Abstract
Purpose
To objectively measure changes in the human ciliary muscle dimensions in vivo following instillation of topical phenylephrine, a mydriatic and vasodilating agent.
Methods
A cross-sectional study of 25 healthy young adults was conducted. Measurements of pupil size, accommodation, and ciliary muscle thickness were made both before and 30 minutes after instillation of 1% proparacaine and 2.5% phenylephrine. Accommodation was measured three ways: subjectively using a push-up technique and Royal Air Force (RAF) rule, and objectively using both the Grand Seiko autorefractor and PowerRefractor. Images of the temporal ciliary muscle were made using the Visante Anterior Segment Optical Coherence Tomographer (OCT). Ciliary muscle images were objectively analyzed using a computer-based segmentation technique.
Results
Amplitude of accommodation with the push-up test was reduced by about 1D with phenylephrine (p < 0.001). Phenylephrine did not change the accommodative response to a 4-D Badal target as measured by either autorefraction or photorefraction (p > 0.30). There was statistically significant thickening of the anterior region and thinning of the posterior region of the ciliary muscle with accommodation (p < 0.001, all locations). Phenylephrine did not affect either baseline ciliary muscle thickness, or the accommodative contraction of the muscle (p > 0.09).
Conclusions
Low-dose phenylephrine does not affect ciliary muscle dimensions, ciliary muscle contractility, or accommodative response to a 4 D near target.
Keywords: Optical coherence tomography, anterior segment imaging, ciliary muscle, accommodation, phenylephrine, presbyopia
In the study of presbyopia, we and others have been faced with the challenge of recording the accommodative response or imaging the crystalline lens through a small pupil and thus have relied on phenylephrine to dilate the pupil.1–9 Research has proceeded with limited understanding of the drug’s effect on the ciliary muscle itself. Recent advances in objective imaging technology have allowed a study of the direct effect of phenylephrine on the human ciliary muscle dimensions or contractility.
The ciliary muscle is predominantly under the control of the parasympathetic nervous system and antimuscarinic drugs such as atropine and cyclopentolate temporarily paralyze the ciliary muscle, abolishing accommodation.10 There is evidence that drugs acting on the sympathetic nervous system can also influence accommodation through noradrenaline’s action on two subclasses of postsynaptic receptors: α and β adrenoceptors.10 There are more β than α adrenoceptors in human ciliary muscle.11, 12 Phenylephrine is primarily an α-agonist and should have little influence on the β receptors in ciliary muscle, but it is possible that phenylephrine may alter ciliary muscle size or function via α receptors.10
Previous research suggests that phenylephrine reduces the subjectively measured amplitude of accommodation;13 however, it is unclear whether this effect is mediated by sympathetic receptors in the ciliary muscle, an indirect effect of the vasoconstrictive properties of the drug, or a side effect of the increased ocular aberrations or decreased depth of focus associated with a dilated pupil. Garner et al. reported that phenylephrine had no effect on the resting focus of accommodation but Culhane and coworkers found that phenylephrine affected dynamic aspects of accommodation.14, 15 More recently, Ostrin and Glasser showed that phenylephrine did not affect Edinger-Westphal-stimulated accommodative amplitude or dynamics in rhesus monkeys.16 To date, multiple studies have used up to 10% phenylephrine in human studies of accommodation without a clear understanding of its effect on the ciliary muscle.1–9
Advances in technology now allow in vivo measurements of the accommodative structures of the human eye coupled with objective measurements of accommodation. Specifically, photorefraction17, 18 and autorefraction19 afford fast, precise measurements of the accommodative response, and optical coherence tomography (OCT) allows non-invasive imaging of the ciliary muscle in vivo.20 These techniques have already been used to demonstrate ciliary muscle contraction with accommodation.21, 22 The difficulty, however, lies in using the combination of these technologies to measure the ciliary muscle in ageing eyes with very small pupils. We knew from data collection in a separate study that 2.5% phenylephrine would allow us to achieve sufficient pupil dilation for photorefraction in a presbyopic population. The purpose of this study was to determine if, in response to topical administration of 2.5% phenylephrine, there are (1) changes in the dimensions of the ciliary muscle and (2) changes in the accommodative contraction of the ciliary muscle.
METHODS
Enrollment and Overview
A cross-sectional study of healthy young adults was conducted to assess ciliary muscle and accommodative changes with 2.5% phenylephrine. Enrollment criteria for the study were: age 18 to 40 years; no prior history of accommodative dysfunction; visual acuity correctable to at least 20/25 in each eye with no strabismus; and habitual refractive error less than 5.00 D in either meridian with less than 1.00 D of astigmatism. Subjects in this age range, rather than presbyopic subjects, were chosen because it was thought that the effects of the 2.5% phenylephrine on accommodation, if present, would be more readily observable in a younger age range. Subjects were either emmetropic or, if ametropic, were required to wear their habitual soft contact lens correction during testing.
In a single visit, pupil size and accommodative function were measured, and the ciliary muscle was imaged in relaxed and accommodated states (0 and 4 D targets, described below). Measurements were repeated 30 minutes after topical administration of one drop of 1% proparacaine and one drop of 2.5% phenylephrine hydrochloride. Proparacaine was used both to increase patient comfort and because application of proparacaine has been shown to increase the effect of phenylephrine, especially in patients with dark irides.13 All testing was conducted on the right eye only with the left eye occluded. The Ohio State University’s Biomedical Sciences Institutional Review Board, in accordance with the tenets of the Declaration of Helsinki, approved the study protocol. Subjects were educated on the purpose of the study, and informed consent was obtained from each subject before beginning the study.
Measures of Accommodative Function and Pupil Size
Accommodative function was measured in three ways: subjective maximum accommodation using an RAF near point rule (Haag-Streit England, Essex, UK); objective accommodative response using the Grand Seiko WV 500 Auto-Refractor (Grand Seiko, Ltd., Hiroshima, Japan) and objective accommodative response using the PowerRefractor II (MultiChannelSystems, Reutlingen, Germany). For subjective testing, maximum monocular accommodation was determined using the push-up to blur technique and a 1 mm letter target.23 Objective static accommodative response was measured with the Grand Seiko autorefractor and a 2 mm letter target on a Badal lens track. The accommodative response was measured at 0 and 4 D stimulus levels, and an average of five measurements was made of the response at each level. During ciliary muscle imaging, accommodative response was confirmed with the PowerRefractor, using the built-in calibration (Figure 1). Subjects viewed a Maltese cross target at 0 and 4 D stimulus levels. As the PowerRefractor acquires images at a rate of 25 Hz, the mean accommodative response under the imaging conditions was used in data analysis. Up to 20 seconds of data were averaged. The PowerRefractor also provided a measurement of pupil size, and the average pupil diameter over the recording time was used for analysis.
Figure 1.
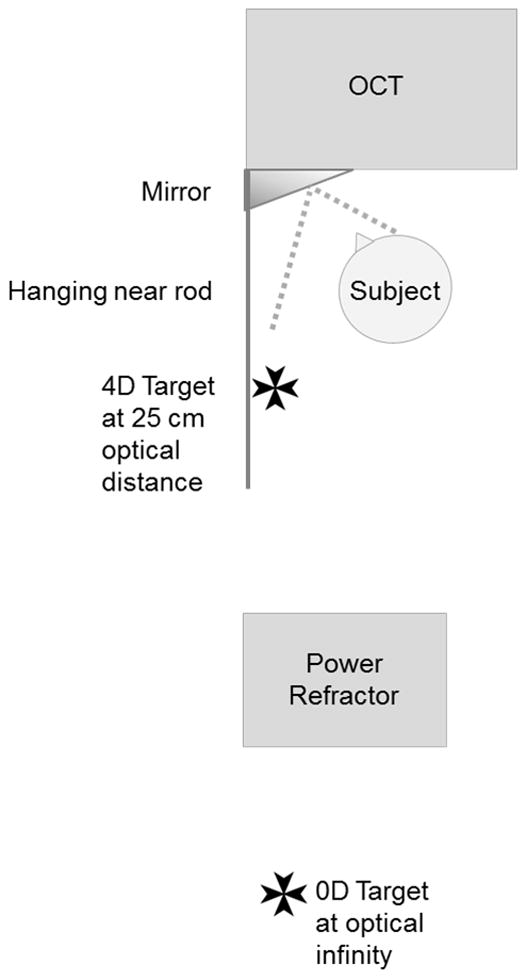
Diagram of OCT and PowerRefractor set-up. The OCT images of the ciliary muscle were taken while the subject focused through a mirror attached to the machine on either a target attached to the far wall or hanging from a near point rod.
Ciliary Muscle Imaging Acquisition and Analysis
The Visante Anterior Segment OCT (Carl Zeiss Meditec Dublin, CA, USA) was used to image the ciliary muscle as described previously.24 Briefly, the subject was seated in front of the instrument with the right eye viewing a Maltese cross target on the far wall (0 D stimulus) and then on a rod at 25 cm (4 D stimulus) (Figure 1). The left eye was occluded. The temporal ciliary muscle of each subject was imaged through the sclera. Images were obtained in “Enhanced High-Resolution Corneal Mode” using the Visante 2.0 system software. Our previous work used steel ball calibration to verify that the resolution was within 5% of the manufacturer’s reported 128 pixel/mm.24 Four images of the ciliary muscle were obtained at each stimulus level, as previous research indicated that three repeat measurements provided an intraclass correlation over 0.8 for cross-sectional thickness measurements.24
Images were analyzed using an objective image segmentation algorithm.24 The program provided cross-sectional thickness measurements at 1-, 2-, and 3-mm posterior to the scleral spur (CMT1, CMT2, CMT3), plus a maximum thickness value (CMTMAX) (Figure 2).
Figure 2.
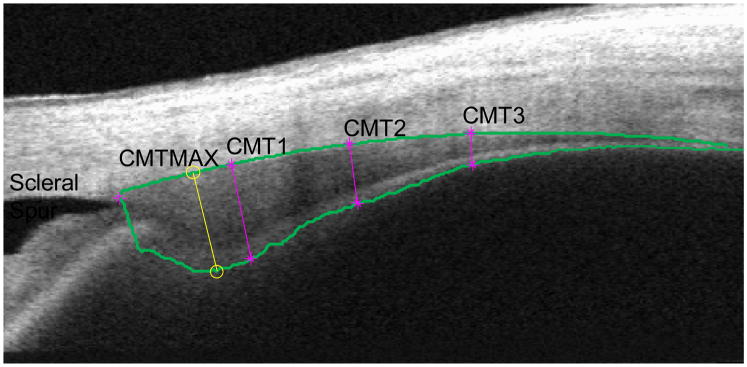
Computer-based image segmentation analysis that provides cross-sectional ciliary muscle thickness (CMT) measurements at 1, 2, and 3 mm posterior to the scleral spur, and a maximum thickness measurement. The image was taken while the subject focused on the 4 D stimulus.
Data Analysis
Differences in the accommodative response, pupil size and ciliary muscle dimensions before and after phenylephrine were compared using a repeated measures regression analysis. As there was only a single measure of maximum subjective amplitude of accommodation (not at two stimulus levels), pre- and post-phenylephrine amplitude was compared using a paired t-test. The estimated change in the ciliary muscle per diopter of accommodative response was calculated by dividing the change in thickness by the subject’s accommodative response (4D – 0D) as measured by the Grand Seiko Autorefractor.
RESULTS
Twenty-five subjects between 20 and 27 years of age were enrolled. Just over half were female and all but one subject was Caucasian. Iris color was not recorded. The accommodative responses with and without phenylephrine are shown in Table 1. The maximum accommodative response, as measured using the push-up method, was reduced by about 1 D following instillation of phenylephrine (p < 0.001). On average, subjects accommodated about 3 D to the 4 D stimulus, as measured by both autorefraction and PowerRefractor. In a repeated measures regression analysis, the interaction term between phenylephrine and accommodation was not statistically significant for either the autorefractor or PowerRefractor responses and was removed from the model (p > 0.30). Phenylephrine did not affect either the objectively measured accommodative response (autorefractor p = 0.27, PowerRefractor p = 0.97).
Table 1.
Accommodation and pupil size pre- and post-phenylephrine (mean ± SD). Positive values indicate increasing accommodation.
| Accommodative Response (D)
|
Pupil Size (mm) | ||||
|---|---|---|---|---|---|
| Push-up | Autorefractor | PowerRefractor | |||
| Pre-PE | 0D Stimulus | -- | 0.33 ± 0.82 | − 0.26 ± 0.36 | 6.15 ± 0.85 |
| 4D Stimulus | -- | 3.06 ± 0.26 | 3.02 ± 0.87 | 5.65 ± 0.84 | |
| Maximum | 8.99 ± 1.37 | -- | -- | -- | |
|
| |||||
| Post-PE | 0D Stimulus | -- | 0.33 ± 0.77 | 0.04 ± 0.55 | 7.13 ± 0.84 |
| 4D Stimulus | -- | 3.02 ± 0.26 | 3.04 ± 0.82 | 6.62 ± 1.06 | |
| Maximum | 7.78 ± 1.54 | -- | -- | -- | |
Pupil size increased approximately 1 mm with phenylephrine and decreased about 0.5 mm with accommodation (Table 1). The addition of phenylephrine did not abolish the accommodative pupil response. In the regression model, the interaction term between phenylephrine and accommodation was not statistically significant (p = 0.99), but both phenylephrine and accommodation were statistically significant factors (phenylephrine: p < 0.001; accommodation: p < 0.001).
The effect of accommodation and phenylephrine on ciliary muscle thickness is presented in Table 2. In the regression of each location, the interaction term between phenylephrine and accommodation was not statistically significant (all p > 0.09). The effect of phenylephrine alone on ciliary muscle thickness was also not statistically significant (all p > 0.43). The estimated effects sizes of phenylephrine were 1 to 3 μm and were not consistent in direction of muscle change. Confidence intervals for the estimated effect sizes of phenylephrine were on the order of ±10 μm. With accommodation, there was anterior thickening and posterior thinning of the ciliary muscle, consistent with an anterior-inward shift of the sphincter muscle (all p < 0.001). The average observed change in ciliary muscle thickness per diopter of accommodative response (CMT / accommodative response on the autorefractor) was (mean ± SE) 21 ± 3 μm/D thickening at CMTMAX, 15 ± 2 μm/D thickening at CMT1, 11 ± 2 μm/D thinning at CMT2 and 17 ± 3 μm/D thinning at CMT3.
Table 2.
Ciliary muscle thickness (μm) (mean ± SD) pre- and post-phenylephrine (PE).
| CMTMAX | CMT1 | CMT2 | CMT3 | ||
|---|---|---|---|---|---|
| Pre-PE | 0D Stimulus | 825 ± 90 | 796 ± 91 | 598 ± 103 | 367 ± 72 |
| 4D Stimulus | 893 ± 89 | 844 ± 87 | 562 ± 106 | 312 ± 63 | |
|
| |||||
| Post-PE | 0D Stimulus | 832 ± 74 | 797 ± 81 | 586 ± 100 | 371 ± 82 |
| 4D Stimulus | 890 ± 82 | 842 ± 88 | 568 ± 115 | 315 ± 75 | |
CMT1, CMT2 and CMT3 are cross-sectional ciliary muscle thickens measurements at 1-, 2-, and 3-mm posterior to the scleral spur and CMTMAX is the maximum cross-sectional thickness measurement of the muscle.
DISCUSSION
There was a 1 D decrease in the subjectively-measured amplitude of accommodation following instillation of phenylephrine. This finding could either be due to a direct effect of phenylephrine on the ciliary muscle, a secondary effect of the increase in pupil dimensions increasing ocular aberrations and decreasing depth of focus, or an artifact of the limited repeatability of this measurement.13, 14, 25 Previous work suggests that a 1 mm increase in pupil size would account for less than a 0.5 D shift in subjective depth of focus.26, 27 The repeatability of the push-up test is about 1.50 D.28 This knowledge, coupled with the lack of objectively-measured change in accommodation suggests that the subjective decrease in accommodation was within the limits of the measurement technique.
In this study, the objectively-measured accommodative response and cross-sectional ciliary muscle dimensions and contractility to a 4 D stimulus were not altered by the instillation of 2.5% phenylephrine. This accommodative finding is in agreement with Ostrin and Glasser’s work on non-human primates, which demonstrated no systematic difference in the objectively measured accommodative response before and after two drops of 10% phenylephrine.16 While Ostrin and Glasser’s study was conducted using Edinger-Westphal stimulation, not an accommodative response to blur, it does suggest that there was no physiological effect on the accommodative system. Because of the current limitations of our 6-D Badal lens system on the Grand Seiko Autorefractor and the range of powers that the PowerRefractor can accurately measure, it was not possible to objectively measure the full accommodative response of this sample of young adults.17, 18 Other research has been conducted on the effect of phenylephrine at higher accommodative levels and with fixed pupil size, and researchers have examined changes in the ocular aberrations with increased pupil size; however, the primary aim of this study was to determine the effect of phenylephrine on static accommodation and ciliary muscle dimensions at accommodative levels relevant to the study of presbyopia in humans.13, 14, 25, 29
The anterior ciliary muscle thickening and posterior thinning with accommodation found in this study agree with previous reports.21, 30 Sheppard and Davies studied a population of 50 subjects aged 19 to 34 years and used the Visante OCT to demonstrate a change in ciliary muscle thickness with accommodation.21 While some of their measurement locations were based on an estimated length of the muscle, the location of their “CM25” would roughly correspond with our CMT1. They reported a 7.1 ± 6.4 μm/D thickening at CM25 and a 2.2 ± 11 μm/D thinning at CMT2 with accommodation. Sheppard and Davies made manual caliper measurements and adjusted the refractive index of the tissue post-hoc, and oblique to the scanning direction, thus their results may not be directly comparable.31 Lossing et al. used the same technique and found anterior thickening of 18 μm/D at the CMTMAX.22 They reported a thinning of 12 μm/D at the 3-mm location. These findings agree well with our anterior thickening of 15 and 21 μm/D and posterior thinning of 11 and 17 μm/D.
Phenylephrine did not affect ciliary muscle dimensions at either the 0 or 4 D stimulus levels. Furthermore, we found no interaction between phenylephrine and contraction of the ciliary muscle. To our knowledge, this is the first study to examine the effect of phenylephrine on the human ciliary muscle in vivo.
There are limitations to this study and future work could examine the effect of higher doses of phenylephrine and the effect of 2.5% phenylephrine at higher accommodative levels. We elected to use 2.5% phenylephrine because it is widely used and available in the US and because we wanted to use the lowest dose required to facilitate measurements. We know from other, unpublished studies from our laboratory that the 2.5% phenylephrine dose is sufficient to achieve the required pupillary dilation for measuring presbyopic subjects. There was a potential for bias in the measurement of amplitude of accommodation, and other objective measures should be considered. Researchers should consider conducting similar studies to understand the effect of other topical agents commonly used in research and clinical care. Finally, the sample size was only 25 subjects. The parameter estimates for the effect size of phenylephrine on muscle thickness were small (1–3 μm); however, the confidence intervals of 10 μm approach clinical relevance. Despite these limitations, our findings provide support that 2.5% topical phenylephrine does not significantly affect ciliary muscle dimensions or contractile response to a 4 D target and can be used in studies of presbyopia when pupil size is a concern.
Acknowledgments
This research was supported by: National Institutes of Health NEI K23EY019097 (Richdale), NEI U10-EY08893 (Zadnik), KL2-RR025754 (Bailey), National Science Foundation DMS-0811003 (Kao), an Alfred P. Sloan Fellowship (Kao), and an American Academy of Optometry Ezell Fellowship (Richdale). Mark Bullimore is a consultant for Alcon Inc., Elenza Inc., and Carl Zeiss Meditec.
Footnotes
All other authors have no disclosures in relation to this work.
References
- 1.Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye. 1: Evaluation of in vivo measurement techniques. Appl Opt. 1989;28:1097–102. doi: 10.1364/AO.28.001097. [DOI] [PubMed] [Google Scholar]
- 2.Dubbelman M, van der Heijde GL, Weeber HA. Change in shape of the aging human crystalline lens with accommodation. Vision Res. 2005;45:117–32. doi: 10.1016/j.visres.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Drexler W, Baumgartner A, Findl O, Hitzenberger CK, Fercher AF. Biometric investigation of changes in the anterior eye segment during accommodation. Vision Res. 1997;37:2789–800. doi: 10.1016/s0042-6989(97)00066-7. [DOI] [PubMed] [Google Scholar]
- 4.Dubbelman M, van der Heijde GL, Weeber HA, Vrensen GF. Changes in the internal structure of the human crystalline lens with age and accommodation. Vision Res. 2003;43:2363–75. doi: 10.1016/s0042-6989(03)00428-0. [DOI] [PubMed] [Google Scholar]
- 5.Rosales P, Dubbelman M, Marcos S, van der Heijde R. Crystalline lens radii of curvature from Purkinje and Scheimpflug imaging. J Vis. 2006;6:1057–67. doi: 10.1167/6.10.5. [DOI] [PubMed] [Google Scholar]
- 6.Koretz JF, Cook CA, Kaufman PL. Aging of the human lens: changes in lens shape at zero-diopter accommodation. J Opt Soc Am (A) 2001;18:265–72. doi: 10.1364/josaa.18.000265. [DOI] [PubMed] [Google Scholar]
- 7.Dubbelman M, van der Heijde GL, Weeber HA. The thickness of the aging human lens obtained from corrected Scheimpflug images. Optom Vis Sci. 2001;78:411–6. doi: 10.1097/00006324-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Koretz JF, Cook CA, Kaufman PL. Aging of the human lens: changes in lens shape at zero-diopter accommodation. J Opt Soc Am (A) 2001;18:265–72. doi: 10.1364/josaa.18.000265. [DOI] [PubMed] [Google Scholar]
- 9.Hermans E, Dubbelman M, van der Heijde R, Heethaar R. The shape of the human lens nucleus with accommodation. J Vis. 2007;7:161–0. doi: 10.1167/7.10.16. [DOI] [PubMed] [Google Scholar]
- 10.Gilmartin B. A review of the role of sympathetic innervation of the ciliary muscle in ocular accommodation. Ophthalmic Physiol Opt. 1986;6:23–37. [PubMed] [Google Scholar]
- 11.Tornqvist G. The relative importance of the parasympathetic and sympathetic nervous systems for accommodation in monkeys. Invest Ophthalmol. 1967;6:612–7. [PubMed] [Google Scholar]
- 12.Tamm ER, Lutjen-Drecoll E. Ciliary body. Microsc Res Tech. 1996;33:390–439. doi: 10.1002/(SICI)1097-0029(19960401)33:5<390::AID-JEMT2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Mordi JA, Lyle WM, Mousa GY. Effect of phenylephrine on accommodation. Am J Optom Physiol Opt. 1986;63:294–7. doi: 10.1097/00006324-198604000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Culhane HM, Winn B, Gilmartin B. Human dynamic closed-loop accommodation augmented by sympathetic inhibition. Invest Ophthalmol Vis Sci. 1999;40:1137–43. [PubMed] [Google Scholar]
- 15.Garner LF, Brown B, Baker R, Colgan M. The effect of phenylephrine hydrochloride on the resting point of accommodation. Invest Ophthalmol Vis Sci. 1983;24:393–5. [PubMed] [Google Scholar]
- 16.Ostrin LA, Glasser A. The effects of phenylephrine on pupil diameter and accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:215–21. doi: 10.1167/iovs.03-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen PM, Radhakrishnan H, O’Leary DJ. Repeatability and validity of the PowerRefractor and the Nidek AR600-A in an adult population with healthy eyes. Optom Vis Sci. 2003;80:245–51. doi: 10.1097/00006324-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Choi M, Weiss S, Schaeffel F, Seidemann A, Howland HC, Wilhelm B, Wilhelm H. Laboratory, clinical, and kindergarten test of a new eccentric infrared photorefractor (PowerRefractor) Optom Vis Sci. 2000;77:537–48. doi: 10.1097/00006324-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Gwiazda J, Weber C. Comparison of spherical equivalent refraction and astigmatism measured with three different models of autorefractors. Optom Vis Sci. 2004;81:56–61. doi: 10.1097/00006324-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Kao CY, Hofer M, Sapiro G, Stem J, Rehm K, Rottenberg DA. A geometric method for automatic extraction of sulcal fundi. IEEE Trans Med Imaging. 2007;26:530–40. doi: 10.1109/TMI.2006.886810. [DOI] [PubMed] [Google Scholar]
- 21.Sheppard AL, Davies LN. In vivo analysis of ciliary muscle morphologic changes with accommodation and axial ametropia. Invest Ophthalmol Vis Sci. 2010;51:6882–9. doi: 10.1167/iovs.10-5787. [DOI] [PubMed] [Google Scholar]
- 22.Lossing LA, Sinnott LT, Kao CY, Richdale K, Bailey MD. Measuring changes in ciliary muscle thickness with accommodation in young adults. Optom Vis Sci. 2012;89:719–26. doi: 10.1097/OPX.0b013e318252cadc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stark LR, Atchison DA. Subject instructions and methods of target presentation in accommodation research. Invest Ophthalmol Vis Sci. 1994;35:528–37. [PubMed] [Google Scholar]
- 24.Kao CY, Richdale K, Sinnott LT, Grillott LE, Bailey MD. Semiautomatic extraction algorithm for images of the ciliary muscle. Optom Vis Sci. 2011;88:275–89. doi: 10.1097/OPX.0b013e3182044b94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awwad ST, El-Kateb M, McCulley JP. Comparative higher-order aberration measurement of the LADARWave and Visx WaveScan aberrometers at varying pupil sizes and after pharmacologic dilation and cycloplegia. J Cataract Refract Surg. 2006;32:203–14. doi: 10.1016/j.jcrs.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 26.Marcos S, Moreno E, Navarro R. The depth-of-field of the human eye from objective and subjective measurements. Vision Res. 1999;39:2039–49. doi: 10.1016/s0042-6989(98)00317-4. [DOI] [PubMed] [Google Scholar]
- 27.Atchison DA, Charman WN, Woods RL. Subjective depth-of-focus of the eye. Optom Vis Sci. 1997;74:511–20. doi: 10.1097/00006324-199707000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfield M, Cohen AS. Repeatability of clinical measurements of the amplitude of accommodation. Ophthalmic Physiol Opt. 1996;16:247–9. [PubMed] [Google Scholar]
- 29.Jenkins TC. Aberrations of the eye and their effects on vision: 1. Spherical aberration. Br J Physiol Opt. 1963;20:59–91. [PubMed] [Google Scholar]
- 30.Stachs O, Martin H, Kirchhoff A, Stave J, Terwee T, Guthoff R. Monitoring accommodative ciliary muscle function using three-dimensional ultrasound. Graefes Arch Clin Exp Ophthalmol. 2002;240:906–12. doi: 10.1007/s00417-002-0551-2. [DOI] [PubMed] [Google Scholar]
- 31.Bailey MD. How should we measure the ciliary muscle? Invest Ophthalmol Vis Sci. 2011;52:1817–8. doi: 10.1167/iovs.11-7313. [DOI] [PubMed] [Google Scholar]


