasma membrane could be detected at the distal pole in the differential interference contrast images. Data were routinely analyzed by two investigators independently to ensure the reliability of this analysis.
Bone marrow reconstitution experiments
Bone marrow cells were isolated from donor Themis1−/− mice (B6.129S-Themistm1Gasc; Jackson Laboratory Stock no. 010919) (1), which were pretreated with 5-fluorouracil 5 d before isolation. A total of 2 × 106 bone marrow cells were cultured in 1 ml DMEM supplemented with 10% FBS, Pen/Strep/Glut, 2-ME, and nonessential amino acids in one well of a 24-well plate. Cytokines (PeproTech) were also added to the media as IL-3 (20 ng/ml), IL-6 (25 ng/ml), and stem cell factor (100 ng/ml). For virus generation, retroviral vectors (i.e., empty vector, WT-Themis vector, or mutated Themis vector) were transfected into Plat-E packaging cells that were preseeded 1 d before (as 5 × 106 cells per 10-cm petri dish). Twenty-four hours posttransfection, spent media were aspirated and replaced with 5 ml fresh media. At 48 and 72 h posttransfection, the derived viral supernatants were collected and concentrated by centrifugation with Amicon Ultra4 Centrifugal Filter Unit (UFC810024). Concentrated viral supernatants were used to spin-infect cultured bone marrow cells in the presence of 8 mg/ml Polybrene. The spin infection was carried out at 2500 rpm, 32°C for 2 h. The infection efficiency of bone marrow cells was determined by analyzing the percentage of GFP+ cells with flow cytometry. A typical infection efficiency falls between 30 and 70%. Infected bone marrow cells were i.v. injected into lethally irradiated (i.e., 1100 rads in two equally split doses) B6.SJL recipient mice (CD45.1+ versus CD45.2+ in donor cells). Eight weeks postreconstitution, mice bearing >5% CD45.2+GFP+ thymocytes were included and phenotyped for thymocyte and mature T cell development.
Protein expression and purification
GST and GST-Grb2 fusion proteins (both full-length and SH3C) were expressed in Escherichia coli growing in terrific broth medium at 18°C after induction with 50 μM IPTG. Cells were lysed by sonication in TPE lysis buffer (1% Triton X-100, PBS, and 100 mM EDTA) with a protease inhibitor mixture added. The mixture was centrifuged at 48,000 × g for 1 h, and the soluble fraction was incubated overnight with glutathione Sepharose beads. The next day, beads were washed extensively with cold wash buffer (50 mM Tris pH 7.5, 100 mM EDTA, and 0.1% Tween 20) before overnight incubation with elution buffer (100 mM reduced glutathione brought to pH 8.0 with a concentrated Tris buffer stock, pH 8.8). The eluted GST fusion proteins were further purified by gel filtration chromatography on a Superdex 75 column (GE Healthcare) in running buffer (20 mM Tris pH 7.5, 150 mM NaCl). Finally, purified proteins were dialyzed extensively against 5 mM Tris pH 7.5 and concentrated to >10 mg/ml before storage at −80°C.
Peptide arrays
The full amino acid sequence of human THEMIS (Uniprot code: Q8N1K5, isoform 1) was chemically synthesized as an array of spots of overlapping peptides (Multipep synthesizer; Intavis) with a peptide length of 29 amino acids, sliding 3 residues along the sequence with each consecutive peptide spot. Membranes were incubated for 4 h in blocking buffer (3% OVA, 20 mM Tris-HCl pH 7.5, 100 mM NaCl, 0.1% Tween 20, 2 mM DTT, 1 mM sodium molybdate, 1 mM sodium orthovanadate) and probed first with 0.1 μM GST overnight, followed by incubation with anti-GST primary Ab, and then HRP-coupled secondary Ab, and finally visualized by ECL detection to confirm no background binding. The same membranes were then incubated again in blocking buffer before reprobing overnight with 0.1 μM purified GST-Grb2 (full-length) or GST-Grb2 SH3C followed by detection, as described earlier, of Grb2-interacting epitopes. The Grb2 SH3C binding motif in THEMIS harboring the RxxK motif was incorporated into additional membrane spot-synthesized peptides. These feature an alanine scan and sequence truncations for the mapping of key binding determinants; multiple mutants of the proximal double tyrosine motif (Y540 and Y541) and peptides both without tyrosine phosphorylation and singly tyrosine phosphorylated. Membrane probing and detection was as described earlier for the scanning array.
Results
GRB2 binding is required for THEMIS recruitment via LAT to the IS
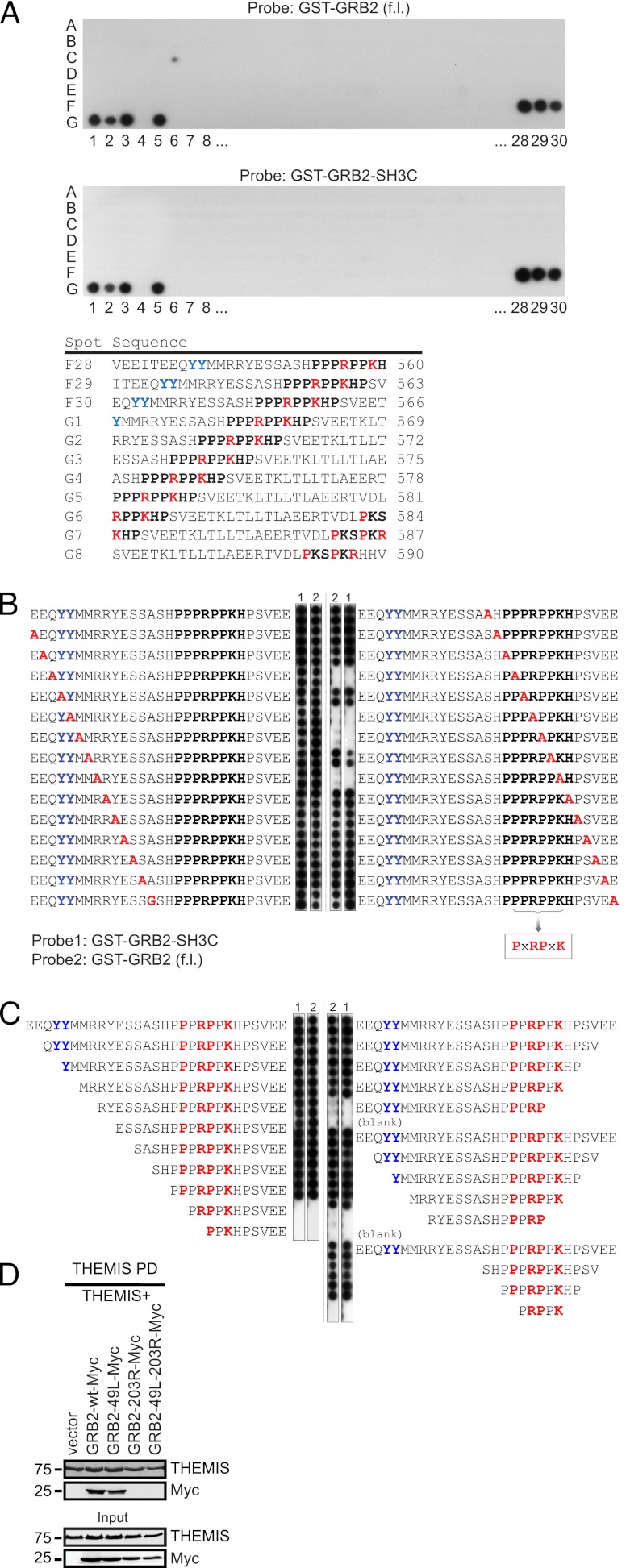
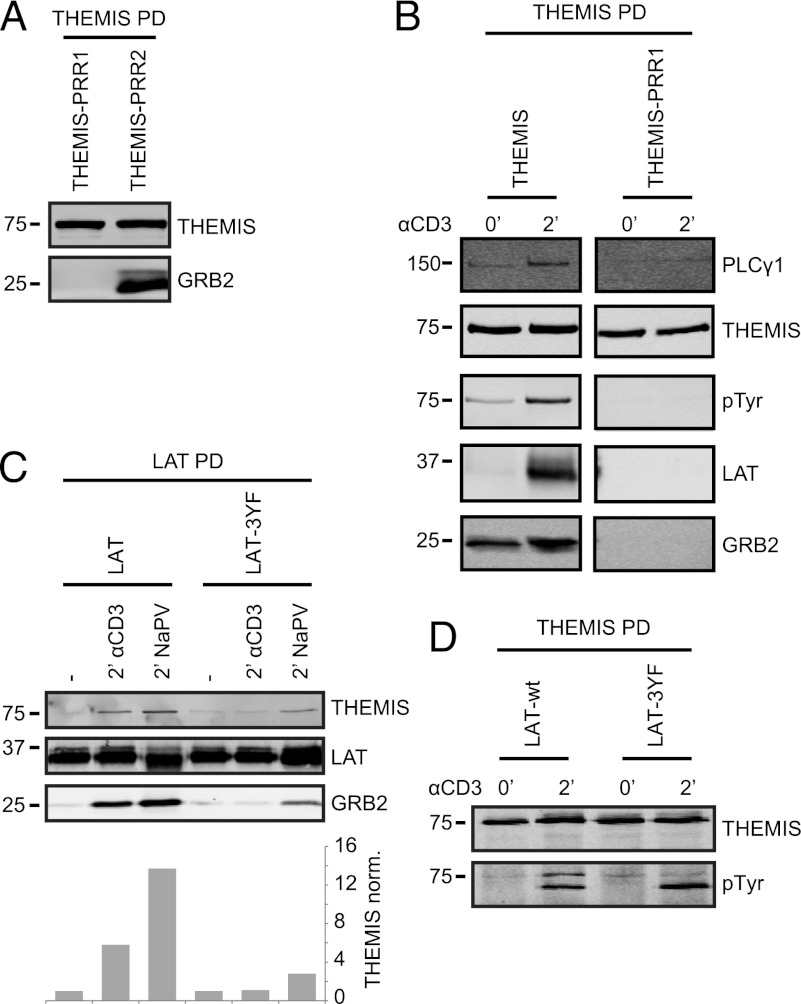
The highly conserved proline-rich sequence PPPRPPKHP (residues 553–561, Supplemental Fig. 1A, 1B) of THEMIS (here designated PRR1) resembles consensus binding sites for the SH3C of GRB2 (GRB2-SH3C) on Gab proteins and SLP76 lacking the typical PxxP core motif (12–14). To directly verify this prediction, we assessed binding of recombinant GST-fusion proteins of GRB2-SH3C and full-length GRB2 to an array of 29-mer peptides scanning the entire THEMIS sequence (Fig. 1A). Binding of full-length GRB2 and GRB2-SH3C was observed only to 29-mers containing the intact PRR1 sequence. A core-binding motif of PxRPxK was defined by alanine-scanning substitution and successive truncations (Fig. 1B, 1C). Pull-down experiments of Strep-tagged THEMIS from transfected HEK293 cells confirmed that binding of GRB2 to THEMIS was mainly mediated via GRB2-SH3C, as mutation of the N-terminal SH3 domain (SH3N) had only a minimal effect (Fig. 1D). This agrees with RxxK motifs having considerably higher affinity for GRB2-SH3C than for SH3N (12, 15). In agreement with a single GRB2-binding site, THEMIS carrying alanine substitutions at P555 and P558 of PRR1 (THEMIS-PRR1) expressed in Jurkat cells showed defective binding to GRB2, whereas mutating PRR2 had no effect (Fig. 2A). THEMIS-PRR1 showed a loss of TCR-induced association with LAT, was not tyrosine phosphorylated, and exhibited decreased interaction with PLCγ1 (Fig. 2B), a key component of the LAT–SLP76 complex (16). The N-terminal moiety of LAT contains three (two YVNV and one YENL) binding motifs for the SH2 domain of GRB2 and may thus recruit the THEMIS–GRB2 complex. When a LAT construct with all three tyrosines at these sites mutated to phenylalanine (Y171F, Y191F, and Y226F, i.e., LAT-3YF) was expressed in the LAT-deficient Jurkat line J.CaM2.5, the TCR-induced LAT association to GRB2 and THEMIS was lost (Fig. 2C), as was THEMIS phosphorylation on tyrosine (Fig. 2D). Taken together, these data strongly suggested that constitutive binding of GRB2-SH3C to THEMIS serves to allow THEMIS recruitment onto the LAT signalosome after TCR stimulation.
FIGURE 1.
GRB2-SH3C binds an RxxK motif on THEMIS. (A) A peptide scanning array covering full-length human THEMIS (Uniprot Q8N1K5 isoform 1) was spot synthesized as 29 aa peptides, sliding 3 aa with each step. (Top panel) Membrane probed with 0.1 μM GST-GRB2 (full length); (bottom panel) 0.1 μM GST-GRB2 SH3C domain. Peptides surrounding the single binding region are displayed to the right with the probable binding region emphasized in bold type. (B) Alanine scanning peptide array through the identified binding region. wt residues were sequentially mutated to alanine (or glycine, when alanine present). Peptides were spotted in duplicates and the array was probed as in (A). Key GRB2 binding residues are indicated in the box. (C) Stepwise N- and C-terminal truncations of the THEMIS peptide used for alanine-scanning substitution as seen in (B). (D) HEK293 cells were cotransfected with a human THEMIS construct carrying a C-terminal OneStrepTag (THEMIS-Strep) and either empty vector, GRB2-wt-Myc, GRB2-49L-Myc (SH3N mutant), GRB2-203R-Myc (SH3C mutant), or GRB2-49L-203R-Myc (SH3C and N mutant). THEMIS was precipitated by Streptactin pull down and analyzed for bound GRB2 by immunoblotting. Data are representative of three independent experiments.
FIGURE 2.
GRB2 binding is required for THEMIS recruitment to the LAT signalosome. (A) Themis-Strep mutants of PRR1 and 2 (P555/558A and P582/585A, respectively) expressed in Jurkat cells were pulled down with Streptactin-Sepharose and probed for GRB2 association by immunoblotting. (B) As in (A), but from resting and CD3 mAb stimulated cells and analyzed for tyrosine phosphorylation, GRB2, LAT, and PLCγ1 association. (C) LAT-deficient Jurkat J.CaM2.5 were reconstituted with LAT-Strep wt or 3YF (Y171/191/226F) mutant. LAT pull downs of CD3 Ab or NaPV-treated cells were probed for GRB2 and THEMIS binding. Relative amounts of THEMIS pulled down and normalized to LAT are shown. (D) LAT-deficient Jurkat J.CaM2.5 were transduced with THEMIS-Strep and either LAT wt or 3YF (Y171F, Y191F, and Y226F) mutant. THEMIS was pulled down from resting and stimulated cells, and assessed for tyrosine phosphorylation by immunoblotting. Data are representative of three independent experiments.
An earlier study failed to detect changes in the distribution of THEMIS upon TCR triggering (5). In light of the data presented earlier, we re-examined this aspect by live-cell imaging using GFP reporters in primary T cells. We used primed T cells from 5C.C7 TCR transgenic mice recognizing a peptide from moth cytochrome c (MCC; aa 82–103) in the context of I-Ek (11). Activated 5C.C7 T cells were transduced with retroviral vectors expressing GFP fusion constructs for murine THEMIS-wt or THEMIS-PRR1 (P557A/P560A), the latter showing no detectable binding to GRB2 (Fig. 3A). Upon conjugate formation with MCC peptide-pulsed murine CH27 B cell lymphoma cells, THEMIS-wt-GFP exhibited a rapid recruitment to the T cell–APC interface, mostly in a diffuse and lamellal pattern (Fig. 3B, see Supplemental Fig. 2 for description of the individual patterns.). By contrast, interface recruitment of THEMIS-PRR1-GFP was significantly reduced (Fig. 3C). The distribution of THEMIS at the T–APC interface was less central than LAT (11), suggesting THEMIS localization with peripheral signaling TCRs. These data demonstrated that upon TCR stimulation, THEMIS is rapidly recruited from an intracellular pool to the T cell–APC interface in a GRB2-dependent manner, likely via LAT, supporting the idea that THEMIS has a role in regulating TCR signaling.
FIGURE 3.
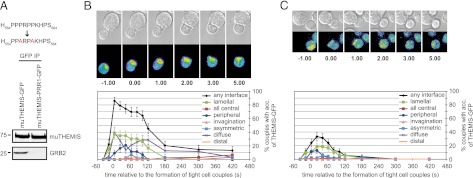
Recruitment of THEMIS to the IS is mediated via GRB2. (A) GRB2 association to murine THEMIS wt and PRR1 mutant (P557/560A) GFP constructs in HEK293 cells. Data are representative of three independent experiments. (B and C) Live-cell imaging of conjugates between 5C.C7 T cells (MCC82-103 in context of I-Ek) transduced with muTHEMIS-GFP (B) or muTHEMIS-PRR1-GFP (C) with MCC-pulsed (10 μM) CH27 B cells. Time point zero marks the formation of a tight cell contact. Differential interference contrast images are shown in the top rows, with top-down, maximum projections of three-dimensional fluorescence data in the bottom rows (intensities in rainbow-like false-color scale). The graphs display the accumulation of GFP sensors with the indicated patterns expressed as percentages of the total number of tight cell couples formed over time (see Supplemental Fig. 2 for description of the individual patterns). Forty-three and 54 couples were analyzed for THEMIS-wt-GFP and THEMIS-PRR1-GFP, respectively.
THEMIS is a target of Lck and ZAP70-mediated phosphorylation
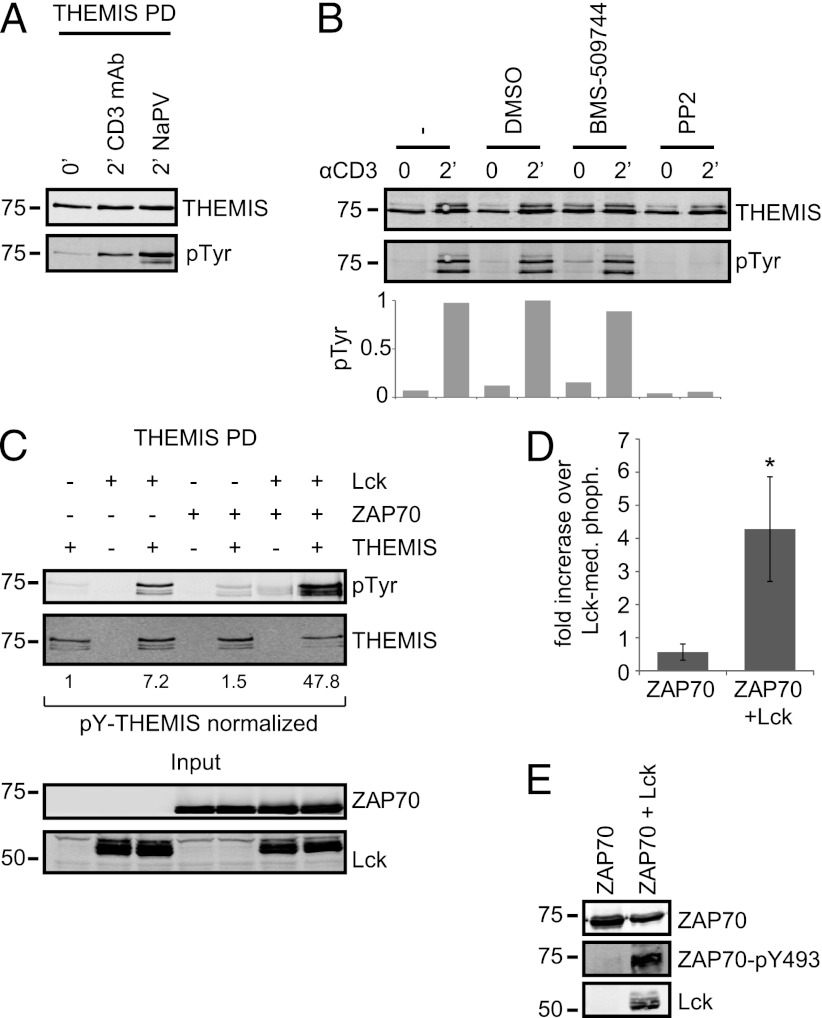
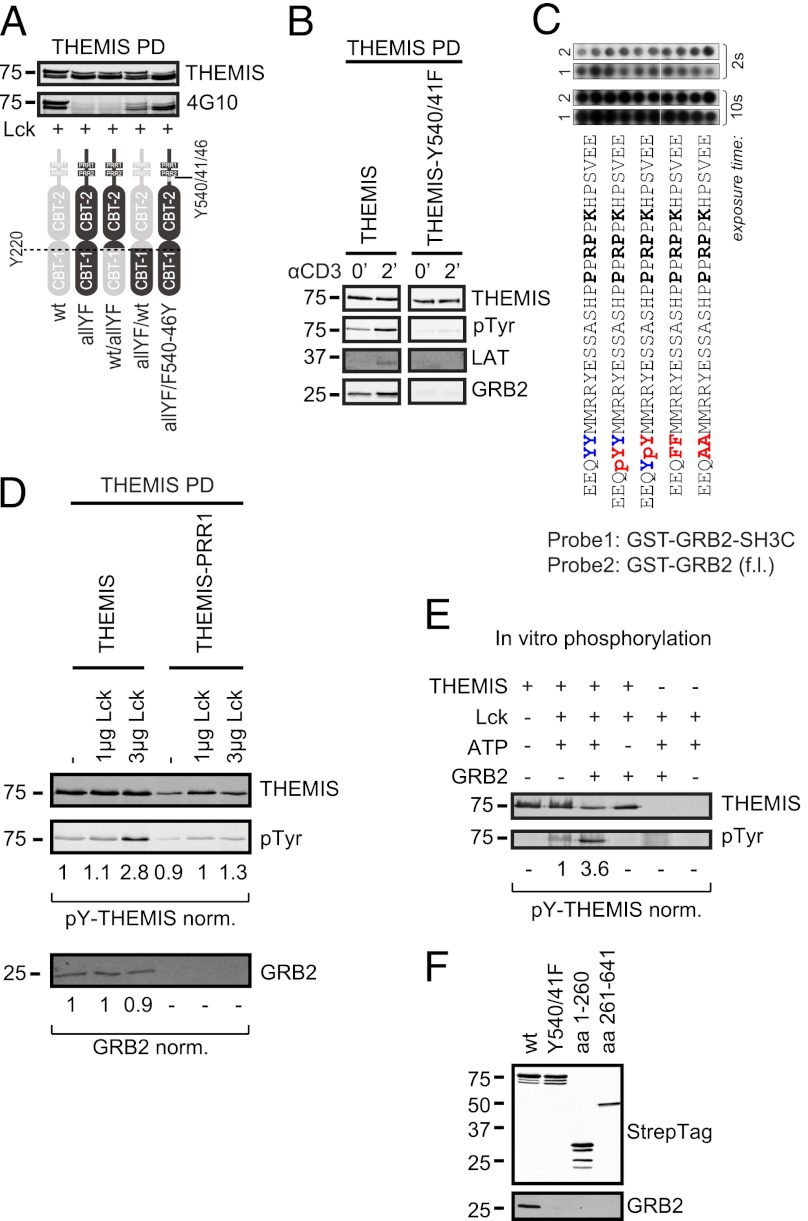
Tyrosine phosphorylation of THEMIS may generate binding sites for interacting partners and/or help induce conformational changes required for protein activation. Three major protein tyrosine kinases, Lck, ZAP70, and IL-2–inducible T cell kinase (Itk), control tyrosine phosphorylation during the earliest phases of TCR signaling. In Jurkat T cells, THEMIS was rapidly tyrosine phosphorylated after CD3 stimulation or sodium pervanadate (NaPV) addition (Fig. 4A). An involvement of Itk could be ruled out by using the specific inhibitor BMS-509744 (17), which, in contrast with the Src family kinase inhibitor PP2, had no effect on THEMIS phosphorylation (Fig. 4B). To investigate whether Lck and/or ZAP70 phosphorylated THEMIS, we performed coexpression experiments in HEK293 cells. The data showed that Lck and ZAP70 together induced stronger phosphorylation of THEMIS than either kinase alone (Fig. 4C, 4D), an effect likely caused by Lck-mediated activation of ZAP70 (18), as evidenced by a strong ZAP70-pY493 signal only when Lck was coexpressed (Fig. 4E). We deduce that THEMIS can be a substrate of both Lck and ZAP70. Y34, Y95, Y174, Y540, and Y541 were predicted as possible target sites by Netphos 2.0 (score > 0.8) (19). Recombinant THEMIS was phosphorylated in vitro by recombinant Lck or ZAP70, and phospho-sites Y95, Y174, Y220, Y353, Y429, Y540, and Y541 were detected with high confidence by MS (Supplemental Table I). In agreement with this, Y540 and Y541 were predicted as potential Src phosphorylation sites by the NetphosK1.0 tool and as potential Lck and ZAP70 target sites by the group-based phosphorylation scoring system (19, 20). These data were corroborated by chimeric swap constructs between wt and an all-YF mutant (all 19 tyrosines of THEMIS mutated to phenylalanine) cotransfected with Lck in HEK293 cells (Fig. 5A). An all-YF mutant except for Y540/541/546 was still efficiently tyrosine phosphorylated. In addition, considering conservation of tyrosines both on the interspecies and intraspecies level (i.e., between THEMIS and THEMIS2 [21]; Supplemental Table I) led us to conclude that Y540 and Y541 are major TCR-induced phosphorylation sites. Attempts to detect pY540/pY541-containing tryptic peptide(s) by MS from THEMIS isolated from TCR-stimulated Jurkat cells failed maybe because of inefficient trypsin digestion as previously noted at other phosphorylation sites (22). TCR-induced tyrosine phosphorylation of a double Y540/541F THEMIS mutant expressed in Jurkat cells was profoundly affected (Fig. 5B). However, surprisingly, THEMIS-Y540/541F lost constitutive association with GRB2 and could no longer bind to LAT. Substitutions, deletions, or phosphorylation of Y540 or Y541 in peptide-array analysis did not affect GRB2 binding (Fig. 1B, 1C, 5C), nor could we detect any obvious changes in GRB2 associated to increasingly phosphorylated THEMIS in coexpression studies with Lck in HEK293 cells (Fig. 5D). It seems therefore unlikely that tyrosine phosphorylation directly modulates THEMIS–GRB2 complex formation. Nevertheless, our data suggested that a secondary/tertiary structure connecting Y540/Y541 and PRR1 makes them mutually sensitive to mutations. Indeed, tyrosine phosphorylation of THEMIS-PRR1 by Lck in HEK293 was substantially reduced (Fig. 5D). Moreover, recombinant THEMIS purified as a monomer without GRB2 was poorly phosphorylated by Lck in vitro, whereas addition of recombinant GRB2 reconstituted THEMIS tyrosine phosphorylation (Fig. 5E). In this context, it is of interest that a soluble fragment of THEMIS lacking the CABIT-1 domain did not bind GRB2 (Fig. 5F). This suggests that the PRR1 motif and/or its surrounding region are somehow connected to a distal region of THEMIS, hinting at a global compact structure of the entire protein. Thus, GRB2 association might keep THEMIS accessible to protein tyrosine kinases.
FIGURE 4.
THEMIS is a target of Lck and ZAP70-mediated phosphorylation. (A) THEMIS-Strep was pulled down from Jurkat cells treated as indicated and tyrosine phosphorylation was probed by immunoblotting. (B) Jurkat cells were left untreated or pretreated as indicated with either BMS-509744 (10 μM), PP2 (50 μM), or vehicle control (DMSO) for 30 min before CD3 mAb stimulation and THEMIS immunoprecipitation. THEMIS tyrosine phosphorylation was assessed by immunoblotting. Compiled data of two independent experiments can be found in Supplemental Fig. 3. (C) HEK293 cells were transfected with THEMIS-Strep alone or in combination with Lck and/or ZAP70. Tyrosine phosphorylation of pulled-down THEMIS was assessed by immunoblotting. (D) Quantification of THEMIS tyrosine phosphorylation by ZAP70 alone or in combination with Lck across multiple experiments expressed as fold increase over Lck-mediated phosphorylation (n = 4; *p < 0.05, Student t test). (E) As in (C), but probed for ZAP70-pY493. Data are representative of at least three independent experiments, except for (B), which has been repeated twice.
FIGURE 5.
Interdependence of Y540 and Y541 phosphorylation and PRR1 function. (A) HEK293 cells were cotransfected with Lck and either THEMIS-Strep wt, an all tyrosine to Phe mutant (19YF) and swap mutants between wt and 19YF (residue 220 as swapping boundary). Tyrosine phosphorylation of THEMIS pull-downs was assessed by immunoblotting. (B) THEMIS-Strep wt or Y540 and Y541 to Phe mutant were isolated from Jurkat cells treated as indicated and probed for tyrosine phosphorylation, GRB2, and LAT association. (C) Peptide scanning array as in Fig. 1A with phosphorylation and substitutions at Y540 and Y541. (D) HEK293 cells were cotransfected with THEMIS-Strep wt or PRR1 mutant and increasing amounts of Lck. Precipitated THEMIS was probed for tyrosine phosphorylation and GRB2 association. (E) A total of 500 ng recombinant THEMIS-Strep was phosphorylated in vitro with 25 ng recombinant Lck. Where indicated, THEMIS-Strep was preincubated with recombinant GST-GRB2 (10 μM) for 1 h. THEMIS tyrosine phosphorylation was quantified by immunoblotting. (F) HEK293 cells were transduced with C-terminally Strep-tagged lentiviral constructs of either full-length wt and Y540/541F mutant THEMIS or truncation constructs consisting of CABIT domain 1 (aa 1–260) and CABIT domain 2 including the tail sequence (aa 261–641). After Streptactin pull downs, THEMIS constructs were assessed for GRB2 binding by immunoblotting. Data are representative of three independent experiments.
PRR1 is crucial for THEMIS function in vivo
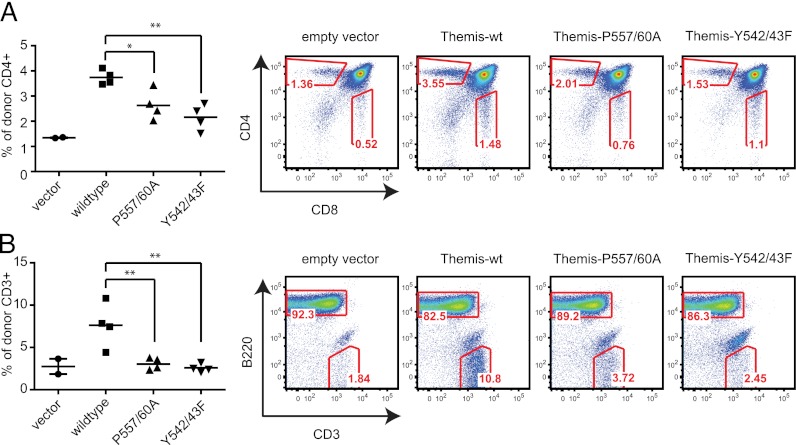
Finally, we determined whether the constitutive THEMIS–GRB2 complex was relevant in thymocyte development. PRR1 in murine THEMIS was disrupted by point mutations (muTHEMIS-PRR1; see Fig. 3A). Lethally irradiated B6.SJL (CD45.1+) recipient mice were reconstituted with Themis−/− bone marrow cells (CD45.2+) retrovirally transduced either with empty vector, muTHEMIS-wt, or muTHEMIS-PRR1. GFP expression driven by an IRES-GFP cassette on the retroviral vector allowed gating on “truly” transduced cells. THEMIS-wt–transduced donor bone marrow cells reconstituted SP thymocyte development (Fig. 6A) and the peripheral T cell compartment (Fig. 6B). By contrast, perturbations of THEMIS-PRR1 led to a severe reduction in CD4 SP thymocytes and peripheral T cells. In line with the critical role of Y540/41 (Y542 and Y543 in mouse) in THEMIS phosphorylation and GRB2 binding (Fig. 6B), muTHEMIS-Y542/43F failed to reconstitute normal T cell development. Taken together, our findings demonstrate that TCR-proximal positioning of THEMIS via GRB2-LAT is physiologically important for thymocyte development.
FIGURE 6.
Critical role of the THEMIS–GRB2 complex for thymocyte development. (A) Development of wt or PRR1-mutant THEMIS expressing thymocytes in bone marrow chimeric mice. Irradiated B6.SJL recipient mice (CD45.1+) were reconstituted with Themis−/− bone marrow cells (CD45.2+) transduced with a bicistronic GFP-based retroviral vector harboring either no insert, muTHEMIS-wt, muTHEMIS-PRR1 (P557/560A), or muTHEMIS-Y542/43F. Four mice were injected per experiment. Eight weeks postreconstitution, only recipient mice bearing >5% CD45.2+GFP+ thymocytes were included and analyzed by flow cytometry. In the far left scheme, each symbol represents an individual mouse. Shown are CD4 versus CD8 stains after gating on CD45.2+GFP+ (transgene expressing) thymocytes. (B) Reconstitution of the peripheral T cell compartment in the bone marrow chimeric mice described in (A). Shown are B220 versus CD3 FACS stains after gating on CD45.2+GFP+ splenocytes. Data shown are representative of two independent experiments. Statistical analysis of THEMIS mutants versus THEMIS-wt as control is shown. *p ≤ 0.05, **p ≤ 0.01, one-way ANOVA (Dunnett’s post hoc test, THEMIS-wt as control). Vector control reconstitutions frequently fail because of low cellularity (failed thymus reconstitution) and only serve to illustrate the background signal of the analysis.
Discussion
Although the essential role of THEMIS in conventional T cell development is well established, its molecular function remains elusive. Protein components that channel and tune TCR-proximal signaling show stereotypical molecular signatures. Thus, soon after TCR engagement, they are recruited directly to the TCR or LAT and are phosphorylated on tyrosine residues (23), inevitably localizing at the IS. Our study shows that these three properties are satisfied by THEMIS, conclusively defining it as an element of the TCR-proximal signaling machinery. We demonstrate that THEMIS requires constitutive association with the adaptor GRB2, which permits recruitment onto LAT, followed by THEMIS tyrosine phosphorylation by active Lck and ZAP70. Consistently, mutations affecting GRB2 binding strongly affected THEMIS accumulation at the IS and T cell development in THEMIS-deficient mice.
We defined GRB2-SH3C binding to THEMIS at the conserved PRR1 site, mediated by the core-binding motif of PxRPxK. Our data contradict recent reports suggesting that GRB2-SH3N mediates binding to THEMIS (5, 21). Although the reason for this discrepancy is unclear, our data do agree with published reports indicating that GRB2-SH3N preferentially binds to motifs conforming to the consensus PxxPxR (15), such as the type II polyproline helix in SOS (24), and displays only negligible affinities toward RxxK motifs (12, 15). This leaves open the possibility that GRB2, via SH3N, helps in bridging LAT and THEMIS to an unknown partner. Live-cell imaging of THEMIS-GFP showed GRB2-dependent dynamic recruitment not exclusively at the center of the IS but also into lamellar structures that transiently cover the entire interface. Such structures appear to be active sites of membrane signaling as defined by the presence of LAT and active signaling proteins.
Our data suggest that when bound to LAT, THEMIS becomes a substrate for Lck and ZAP70, which prominently phosphorylate Y540 and Y541. Database searches found no apparent consensus binding motifs surrounding pY540 and/or Y541, suggesting that these pTyr may serve another function. Enhanced tyrosine phosphorylation of THEMIS when in complex with GRB2 was somewhat surprising, as was the dependency of GRB2 binding on Y540 and Y541. These data evoke similarity to GAREM, a protein regulating EGFR-proximal signaling that contains a single CABIT domain and constitutively associates to GRB2. Mutation of a tyrosine proximal to the GRB2 binding site also abolished GAREM-GRB2 association (25). These perturbations at the GRB2 binding site (a short, supposedly poorly structured sequence), induced by medium- and long-range distal alterations in THEMIS structure, indirectly suggest the existence of a complex network of intramolecular interactions. Thus, Y540/541 may be gatekeepers at a CABIT domain–proximal region undergoing conformational changes upon their phosphorylation. This may be indicative of a “closed” structure that could be unleashed for functional activation, similar to tyrosine phosphorylation–dependent mechanism that releases autoinhibition in Vav proteins (26). Further studies using recombinant THEMIS should allow testing of this model.
Notably, the THEMIS PRR1 mutant construct tested in this study gave an intermediate phenotype between wt and empty vector in thymocytes. This is in apparent contradiction to the biochemical data where we observed a complete loss of GRB2 binding. However, using a more sensitive imaging approach (Fig. 3B, 3C), we saw severely reduced and delayed, but still detectable, IS recruitment of THEMIS-PRR1 when compared with THEMIS-wt. A residual binding affinity for GRB2 of the disturbed, but not deleted, RXXK region might yield the intermediate phenotype of THEMIS-PRR1, perhaps because of the innate flexibility of this poorly structured region. Of note, the incomplete loss of function was only evident in the thymus, but not in peripheral T cells.
Our data might help explain the role of THEMIS during thymocyte development. Indeed, it is of particular interest that both THEMIS–GRB2 and SOS–GRB2 complexes can bind to the same sites on LAT, and that in immature CD4+8+ thymocytes, THEMIS and SOS1 expression substantially increases or decreases, respectively, relative to their expression in earlier stage CD4−8− thymocytes (1, 27). During positive selection, association of the THEMIS–GRB2 complex to LAT may prevail over SOS1–GRB2, the former favoring a LAT-SLP76 signalosome configuration triggering the weak but sustained ERK and calcium signaling required for positive selection (28). Agonist-driven negative selection (that elicits strong and transient ERK activation) (28) might instead favor SOS1-GRB2/LAT-type signalosomes during the late phases of (or post) positive selection, when THEMIS levels decrease, thus favoring cell death. The observation that positive section requires THEMIS (1–3), but not SOS proteins (27), and recent data that SOS1 is instead required for negative selection (29) agree with this model. A functional role of constitutive THEMIS–GRB2 association in thymocyte positive selection agrees with data on conditional ablation of GRB2 in thymocytes (30), which showed that GRB2–SH2 and –SH3C (required for association to THEMIS) were found to be indispensable for thymocyte development (30).
In conclusion, our data firmly establish that thymocyte development crucially depends on THEMIS as a component of the TCR-proximal signaling machinery. It will be important to identify other THEMIS interacting partners, and the molecular basis by which it regulates the TCR signaling cascade.
Acknowledgments
We thank V. Cerundolo, R.A. Weinberg, and L.E. Samelson for plasmids, B. Thomas for help with MS data analysis, and all the members of the T cell signaling laboratory for helpful discussions. We are grateful to Dr. Gottfried Baier for providing the Itk inhibitor BMS-509744.
This work was supported by Wellcome Trust Grant GR076558MA (to O.A.); EU-FP7 “Sybilla” Grant 201106 (to O.A.); National Institutes of Health Grants AI073870 (to N.R.J.G.), GM065230 (to N.R.J.G.), and T32 AI007244 (to J.A.H.H.); the Erwin Schroedinger Fellowship from the Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung; to W.P.); EU-FP7 “Targetbinder” (S.M.F.); the Breast Cancer Campaign (S.M.F.); the Oxford Cancer Research Centre Development Fund (S.M.F.); and the Irving S. Sigal Postdoctoral Fellowship (to J.A.H.H.). This is manuscript number 21958 from The Scripps Research Institute.
The online version of this article contains supplemental material.
- CABIT
- cysteine-containing all β in THEMIS
- CPFP
- central proteomics facility pipeline
- DP
- double-positive
- GRB2
- growth factor receptor–bound protein 2
- HEK293
- human embryonic kidney epithelial cells
- IS
- immunological synapse
- Itk
- IL-2–inducible T cell kinase
- LAT
- linker for activation of T cells
- MCC
- moth cytochrome c
- MS
- mass spectrometry
- muTHEMIS-PRR1
- proline-rich region 1 in murine thymocyte-expressed molecule involved in selection disrupted by point mutations
- NaPV
- sodium pervanadate
- PLC
- phospholipase C
- PRR
- proline-rich region
- SH
- Src homology
- SH3C
- C-terminal SH3 domain
- SH3N
- N-terminal SH3 domain
- SLP76
- Src homology 2 domain-containing leukocyte protein of 76 kDa
- SOS
- son of sevenless
- THEMIS
- thymocyte-expressed molecule involved in selection
- THEMIS-PRR1
- THEMIS carrying alanine substitutions at P555 and P558 of PRR1
- wt
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Fu G., Vallée S., Rybakin V., McGuire M. V., Ampudia J., Brockmeyer C., Salek M., Fallen P. R., Hoerter J. A., Munshi A., et al. 2009. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat. Immunol. 10: 848–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson A. L., Aravind L., Shulzhenko N., Morgun A., Choi S. Y., Crockford T. L., Lambe T., Domaschenz H., Kucharska E. M., Zheng L., et al. 2009. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat. Immunol. 10: 831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lesourne R., Uehara S., Lee J., Song K. D., Li L., Pinkhasov J., Zhang Y., Weng N. P., Wildt K. F., Wang L., et al. 2009. Themis, a T cell-specific protein important for late thymocyte development. Nat. Immunol. 10: 840–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gascoigne N. R., Palmer E. 2011. Signaling in thymic selection. Curr. Opin. Immunol. 23: 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick M. S., Oda H., Hayakawa K., Sato Y., Eshima K., Kirikae T., Iemura S., Shirai M., Abe T., Natsume T., et al. 2009. Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proc. Natl. Acad. Sci. USA 106: 16345–16350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chabod M., Pedros C., Lamouroux L., Colacios C., Bernard I., Lagrange D., Balz-Hara D., Mosnier J. F., Laboisse C., Vergnolle N., et al. 2012. A spontaneous mutation of the rat Themis gene leads to impaired function of regulatory T cells linked to inflammatory bowel disease. PLoS Genet. 8: e1002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brockmeyer C., Paster W., Pepper D., Tan C. P., Trudgian D. C., McGowan S., Fu G., Gascoigne N. R., Acuto O., Salek M. 2011. T cell receptor (TCR)-induced tyrosine phosphorylation dynamics identifies THEMIS as a new TCR signalosome component. J. Biol. Chem. 286: 7535–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trudgian D. C., Thomas B., McGowan S. J., Kessler B. M., Salek M., Acuto O. 2010. CPFP: a central proteomics facilities pipeline. Bioinformatics 26: 1131–1132 [DOI] [PubMed] [Google Scholar]
- 9. Trudgian, D. C., R. Singleton, M. E. Cockman, P. J. Ratcliffe, and B. M. Kessler. 2012. ModLS: Post-translational modification localization scoring with automatic specificity expansion. J. Proteomics Bioinform. 5: 283–289.
- 10.Beausoleil S. A., Villén J., Gerber S. A., Rush J., Gygi S. P. 2006. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 24: 1285–1292 [DOI] [PubMed] [Google Scholar]
- 11.Singleton K. L., Roybal K. T., Sun Y., Fu G., Gascoigne N. R., van Oers N. S., Wülfing C. 2009. Spatiotemporal patterning during T cell activation is highly diverse. Sci. Signal. 2: ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewitzky M., Kardinal C., Gehring N. H., Schmidt E. K., Konkol B., Eulitz M., Birchmeier W., Schaeper U., Feller S. M. 2001. The C-terminal SH3 domain of the adapter protein Grb2 binds with high affinity to sequences in Gab1 and SLP-76 which lack the SH3-typical P-x-x-P core motif. Oncogene 20: 1052–1062 [DOI] [PubMed] [Google Scholar]
- 13.Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. 1994. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell 76: 933–945 [DOI] [PubMed] [Google Scholar]
- 14.Harkiolaki M., Tsirka T., Lewitzky M., Simister P. C., Joshi D., Bird L. E., Jones E. Y., O’Reilly N., Feller S. M. 2009. Distinct binding modes of two epitopes in Gab2 that interact with the SH3C domain of Grb2. Structure 17: 809–822 [DOI] [PubMed] [Google Scholar]
- 15.Lewitzky M., Harkiolaki M., Domart M. C., Jones E. Y., Feller S. M. 2004. Mona/Gads SH3C binding to hematopoietic progenitor kinase 1 (HPK1) combines an atypical SH3 binding motif, R/KXXK, with a classical PXXP motif embedded in a polyproline type II (PPII) helix. J. Biol. Chem. 279: 28724–28732 [DOI] [PubMed] [Google Scholar]
- 16.Balagopalan L., Coussens N. P., Sherman E., Samelson L. E., Sommers C. L. 2010. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb. Perspect. Biol. 2: a005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin T. A., McIntyre K. W., Das J., Liu C., O’Day K. D., Penhallow B., Hung C. Y., Whitney G. S., Shuster D. J., Yang X., et al. 2004. Selective Itk inhibitors block T-cell activation and murine lung inflammation. Biochemistry 43: 11056–11062 [DOI] [PubMed] [Google Scholar]
- 18.Brdicka T., Kadlecek T. A., Roose J. P., Pastuszak A. W., Weiss A. 2005. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol. Cell. Biol. 25: 4924–4933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blom N., Gammeltoft S., Brunak S. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294: 1351–1362 [DOI] [PubMed] [Google Scholar]
- 20.Xue Y., Zhou F., Zhu M., Ahmed K., Chen G., Yao X. 2005. GPS: a comprehensive www server for phosphorylation sites prediction. Nucleic Acids Res. 33(Web Server issue): W184–W187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesourne R., Zvezdova E., Song K. D., El-Khoury D., Uehara S., Barr V. A., Samelson L. E., Love P. E. 2012. Interchangeability of Themis1 and Themis2 in thymocyte development reveals two related proteins with conserved molecular function. J. Immunol. 189: 1154–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nika K., Soldani C., Salek M., Paster W., Gray A., Etzensperger R., Fugger L., Polzella P., Cerundolo V., Dushek O., et al. 2010. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32: 766–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acuto O., Di Bartolo V., Michel F. 2008. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat. Rev. Immunol. 8: 699–712 [DOI] [PubMed] [Google Scholar]
- 24.Terasawa H., Kohda D., Hatanaka H., Tsuchiya S., Ogura K., Nagata K., Ishii S., Mandiyan V., Ullrich A., Schlessinger J., et al. 1994. Structure of the N-terminal SH3 domain of GRB2 complexed with a peptide from the guanine nucleotide releasing factor Sos. Nat. Struct. Biol. 1: 891–897 [DOI] [PubMed] [Google Scholar]
- 25.Tashiro K., Tsunematsu T., Okubo H., Ohta T., Sano E., Yamauchi E., Taniguchi H., Konishi H. 2009. GAREM, a novel adaptor protein for growth factor receptor-bound protein 2, contributes to cellular transformation through the activation of extracellular signal-regulated kinase signaling. J. Biol. Chem. 284: 20206–20214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghazadeh B., Lowry W. E., Huang X. Y., Rosen M. K. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102: 625–633 [DOI] [PubMed] [Google Scholar]
- 27.Kortum R. L., Sommers C. L., Alexander C. P., Pinski J. M., Li W., Grinberg A., Lee J., Love P. E., Samelson L. E. 2011. Targeted Sos1 deletion reveals its critical role in early T-cell development. Proc. Natl. Acad. Sci. USA 108: 12407–12412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniels M. A., Teixeiro E., Gill J., Hausmann B., Roubaty D., Holmberg K., Werlen G., Holländer G. A., Gascoigne N. R., Palmer E. 2006. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 444: 724–729 [DOI] [PubMed] [Google Scholar]
- 29.Kortum R. L., Sommers C. L., Pinski J. M., Alexander C. P., Merrill R. K., Li W., Love P. E., Samelson L. E. 2012. Deconstructing Ras signaling in the thymus. Mol. Cell. Biol. 32: 2748–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang I. K., Zhang J., Chiang Y. J., Kole H. K., Cronshaw D. G., Zou Y., Gu H. 2010. Grb2 functions at the top of the T-cell antigen receptor-induced tyrosine kinase cascade to control thymic selection. Proc. Natl. Acad. Sci. USA 107: 10620–10625 [DOI] [PMC free article] [PubMed] [Google Scholar]