Abstract
Background
Severe malarial anemia (SMA) resulting from Plasmodium falciparum infections is one of the leading causes of childhood mortality in sub-Saharan Africa. The innate immune mediator, macrophage migration inhibitory factor (MIF) plays a critical role in the pathogenesis of SMA.
Methods
To investigate the influence of MIF genetic variation on susceptibility to SMA, haplotypes of the MIF-173(G/C) and -794CATT5–8 polymorphisms were examined in a cohort of Kenyan children.
Results
A statistically significant relationship between increasing frequencies of longer CATT repeats at -794 and increasing malarial anemia severity was observed. In addition, there was a strong association between lower MIF concentrations and longer CATT repeats. Multivariate logistic regression analyses demonstrated that the MIF-794CATT6/-173G (6G) haplotype was associated with protection against SMA while carriers of 7C or 8C haplotypes had increased risk of developing SMA. Furthermore, carriers of the 7C or 8C haplotypes had reduced plasma MIF levels during acute disease.
Conclusions
The findings demonstrate that variation in the MIF promoter influences susceptibility to SMA and peripheral MIF production. However, the MIF-173 and -794 polymorphisms appear to have both independent, as well as interactive effects on different measures of disease severity, suggesting a complex role for MIF in malarial pathogenesis.
Keywords: Plasmodium falciparum, severe malaria, anemia, Macrophage migration inhibitory factor (MIF), promoter polymorphisms
INTRODUCTION
Life-threatening complications in children with Plasmodium falciparum malaria include: cerebral malaria (CM); high-density parasitemia (HDP); respiratory distress; and severe malarial anemia (SMA) [1–3]. Clinical presentations of pediatric severe malaria vary markedly across regions with differing transmission intensities, with CM primarily occurring in lower transmission regions and SMA being most prevalent in holoendemic areas [4]. However, differences in disease severity among children of similar ages, residing in a given transmission area, are likely due to variation in the host immune response that is conditioned by genetic factors.
Our previous studies, as well as those of others, have demonstrated that the nature and magnitude of innate immune mediator production [3, 5–7] and genetic variation in host immune response genes [8–11] are important determinants of the development and outcomes of severe malaria. Since macrophage migration inhibitory factor (MIF) is a central regulator of innate immune responses to bacterial and parasitic infections [12–17], investigations in our laboratory have focused on the role of MIF in malarial pathogenesis. Although studies in murine models of malaria suggest that increased MIF production causes suppression of erythropoiesis and development of SMA [18, 19], we have recently shown that circulating MIF concentrations progressively decline with increasing anemia severity in Kenyan children with falciparum malaria [20]. These results are consistent with our previous findings in Gabon [21] showing lower MIF levels in children with acute malaria relative to healthy controls, and recent data from experimental human malaria [22] demonstrating that peripheral blood MIF production significantly decreased during the course of P. falciparum infection.
Of potential importance for influencing patterns of MIF expression and disease severity in children with malaria is genetic variation in the MIF promoter. A tetranucleotide short tandem repeat polymorphism (STRP), MIF-794 CATT5–8, and a single nucleotide polymorphism (SNP), MIF-173G/C, functionally influence susceptibility and severity to inflammatory diseases, including arthritis, atopy, lung disease, and scleroderma [23–29]. In general, longer CATT repeats (>5) at -794 and the -173C allele are associated with elevated MIF production and increased susceptibility to inflammatory diseases [23–27, 30]. However, some investigations in chronic inflammatory diseases have observed an opposite pattern of MIF production and disease susceptibility [31, 32]. A previous study in Zambian children revealed that carriers of >5 CATT repeat alleles at -794 had a higher risk of developing HDP relative to those with the 5-repeat allele [33]. In addition, our recent investigation in a large cohort of Kenyan children demonstrated that a CC genotype at -173 was associated with increased susceptibility to HDP [9]. Although several studies demonstrated linkage disequilibrium (LD) between polymorphisms at MIF-794 and -173, with haplotypes of the two loci being stronger predictors of disease risk than either one alone [23, 24, 26, 34], the role of MIF promoter haplotypes in conditioning susceptibility to SMA has not been reported.
To comprehensively investigate the relationship between MIF promoter polymorphisms and SMA pathogenesis, we examined the MIF-794 STRP in the cohort of children in whom we previously analyzed the MIF-173 SNP [9], and constructed haplotypes for the two loci. Data presented here show a significant relationship between malaria disease severity and the -794 STRP, independent of the -173 SNP. In addition, results presented here demonstrate, for the first time, an association between MIF-794 and -173 haplotypes, susceptibility to SMA, and peripheral blood MIF production that appears independent of the effect of MIF polymorphisms on HDP.
STUDY PARTICIPANTS AND METHODS
Study site
This study was conducted as part of our ongoing investigations at the Siaya District Hospital, western Kenya, examining the genetic and immunological basis of pediatric SMA [35]. This community has holoendemic P. falciparum transmission where residents receive up to 300 infective mosquito bites per annum, and the prevalence of falciparum infection is 83% in children one to four years of age, with SMA and HDP being the primary clinical manifestations of severe malaria [35, 36].
Study participants
Children less than three years of age (n=643) presenting at the hospital with acute malaria (or for routine immunizations) were recruited into the study. All study participants were from the Luo ethnic group [35], thus, providing a homogenous population for the genetic investigations. After obtaining informed written consent from the parents/guardians, capillary blood was collected from study participants for determination of parasitemia and initial hemoglobin (Hb) levels. Children with detectable P. falciparum parasitemia (acute malaria, n=519) were categorized into two groups according to their anemia status: non-SMA (Hb≥6.0 g/dL, n=309) and SMA (Hb<6.0 g/dL, n=210). The definition of SMA was based on the distribution of anemia determined by >14,000 longitudinal Hb measurements in age- and gender-matched children from the same geographic location [37]. In addition, HDP (≥10,000 parasites/μL) was based on the distribution of parasitemia in the population determined in previous studies [35, 38]. Aparasitemic controls (AC, n=124) were children with P. falciparum-negative blood smears and free from fever or other symptoms of malaria in the preceding two weeks prior to enrollment. Exclusion criteria included those children co-infected with other species of Plasmodium, prior hospitalization and/or transfusion for any cause, and CM.
The study was approved by the Ethics Committees of the Kenya Medical Research Institute, University of Pittsburgh Institutional Review Board, and University of New Mexico Institutional Review Board. Informed written consent was obtained from the parent/legal guardian of all participating children.
Sample collection and laboratory evaluation
Prior to administration of anti-malarial therapy and supportive care, venous blood was collected into tubes containing the anticoagulant EDTA. The blood sample was used for a complete blood count and Hb determination (Coulter® AcT diff2™, Beckman Coulter Corporation), plasma preparation, and HIV-1 testing. Peripheral blood smears were stained with Giemsa reagents and examined for Plasmodial parasites under oil immersion. The number of parasites per 300 white blood cells (WBC) was determined, and parasitemia per/μL of blood was calculated using the total WBC count obtained from the automated hematology analyzer. Sickle-cell status was determined by alkaline cellulose acetate electrophoresis on Titan III plates (Helena BioSciences, UK) according to the manufacturers’ recommendations. HIV-1 status was determined using two serological methods followed by proviral DNA PCR, as described previously [39]. All parents/guardians of the study participants received pre- and post-test HIV/AIDS counseling. At the time of enrollment, none of the children had been started on anti-retroviral therapy.
Genetic analyses
Blood spots were collected on FTA Classic® cards (Whatman Inc., USA) and DNA was extracted using the Gentra System (Gentra System Inc., USA). Samples were genotyped for the MIF-794 STRP using previously described methods [27] and the MIF-173 SNP according to our previous methods [9].
Determination of MIF concentrations
MIF concentrations in plasma samples were determined using an ELISA with a matched anti-MIF antibody pair (R&D systems, USA) according to manufacturer’s recommendations. The limit of detection was >31.25 pg/mL.
Data analyses
Comparison of variables across the three clinical groups (AC, non-SMA and SMA) were conducted using the Kruskal-Wallis test, and where significant differences were obtained, Mann-Whitney U tests were used for pairwise comparisons. Circulating MIF levels between groups were compared using Mann-Whitney U tests. The association between CATT repeat length and malarial anemia severity was determined using the Cochran-Armitage trend test for proportions [40] as implemented in the coin package [41], available for the R statistical program [42], while the association between repeat length and MIF concentration was determined using the Cochran-Armitage trend test for counts [43]. MIF promoter haplotypes were constructed from the -173G/C and -794CATTn genotype data using the HPlus software program (Fred Hutchinson Cancer Research Center, USA). Agreement with Hardy-Weinberg equilibrium (HWE) was tested using the procedure of Guo and Thompson [44] as implemented in the software program Arlequin [45]. This program was also used to measure LD between the -173 and -794 loci, using a likelihood ratio test. For analysis of association between disease and genetic variants, three primary outcomes were defined: presence of parasitemia, HDP, and SMA. Multivariate logistic regression analyses were conducted for each of these outcomes using genotype/haplotype, age, gender, sickle-cell trait, and HIV-1 status as independent predictors. HIV status was entered as a dichotomoous variable for exposed/non-exposed, where by children with a positive PCR test and/or a positive serological test were considered to be exposed.
RESULTS
Characteristics of study participants
Children (age: 3–32 mos.) were categorized into three clinical groups: AC (n=124); non-SMA (n=309); and SMA (n=210). Differences in gender distribution across the groups were not statistically significant (P=0.217, Table 1). However, age differed across the groups (P=0.001), with children in the non-SMA group being older than those in the other two groups (P<0.001 for both comparisons). Axillary temperature differed across the groups (P=0.003) and was elevated in the acute malaria groups relative to the AC group (P<0.001 for both comparisons). Hemoglobin, which was used in the categorization criteria, as expected, differed across the groups (P<0.001). However, parasitemia, as well as the prevalence of HDP, did not significantly differ between the non-SMA and SMA groups (P=0.138, and P=0.307, respectively).
Table 1.
Demographical, clinical, and parasitological characteristics of the study participants.
| Characteristic | AC | Acute malaria
|
P | |
|---|---|---|---|---|
| Non-SMA | SMA | |||
| Number (n) | 124 | 309 | 210 | |
| Gender (n, %) | ||||
| Female | 72 (58) | 151 (49) | 106 (50) | 0.217 |
| Male | 52 (42) | 158 (51) | 104 (50) | |
| Age (mos.) | 8.0 (4.2–15.0) | 10.0 (6.0–16.0) | 8.0 (5.0–13.0) | 0.001 |
| Axillary temperature (oC) | 37.0 (36.5–37.8) | 37.5 (36.7–38.4) | 37.4 (36.8–38.2) | 0.003 |
| Hemoglobin (g/dL) | 10.0 (6.5–11.4) | 8.2 (6.9–9.6) | 4.9 (4.2–5.5) | <0.001 |
| Parasitemia (/μL) | 0 | 21360 (6154–49956) | 17489 (4005–41071) | 0.138* |
| HDP (n, %) | - | 205 (66) | 124 (59) | 0.307 |
Children less than three years of age were recruited from the Siaya district hospital in Western Kenya and categorized into three groups: aparasitemic controls (AC), malaria without severe anemia (non-SMA), and SMA. Data are presented as median (lower quartile-upper quartile) or n (%). P-values were obtained from Kruskal-Wallis or Chi-square tests, respectively, for differences across groups. *Comparison of parasitemia in non-SMA vs. SMA groups.
Distribution of MIF-794 genotypes and alleles
To investigate the association between variation in the MIF-794 CATT repeat and malarial pathogenesis, frequency distributions of the MIF-794 genotypes and alleles were determined in three groups of children: AC, non-SMA, and SMA. The 5,6 genotype was the most frequent in all three groups (23–34%), while the 5,8; 7,8; and 8,8 were the rarest genotypes (over all frequencies <1%, Table 2). The most prevalent -794 allele in the clinical groups was the 5-repeat (42–48%), while the 8-repeat showed the lowest frequency (1–3%, Table 2). Frequencies of the 5- and 6-repeat alleles decreased across the groups with increasing malarial anemia severity, while the 7-and 8-repeat alleles were increased (Table 2). A trend analyses using the Cochran-Armitage test for proportions [40] demonstrated a statistically significant relationship (P=0.007) between increasing frequencies of longer repeat lengths (and decreasing frequencies of shorter repeats) and increasing malarial anemia severity (AC<non-SMA<SMA), suggesting an association between MIF-794 alleles and susceptibility to SMA.
Table 2.
MIF-794 genotype and allele frequencies according to clinical categories.
| MIF-794 genotypes | Frequencies, n (%)
|
|||
|---|---|---|---|---|
| AC | Non-SMA | SMA | Total | |
| 5,5 | 32 (25.8) | 83 (26.9) | 45 ( 21.4) | 160 (24.9) |
| 5,6 | 42 (33.9) | 88 (28.5) | 49 (23.3) | 179 (27.8) |
| 5,7 | 11 (8.9) | 30 (9.7) | 34 (16.2) | 75 (11.7) |
| 5,8 | 1 (0.8) | 0 (0) | 2 (0.9) | 3 (0.5) |
| 6,6 | 23 (18.5) | 57 (18.4) | 39 (18.6) | 119 (18.5) |
| 6,7 | 8 (6.4) | 31 (10.0) | 27 (12.9) | 66 (10.3) |
| 6,8 | 1 (0.8) | 6 (1.9) | 2 (0.9) | 9 (1.4) |
| 7,7 | 6 (4.8) | 12 (3.9) | 8 (3.8) | 26 (4.0) |
| 7,8 | 0 (0) | 2 (0.6) | 1 (0.5) | 3 (0.5) |
| 8,8 | 0 (0) | 0 (0) | 3 (1.4) | 3 (0.5) |
| total | 124 (100) | 309 (100) | 210 (100) | 643 (100) |
|
MIF-794 alleles
|
||||
| 5 | 118 (47.6) | 284 (45.9) | 175 (41.7) | 577 (44.9) |
| 6 | 97 (39.1) | 239 (38.7) | 156 (37.1) | 492 (38.3) |
| 7 | 31 (12.5) | 87 (14.1) | 78 (18.6) | 196 (15.2) |
| 8 | 2 (0.8) | 8 (1.3) | 11 (2.6) | 21 (1.6) |
| total | 248 (100) | 618 (100) | 420 (100) | 1286 (100) |
DNA samples obtained from study participants were genotyped for the STRP (CATT5–8) at -794 of the MIF promoter by amplifying the target region with flanking primers and resolving the products using capillary electrophoresis. Genotypic and allelic frequencies are expressed as percent of each clinical category.
Association of MIF -794 genotypes with disease
Based on the frequency distribution of the -794 repeat in our cohort (see Table 2), and previous studies demonstrating that an increasing number of CATT repeats are associated with enhanced susceptibility to inflammatory diseases [26, 27, 33], children were divided into three genotypic groups: XX (n=458), XY (n=153), and YY (n=32), where X=5- or 6-repeat alleles, and Y=7- or 8-repeat alleles. Multivariate logistic regression analyses revealed that relative to individuals with the XX genotype, there was an 82% increase in the risk of parasitemia in children with the XY genotypes (P=0.029), but only a 5% increased risk of parasitemia in individuals with the YY genotypes (P=0.923, Table 3). The model further illustrated that there was no evidence of an association between genotypic groups and the risk of HDP (Table 3). However, XY and YY individuals had a 63% and 59% greater risk of SMA relative to the XX group, respectively, with differences being statistically significant for the XY group (P=0.024), but not for the YY group (P=0.270, Table 3). Taken together, these results suggest that an increasing number of CATT repeats at MIF-794 may be associated with enhanced susceptibility to parasitemia and SMA.
Table 3.
Association of MIF-794 genotypic categories with malaria outcomes.
| Disease category | Risk relative to XX genotype
|
|
|---|---|---|
| XY | YY | |
| Parasitemic | ||
| Odds ratio | 1.82 | 1.05 |
| 95% CI | 1.06 – 3.10 | 0.41 – 2.66 |
| P | 0.029 | 0.923 |
| High-density parasitemia | ||
| Odds ratio | 1.11 | 0.85 |
| 95% CI | 0.73 – 1.70 | 0.37 – 1.95 |
| P | 0.619 | 0.700 |
| Severe Malarial Anemia | ||
| Odds ratio | 1.63 | 1.59 |
| 95% CI | 1.07 – 2.49 | 0.70 – 3.65 |
| P | 0.024 | 0.270 |
The four CATT repeat alleles were categorized into two artificial alleles (X=5, 6 alleles and Y=7,8 alleles). The risk of disease in XY (n=153) and YY (n=32) carriers relative to individuals with the XX genotype (n=458) was determined by multivariate logistic regression, controlling for age, gender, HIV-1 status, and sickle-cell trait. The results are presented as odds ratios, 95% confidence intervals (CI), and P values.
Relationship between MIF -794 CATT repeat length and circulating MIF levels
The relationship between CATT repeat length and MIF production was investigated by examining circulating MIF levels in carriers of the different alleles. In contrast to previously reported patterns [24, 27], these analyses showed a progressive decrease in circulating MIF concentrations with carriage of longer CATT repeats (P=0.039 across groups, Table 4). Additional analyses using the Cochran-Armitage trend test for counts [43] showed a strong association between lower MIF concentrations and longer CATT repeats (P<0.001), suggesting that carriers of the disease-associated alleles have decreased peripheral blood MIF production.
Table 4.
Circulating MIF levels in Kenyan children according to -794 alleles.
| MIF-794 alleles | MIF concentration (pg/mL) |
|---|---|
| 5 (n=296) | 4433 (2987–6692) |
| 6 (n=232) | 4094 (2588–6258) |
| 7 (n=100) | 3731 (2150–6004) |
| 8 (n=12) | 3137 (978–4801) |
Plasma MIF concentrations in study participants were determined by ELISA and presented according to their carriage of MIF-794 alleles. MIF levels are shown as median (lower quartile-upper quartile). A Kruskal-Wallis test showed a statistically significant (P=0.039) difference in MIF concentrations across groups.
MIF promoter haplotypes
To examine the impact of interactions between the -794 and -173 loci and susceptibility to severe malaria, haplotypes of the two polymorphic sites were constructed. In contrast to previous studies from other regions in which the 5C haplotype (i.e., -794CATT5 /-173 C) was rare [26, 31, 34], the most common haplotype in the cohort was 5C (31%), while the 8G haplotype had the lowest frequency (<1%, Table 5). The distribution of haplotypes showed significant departure from HWE (P<0.001). In addition, there was significant evidence of LD between the -794 and -173 polymorphic sites (likelihood ratio χ2=42.4, P<0.001). Of particular interest, the proportion of 5G to 5C and 6G to 6C differed ~2-fold, while there was a disproportionately higher prevalence of 7C and 8C relative to 7G and 8G (i.e., 9-fold and 4-fold difference, respectively, Table 5), suggesting potential interactions between the two polymorphic sites.
Table 5.
MIF-794/-173 haplotypic frequencies.
| MIF-794/ -173 haplotypes | Haplotype frequencies, n (%) |
|---|---|
| 5G | 126 (14.1) |
| 5C | 274 (30.6) |
| 6G | 233 (26.0) |
| 6C | 107 (11.9) |
| 7G | 14 (1.6) |
| 7C | 127 (14.2) |
| 8G | 3 (0.3) |
| 8C | 12 (1.3) |
| total | 896 (100) |
MIF promoter haplotypes were constructed from the -794 STRP data in Table 2 and -173 SNP genotypes determined previously [9] using HPlus software. Complete data for both loci were available for 448 children resulting in a total of 896 haplotypes. The frequencies of individual haplotypes are expressed as percent of total haplotypes.
Association of MIF promoter haplotypes with disease
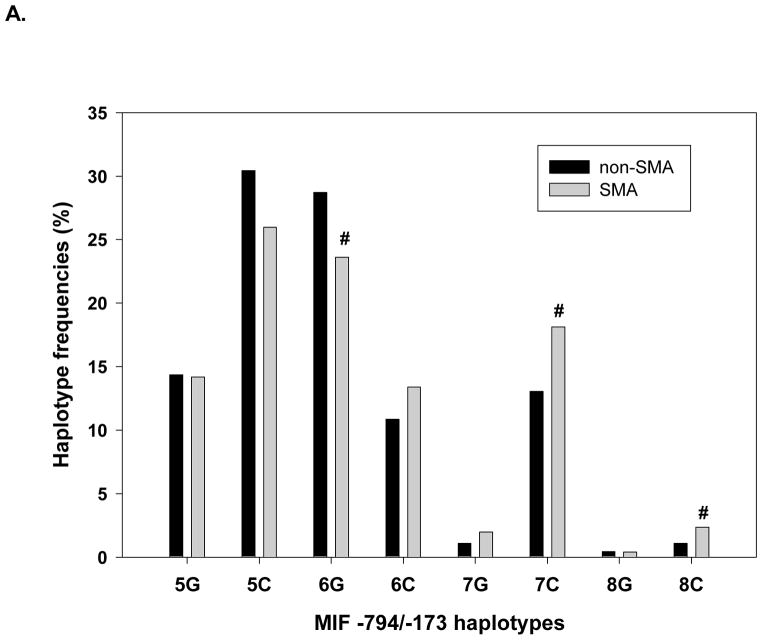
To determine if MIF haplotypes were associated with susceptibility to malaria, relationships between haplotypes and primary disease outcome variables were analyzed. These analyses revealed that MIF haplotypes were significantly associated with SMA (P=0.020), but not presence of parasitemia (P=0.220), or susceptibility to HDP (P=0.830). The association of MIF haplotypes with SMA was, therefore, further examined by comparing frequencies of individual haplotypes in the non-SMA vs. SMA groups to identify potential severe disease-associated haplotypes (Figure 1A). The frequency of the 6G haplotype was substantially under-represented in children with SMA, while the 7C and 8C haplotypes appeared to be over-represented in the SMA group (P≤0.100 for all comparisons, Figure 1), suggesting possible associations with susceptibility to SMA. Additional analyses using the logistic regression model demonstrated that children with the 6G haplotype were 37% less susceptible to SMA than individuals without this haplotype (P=0.050, Table 6). Conversely, children with the 7C haplotype had a 60% increased risk of SMA relative to those without 7C, although this difference did not reach statistical significance (P=0.064, Table 6). When carriers of the 7C or 8C haplotypes were combined however, these individuals had a 71% increased risk of SMA relative to children without 7C and 8C (P=0.031, Table 6). Since children with CC at MIF-173 have significantly increased susceptibility to HDP [9], it was of interest to determine if the risk of SMA was further exacerbated in the subset of individuals with the -173 CC genotype who carried a 7C or 8C haplotype. These analyses revealed that the risk of SMA was nearly 2.5-fold higher in this subset of children [odds ratio (95% confidence interval) = 2.46 (1.37–4.43), P=0.003], demonstrating an even further increase over the risk observed in the 7C/8C haplotype group as a whole. Thus, genetic variation within the MIF promoter at the -173 and -794 polymorphisms can both confer protection against SMA, or condition increased susceptibility to SMA in children with P. falciparum.
Figure 1.
A. Association of MIF promoter haplotypes with susceptibility to SMA. Haplotypes were constructed from the MIF-794 CATT5–8 and -173 G/C genotypic data using HPlus software, and the frequencies of each haplotype in children with severe malarial anemia (SMA) or malaria without severe anemia (non-SMA) are expressed as percentages. Differences in haplotype frequencies between the two groups were compared using Fisher’s test. #P<0.10, compared to the non-SMA group.
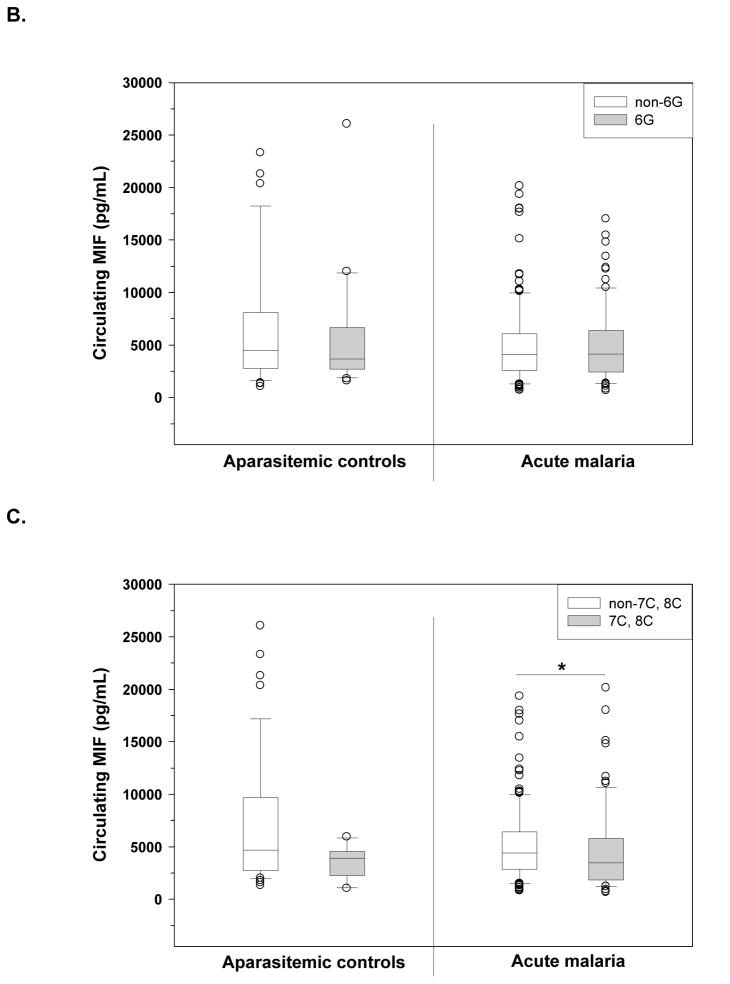
Figure B and C: Functional relationship of SMA-associated haplotypes with circulating MIF levels. Plasma levels of MIF were determined in children with acute malaria and aparasitemic controls using ELISA. B) MIF levels in the control (non-6G, n=33; 6G, n=23) and malaria (non-6G, n=137; 6G, n=102) groups presented according to carriage of the MIF -794/-173 6G haplotype. C) MIF levels in the control (non-7C, 8C, n=45; 7C, 8C, n=11) and malaria (non-7C, 8C n=165; 7C, 8C, n=74) groups presented according to carriage of the MIF-794/-173 7C or 8C haplotypes. Boxes represent the interquartile range, the line through the box represents the median, whiskers illustrate the 10th and 90th percentiles, and symbols represent outliers. *Differences between groups were statistically significant by Mann-Whitney U test (P<0.05).
Table 6.
Association between MIF-794/-173 haplotype carriage and susceptibility to SMA.
| Risk of SMA according to MIF -794/-173 haplotype carriage | |||
|---|---|---|---|
|
| |||
| 6G haplotype | 7C haplotype | 7C or 8C haplotypes | |
|
|
|||
| Odds ratio | 0.63 | 1.60 | 1.71 |
| 95% CI | 0.40 – 1.00 | 0.97 – 2.65 | 1.05 – 2.77 |
| P | 0.050 | 0.064 | 0.031 |
Association between MIF promoter haplotypes and susceptibility to SMA was determined using multivariate logistic regression controlling for age, gender, HIV-1 status, and sickle-cell trait. Odds ratios with 95% confidence intervals (CI), and P values represent the risk of SMA in carriers of given haplotypes compared to non-carriers.
Functional relationship between MIF haplotypes and MIF production
Since P. falciparum infection causes dysregulation in MIF production [18–21], the relationship between disease-associated haplotypes and circulating MIF levels were examined separately in aparasitemic children and children with acute malaria. Circulating levels of MIF were not significantly different between children with and without the 6G haplotype in either the aparasitemic or acute malaria categories (P=0.739, and P=0.796, respectively, Figure 1B). However, possession of the 7C or 8C haplotype was associated with lower circulating MIF levels in both aparasitemic children (P=0.103), and among children with acute malaria (P=0.034, Figure 1C) relative to non-7C/8C children. These data suggest that MIF promoter haplotypes associated with susceptibility to SMA appear to be functionally related to peripheral blood MIF production.
DISCUSSION
We recently demonstrated that variation at MIF-173 was associated with susceptibility to HDP, but not SMA, in the cohort of Kenyan children examined here [9]. In the current study, a more comprehensive examination of the role of genetic variation in the MIF promoter on conditioning susceptibility to severe childhood malaria was conducted by analyzing the MIF-794 STRP, and by examining haplotypes of MIF-794 and -173. Consistent with previous observations in Zambian children [33], the 5-repeat was the most prevalent MIF-794 allele in the Kenyan cohort. This distribution of -794 alleles differs from patterns reported in Caucasian [27] and Northeast Asian [34] populations, where the 6-repeat was predominant. Thus, there appears to be significant geographic variation in the distribution of the MIF-794 STRP, as previously documented for the MIF-173 SNP [9, 28, 33].
In holoendemic transmission areas, such as our current study site in western Kenya, nearly all of the children experience multiple clinical episodes of malaria during the first five years of life [4]. While a majority of these infected children present with only mild forms of malaria, others experience severe life-threatening complications that predominantly manifest as SMA [35]. Therefore, identifying gene variants associated with susceptibility to severe disease is best accomplished by comparing the genetic backgrounds of P. falciparum-infected children who develop SMA versus those that do not develop SMA. The impact of MIF promoter polymorphisms on susceptibility to SMA was investigated by examining MIF variants in three clinically distinct groups: healthy aparasitemic controls; P. falciparum-infected children without SMA; and children with SMA. Trend analyses showed a significant pattern of increased malarial anemia severity in carriers of longer CATT (7 and 8) repeat alleles, suggesting a potential relationship with susceptibility to SMA.
Additional multivariate logistic regression analyses controlled for the confounding effects of age, gender, sickle-cell trait, and HIV-1 status. Although many factors, including nutritional indices, co-infections with other pathogens, and parents’ socio-economic status could influence disease outcomes, only those factors that were found to significantly impact the pathogenesis of SMA in this population [35, 38, 39] were included in the multivariate analyses models. In addition, since data presented here, as well as in our previous studies [9, 20, 35, 46], demonstrate that SMA is not significantly associated with concomitant parasitemia, HDP and SMA were examined as separate outcome variables. These analyses identified a relationship between the presence of longer repeat alleles and susceptibility to SMA, but not HDP. Interestingly, this association was statistically significant for heterozygous longer repeat carriers (XY) but not homozygous individuals (YY), perhaps due to the limited sample size of the homozygous group. Children in the XY group had a significantly increased likelihood of being parasitemic (any density) compared to the XX group, suggesting that carriage of a shorter number of repeats may protect against acquisition of P. falciparum infections. However, since the parasitemic status of children in areas of high transmission intensity is dynamic, longitudinal studies are required to definitively show an association between MIF genetic variants and susceptibility to infection over extended periods of exposure.
Although the MIF-794 STRP and -173 SNP are independently associated with various diseases [28], haplotypes of the two polymorphic sites are more strongly associated with functional gene expression and susceptibility to inflammatory disease [24, 26, 34]. Therefore, MIF promoter haplotypes were constructed and the association between the various haplotypes and malarial anemia severity was investigated. Since the distribution of the 7C and 8C haplotypes showed similar patterns in the SMA and non-SMA groups, these two haplotypes were combined for increased statistical power. Despite the lack of an independent association between the -173 SNP and SMA, haplotypic analyses revealed that the 6G haplotype was protective against SMA, while the 7C or 8C haplotypes were associated with increased susceptibility to SMA. Although the 7C haplotype is associated with several inflammatory diseases, including scleroderma [47], atopy [34] and inflammatory arthritis [26], to our knowledge, this is the first report of an association between the 6G haplotype and protection from any disease. Moreover, the role of the 8C haplotype has not previously been examined due to the extremely low frequency of this haplotype in most populations [28, 31]. Additional analyses revealed that susceptibility to SMA was further increased in children who had the SMA-susceptible haplotype (i.e., 7C or 8C) in combination with the HDP-susceptible CC genotype at -173 [9], suggesting that the impact of these disease susceptibility traits may be synergistic.
The mechanism(s) by which MIF promoter variants influence susceptibility to SMA was further investigated by examining peripheral blood MIF production in aparasitemic and parasitemic children separately, allowing us to distinguish the potential impact of genetic variation on basal MIF production from changes that result from host-parasite interactions. Overall, there was a progressive decline in MIF levels in children with increasing length of CATT repeats. Furthermore, while the 6G haplotype showed no significant relationship with MIF production, carriage of the 7C or 8C haplotypes was associated with decreased MIF production during acute disease. These observations are in contrast to previous in vitro studies in various cell types demonstrating that increased MIF production is associated with an increasing number of CATT repeats [24, 27], and in individuals with the 7C haplotype relative to those with non-7C haplotypes [47]. However, results presented here are consistent with previous investigations demonstrating that MIF expression is significantly suppressed in 7C promoter constructs compared to 5G and 6G constructs [34], illustrating the complexity of MIF transcriptional regulation. Results presented here showing that the 7C and 8C haplotypes are associated with increased susceptibility to SMA and reduced MIF expression are in agreement with our previous investigations demonstrating decreased MIF production in Kenyan children with SMA [20]. These data also support our recent study illustrating that the MIF production in response to malaria is influenced by variation in the MIF promoter [9], suggesting that adequate MIF production may be important in mediating protective immune responses to P. falciparum infection.
Acknowledgments
The study was funded from a National Institutes of Health (NIH) Grant 2 R01AI51305 (DJP), Fogarty International Center (FIC) Training Grant 1 D43TW05884 (DJP), and AI51306 (RB).
We offer our sincere gratitude and appreciation for all of the parents, guardians, and children from the Siaya District community for their participation in this study. We are also grateful to the staff at the University of New Mexico/KEMRI laboratories and the Siaya District Hospital for their support during the study. These data are published with the approval of the Director of the Kenya Medical Research Institute.
List of uncommon abbreviations
- AC
aparasitemic controls
- CM
cerebral malaria
- HDP
high-density parasitemia
- HWE
Hardy-Weinberg equilibrium
- LD
linkage disequilibrium
- MIF
macrophage migration inhibitory factor
- SMA
severe malarial anemia
- SNP
single nucleotide polymorphism
- STRP
short tandem repeat polymorphism
Footnotes
The study was approved by the Ethics Committees of the Kenya Medical Research Institute, University of Pittsburgh Institutional Review Board, and University of New Mexico Institutional Review Board. Informed written consent was obtained from the parent/legal guardian of all participating children.
Yale University has applied for a patent describing the utility of MIF genotyping in the diagnosis of chronic anemia.
References
- 1.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 2.Mockenhaupt FP, Ehrhardt S, Burkhardt J, et al. Manifestation and outcome of severe malaria in children in northern Ghana. Am J Trop Med Hyg. 2004;71:167–72. [PubMed] [Google Scholar]
- 3.Awandare GA, Goka B, Boeuf P, et al. Increased Levels of Inflammatory Mediators in Children with Severe Plasmodium falciparum Malaria with Respiratory Distress. J Infect Dis. 2006;194:1438–46. doi: 10.1086/508547. [DOI] [PubMed] [Google Scholar]
- 4.Snow RW, Omumbo JA, Lowe B, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–4. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 5.Keller CC, Kremsner PG, Hittner JB, Misukonis MA, Weinberg JB, Perkins DJ. Elevated nitric oxide production in children with malarial anemia: hemozoin-induced nitric oxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect Immun. 2004;72:4868–73. doi: 10.1128/IAI.72.8.4868-4873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkins DJ, Weinberg JB, Kremsner PG. Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J Infect Dis. 2000;182:988–92. doi: 10.1086/315762. [DOI] [PubMed] [Google Scholar]
- 7.McDevitt MA, Xie J, Gordeuk V, Bucala R. The anemia of malaria infection: role of inflammatory cytokines. Curr Hematol Rep. 2004;3:97–106. [PubMed] [Google Scholar]
- 8.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–92. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awandare GA, Ouma C, Keller CC, et al. A macrophage migration inhibitory factor promoter polymorphism is associated with high-density parasitemia in children with malaria. Genes Immun. 2006;7:568–575. doi: 10.1038/sj.gene.6364332. [DOI] [PubMed] [Google Scholar]
- 10.Ouma C, Davenport GC, Awandare GA, et al. Polymorphic variability in the interleukin (IL)-1beta promoter conditions susceptibility to severe malarial anemia and functional changes in IL-1beta production. J Infect Dis. 2008;198:1219–26. doi: 10.1086/592055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouma C, Davenport GC, Were T, et al. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum Genet. 2008;124:515–24. doi: 10.1007/s00439-008-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juttner S, Bernhagen J, Metz CN, Rollinghoff M, Bucala R, Gessner A. Migration inhibitory factor induces killing of Leishmania major by macrophages: dependence on reactive nitrogen intermediates and endogenous TNF-alpha. J Immunol. 1998;161:2383–90. [PubMed] [Google Scholar]
- 13.Bozza M, Satoskar AR, Lin G, et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–6. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calandra T, Echtenacher B, Roy DL, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–70. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 15.Koebernick H, Grode L, David JR, et al. Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc Natl Acad Sci U S A. 2002;99:13681–6. doi: 10.1073/pnas.212488699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes JL, Terrazas LI, Espinoza B, et al. Macrophage migration inhibitory factor contributes to host defense against acute Trypanosoma cruzi infection. Infect Immun. 2006;74:3170–9. doi: 10.1128/IAI.01648-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martiney JA, Sherry B, Metz CN, et al. Macrophage migration inhibitory factor release by macrophages after ingestion of Plasmodium chabaudi-infected erythrocytes: possible role in the pathogenesis of malarial anemia. Infect Immun. 2000;68:2259–67. doi: 10.1128/iai.68.4.2259-2267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDevitt MA, Xie J, Shanmugasundaram G, et al. A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J Exp Med. 2006;203:1185–96. doi: 10.1084/jem.20052398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awandare GA, Ouma Y, Ouma C, et al. Role of monocyte-acquired hemozoin in suppression of macrophage migration inhibitory factor in children with severe malarial anemia. Infect Immun. 2007;75:201–10. doi: 10.1128/IAI.01327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awandare GA, Hittner JB, Kremsner PG, et al. Decreased circulating macrophage migration inhibitory factor (MIF) protein and blood mononuclear cell MIF transcripts in children with Plasmodium falciparum malaria. Clin Immunol. 2006;119:219–25. doi: 10.1016/j.clim.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.De Mast Q, Sweep FC, McCall M, et al. A decrease of plasma macrophage migration inhibitory factor concentration is associated with lower numbers of circulating lymphocytes in experimental Plasmodium falciparum malaria. Parasite Immunol. 2008;30:133–8. doi: 10.1111/j.1365-3024.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 23.Donn R, Alourfi Z, De Benedetti F, et al. Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:2402–9. doi: 10.1002/art.10492. [DOI] [PubMed] [Google Scholar]
- 24.Donn R, Alourfi Z, Zeggini E, et al. A functional promoter haplotype of macrophage migration inhibitory factor is linked and associated with juvenile idiopathic arthritis. Arthritis Rheum. 2004;50:1604–10. doi: 10.1002/art.20178. [DOI] [PubMed] [Google Scholar]
- 25.Donn RP, Shelley E, Ollier WE, Thomson W. A novel 5′-flanking region polymorphism of macrophage migration inhibitory factor is associated with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2001;44:1782–5. doi: 10.1002/1529-0131(200108)44:8<1782::AID-ART314>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Barton A, Lamb R, Symmons D, et al. Macrophage migration inhibitory factor (MIF) gene polymorphism is associated with susceptibility to but not severity of inflammatory polyarthritis. Genes Immun. 2003;4:487–91. doi: 10.1038/sj.gene.6364014. [DOI] [PubMed] [Google Scholar]
- 27.Baugh JA, Chitnis S, Donnelly SC, et al. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3:170–6. doi: 10.1038/sj.gene.6363867. [DOI] [PubMed] [Google Scholar]
- 28.Renner P, Roger T, Calandra T. Macrophage migration inhibitory factor: gene polymorphisms and susceptibility to inflammatory diseases. Clin Infect Dis. 2005;41 (Suppl 7):S513–9. doi: 10.1086/432009. [DOI] [PubMed] [Google Scholar]
- 29.Mizue Y, Ghani S, Leng L, et al. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci U S A. 2005;102:14410–5. doi: 10.1073/pnas.0507189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radstake TR, Sweep FC, Welsing P, et al. Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum. 2005;52:3020–9. doi: 10.1002/art.21285. [DOI] [PubMed] [Google Scholar]
- 31.Donn RP, Ray DW. Macrophage migration inhibitory factor: molecular, cellular and genetic aspects of a key neuroendocrine molecule. J Endocrinol. 2004;182:1–9. doi: 10.1677/joe.0.1820001. [DOI] [PubMed] [Google Scholar]
- 32.Miterski B, Drynda S, Boschow G, et al. Complex genetic predisposition in adult and juvenile rheumatoid arthritis. BMC Genet. 2004;5:2. doi: 10.1186/1471-2156-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong XB, Leng L, Beitin A, et al. Simultaneous detection of microsatellite repeats and SNPs in the macrophage migration inhibitory factor (MIF) gene by thin-film biosensor chips and application to rural field studies. Nucleic Acids Res. 2005;33:e121. doi: 10.1093/nar/gni123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hizawa N, Yamaguchi E, Takahashi D, Nishihira J, Nishimura M. Functional polymorphisms in the promoter region of macrophage migration inhibitory factor and atopy. Am J Respir Crit Care Med. 2004;169:1014–8. doi: 10.1164/rccm.200307-933OC. [DOI] [PubMed] [Google Scholar]
- 35.Ong’echa JM, Keller CC, Were T, et al. Parasitemia, Anemia, and Malarial Anemia in Infants and Young Children in a Rural Holoendemic Plasmodium falciparum Transmission Area. Am J Trop Med Hyg. 2006;74:376–385. [PubMed] [Google Scholar]
- 36.Bloland PB, Ruebush TK, McCormick JB, et al. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission I. Description of study site, general methodology, and study population. Am J Trop Med Hyg. 1999;60:635–40. doi: 10.4269/ajtmh.1999.60.635. [DOI] [PubMed] [Google Scholar]
- 37.McElroy PD, ter Kuile FO, Lal AA, et al. Effect of Plasmodium falciparum parasitemia density on hemoglobin concentrations among full-term, normal birth weight children in western Kenya, IV. The Asembo Bay Cohort Project. Am J Trop Med Hyg. 2000;62:504–12. doi: 10.4269/ajtmh.2000.62.504. [DOI] [PubMed] [Google Scholar]
- 38.Aidoo M, Terlouw DJ, Kolczak MS, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–2. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 39.Otieno RO, Ouma C, Ong’echa JM, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. Aids. 2006;20:275–80. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 40.Williams PL. Trend Test for Counts and Proportions. In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics. 2. Wiley Interscience; 2005. [Google Scholar]
- 41.Hothorn T, Hornik K, van de Wiel MA, Zeileis A. Implementing a Class of Permutation Tests: The coin Package. Journal of Statistical Software. 2008;28:1–23. [Google Scholar]
- 42.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 43.Piegorsch WW, Bailer AJ. Statistics for Environmental Biology and Toxicology. CRC Press/Chapman and Hall; 1997. [Google Scholar]
- 44.Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–72. [PubMed] [Google Scholar]
- 45.Excoffier L, Laval G, Schneider S. Arlequin ver. 3. 0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- 46.Ouma C, Keller CC, Opondo DA, et al. Association of FC{gamma} receptor IIA (CD32) polymorphism with malarial anemia and high-denisty parasitemia in infants and young children. Am J Trop Med Hyg. 2006;74:573–577. [PubMed] [Google Scholar]
- 47.Wu SP, Leng L, Feng Z, et al. Macrophage migration inhibitory factor promoter polymorphisms and the clinical expression of scleroderma. Arthritis Rheum. 2006;54:3661–9. doi: 10.1002/art.22179. [DOI] [PubMed] [Google Scholar]