Abstract
Objectives
PRAME (Preferentially Expressed Antigen in Melanoma) is a tumor-associated antigen recognized by immunocytes, and it induces cytotoxic T cell-mediated responses in melanoma. PRAME expression in tumors interferes with retinoic acid receptor (RAR) signaling thus promoting tumor progression. Here, we study PRAME expression in head and neck squamous cell carcinoma (HNSCC) to determine its potential clinical significance.
Materials and Methods
PRAME expression in HNSCC was evaluated by immunohistochemistry in tissue microarrays of primary tumors (n=53), metastatic lymph nodes (n=8) and normal oral mucosa (n=11). Biopsies of dysplastic oral lesions (n=12) were also examined. PRAME expression levels in tissues were correlated with markers of poor prognosis in HNSCC. PRAME mRNA in HNSCC cell lines and in normal immortalized human keratinocytes (HaCaT cell line) was measured by qRT-PCR, and the protein expression by flow cytometry and western blots.
Results
PRAME was expressed in HNSCC cell lines and HNSCC lesions. PRAME expression in dysplastic mucosa was variable. No or only weak expression was found in normal cells or tissues. PRAME expression levels significantly correlated with the tumor grade, size, nodal involvement and the clinical status of HNSCC patients.
Conclusions
Elevated PRAME expression associates with clinicopathologic markers of poor outcome in HNSCC and might identify potential candidates with pre-cancerous lesions for chemoprevention with retinoids.
Keywords: head and neck cancer, PRAME overexpression, larynx dysplasia, oral mucosa, immunohistochemistry, clinical association
Introduction
Head and neck squamous cell cancers (HNSCC) represent a group of diseases with a considerable socioeconomic and clinical impact. HNSCC accounts for 40,000 new cases per year in the United States and 500,000 cases worldwide [1]. Although early detection and treatment have improved in the last decades, the 5-year survival still remains below 50% primarily because of local cancer recurrence and/or the appearance of second primary tumors [1]. While an improvement in outcome of HNSCC patients has been made due to a broader use of chemoradiotherapies recurrence remains a major problem [2, [Posner, 2007 #3, 3]. To date, no biomarkers predicting favorable outcome or response to definitive chemoradiotherapy are available. Traditional biomarkers, such as tumor size or extracapsular spread, appear to be inadequate for the risk stratification of patients with HNSCC or for predicting recurrence. There is a growing need for clinically-relevant and practical biomarkers that will allow the appropriate selection of high-risk patients who might benefit from more aggressive treatment strategies.
Several prognostic biomarkers in HNSCC have been described, including, e.g., beta-tubulin 2, glutathione S-transferase, p53, ERCC1 expresssion, ERC1 polymorphism, RRM1, HPV, K-ras mutations, EGFR, EGFRvIII or EGFR kinase domain mutations [4-9]. However, none of these biomarkers has been validated or shown to have a significant impact on improving HNSCC outcome. More recently, a new category of tumor associated antigens (TAA), cancer D testis antigens, have emerged as potentially important targets for antigen-specific cancer immunotherapy [10]. PRAME (Preferentially Expressed Antigen in Melanoma) was found to be responsible for triggering cytotoxic T cell-mediated immune responses in melanoma [11]. PRAME expression has been described in other solid tumors, and has been extensively evaluated in leukemias [12-14]. PRAME is weakly expressed or not expressed in normal tissues [15]. Recent data indicate that PRAME may contribute to disease progression by interfering with the metabolic pathway of all-trans retinol (vitamin A) and its active metabolites, collectively called retinoids [15].
Retinoic acid (RA) is known to regulate many aspects of cell proliferation, differentiation, apoptosis and vertebrate development [15-19]. Biologically active retinoid all-trans retinoic acid (RA) and its metabolites signal upon binding to the retinoic acid receptors (RARa, b, and g isoforms) and the retinoid X receptors (RXRa, b, and g isoforms) expressed on various cells [15]. The protein PRAME (Gene ID: 75829) is a dominant repressor of RAR signaling: upon binding to RAR in the presence of RA, PRAME prevents ligand-induced receptor activation, antagonizing RAR signaling [16]. Overexpression of PRAME in human cancer cells confers growth or survival advantages and promotes malignant differentiation of stem cells [4]. Thus, a loss of RA responsiveness induced by PRAME overexpression in cancer might be beneficial not only for malignant but also for pre-cancerous cells.
The presence of the PRAME in HNSCC cells could explain a lack of the past success in chemoprevention with retinoids in patients with pre-cancerous oral lesions such as leukoplakia or erythroplakia [20, 21].
This study analyzes the PRAME mRNA and protein expression in HNSCC cell lines and PRAME protein levels in tumor tissues of patients with HNSCC and in pre-cancerous oral lesions. To the best of our knowledge, this is the first study correlating the immunohistochemical analysis of PRAME expression in HNSCC with conventional markers of poor prognosis in HNSCC and with the severity of dysplasia.
Materials and Methods
Patients and controls
Tables 1-2 summarize characteristics of the patients included in this study. The patients whose tumor specimens were used for preparation of TMAs included 47 males and 6 females (median age, 58 years; range, 40-90 years). All patients had histopathologically confirmed squamous cell carcinomas. The cohort of patients with lymph node metastases included 6 males and 2 females (median age, 51 years; range, 40-67 years). The group of patients with dysplasia of the larynx, whose tumors were sectioned for IHC, included 9 males and 3 females (median age, 55 years; range, 33-76 years). The group of normal healthy controls comprised seven men and five women (median age, 32 years; range, 20–50 years).
Table 1. Clinicopathological characteristics of the HNSCC patients with primary tumors as well as genders and ages of healthy donors included in this study1.
| Characteristics | HNSCC patients (n=53) | Lymph node mets (n=8) | Healthy Controls (n=11) |
|---|---|---|---|
| Sex: | |||
| Male | 47 | 6 | 6 |
| Female | 6 | 2 | 5 |
| Age: | |||
| Range | 40-90 | 40-67 | 19-48 |
| Median | 58 | 51 | 30 |
| Tumor site: | |||
| Oral cavity | 12 | 3 | |
| Pharynx | 2 | 1 | |
| Larynx | 27 | 4 | |
| Paranasal | 12 | ||
| sinuses | |||
| Tumor differentiation: | |||
| Well (G1) | 11 | 2 | |
| Moderate (G2) | 33 | 2 | |
| Poor (G3) | 17 | 4 | |
| Tumor size: | |||
| T1 | 4 | ||
| T2 | 28 | ||
| T3 | 12 | ||
| T4 | 9 | ||
| Nodal involvement: | |||
| N- | 37 | ||
| N+ | 16 | ||
| Stage: | |||
| I | 4 | ||
| II | 20 | ||
| III | 17 | ||
| IV | 12 | ||
| Metastasis: | |||
| None (M0) | 51 | ||
| Distatnt (M1) | 2 |
The patients included in this study received no radiotherapy or chemotherapy before surgery. The data for 8 patients with nodal metastases are not included.
Table 2.
Clinicopathological characteristics of the dysplasia patients included in this study.1
| Characteristics | Patients (n=12) |
|---|---|
| Sex: | |
| Male | 9 |
| Female | 3 |
| Age: | |
| Range | 33-76 |
| Median | 55 |
| Dysplasia Site: | |
| Vocal cords of the larynx | 12 |
| Grade of dysplasia: | |
| Mild (SIN I) | 7 |
| Moderate (SIN II) | 3 |
| Severe dysplasia (SIN III) | 2 |
| Carcinoma in situ (SIN III) | 0 |
Classification systems that categorize intraepithelial head and neck lesions, according to WHO classification (2005). (SIN; squamous intraepithelial neoplasia)
Tissues
Formalin-fixed paraffin-embedded commercially available tissue microarrays (TMA) (US Biomax Inc, Rockville, MD) contained duplicate cores per case of primary head and neck cancer lesions (n=53), lymph node metastases unmatched with primary lesions (n=8) and normal oral mucosa (n=11) obtained from normal donors who underwent uvulopalatopharyngoplasty due to obstructive sleep apnea syndrome.. The HNSCC patients included in this study received no radiotherapy or chemotherapy before surgery. Pre-cancerous tissue samples were obtained from 12 patients diagnosed and treated for different grades dysplasia of the laryngeal vocal cords at the Department of Otolaryngology, University of Medical Sciences, Poznan, Poland. The study was approved by the Ethics Committee at the University of Medical Sciences in Poznan and all patients signed informed consent forms. The stained sections were evaluated using a Olympus BX-41 microscope. The characteristics of the HNSCC patients, lymph node metastases, normal controls or patients with pre-malignant lesions included in this study are presented in Table 1 or Table 2, respectively.
Cell lines
HNSCC cell lines (PCI-1, PCI-13, and PCI-30) were established at the University of Pittsburgh Cancer Institute and maintained as previously described [22, 23]. PCI-1 was derived from a T2N0M0 moderately-well differentiated recurrent tumor of the larynx. PCI-13 was derived from a T4N1M0 poorly differentiated tumor of the oral cavity. PCI-30 was derived from a T3N1 M0 moderately-well differentiated primary tumor of the tongue [22-25]. Normal immortalized human kerationocytes (HaCaT) were purchased from ATCC. Cells were cultured in RPMI 1640 supplemented with 10% (v/v) FCS, L-glutamine, and antibiotics (all from Invitrogen). Cells used for our experiments were in the log phase of growth and were negative for Mycoplasma and endotoxin, as confirmed by PCR (Mycoplasma Tissue Culture Detection kit, Gen-Probe) and Limulus Amebocyte Lysate assay (Cambrex), respectively.
Reverse-transcription and quantitative real-time PCR (qRT-PCR) analysis of PRAME transcripts
Total RNA from cell lines was isolated using TRI Reagent (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany) as previously described [26]. RNA integrity was confirmed by denaturing agarose gel electrophoresis, and the concentration was quantified by measuring the optical density (OD) at 260 nm using BioPhotometer (Eppendorf AG, Hamburg, Germany). RNA samples were treated with DNase I and reverse-transcribed into cDNA using oligo-dT primers. Reverse transcription was performed using M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The qRT-PCR assay was performed using the LightCycler 480 II detection system (Roche Diagnostics GmbH, Mannheim, Germany) and the LightCycler 480 SYBR Green I Master as the detection dye. Primers used to detect PRAME were: 5′- TCACCTCTCAGTTCCTCAGTC-3′ and 5′-AGGGTTTCCAAGGGGTTCATC-3′(ENST#: 00000398743, product size 118bp). Primers used to detect PBGD (porphobilinogen deaminase), a housekeeping gene, were: 5′-GCCAAGGACCAGGACATC-3′ and 5′-TCAGGTACAGTTGCCCATC-3′(ENST#: 00000278715, product size 160bp). The quantity of PRAME transcripts in each sample was standardized by PBGD transcript level. For amplification, 5μl of LightCycler 480 SYBR Green I Master, 1μl of primers (Oligo, Warsaw, Poland), 3μl of water (Sigma-Aldrich Chemie, Steinheim, Germany) and 1μl of cDNA solution were mixed together. One RNA sample of each preparation was processed without RT-reaction to provide a negative control in subsequent PCR. Sample amplification included a hot start (95°C, 5 minutes) followed by 50 cycles of denaturation at 95°C for 6 seconds, annealing at 60°C for 6 seconds, and extension at 72°C for 6 seconds. After amplification, Melt Curve analysis was performed to analyze the product melting temperature. The amplification products were also resolved by 3% agarose gel electrophoresis and visualized by ethidium bromide staining.
Flow cytometry
Flow cytometry was used to evaluate PRAME expression in PCI cells. A Becton Dickinson flow cytometer equipped with FACSDiva v6.1.2 software was used. At least 2 × 104 PCI cells where acquired for analysis. The following antibodies were used for staining: polyclonal rabbit anti-human PRAME (Sigma-Aldrich) and secondary antibody FITC-labeled donkey anti-rabbit IgG (Jackson ImmunoResearch). Before staining, all Abs were pre-titrated using PCI-13 cells to establish optimal staining dilutions. Briefly, samples were permeabilized with PBS containing 0.5% bovine serum albumin and 0.1% (v/v) saponin, and stained with anti-PRAME Ab for 30 min at room temperature. Cells were further washed twice with PBS containing 0.5% bovine serum albumin and 0.2% (v/v) saponin, and then stained with goat anti-rabbit FITC-labeled secondary Ab, resuspended in buffer, and immediately analyzed by flow cytometry. Appropriate isotype controls were included for each sample.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analyses
Cells were harvested and treated in lysis RIPA buffer (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany) supplemented with 10% of proteases cocktail inhibitor (Roche Diagnostics GmbH, Mannheim, Germany). Protein concentration was quantified using Bradford method according to manufacturer protocol (Bio-Rad Laboratories GmbH, Munchen, Germany). Next, 30μg of protein were resuspended in sample buffer and separated on 10% Tris-glycine gel using SDS-PAGE. Gel proteins were semidry transferred to PVDF membrane (Roche Diagnostics, Mennheim, Germany), which was blocked with 5% milk in Tris-buffered saline/Tween. Immunodetection was performed with PRAME Ab (1:1000), followed by incubation with goat anti-rabbit IgG-HRP-conjugated Ab (1:2000). As a loading control, the blots were stripped and reprobed with a goat anti-GAPDH Ab (1:3000), followed by incubation with goat anti-rabbit IgG-HRP-conjugated Ab (1:10000). Bands were revealed using SuperSignal West Femto Chemiluminescent Substrate, Thermo Fisher Scientific (Rockford, IL) and Biospectrum Imaging System 500, UVP Ltd. (Upland, CA, USA).
Immunostaining
The following primary antibodies were used for immunostaining: rabbit anti-human PRAME (Sigma-Aldrich, Poznan, Poland) (5ug/mL diluted in Ab diluent) and isotype control rabbit IgG (DAKO, Gdynia, Poland). Paraffin sections of tumor tissues or normal mucosa were stained as previously described [27, 28]. After standard deparaffinization, the EnVision+ System (Dako) was used for staining according to the manufacturer's instructions. In short, after an overnight incubation with the Abs, sections were first incubated with labeled polymer-horseradish peroxidase (HRP) anti-rabbit antibody and then with 3,3′-diaminobenzidine. To eliminate nonspecific binding of the secondary antibody, tissue sections were incubated with a serum-free protein blocker before adding the primary antibodies. Sections were counterstained with Meyer's hematoxylin and mounted in glycerol jelly. Slides were evaluated in a light microscope under 200× magnification. For digital image analysis, the software AnalySISˆB was used. All stained sections were analyzed and scored by two independent investigators (MJS and GD) to avoid bias, and the two scores were averaged and recorded. Each duplicate cores per case of TMA was entirely scored. The sections were scored according to the % of tumor cells staining (POSITIVITY) (<25%= 0; 25–75%= 50; and >75%= 100). The level of staining INTENSITY was recorded as none = 0, weak = 1, moderate =2, or strong = 3).
Statistical analysis
Data were summarized by descriptive statistics (means ± SD for continued variables and frequency or percentage for categorical variables). The flow cytometry results were shown as mean fluorescence intensity (MFI). Fisher's exact tests were used to determine if there was a difference in PRAME expression among the tissue types. To examine associations between patient characteristics and PRAME expression, Jonckheere Terpstra Tests for ordered differences (grade, stage, tumor size and nodal involvement) were used. Adjustments to p-values were made using the Bonferroni step-down procedure. P<0.05 was considered to be significant.
Results
PRAME expression in cell lines and tissues
Initially, PRAME expression at mRNA and protein levels was evaluated in HNSCC cell lines (PCI-1, PCI-13, PCI30) and HaCaT cells. By qRT-PCR, the message for PRAME was found to be very strongly expressed in PCI-1 cells, considerably less well expressed in PCI30 cells, and only weakly expressed in PCI 13 cells (Fig. 1A). PRAME mRNA expression in HaCaT cells was negligible relative to that in PCI cells. These results suggested that cell lines derived from well or moderately differentiated HNSCC (PCI-1 and PCI-30) expressed higher levels of PRAME mRNA than poorly differentiated tumor cells (PCI-13). However, additional cell line need to be tested to confirm this preliminary observation.
Figure 1. PRAME expression in PCI-1, PCI-13, PCI-30 and HaCaT cell lines.
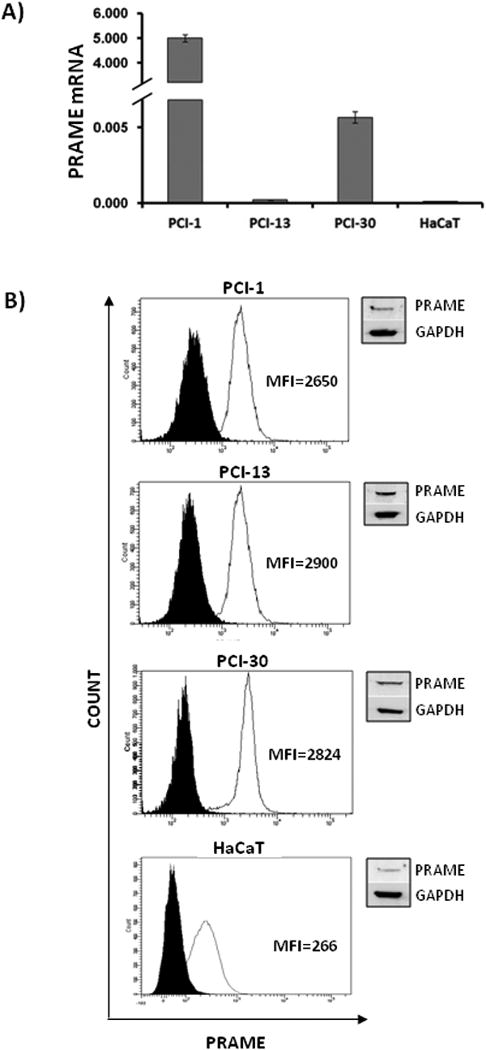
(A) Expression of PRAME mRNA. The PRAME transcript levels were determined by qRT-PCR analysis of cDNA. The cells were maintained in complete RPMI-1640 medium. After trypsinization and cell collection, total RNA was isolated and treated with DNase I, quantified, and reverse-transcribed into cDNA. The levels of PRAME transcripts were standardized against PBGD cDNA level, and are expressed as relative levels per 1μg of total RNA. Each sample was measured in triplicate, and the results represent means ± SD from three experiments; (B) Expression of the PRAME protein as measured by flow cytometry using permeabilized PCI or HaCaT cells. The data are expressed as mean fluorescence intensity (MFI) (open peaks). Black peaks show control cells stained with isotype control Abs. The inserts on the upper right show results of western blots for PRAME. Representative data for one of three experiments performed with each cell line are shown.
PRAME protein expression studied in HNSCC cell lines and HaCaT cells by flow cytometry was found to be somewhat stronger in PCI-13 (MFI=2432±467) than in PCI-1 (MFI=2110±540) or PCI-30 cells (MFI=2069±755) (Fig. 1B), and the weakest protein expression was found in HaCaT cells (MFI=266±45). These results were confirmed by western blots (Fig. 1B, inserts). The obvious lack of correlation between PRAME mRNA and protein expression levels in HNSCC cell lines is not surprising. mRNA may be a subject to degradation by cellular nucleases or may be retained in nucleus and not translated, leading to their overestimation relative to protein levels [29, 30]. During the transition from a gene to protein, a number of posttranscriptional or posttranslational regulation steps might occur, including alternative splicing, polyribosomes activity, micro-RNA effects or changes in protein stabilization. For this reason, expression of PRAME protein rather than mRNA is more biologically important.
In tissues, PRAME was localized in cytoplasm and/or nuclei. PRAME expression was significantly greater in HNSCC tissues (p<0.0001) or lymph node metastases (p<0.0006) than in control normal mucosa (Fig. 2). PRAME expression in normal mucosa was negative. However, in one case, normal mucosa was weakly positive for PRAME, and its expression was confined to the basal layer of the epithelium (Fig. 2A, B). PRAME was detected in all primary tumors and in metastatic lymph nodes (Fig. 2). The staining intensity ranged from weak to strong. No differences were found in PRAME expression between primary tumors vs. lymph node metastases (Fig. 2E, F).
Figure 2. PRAME expression in normal control (NC) oral mucosa obtained from normal donors (NC), HNSCC lesions or metastatic lymph nodes (LN).
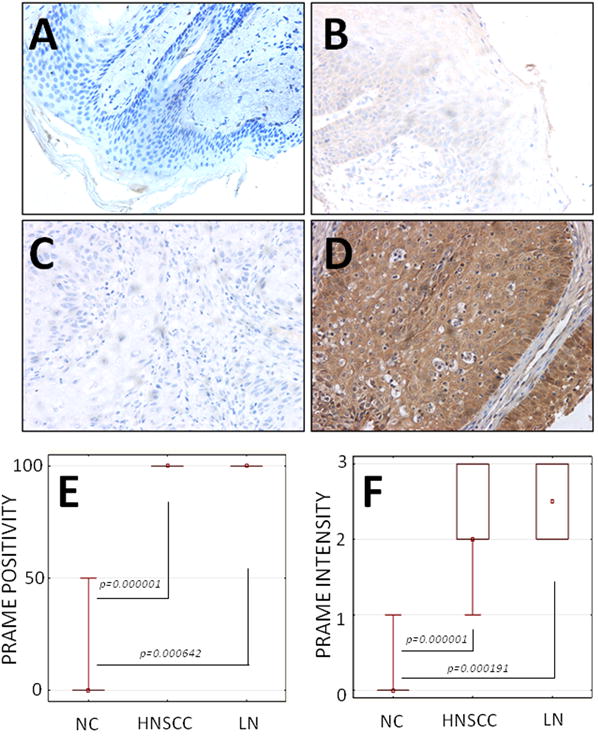
(A) Isotype Ab control in a specimen of NC mucosa (× 200); (B) No or only weak expression of PRAME in a specimen of NC mucosa (× 200); (C) Negative (isotype Ab) control in a specimen of HNSCC (× 200); (D) Strong expression of PRAME in a representative specimen of HNSCC (× 200); (E) PRAME expression (% POSITIVE CELLS) in NC vs. HNSCC or LN, respectively. All HNSCC and LN were scored at >75% positivity; (F) PRAME expression (INTENSITY, measured as described in Methods) in NC vs. HNSCC or LN, respectively flow cytometry.
PRAME expression correlates with tumor grade
PRAME staining intensity was found to correlate with tumor grade: the highest PRAME expression was found in poorly differentiated tumors (G3) and the lowest PRAME expression in well differentiated tumors (G1). These staining results corresponded to expression of the PRAME protein in the HNSCC cell lines (Fig. 1B), where the highest level of PRAME protein was found in PCI-13. There was a significant difference in PRAME expression in well-differentiated tumors vs. moderately differentiated or poorly differentiated tumors (p=0.0169 or p=0.0073, respectively). We did not find significant difference in PRAME expression levels between moderately differentiated tumors (G2) and poorly differentiated tumors (G3) (Fig. 3A, B, C).
Figure 3. PRAME expression in HNSCC tissues.
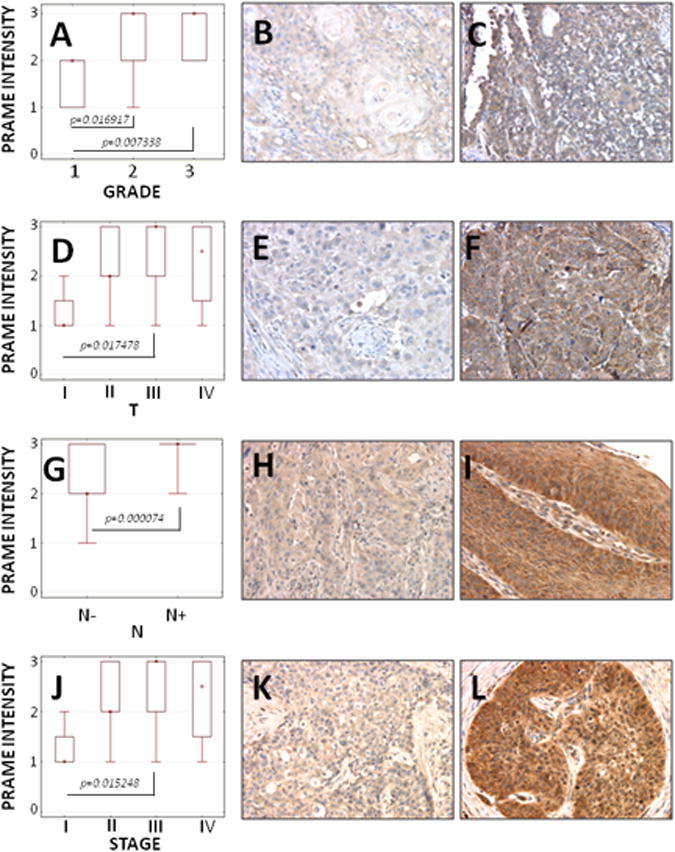
(A) PRAME staining intensity vs. tumor grade in all tumors; (B, C) representative PRAME expression in G1 tumor vs. G3 tumor (× 200); (D) PRAME staining intensity vs. tumor size for all tumors; (E, F) representative PRAME expression in T1 tumor vs. T3 tumor (× 200); (G) PRAME staining intensity vs. nodal involvement for N- or N+ specimens; (H, I) representative PRAME expression in tumor without nodal involvement (N-) vs. tumor with nodal involvement (N+) (× 200); (J) PRAME staining intensity vs. clinical stage for all tumors; (K, L) representative PRAME expression in stage I vs. stage III (× 200).
PRAME expression correlates with tumor size
The lowest PRAME expression levels were found in T1 tumors (p<0.05) vs. relatively higher PRAME expression in T2, T3 or T4 tumors (Fig. 3D, E, F).
PRAME expression correlates with the presence of lymph node metastases
The PRAME expression levels also correlated with lymph node metastases. The highest level of expression was found in patients with lymph nodes involvement (p<0.05) (Fig. 3G, H, I).
PRAME expression correlates with the disease progression
PRAME was found to be highly expressed in patients with advanced diseases, as there was a significant difference in PRAME expression levels between stage I vs. II, III and IV disease. No significant differences in PRAME expression levels were seen between HNSCC patients with stage II vs. III vs. IV disease (Fig. 3J, K, L).
PRAME expression in pre-cancerous lesions conditions of HNSCC
PRAME expression was evaluated in dysplastic mucosa of different grades in the larynx. Its expression was found in 8/12 (66%) cases, ranging in intensity from weak to strong. There was no correlation between PRAME expression levels and the severity of dysplasia. However, in 4/12 lesions (33%) PRAME was not detectable (Fig. 4). Two out of four negative patients had moderate grade (SIN II) dysplasia, and another two had mild (SIN I) or severe grade of dysplasia (SIN III), respectively.
Figure 4. PRAME expression in dysplastic oral mucosa.
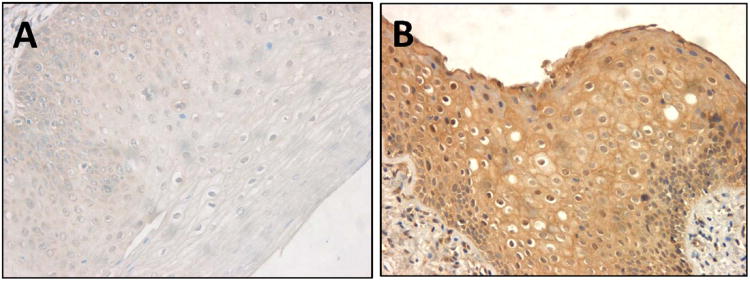
(A) Negative/weak expression of PRAME in a specimen of the mild grade dysplasia (SIN II). (B) High PRAME expression in a specimen of the moderate grade dysplasia. (× 200). Representative data.
Discussion
Cancer testis (CT) antigens are considered to be promising targets for immunotherapy of cancers, because of their tumor-specific expression and their ability to elicit autologous T-cell responses. To date, a limited number of TAA that are both tumor specific and immunogenic have been identified. Among them are TAA that commonly serve as targets for immune interventions, including non-mutated self-proteins such as aldehyde dehydrogenase 1A1 (ADHL1-A1), chondroitin sulfate proteoglycan 4 (CSPG-4), MAGE, cyclin B1, SART-1, SART-3, survivin, HER-2 (EGF-R), CEA, and MUC-1 or mutated p53 protein. These TAA have been used as immunogens in anti-cancer vaccine clinical trials for various types of solid tumors [9, 31-33]. Some of these TAA have also been shown to have a prognostic value. For example, MAGE-A was shown to be associated with poor overall survival and a high risk of metastases in patients with primary osteosarcoma or melanoma [34, 35]. Among CT antigens expressed in human tumor cells, PRAME represses the RA signaling pathway, which is implicated in cell proliferation, differentiation and apoptosis. The expression of this antigen in RA-sensitive cells enables them to escape from RA-induced cell growth arrest, differentiation and apoptosis. This ability to repress the RA pathway might explain positive selection of PRAME overexpressing cells during oncogenesis and its expression in pre-malignant cells. Moreover, the restoration of sensitivity to RA in RA-resistant cells can be obtained by the knockdown of PRAME by RNA interference [15].
Our findings on PRAME expression in HNSCC are consistent with the existing literature reports for other solid tumors, including ovarian cancer, lung cancer, melanoma and others [13, 21]. It has also been reported that many CT antigens are expressed in HNSCC at the mRNA or protein levels. In addition to PRAME, these CT antigens include melanoma antigen-1 (MAGE-1), MAGE-4, MAGE-10, MAGE-12, B-melanoma antigen, CTL-recognized melanoma antigen (CT antigen 2) [LAGE], New York esophageal squamous cell carcinoma antigen (CT antigen 1), SSX-2, SSX-4, BAGE, GAGE-1/2, GAGE-3/4. Further MAGE-A4 expression at the mRNA level predicted poor outcome independently of clinical parameters in HNSCC [36, 37]. We found PRAME to be expressed in all tumor samples and HNSCC cell lines. However, its expression pattern and levels were variable and dependent on pathological characteristics of the tumor. Importantly, in our study, PRAME expression at the protein level correlated with the clinicopathological criteria known to indicate high risk or poor outcome in HNSCC. This finding suggests that PRAME may have a prognostic value in HNSCC and should be further evaluated as a potential diagnostic, prognostic and perhaps therapeutic target in this type of cancer.
A novel finding of the current study relates to the heterogeneous expression of PRAME in pre-cancerous dysplastic lesions in the larynx. It is possible that the outcome of RA therapy in premalignant oral diseases may depend on the individual level of PRAME expression. Therefore, it might be beneficial to select patients for RA treatments with based on the presence of the PRAME protein in pre-malignant lesions. Our results indicate that one third of patients with pre-cancerous lesions lack PRAME and, consequently, are potential candidates for the use of RA in prevention therapy. Ambiguous results of the previous clinical trials evaluating the usefulness of retinoids in chemoprevention could perhaps be explained by the failure to recognize PRAME as a factor implicated in RA resistance. Selection of PRAME-negative patients for RA trials could result in better clinical responses. Furthermore, future iRNA-based strategies targeting PRAME followed by RA treatment could further increase its effectiveness. In addition, results of our recent in vitro studies (data not shown) provide preliminary evidence that silencing PRAME either with siRNA or with lentivirus particles followed by treatment with RA of PCI cells, inhibits tumor growth, induces apoptosis of tumor cells and down-regulates the expression of epidermal growth factor receptor (EGFR) on these cells
The frequent and high expression of PRAME detected in HNSCC might indicate that this protein plays important role in the biology of head and neck cancers. Furthermore, the identification of PRAME proteins as predictors of tumor progression and poor clinical prognosis raises the interesting possibility that PRAME may serve as a novel biomarker for diagnosis and as a target of immunotherapy. However, limitations of the current study due to small cohort with the pre-malignant lesions prevented us from identifying PRAME as biomarker in this group of patients. Clearly, PRAME expression and function in dysplasia should be elucidated further.
Acknowledgments
This work was supported in part by the NIH grant PO-1 CA109688 to TLW and by the Polish Ministry of Science and Higher Education (NN401047738) and the Foundation for Polish Science (Homing Plus/2010-1/13) grants to MJS. BS and JM were supported by the Foundation for Polish Science “Homing Plus/2010-1/13” scholarship grant (MJS). We thank Andrzej Kluk for technical assistance. The authors wish to thank Professor Witold Szyfter for his useful comments.
Abbreviations
- PRAME
Preferentially Expressed Antigen in Melanoma
- RA
retinoic acid
- RAR
retinoic acid receptor
- HNSCC
head and neck squamous cell carcinoma
- TAA
tumor associated antigens
- ALDH
aldehyde dehydrogenase
- RT-PCR
reverse transcription polymerase chain reaction
- ABs
antibodies
- HRP
horseradish peroxidase
- MFI
mean fluorescence intensity
- MAGE
melanoma antigen genes
- HER (EGF-R)
epidermal growth factor receptor
- CEA
carcinoembrionic antigen
- MUC
mucin
- ERCC1
excision repair cross-complementation group 1
- RRM1
ribonucleotide reductase M1
- HPV
human papillomavirus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 2.Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol. 2011;12:153–9. doi: 10.1016/S1470-2045(10)70279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 4.Argiris A, Lee SC, Feinstein T, Thomas S, Branstetter BFT, Seethala R, et al. Serum biomarkers as potential predictors of antitumor activity of cetuximab-containing therapy for locally advanced head and neck cancer. Oral Oncol. 2011;47:961–6. doi: 10.1016/j.oraloncology.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calli C, Calli A, Pinar E, Oncel S, Tatar B. Prognostic significance of p63, p53 and ki67 expression in laryngeal basaloid squamous cell carcinomas. B-ENT. 2011;7:37–42. [PubMed] [Google Scholar]
- 6.Cullen KJ, Schumaker L, Nikitakis N, Goloubeva O, Tan M, Sarlis NJ, et al. beta-Tubulin-II expression strongly predicts outcome in patients receiving induction chemotherapy for locally advanced squamous carcinoma of the head and neck: a companion analysis of the TAX 324 trial. J Clin Oncol. 2009;27:6222–8. doi: 10.1200/JCO.2009.23.0953. [DOI] [PubMed] [Google Scholar]
- 7.Lee KD, Lee HS, Jeon CH. Body fluid biomarkers for early detection of head and neck squamous cell carcinomas. Anticancer Res. 2011;31:1161–7. [PubMed] [Google Scholar]
- 8.Wu Y, Posner MR, Schumaker LM, Nikitakis N, Goloubeva O, Tan M, et al. Novel biomarker panel predicts prognosis in human papillomavirus-negative oropharyngeal cancer: an analysis of the TAX 324 trial. Cancer. 2011;118:1811–7. doi: 10.1002/cncr.26485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer CJ. Exploring biomarkers in head and neck cancer. Cancer. 2012 doi: 10.1002/cncr.26718. [DOI] [PubMed] [Google Scholar]
- 10.Rhee JC, Khuri FR, Shin DM. Advances in chemoprevention of head and neck cancer. Oncologist. 2004;9:302–11. doi: 10.1634/theoncologist.9-3-302. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda H, Lethe B, Lehmann F, van Baren N, Baurain JF, de Smet C, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. doi: 10.1016/s1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 12.Atanackovic D, Luetkens T, Kloth B, Fuchs G, Cao Y, Hildebrandt Y, et al. Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am J Hematol. 2011;86:918–22. doi: 10.1002/ajh.22141. [DOI] [PubMed] [Google Scholar]
- 13.Epping MT, Bernards R. A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res. 2006;66:10639–42. doi: 10.1158/0008-5472.CAN-06-2522. [DOI] [PubMed] [Google Scholar]
- 14.Zou C, Shen J, Tang Q, Yang Z, Yin J, Li Z, et al. Cancer-testis antigens expressed in osteosarcoma identified by gene microarray correlate with a poor patient prognosis. Cancer. 2011;118:1845–55. doi: 10.1002/cncr.26486. [DOI] [PubMed] [Google Scholar]
- 15.Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–47. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011;226:322–30. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolle P. Developmental expression of retinoic acid receptors (RARs) Nucl Recept Signal. 2009;7:e006. doi: 10.1621/nrs.07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Means AL, Gudas LJ. The roles of retinoids in vertebrate development. Annu Rev Biochem. 1995;64:201–33. doi: 10.1146/annurev.bi.64.070195.001221. [DOI] [PubMed] [Google Scholar]
- 20.Freemantle SJ, Dragnev KH, Dmitrovsky E. The retinoic acid paradox in cancer chemoprevention. J Natl Cancer Inst. 2006;98:426–7. doi: 10.1093/jnci/djj116. [DOI] [PubMed] [Google Scholar]
- 21.Papadimitrakopoulou VA, Lee JJ, William WN, Jr, Martin JW, Thomas M, Kim ES, et al. Randomized trial of 13-cis retinoic acid compared with retinyl palmitate with or without beta-carotene in oral premalignancy. J Clin Oncol. 2009;27:599–604. doi: 10.1200/JCO.2008.17.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heo DS, Snyderman C, Gollin SM, Pan S, Walker E, Deka R, et al. Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res. 1989;49:5167–75. [PubMed] [Google Scholar]
- 23.Lansford CD, Grenman R, Bier H, Somers KD, Kim SY, Whiteside TL, et al. Human Cell Culture, Volume II, Cancer Cell Lines Part 2. Vol. 2. Dordrecht: Kluwer Academic Publishers; 1999. Head and Neck Cancers; pp. 185–255. [Google Scholar]
- 24.Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, et al. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck. 2007;29:163–88. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 25.Meissner M, Reichert TE, Kunkel M, Gooding W, Whiteside TL, Ferrone S, et al. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11:2552–60. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 27.Szajnik M, Szczepanski MJ, Czystowska M, Elishaev E, Mandapathil M, Nowak-Markwitz E, et al. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28:4353–63. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szczepanski MJ, Czystowska M, Szajnik M, Harasymczuk M, Boyiadzis M, Kruk-Zagajewska A, et al. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–13. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gry M, Rimini R, Stromberg S, Asplund A, Ponten F, Uhlen M, et al. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics. 2009;10:365. doi: 10.1186/1471-2164-10-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat Struct Mol Biol. 2004;11:121–7. doi: 10.1038/nsmb724. [DOI] [PubMed] [Google Scholar]
- 31.Visus C, Wang Y, Lozano-Leon A, Ferris RL, Silver S, Szczepanski MJ, et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8(+) T cells. Clin Cancer Res. 2011;17:6174–84. doi: 10.1158/1078-0432.CCR-11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiteside TL. Immunobiology and immunotherapy of head and neck cancer. Curr Oncol Rep. 2001;3:46–55. doi: 10.1007/s11912-001-0042-3. [DOI] [PubMed] [Google Scholar]
- 33.Whiteside TL. Immunobiology of head and neck cancer. Cancer Metastasis Rev. 2005;24:95–105. doi: 10.1007/s10555-005-5050-6. [DOI] [PubMed] [Google Scholar]
- 34.Sudo T, Kuramoto T, Komiya S, Inoue A, Itoh K. Expression of MAGE genes in osteosarcoma. J Orthop Res. 1997;15:128–32. doi: 10.1002/jor.1100150119. [DOI] [PubMed] [Google Scholar]
- 35.Velazquez EF, Jungbluth AA, Yancovitz M, Gnjatic S, Adams S, O'Neill D, et al. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)--correlation with prognostic factors. Cancer Immun. 2007;7:11. [PMC free article] [PubMed] [Google Scholar]
- 36.Cuffel C, Rivals JP, Zaugg Y, Salvi S, Seelentag W, Speiser DE, et al. Pattern and clinical significance of cancer-testis gene expression in head and neck squamous cell carcinoma. Int J Cancer. 2011;128:2625–34. doi: 10.1002/ijc.25607. [DOI] [PubMed] [Google Scholar]
- 37.Figueiredo DL, Mamede RC, Proto-Siqueira R, Neder L, Silva WA, Jr, Zago MA. Expression of cancer testis antigens in head and neck squamous cell carcinomas. Head Neck. 2006;28:614–9. doi: 10.1002/hed.20380. [DOI] [PubMed] [Google Scholar]


