Summary
Carabids and other epigeal arthropods make important contributions to biodiversity, food webs and biocontrol of invertebrate pests and weeds. Pitfall trapping is widely used for sampling carabid populations, but this technique yields biased estimates of abundance (‘activity‐density’) because individual activity – which is affected by climatic factors – affects the rate of catch. To date, the impact of temperature on pitfall catches, while suspected to be large, has not been quantified, and no method is available to account for it. This lack of knowledge and the unavailability of a method for bias correction affect the confidence that can be placed on results of ecological field studies based on pitfall data.
Here, we develop a simple model for the effect of temperature, assuming a constant proportional change in the rate of catch per °C change in temperature, r, consistent with an exponential Q10 response to temperature. We fit this model to 38 time series of pitfall catches and accompanying temperature records from the literature, using first differences and other detrending methods to account for seasonality. We use meta‐analysis to assess consistency of the estimated parameter r among studies.
The mean rate of increase in total catch across data sets was 0·0863 ± 0·0058 per °C of maximum temperature and 0·0497 ± 0·0107 per °C of minimum temperature. Multiple regression analyses of 19 data sets showed that temperature is the key climatic variable affecting total catch. Relationships between temperature and catch were also identified at species level. Correction for temperature bias had substantial effects on seasonal trends of carabid catches.
Synthesis and Applications. The effect of temperature on pitfall catches is shown here to be substantial and worthy of consideration when interpreting results of pitfall trapping. The exponential model can be used both for effect estimation and for bias correction of observed data. Correcting for temperature‐related trapping bias is straightforward and enables population estimates to be more comparable. It may thus improve data interpretation in ecological, conservation and monitoring studies, and assist in better management and conservation of habitats and ecosystem services. Nevertheless, field ecologists should remain vigilant for other sources of bias.
Keywords: activity‐density, Arrhenius equation, Carabidae, differencing, meta‐analysis, model estimation, monitoring, pitfall traps
Short abstract
The effect of temperature on pitfall catches is shown here to be substantial and worthy of consideration when interpreting results of pitfall trapping. The exponential model can be used both for effect estimation and for bias correction of observed data. Correcting for temperature‐related trapping bias is straightforward and enables population estimates to be more comparable. It may thus improve data interpretation in ecological, conservation and monitoring studies, and assist in better management and conservation of habitats and ecosystem services. Nevertheless, field ecologists should remain vigilant for other sources of bias.
Introduction
Epigeal arthropods play a vital role in ecosystem functioning, due to their high abundance and taxonomic as well as functional diversity (Kromp 1999; Holland 2002). Among this fauna, carabid beetles (Coleoptera: Carabidae) are numerically dominant. They provide valuable ecosystem services such as predation on crop pests and weed seeds, and food for farmland birds (Thiele 1977; Kromp 1999; Holland 2002). Carabids are widely used as indicator species in studies on diversity, ecosystem functioning and environmental quality (Leslie et al. 2007; Bohan et al. 2011; Kotze et al. 2011).
Pitfall traps are widely used for sampling carabids and other epigeal arthropods (Southwood & Henderson 2000). Advantages are low cost and ease of use. However, interpretation of pitfall trap data is contentious because the size of the catch is not only affected by density, but also by the activity of the sampled organisms. Hence, pitfall trap catches have been described as ‘activity‐density’ (Heydemann 1953). Weather, and especially temperature, is suspected to have a large effect on the activity of epigeal arthropods (Messenger 1959; Mitchell 1963; Honek 1988, 1997a). While there is a large body of literature on the effects of weather on catches of flying insects (Williams 1940; Taylor 1963; Briers, Carsiss & Gee 2003), there is little quantitative evidence for the effect of weather on trap catch rates of epigeal arthropods. Correlations between temperature and carabid catch have been documented (Dempster 1967; Jones 1976; Hatten et al. 2007), but these may reflect parallel seasonal patterns in temperature and species emergence, activity and motivation rather than the direct effect of temperature on catch (Johnson 1969). Honek (1997b) was the only one who conducted a methodologically rigorous study on the effect of temperature on carabid catches. Unfortunately, it is difficult to generalize from his analysis because it was based on a short time series of catches of only a single carabid species.
Here, we propose a simple exponential model to describe the relationship between catch rate and temperature, and use 38 published data sets from 4 countries to fit this model to data. The estimation method accounts for seasonal trends in the data by analysing differences rather than raw data (Cormac & Ord 1979). We compare three methods of differencing. The resulting estimates of temperature effect are then analysed in a meta‐analysis framework to calculate the average effect of temperature as accurately as possible, and assess consistency among studies. We then describe an approach to correct time series of carabid pitfall catches for temperature bias and show practical examples of the effect of such correction. Furthermore, we study species‐specific temperature responses and explore the influence of other weather factors than temperature.
The following research questions were addressed: (i) is there a consistent direct temperature effect on carabid trap catches across data sets at the total catch and/or species level, and if so, how large? (ii) do climatic factors other than temperature affect catch, and how important are they, compared to temperature? (iii) can temperature‐related biases associated with pitfall catch data be corrected for, and would such corrections affect estimated seasonal trends in carabid abundance?
Materials and methods
Data
We assembled 38 time series of carabid catches with associated weather data from the Czech Republic, the Netherlands, the UK and the USA. All data sets comprised at least 15 consecutive pitfall samples at a single location. Most data sets (28) are from arable crop systems, one was from a field edge, four from perennial grassland and five from apple orchards (details in Tables S1 and S2 in Supporting Information). The data sets were collected from 1974 to 2010. Data originating from the same area and year but from different types of vegetation were analysed separately because differences in vegetation structure affect microclimate and trap catch (Crist & Ahern 1999; Hatten et al. 2007). Data sets were standardized by calculating the rate of catch as numbers caught per trap per day. As the analysis entails taking logarithms, we added 1 to all data points to account for zeros.
Pitfall traps are usually placed and emptied in the morning. Accordingly, the mean minimum and maximum temperatures experienced during a sample interval were calculated from the first day of the sampling period until (and including) the day before emptying (Hemerik & Brussaard 2002).
Model for the relationship between temperature and catch rate
As a basis for our analysis, we postulate that an absolute change in temperature will result in a relative change in daily catch and that this relative change in daily catch per unit of temperature is constant over an ecologically relevant range of temperature (Williams 1940). Mathematically:
| (eqn 1) |
where T is temperature in °C, and is the daily catch, that is, the number of individuals caught daily at temperature T, and the estimated parameter r represents the rate of change in relative catch rate predicted to occur at a given temperature. As an example, if r = 0·04, an increase in 1 °C will lead to an increase of exp(r) = 1·0408 in catch, that is, 4·08%.
The relative character of the parameter r with respect to the measurement of catch is critical because details of pitfall‐trapping method vary by study (i.e. they differ in size, material, liquid in the pitfall, cover, et cetera; see Table S1). If the effect of temperature was expressed as an absolute change in the catch, effects of the pitfall design would enter into the estimate of the parameter r and make the result less generic. Moreover, the use of a relative change in the catch implies an exponential relationship, which is characteristic of temperature‐dependent rates in biological systems (Williams 1940; Logan et al. 1976).
The solution to equation (1) is an exponential relationship between the catch and temperature during any two sampling periods, with a multiplication factor of exp(r) per °C:
| (eqn 2) |
where n 1 and n 2 are catch samples from the same data series at any two times 1 and 2, and T 1 and T 2 are the average temperatures during the catch intervals for both catches. Formula (2) can also be expressed as
| (eqn 3) |
where log denotes natural logarithm. Thus, r can be estimated from the data, using the relationship:
| (eqn 4) |
that is, by regressing the difference in natural logarithm of two catches on the temperature difference between two subsequent catch periods, which estimates how an increase or decrease in log(catch) between two dates is related to the difference in temperature. Both minimum and maximum daily temperatures were tested as a predictor of catch, considering that the catch may contain both diurnal and nocturnal species. Maximum temperature data were not available for data set #33; therefore, this data set was analysed for minimum temperature only. Wherever we discuss the relationship between catch rate and temperature in the remainder of this study, this was effectively studied by regressing the difference in the log of the catch rate (+1) on the difference in temperature.
Estimation of the effect of temperature in individual data sets by regression with differences
Time series are prone to showing autocorrelations that may be corrected by detrending. The need to detrend the time series was shown by conducting an autoregression analysis on the catch and temperature data (Table S3). Calculated autoregression coefficients, ar k, were calculated using the ar function in the programming language R, version 2.8.0 (R Development Core Team 2010), where, for example, ar 1 indicates a linear trend, ar 2 a quadratic trend, etc.
Equation 4 was fitted to the data by taking first‐order differences of the log of the catch and of the temperature records through time and regressing one on the other (Cormac & Ord 1979). A difference in catch rate between two periods is therefore compared with the difference in temperature between the same two periods. In the process of taking differences, the effect of seasonal trends in temperature and catch is removed, avoiding the risk of spurious correlation when unrelated time series are regressed against one another (Cormac & Ord 1979). We also tested two other methods for estimating the local (i.e. one point in time) response of catch rate to temperature. These are called ‘two‐point piece‐wise detrending’ and ‘four‐point piece‐wise detrending’, based on the number of time points that is considered in addition to the focal time point (see Supporting information: Appendices S1 and S2). The key difference between the methods is the width of time interval over which reference data are used to estimate the temperature response at a given point in time: two or four time points. Appendix S1 gives theory and Appendix S2 shows an example data analysis. As the three methods of parameter estimation yielded similar results, we focus on results from first‐order differencing, a well‐established statistical method (Cormac & Ord 1979; Shumway & Stoffer 2006).
Synthesizing regression results in individual data sets to an overarching relationship, using meta‐analysis
Following the estimation of the slope of the relationship between Δlog(catch) and Δtemperature in 38 data sets, the overall effect of temperature was assessed by combining in a meta‐analysis, the 37 estimated rate coefficients for maximum temperature and the 38 estimated rate coefficients for minimum temperature. In meta‐analysis, a weighted mean rate is calculated taking into account the variability of the rate estimates in each study. In the first step, it is assumed that all studies are essentially estimating the same rate, and variability among the studies (between study variance) is assumed to be due to sampling error only. This is the fixed‐effects model (Rosenberg et al. 2004; Madden & Paul 2011). On the contrary, the random‐effects model accounts for the possibility that different studies estimate different rates, due to uncontrolled differences in the study designs, for example, the vegetation, the type or size of the trap, duration of sampling interval, the collection fluid, etc.
In the fixed‐effects model, the weight for each study is inversely proportional to the variance of the rate estimate:
| (eqn 5) |
where v i is the variance of the estimated rate in study i. In the random‐effects model, the weights are calculated as:
| (eqn 6) |
where v i is the variance of the estimated rate in study i and is the estimated between‐study variance. Adding in the denominator causes the weights to become more similar to each other than in eqn. 5: the weight of studies with a very accurate estimate of the rate is diminished and the weight of studies with an inaccurate estimate is increased as compared to the fixed‐effects meta‐analysis. This reflects the notion that each study has something to say about the average, because the between study differences are important. The between study variance, , is estimated in a fixed‐effects meta‐analysis as:
| (eqn 7) |
where Q T is the total heterogeneity determined from a fixed‐effects model meta‐analysis (Rosenberg et al. 2004; see below). Although the weights are defined differently for the fixed‐ and random‐effects model, the average rate is calculated for both with the same formula:
| (eqn 8) |
where r i is the rate estimate in study i. If the pooled variance in eqn. 7 is very large as compared to the variance of single study estimates (i.e. large heterogeneity), then all studies have approximately the same weight, and meta‐analysis yields the simple arithmetic average as overall rate estimate. If the pooled variance is small, the studies are weighed according to the precision (as measured by the inverse of the variance) of the estimate of each r i. The average rate has variance (=squared standard error):
| (eqn 9) |
Significance of this average rate (as compared to a value of 0 under the null hypothesis of no relationship between temperature and the catch) is determined by constructing a confidence interval based on the t‐distribution, and determining whether zero is included.
The need for using the random‐effects model is assessed by calculating a measure of heterogeneity between studies in the fixed‐effects model:
| (eqn 10) |
Q T is tested against a χ2 distribution with n‐1 degrees of freedom, where n is the number of studies (Madden & Paul 2011). If there is significant heterogeneity, the random‐effects model is supported, and estimates from the fixed‐effects model are not statistically valid.
The meta‐analysis was performed in MetaWin 2.0 (Rosenberg, Adams & Gurevitch 2000).
Correction of time series for temperature bias
After the size of the temperature effect is estimated from the data, this effect may be corrected for to obtain a standardized catch rate, with all temperature influence removed. The correction can be done using Equation 2, taking n 1 as the corrected catch at reference temperature T 1, while the observed catch is n 2 and the observed temperature T 2. A whole data series can be corrected in this way, where n 2 and T 2 vary according to the chosen time point in the data series, while T 1 is a constant reference temperature. As a result, n 1 is a time series corrected for temperature bias. In this study, we used either the average maximum temperature during an experiment or a constant temperature of 20 °C as reference temperature T 1.
A salient question is whether the rate estimate for bias correction can be taken from the meta‐analysis in the current study (see Results) or should be estimated from an analysis of the relationship between log(catch) and temperature within the time series that is under consideration. The rate estimate from our study (see Results) would be preferable if it has lower uncertainty then the rate estimate from a new study. In the case of the random‐effects model, the standard error of the rate estimate for a new study r new (i.e. prediction error) comprises two components: the variance of the average rate estimate obtained in this study (eqn. 9) and the between study variance (eqn. 7). These are combined as:
| (eqn 11) |
This prediction error can be directly compared to the standard error of a single estimate r i in a study, and to the overall mean error of individual rate estimates (= square root of the mean within study variance. We make these comparisons to assess whether it is advisable to use the average rate from the meta‐analysis for bias correction in future work.
Species‐specific responses
To determine whether we could identify temperature effects in catches of single species, we conducted the analysis for catch series of ‘dominant’ species, that is, species that constituted more than 5% of the total catch in a data set (Table S4). Each species was classified according to its diel activity, if known (e.g. Thiele 1977; Luff 1978; Kegel 1990). A total of 165 data sets (maximum temperature) and 168 data sets (minimum temperature), representing 37 species, were analysed.
Multiple climatic factors
Multivariable effects were investigated using multiple linear regression in 19 data sets (#1 ‐ #17, #37, #38). Before analysis, we first excluded variables showing strong collinearity. For instance, daily heat sum is strongly correlated with irradiation (Crawley 2005). Five weather variables showing minimal collinearity were selected: maximum temperature, daily precipitation, air pressure, air humidity and wind speed. These variables were calculated first as a daily value, and then averaged over the sampling interval. First‐order differences were taken before analysis. We started out by fitting all variables in a full regression model without interactions and then reduced the model by step‐wise removal of insignificant variables on the basis of F‐tests, until a parsimonious model with only significant terms was obtained (Crawley 2005).
Results
Temperature effects at the total catch level
Two‐thirds of the data sets yielded significant regressions between catch rate and temperature. Data and analysis in Fig. 1 exemplifies a common pattern showing that catches are to be higher during episodes with higher than lower temperatures and vice versa (Fig. 1b, c). The distribution of regression slopes for the effect of maximum temperature and minimum temperature on catch rate indicates a sigmoid distribution of the slopes (Fig. 2). Rate estimates for maximum temperature in individual data sets are mostly significant (23 of 37), whereas regressions on minimum temperature are mostly non‐significant (7 of 38 significant). Thus, maximum temperature was in most data sets a better predictor of catch rate than minimum temperature, having greater regression slope r, greater R2 and greater significance of the relationship. Differences in results between detrending methods were minor, with only small differences in estimated slopes, R2 and P‐values of the regressions (Table S5).
Figure 1.
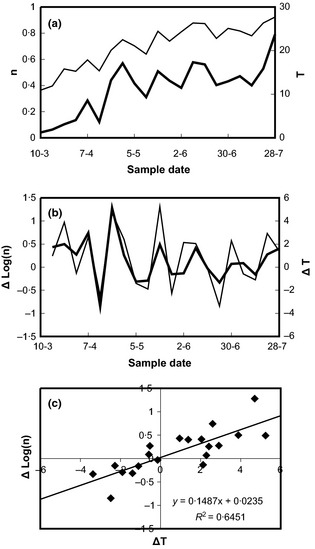
Relationship of time course of temperature and carabid catch rate (#1, Wageningen, the Netherlands 2004, field 8). Panel (a) shows the time trends of the catch (bold line) and of temperature (thin line). Panel (b) shows the same time series in the form of first differences (logs in the case of the catch). Panel (c) displays a significant regression of the difference in the log of the catch on the difference in temperature.
Figure 2.
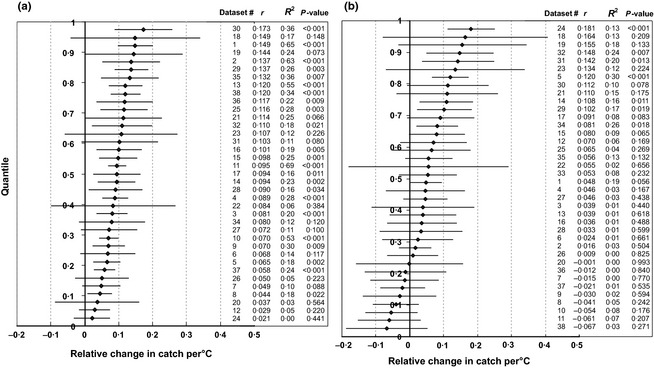
Quantile plots of the temperature effect parameter r (ordered slope parameters r ± 95% confidence intervals). Labels on the side indicate for each point the data set #, the value of the slope parameter r, the coefficient of determination R2 and the P‐value of the regression. Panel (a) is for maximum temperature, panel (b) for minimum temperature.
The regression slopes were analysed in meta‐analysis to assess between study variability, the overall mean effect of temperature across data sets, and its significance. The random‐effects model was supported as shown by large values of heterogeneity: Q T = 51·5, d.f. = 36, P = 0·045 for maximum temperature, and Q T = 75·0, d.f. = 37, P < 0·001 for minimum temperature). Thus, there are significant differences between studies in the effect of temperature on catch rate, and the weights are calculated according to eqn. 6. The mean rate of increase in catch per °C of maximum temperature was 0·0863 ± 0·0058 (t36 = 14·9; P < 0·001), which translates into a Q10 value of exp(10 * 0·0863) = 2·37. The mean rate of increase in catch per °C of minimum temperature was 0·0497 ± 0·0107 (t37 = 4·64; P < 0·001) per °C minimum temperature, which translates into a Q10 value of exp(10* 0·0497) = 1·64 for minimum temperature. Equivalently, the catch doubles for every 8·0 °C increase in maximum temperature or every 14·0 °C increase in minimum temperature. The estimates of the mean rate are very significant (P ≪ 0·001) for both maximum and minimum temperature, corroborating the long suspected influence of temperature on pitfall catches of carabids. The results of the meta‐analysis for two‐point and four‐point piece‐wise detrending were very similar (Table 1).
Table 1.
Meta‐analysis of the temperature effect parameter r, estimated with three methods: differencing, two‐point piece‐wise detrending and four‐point piece‐wise detrending
| Quantity | Maximum temperature | Minimum temperature | |||||
|---|---|---|---|---|---|---|---|
| Differencing | 2 point | 4 point | Differencing | 2 point | 4 point | ||
| Q T (Heterogeneity) | 51·5 | 69·3 | 127·1 | 75·0 | 114·1 | 67·8 | |
| d.f. | 36 | 36 | 36 | 37 | 37 | 37 | |
| P‐value | 0·045 | <0·001 | <0·001 | <0·001 | <0·001 | 0·001 | |
|
|
0·0863 | 0·0900 | 0·0921 | 0·0497 | 0·0459 | 0·0476 | |
|
|
0·0058 | 0·0068 | 0·0081 | 0·0107 | 0·0126 | 0·0105 | |
| σpooled | 0·0181 | 0·0262 | 0·0377 | 0·0441 | 0·0593 | 0·0418 | |
| Square root of the mean within study variance | 0·0440 | 0·0419 | 0·0407 | 0·0595 | 0·0596 | 0·0622 | |
| SE(r new) | 0·0190 | 0·0270 | 0·0385 | 0·0454 | 0·0606 | 0·0431 | |
Q T is a measure for heterogeneity, calculated with eqn. 10. It is tested against a χ2 statistic with reported degrees of freedom and resulting P‐values. is the estimated mean relative rate of change of the catch per °C, calculated with eqns 6–8 (random‐effects model); is the standard error (= standard deviation) of , calculated with eqn. 9 for the random‐effects model taking the square root of the variance; SE(r new) calculated from eqn. 11 is the prediction error that measures the uncertainty of the r estimate for an entirely new study (r new).
Correction for temperature bias
The important question is whether future researchers can use the rate estimates reported here to correct for temperature bias in data series of pitfall catches, or whether it would be more accurate to estimate the rate coefficient from their own site‐specific data. The standard error of a predicted r new for a new data series is 0·0190 when considering maximum temperature and 0·0454 when considering minimum temperature. In only 4 of 37 data sets involving maximum temperature and in 15 of 38 data sets involving minimum temperature, was the standard error of r i smaller in an individual study (Table S5A) than in the meta‐analysis. The r estimates obtained in the current meta‐analysis are therefore in most cases more accurate as a predictor of r for a new study than a new estimate of r made on the basis of the study's data, except when future researchers would collect more extensive and better data than those used in the meta‐analysis. This is further confirmed by comparing the prediction error of r new based on the current study to the square root of the mean within study variance (Table 1). A single r estimate (whether new or any of the r i from this study) has higher expected error than the average r calculated in the meta‐analysis, both for maximum and minimum temperature, although the difference is minor in the case of minimum temperature.
Correction for temperature bias had a substantial effect if there is a large fluctuation in temperature, either as a seasonal trend or as a result of weather variability at shorter time scale. Fig. 3a shows a data series from Wageningen (2004) with a strong seasonal increase in both temperature and carabid catch. When the carabid time series is corrected to the seasonal average temperature (Fig. 3c) or to 20 °C (Fig. 3e), the estimated population density of carabids is much more constant in time than the catch observations (Fig. 3a) would suggest. The seasonal course of the uncorrected catch is therefore diagnosed as heavily influenced by the seasonal course in temperature. A similar conclusion can be drawn from another data set (Newcastle upon Tyne, 1987; Fig. 3b, d, f). Removal of temperature bias indicates that carabids are present in substantial densities during most of the time interval during which measurements were made. The high catches from mid‐July to early September (Fig. 3b) can largely be ascribed to temperature bias and are moderated when the bias is removed (Fig. 3d,f). Obviously, corrections may have little impact in shorter series without distinct temporal trends, which is sometimes the case (data not shown).
Figure 3.
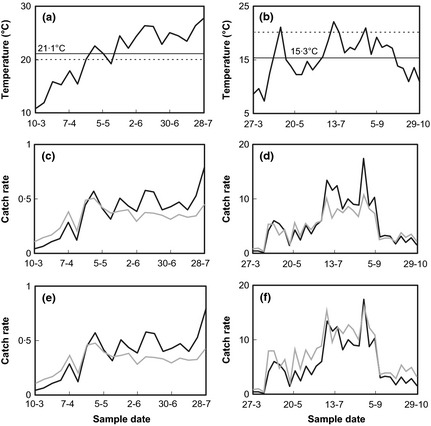
Correction of the catch for temperature bias in two data sets: (a,c,e) Wageningen, the Netherlands 2004 (#1); (b,d,f) Newcastle upon Tyne, UK 1987 (#30). (a,b) actual temperature course (bold), and two fixed reference temperatures: the seasonal average maximum temperature (horizontal solid line) and 20°C (horizontal dashed line); (c,d) actual (black line) and bias‐corrected catch (grey line) for seasonal average maximum temperature; (e,f) observed catch (black line) and bias‐corrected catch (grey line) for 20°C.
Species‐specific responses
Species‐specific responses to maximum or minimum temperature were identified in the majority of data sets: 29 of 37 (Table S6). Of the 168 combinations of data set and species considered, 70 showed a significant temperature response at a confidence level α = 0·05, while another 20 showed a significant response at α = 0·10. Significant responses were found both in data sets that showed a significant relationship between the total catch and temperature and those that did not, and all but two data sets with a significant temperature response at total catch level showed at least one significant species‐level response.
Some patterns were found in the species‐specific responses. For instance, nocturnal Pterostichus madidus (Fabricius) responded more often to minimum temperature than to maximum temperature, while the opposite was the case for diurnal Poecilus cupreus (Linneaus) (Tables 2 and S6). Some other species, for example Harpalus affinis (Schrank) and Pseudoophonus rufipes (DeGeer), responded as often to maximum as to minimum temperature (Tables 2 and S6). Some species did not respond to temperature variation, for example, Brachinus explodens Duftschmid and Nebria brevicollis (Fabricius) (Tables 2 and S6).
Table 2.
Number of data sets out of 38 with a significant regression of the catch of specific species on temperature
| Species | Diel activity | Data sets analysed | Data sets with significant responses | ||||
|---|---|---|---|---|---|---|---|
| Combineda | Maximum temp | Minimum temp | |||||
| +b | −c | +b | −c | ||||
| Harpalus affinis | D/N | 26 | 16 | 15 | 0 | 3 | 1 |
| Pseudoophonus rufipes | N | 25 | 13 | 11 | 0 | 5 | 0 |
| Pterostichus melanarius | D/N | 12 | 6 | 4 | 0 | 4 | 0 |
| Pterostichus madidus | N | 11 | 9 | 2 | 0 | 8 | 0 |
| Nebria brevicollis | N | 8 | 0 | 0 | 0 | 0 | 0 |
| Poecilus cupreus | D | 7 | 5 | 5 | 0 | 0 | 1 |
| Anchomenus dorsalis | N | 6 | 2 | 1 | 0 | 0 | 1 |
| Poecilus lucublandus | N | 6 | 1 | 1 | 0 | 0 | 0 |
| Poecilus scitulus | N | 6 | 1 | 1 | 0 | 1 | 0 |
| Brachinus explodens | ? | 6 | 0 | 0 | 0 | 0 | 0 |
| Amara aenea | D | 5 | 3 | 3 | 0 | 0 | 0 |
| Poecilus versicolor | D | 5 | 3 | 3 | 0 | 0 | 0 |
D – diurnal, N – nocturnal, D/N – inconsistent data in the literature or no clear daily periodicity in activity found (Thiele 1977; Luff 1978; Kegel 1990; Larochelle & Larivière 2003) ? – no literature data.
number of data sets in which significant (P < 0·05) effect of temperature (either maximum, minimum or both) was found, regardless of the slope (positive or negative).
positive effect significant at P < 0·05.
negative effect significant at P < 0·05.
Multiple regressions
In total, 19 data sets were analysed for multivariable effects of weather on the rate of catch to determine whether other factors than temperature were consistent predictors of catch rate. In 12 of the 19 data sets, temperature was a significant predictor (Table 3), and usually it was the most significant predictor as measured by P‐value. Weather variables other than temperature showed occasional significant effects, but the estimated coefficients were not consistent and included negative as well as positive values (Table 3). Thus, the multivariable analysis confirms that temperature is the key weather variable driving short‐term fluctuations in catch rate.
Table 3.
Significance of the effect of weather variables on carabid catch rate in multiple regressions
| Data set # | Simple regression | Multiple regression | ||||
|---|---|---|---|---|---|---|
| Tmax | Tmax | Rain | Pres | Wind | AirHum | |
| 1 | + | + | NS | NS | NS | – |
| 2 | + | + | NS | NS | NS | – |
| 3 | + | + | NS | NS | NS | NS |
| 4 | + | + | NS | NS | + | NS |
| 5 | + | + | NS | NS | + | NS |
| 6 | NS | NS | NS | NS | NS | NS |
| 7 | NS | NS | NS | n.a. | NS | NS |
| 8 | + | NS | NS | n.a. | – | NS |
| 9 | + | NS | NS | n.a. | – | – |
| 10 | + | + | NS | n.a. | NS | NS |
| 11 | + | + | NS | n.a. | NS | NS |
| 12 | NS | NS | NS | n.a. | NS | NS |
| 13 | + | + | – | n.a. | NS | NS |
| 14 | + | + | NS | NS | NS | NS |
| 15 | + | + | NS | NS | NS | NS |
| 16 | + | NS | + | + | NS | NS |
| 17 | + | + | NS | NS | NS | NS |
| 37 | + | + | NS | NS | – | NS |
| 38 | + | NS | NS | NS | – | NS |
Details on data sets are given in Appendices S1 and S2.
Tmax – average maximum temperature; Rain – rainfall sum; Pres – average barometric pressure; Wind – average wind speed; AirHum – average air humidity.
‘+’ significant positive effect; ‘−’ significant negative effect; ‘NS’ not significant at α = 0·05; ‘n.a.’ data not available.
Discussion
This study provides the first unequivocal evidence that temperature affects pitfall catches of carabids. The temperature effect was found across data series from diverse environments, such as croplands, grasslands and orchards. The temperature effect was detected both at the total catch level and at the species level. Temperature was a more consistent and significant predictor of catch rate than other weather variables. In most cases, maximum temperature was a better predictor than minimum temperature. We show that correcting for temperature bias may substantially modify estimates of carabid population changes over time. Therefore, removing temperature bias has the potential to critically modify conclusions from ecological monitoring studies. Estimation results were robust to the detrending method. The estimation method has a strong basis in biological theory about temperature scaling in biological rates and may have wider applicability.
The rate of increase in catch due to maximum temperature is substantial, approximately 0·0863 per °C change, as a relative rate of change. The effect of minimum temperature (0·0497 per °C) was smaller but also significant. Lack of significant association in some data sets may be due to large sampling variability or weak temperature effect on catch rate (Madden & Paul 2011).
Our results provide conclusive evidence that species‐level responses are common, but species‐specific results were more variable than those for total catch. This is expected because sample sizes for individual species are comparatively small, making it harder to identify significant relationships. It is likely that having good enough data (in particular large enough numbers) at the species level is the key to identifying species‐level responses. We expected that diurnal species would respond to maximum temperatures and nocturnal species to minimum temperatures. This held true for some of the common species from our studies, but other species showed a much more variable response to temperature than expected from diel activity or did not respond to temperature at all. Aggregative behaviour in B. explodens (Wautier 1971) may have increased variability in catches and may be responsible for the lack of significant response of this species to temperature. Other intrinsic factors such as thermoregulation, body size, motivation or plasticity in diel rhythms (Baars 1979; Wallin & Ekbom 1994; Atienza, Farinós & Zaballos 1996) and extrinsic factors (e.g. vegetation structure or surface litter; Mitchell 1963; Honek 1988; Hatten et al. 2007) may affect daily activity patterns and thus responses to temperature in both a direct or indirect manner, and mask or confound temperature effects on activity. Data collected simultaneously in different habitats accompanied with on‐site weather records could reveal which factors and conditions alter species‐specific responses to temperature.
We found that temperature was the most important of all studied weather variables. Other weather variables were occasionally significant and in some cases more influential than temperature but their effects on catch rates were not consistent, supporting Jones' (1976) contention that temperature is an important determinant of carabid activity in that temperate environments.
A caveat of our study is that we focused exclusively on the temperature effect in our bias correction. Using our proposed method will not resolve the confounding effects of vegetation density, litter or substrate on catch rates, nor does it correct for the limitations of deficient sampling design or short‐term disturbances such as soil perturbation or vegetation removal.
We conclude that temperature has a major effect on the size of carabid pitfall catches. Our results showed a doubling of catch for every 8 °C increase in maximum temperature, or 14 °C in minimum temperature, based on a comprehensive data analysis, which should prove a useful rule of thumb for researchers and conservationists alike. Correcting for temperature‐related trapping biases of the catch will provide more accurate population estimates and facilitate faunal comparisons when collected from different habitats, environments and/or thermal conditions. Correction for temperature may prevent misinterpretations that can result from temperature bias in pitfall catches. This is especially important if sampling was not simultaneous, for example, when observed differences in catch size resulted mainly from different temperature conditions at the time of sampling. Principles described here might also be fruitfully applied to improving other sampling methodologies in which temperature effects on movement of ectothermic organisms are a concern. Methods developed in this study will therefore make it easier for researchers, ecologists and managers to use and interpret pitfall trap data in ecological, conservation and monitoring studies.
Supporting information
Table S1. Metadata of pitfall catch data
Table S2. Metadata of meteorological data
Table S3. Autoregression analysis of total catch and temperature data
Table S4. Lists of dominant species in 38 pitfall catch data sets
Table S5. Estimated parameters of temperature response of the total carabid catch for three methods of detrending
Table S6. Estimated parameters of species‐specific analyses
Appendix S1. Methods for estimating the parameter r by regression for three methods of detrending
Appendix S2. Worked examples of parameter estimation and correction for temperature bias in MS Excel®
Acknowledgements
The work described here was supported by the project number MZe 0002700604 of the Ministry of Agriculture of the Czech Republic and by a visiting researcher grant to PS by the C.T. de Wit Graduate School for Production Ecology and Resource Conservation, Wageningen University. The Royal Netherlands Meteorological Institute is acknowledged for providing weather data of the Eelde station. We thank E.W. Evans (Utah State University, Logan, USA), D.A. Landis (Michigan State University, East Lansing, USA) and anonymous reviewers for insightful comments.
References
- Atienza, J.C. , Farinós, G.P. & Zaballos, J.P. (1996) Role of temperature in habitat selection and activity patterns in ground beetle Angoleus nitidus. Pedobiologia, 40, 240–250. [Google Scholar]
- Baars, M.A. (1979) Patterns of movement of radioactive carabid beetles. Oecologia, 44, 125–140. [DOI] [PubMed] [Google Scholar]
- Bohan, D.A. , Boursault, A. , Brooks, D.R. & Petit, S. (2011) National‐scale regulation of the weed seedbank by carabid predators. Journal of Applied Ecology, 48, 888–898. [Google Scholar]
- Briers, R.A. , Carsiss, H.M. & Gee, J.H.R. (2003) Flight activity of adult stoneflies in relation to weather. Ecological Entomology, 28, 31–40. [Google Scholar]
- Cormac, R.M. & Ord, J.K. (1979) Spatial and temporal analysis in ecology. International Co‐operative Publishing House, Fairland, USA. [Google Scholar]
- Crawley, M.J. (2005) Statistics: An Introduction using R. John Wiley and Sons, New York, USA. [Google Scholar]
- Crist, T.O. & Ahern, G. (1999) Effects of habitat patch size and temperature on the distribution and abundance of ground beetles (Coleoptera: Carabidae) in an old field. Environmental Entomology, 28, 681–689. [Google Scholar]
- Dempster, J.P. (1967) The control of Pieris rapae with DDT. I. The natural mortality of the young stages of Pieris. Journal of Applied Ecology, 4, 485–500. [Google Scholar]
- Hatten, T.D. , Bosque‐Perez, N.A. , Johnson‐Maynard, J. & Eigenbrode, S.D. (2007) Tillage differentially affects the capture rate of pitfall traps for three species of carabid beetles. Entomologia Experimentalis et Applicata, 124, 177–187. [Google Scholar]
- Hemerik, L. & Brussaard, L. (2002) Diversity of soil macro‐invertebrates in grasslands under restoration succession. European Journal of Soil Biology, 38, 145–150. [Google Scholar]
- Heydemann, B. (1953) Agrarökologische Problematik. Ph.D. thesis, University of Kiel. [Google Scholar]
- Holland, J.M. (2002) The agroecology of carabid beetles. Intercept, Andover, UK. [Google Scholar]
- Honek, A. (1988) The effect of crop density and microclimate on pitfall trap catches of Carabidae, Staphylinidae (Coleoptera), and Lycosidae (Araneae) in cereal fields. Pedobiologia, 32, 233–242. [Google Scholar]
- Honek, A. (1997a) The effect of plant cover and weather on the activity density of ground surface arthropods in a fallow field. Biological Agriculture and Horticulture, 15, 203–210. [Google Scholar]
- Honek, A. (1997b) The effect of temperature on the activity of Carabidae (Coleoptera) in a fallow field. European Journal of Entomology, 94, 97–104. [Google Scholar]
- Johnson, C.G. (1969) Migration and Dispersal of Insects by Flight. Methuen, London, UK. [Google Scholar]
- Jones, M.G. (1976) The arthropod fauna of a winter wheat field. Journal of Applied Ecology, 13, 61–85. [Google Scholar]
- Kegel, B. (1990) Diurnal activity of carabid beetles living on arable land The Role of Ground Beetles in Ecological and Environmental Studies (ed. Stork N.), pp. 65–76. Intercept, Andover, UK. [Google Scholar]
- Kotze, D.J. , Brandmayr, P. , Casale, A. , Duffy‐Richard, E. , Dekoninck, W. , Koivula, M.J. , Lövei, G.L. , Mossakowski, D. , Noordijk, J. , Paarmann, W. , Pizzolotto, R. , Saska, P. , Schwerk, A. , Serrano, J. , Szyszko, J. , Taboada, A. , Turin, H. , Venn, S. , Vermeulen, R. & Zetto, T. (2011) Forty years of carabid beetle research in Europe ‐ from taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. Zookeys, 100, 55–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromp, B. (1999) Carabid beetles in sustainable agriculture: a review on pest control efficacy, cultivation impacts and enhancement. Agriculture, Ecosystems and Environment, 74, 187–228. [Google Scholar]
- Larochelle, A. & Larivière, M.C. (2003) A Natural History of the Ground‐Beetles (Coleoptera: Carabidae) of America North of Mexico. Pensoft, Sofia, BG. [Google Scholar]
- Leslie, T.W. , Hoheisel, G.A. , Biddinger, D.J. , Rohr, J.R. & Fleischer, S.J. (2007) Transgenes sustain epigeal insect biodiversity in diversified vegetable farm systems. Environmental Entomology, 36, 234–244. [DOI] [PubMed] [Google Scholar]
- Logan, J.A. , Wollkind, D.J. , Hoyt, S.C. & Tanigoshi, L.K. (1976) An analytic model for description of temperature dependent rate phenomena in arthropods. Environmental Entomology, 5, 1133–1140. [Google Scholar]
- Luff, M.L. (1978) Diel activity patterns of some field Carabidae. Ecological Entomology, 3, 53–62. [Google Scholar]
- Madden, L.V. & Paul, P.A. (2011) Meta‐analysis for evidence synthesis in plant pathology: an overview. Phytopathology, 101, 16–30. [DOI] [PubMed] [Google Scholar]
- Messenger, P.S. (1959) Bioclimatic studies with insects. Annual Review of Entomology, 4, 183–206. [Google Scholar]
- Mitchell, B. (1963) Ecology of two carabid beetles, Bembidion lampros (Herbst) and Trechus quadristriatus (Schrank) 2. Studies on populations of adults in the field, with special reference to the technique of pitfall trapping. Journal of Animal Ecology, 32, 377–392. [Google Scholar]
- R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Available at: http://www.R-project.org/. [Google Scholar]
- Rosenberg, M.S. , Adams, D.C. & Gurevitch, J. (2000) MetaWin: statistical software for Meta‐Analysis version 2.0, Sinauer Associates, Inc, Sunderland, USA. [Google Scholar]
- Rosenberg, M.S. , Garrett, K.A. , Su, Z. & Bowden, R.L. (2004) Meta‐analysis in plant pathology: synthesizing research results. Phytopathology, 94, 1013–1017. [DOI] [PubMed] [Google Scholar]
- Shumway, R.H. & Stoffer, D.S. (2006) Time Series Analysis and Its Applications With R Examples. Springer Science + Business Media, New York, USA. [Google Scholar]
- Southwood, T.R.E. & Henderson, P.A. (2000) Ecological Methods, 3rd edn. Blackwell Science, Oxford, UK. [Google Scholar]
- Taylor, L.R. (1963) Analysis of the effect of temperature on insects in flight. Journal of Animal Ecology, 32, 99–117. [Google Scholar]
- Thiele, H.‐U. (1977) Carabid Beetles in Their Environments. Springer‐Verlag, Berlin. [Google Scholar]
- Wallin, H. & Ekbom, B. (1994) Influence of hunger level and prey densities on movement patterns in three species of Pterostichus beetles (Coleoptera: Carabidae). Environmental Entomology, 23, 1171–1181. [Google Scholar]
- Wautier, V. (1971) Un phénomèn social chez les Coléoptères: le grégarisme des Brachinus (Caraboidea Brachinidae). Insectes Sociaux, 18 (3 bis), 1–84. [Google Scholar]
- Williams, C.B. (1940) An analysis of four years captures of insects in a light trap. Part II. The effect of weather conditions on insect activity; and the estimation and forecasting of changes in the insect population. Transactions of the Royal Entomological Society of London, 90, 227–306. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Metadata of pitfall catch data
Table S2. Metadata of meteorological data
Table S3. Autoregression analysis of total catch and temperature data
Table S4. Lists of dominant species in 38 pitfall catch data sets
Table S5. Estimated parameters of temperature response of the total carabid catch for three methods of detrending
Table S6. Estimated parameters of species‐specific analyses
Appendix S1. Methods for estimating the parameter r by regression for three methods of detrending
Appendix S2. Worked examples of parameter estimation and correction for temperature bias in MS Excel®


