To the Editor
Protozoan parasites of the genus Plasmodium are the vector-borne causative agents of malaria, responsible for more than 350 million clinical cases and one million deaths annually. Studies using rodent malaria models have shown that during the bite of an infected Anopheles mosquito, Plasmodium sporozoites are transmitted and subsequently traverse a variety of cell types, wounding their membranes on the way1. This allows sporozoites to travel through the dermis into the bloodstream and then pass through the cell layer that lines blood vessels in the liver as well as several hepatocytes before taking up residence in host hepatocytes for further development as exoerythrocytic forms (EEFs)2. Although cell traversal provides a means to cross tissue barriers, it is unclear whether this constitutes its only biological role. A novel function for parasite host cell traversal was proposed by Carrolo et al.3, who showed that cell traversal by sporozoites of the rodent malaria parasite Plasmodium berghei induces secretion of hepatocyte growth factor (HGF). HGF is a soluble factor that activates the receptor tyrosine kinase MET, which is capable of initiating a cascade of signaling events that result in hepatocyte proliferation and survival. Carrolo et al.3 further showed that this signaling cascade is crucial to promote early development of EEFs and thus substantially contributes to transmission success. More recently, the same group demonstrated, again using P. berghei, that MET signaling events are important for the parasite to protect itself from host hepatocyte apoptosis4 and subsequently showed that a dietary supplement, genistein, which inhibits MET activation, can treat liver-stage malaria infection5. A fundamental question, then, is whether this unique function for cell traversal is broadly conserved among Plasmodium species and whether it is found in human malaria parasites.
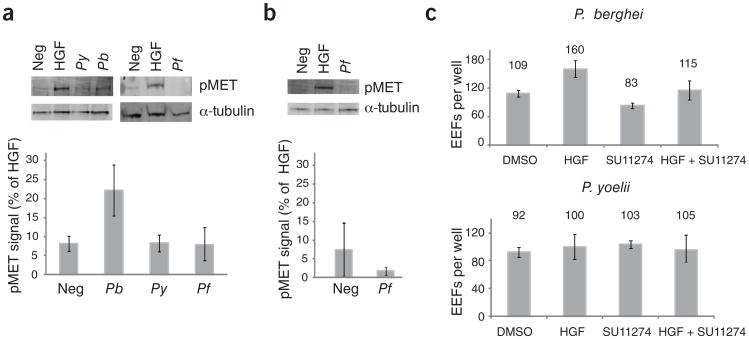
To test the ability of another rodent malaria parasite species, Plasmodium yoelii, and the human malaria parasite, Plasmodium falciparum, to activate MET by cell traversal, we incubated sporozoites of each species with HepG2-CD81 hepatoma cells for 1 h to allow traversal and then collected cellular lysates. Using an antibody specific to the phosphorylated activation loop of MET, we probed the lysates for MET activation. As a negative control, we used lysates from cells that had been incubated with salivary gland extracts from uninfected mosquitoes (Supplementary Methods). We found that cell traversal by P. berghei sporozoites but not P. yoelii or P. falciparum sporozoites led to the activation of MET (Fig. 1a). To ensure that P. falciparum does not fail to activate MET because it is being assayed in a cell type that does not support its EEF development, we allowed P. falciparum sporozoites to traverse HC04 cells, a hepatocyte-derived cell line that does support P. falciparum EEF development6, but, again, we did not detect MET activation (Fig. 1b). To ensure that differences in MET activation were not due strictly to differences in traversal rates, we incubated sporozoites with HepG2-CD81 cells in the presence of labeled dextran. Given that cells that are wounded by traversal take up the labeled dextran, we used FACS to identify traversed cells (Supplementary Methods). We observed comparable rates of traversal for each of the three species. Modest differences in traversal rates between species did not correlate with MET activation. Instead, MET activation was only observed for P. berghei, which had slightly lower traversal rates than P. yoelii, and similar traversal rates when compared to P. falciparum (Supplementary Fig. 1).
Figure 1.
MET signaling is crucial for P. berghei(Pb) but not P. yoelii(Py) or P. falciparum (Pf) hepatocyte infection. (a,b) Cells were incubated with Plasmodium sporozoites for 1 h. Lysates were probed for the presence of activated MET receptor (pY1234/1235) in either HepG2-CD81 cells (a) or HC04 cells (b). Signal was detected in triplicate, normalized to human α-tubulin, and signal produced by 1h of HGF treatment was set to 100. Error bars represent s.d. of triplicate measurements. (c) HepG2-CD81 cells were treated with either HGF or the MET inhibitor SU11274 for 1 h then infected with 1 × 105 P. berghei or P. yoelii sporozoites. Parasites were allowed to develop for 24 h, visualized by immunofluorescence assay and manually counted. Error bars represent s.d. of biological replicate experiments.
To assess whether direct MET activation causes substantial differences in parasite invasion or development rates, we infected HepG2-CD81 cells with P. yoelii or P. berghei after treatment with either HGF or the MET inhibitor SU11274. We found that HGF pretreatment led to an increase in P. berghei EEFs, whereas MET inhibition led to fewer P . berghei EEFs (Fig. 1c and Supplementary Fig. 2), in agreement with Carrolo et al.3. However, no difference in P. yoelii EEF burden was seen after modulation of MET signaling (Fig. 1c and Supplementary Fig. 2).
Taken together, our data suggest that, unlike P. berghei, neither P. yoelii nor P. falciparum activates MET signaling upon cell traversal. Furthermore, P. yoelii host cell invasion and EEF development do not seem to be affected by MET activation or inactivation. All three species traverse HepG2-CD81 cells at comparable rates, suggesting a particular trait unique to P. berghei cell traversal that leads to HGF secretion. Furthermore, P. berghei invasion, early development or both have a stronger dependency on MET. This is particularly interesting in light of the observations that P. berghei is less selective in its host cell than other Plasmodium species. For instance, P. berghei can readily infect HepG2, Hepa1-6 and Huh7 and nonhepatocytic cells, whereas P. yoelii and P. falciparum require CD81 to invade cells7 and only develop in hepatocyte-derived cells. Recently, it has been demonstrated that P. berghei EEFs develop fully inside the skin of the mouse8, but similar evidence for other rodent Plasmodium species is less direct9, and none exists for P. falciparum. One possibility is that P. berghei is able to use receptor tyrosine kinase signaling, which is available in nearly all cell types and lines, to facilitate invasion and early development, whereas other Plasmodium species such as P. yoelii and P. falciparum require more specialized molecules including but not limited to CD81, combinations of which are only provided in a very small number of hepatocyte-derived cell lines or on primary hepatocytes.
Our findings are consistent with the few instances of published data comparing the impact of host signaling in the two rodent parasite species. Cunha-Rodrigues et al.7 recently showed that genistein, a dietary supplement that acts at least in part by inhibiting MET activation, lowers P. berghei liver stage burden in mice. However, higher levels of genistein are required to reduce P. yoelii EEFs in vitro. Identifying previously unknown signaling proteins in the hepatocyte that are required for invasion and liver stage development remains a crucial point of investigation, but caution should be taken to ensure that findings are broadly applicable to malaria parasites in general, including those infecting humans.
Supplementary Material
Footnotes
Competing Financial Interests: The authors declare no competing financial interests.
References
- 1.Mota MM, et al. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan AM, Aly AS, Kappe SH. Cell Host Microbe. 2008;4:209–218. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrolo M, et al. Nat Med. 2003;9:1363–1369. doi: 10.1038/nm947. [DOI] [PubMed] [Google Scholar]
- 4.Leirião P, et al. Cell Microbiol. 2005;7:603–609. doi: 10.1111/j.1462-5822.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 5.Cunha-Rodrigues M, et al. PLoS ONE. 2008;3:e2732. doi: 10.1371/journal.pone.0002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sattabongkot J, et al. Am J Trop Med Hyg. 2006;74:708–715. [PubMed] [Google Scholar]
- 7.Silvie O, et al. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 8.Gueirard P, et al. Proc Natl Acad Sci USA. 2010;107:18640–18645. doi: 10.1073/pnas.1009346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppi A, et al. J Exp Med. 2011;208:341–356. doi: 10.1084/jem.20101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.