Abstract
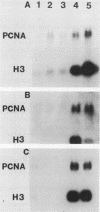
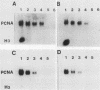
The steady-state mRNA levels of the proliferating cell nuclear antigen (PCNA) gene are growth regulated. In a previous paper (L. Ottavio, C.-D. Chang, M. G. Rizzo, S. Travali, C. Casadevall, and R. Baserga, Mol. Cell. Biol. 10:303-309, 1990), we reported that introns (especially intron 4) participate in growth regulation of the PCNA gene. We have now investigated the role of the 5'-flanking sequence of the human PCNA gene stably transfected into BALB/c 3T3 cells. Promoters of different lengths (from -2856 to -45 upstream of the cap site) were tested. All promoters except the AatII promoter (-45), including a short HpaII promoter (-210), were sufficient for a response to serum, platelet-derived growth factor, and to a lesser extent epidermal growth factor. No construct responded to insulin or platelet-poor plasma. The AatII promoter had little detectable activity. Transcriptional activity was also determined in BALB/c 3T3 cells carrying various constructs of the human PCNA gene by two methods: run-on transcription and reverse transcription-polymerase chain reaction (the latter measuring the heterogeneous nuclear RNA [hnRNA] steady-state levels). There was very little difference in the rate of transcription of the PCNA gene between G0 cells and serum-stimulated cells, although the levels of hnRNA were much higher after stimulation. In G0 cells carrying a human PCNA gene without introns 4 and 5, both transcription rate and hnRNA levels were high. Together with data on the mRNA half-life, these results suggest a posttranscriptional component in the regulation of PCNA mRNA levels after serum stimulation but a transcriptional regulation by intron 4.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen J. B., Broz S. D., Levinson A. D. Expression of the H-ras proto-oncogene is controlled by alternative splicing. Cell. 1989 Aug 11;58(3):461–472. doi: 10.1016/0092-8674(89)90427-3. [DOI] [PubMed] [Google Scholar]
- Cohen J. B., Levinson A. D. A point mutation in the last intron responsible for increased expression and transforming activity of the c-Ha-ras oncogene. Nature. 1988 Jul 14;334(6178):119–124. doi: 10.1038/334119a0. [DOI] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Control of thymidine kinase mRNA during the cell cycle. Mol Cell Biol. 1987 Aug;7(8):2925–2932. doi: 10.1128/mcb.7.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Firak T. A., Subramanian K. N. Minimal transcriptional enhancer of simian virus 40 is a 74-base-pair sequence that has interacting domains. Mol Cell Biol. 1986 Nov;6(11):3667–3676. doi: 10.1128/mcb.6.11.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. A., Byrnes J. J., Miller M. R. Identification of DNA polymerase delta in CV-1 cells: studies implicating both DNA polymerase delta and DNA polymerase alpha in DNA replication. Biochemistry. 1987 Oct 20;26(21):6817–6824. doi: 10.1021/bi00395a035. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. R., Marashi F., Baserga R., Stein J., Stein G. Expression of histone genes in a G1-specific temperature-sensitive mutant of the cell cycle. Biochemistry. 1984 Jul 31;23(16):3731–3735. doi: 10.1021/bi00311a025. [DOI] [PubMed] [Google Scholar]
- Jaskulski D., Gatti C., Travali S., Calabretta B., Baserga R. Regulation of the proliferating cell nuclear antigen cyclin and thymidine kinase mRNA levels by growth factors. J Biol Chem. 1988 Jul 25;263(21):10175–10179. [PubMed] [Google Scholar]
- Jaskulski D., deRiel J. K., Mercer W. E., Calabretta B., Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988 Jun 10;240(4858):1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Jenh C. H., Geyer P. K., Johnson L. F. Control of thymidylate synthase mRNA content and gene transcription in an overproducing mouse cell line. Mol Cell Biol. 1985 Oct;5(10):2527–2532. doi: 10.1128/mcb.5.10.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson K. E., Baserga R. Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9774–9777. doi: 10.1073/pnas.86.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson K. E., Chen S. T., Koniecki J., Ku D. H., Baserga R. S-phase-specific regulation by deletion mutants of the human thymidine kinase promoter. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6848–6852. doi: 10.1073/pnas.86.18.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. T., Gibson C. W., Hirschhorn R. R., Rittling S., Baserga R., Mercer W. E. Expression of thymidine kinase and dihydrofolate reductase genes in mammalian ts mutants of the cell cycle. J Biol Chem. 1985 Mar 25;260(6):3269–3274. [PubMed] [Google Scholar]
- Liu Y. C., Marraccino R. L., Keng P. C., Bambara R. A., Lord E. M., Chou W. G., Zain S. B. Requirement for proliferating cell nuclear antigen expression during stages of the Chinese hamster ovary cell cycle. Biochemistry. 1989 Apr 4;28(7):2967–2974. doi: 10.1021/bi00433a034. [DOI] [PubMed] [Google Scholar]
- Moore K. S., Sullivan K., Tan E. M., Prystowsky M. B. Proliferating cell nuclear antigen/cyclin is an interleukin 2-responsive gene. J Biol Chem. 1987 Jun 25;262(18):8447–8450. [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989 Aug 15;264(23):13856–13864. [PubMed] [Google Scholar]
- Ottavio L., Chang C. D., Rizzo M. G., Travali S., Casadevall C., Baserga R. Importance of introns in the growth regulation of mRNA levels of the proliferating cell nuclear antigen gene. Mol Cell Biol. 1990 Jan;10(1):303–309. doi: 10.1128/mcb.10.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Prelich G., Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988 Apr 8;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Shen Y. M., Hirschhorn R. R., Mercer W. E., Surmacz E., Tsutsui Y., Soprano K. J., Baserga R. Gene transfer: DNA microinjection compared with DNA transfection with a very high efficiency. Mol Cell Biol. 1982 Sep;2(9):1145–1154. doi: 10.1128/mcb.2.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherley J. L., Kelly T. J. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988 Jun 15;263(17):8350–8358. [PubMed] [Google Scholar]
- Shipman P. M., Sabath D. E., Fischer A. H., Comber P. G., Sullivan K., Tan E. M., Prystowsky M. B. Cyclin mRNA and protein expression in recombinant interleukin 2-stimulated cloned murine T lymphocytes. J Cell Biochem. 1988 Nov;38(3):189–198. doi: 10.1002/jcb.240380306. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart P., Ito M., Stewart C., Conrad S. E. Induction of cellular thymidine kinase occurs at the mRNA level. Mol Cell Biol. 1985 Jun;5(6):1490–1497. doi: 10.1128/mcb.5.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Travali S., Ku D. H., Rizzo M. G., Ottavio L., Baserga R., Calabretta B. Structure of the human gene for the proliferating cell nuclear antigen. J Biol Chem. 1989 May 5;264(13):7466–7472. [PubMed] [Google Scholar]
- Tseng B. Y., Prussak C. E., Almazan M. T. Primase p49 mRNA expression is serum stimulated but does not vary with the cell cycle. Mol Cell Biol. 1989 May;9(5):1940–1945. doi: 10.1128/mcb.9.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A. F., Geis A. M., Spain B. H., Wong S. W., Korn D., Wang T. S. Gene expression of human DNA polymerase alpha during cell proliferation and the cell cycle. Mol Cell Biol. 1988 Nov;8(11):5016–5025. doi: 10.1128/mcb.8.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. H., Kelly T. J. Requirement for two DNA polymerases in the replication of simian virus 40 DNA in vitro. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9742–9746. doi: 10.1073/pnas.86.24.9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Weinberg D. H., Virshup D. M., Li J. J., Kelly T. J. Identification of cellular proteins required for simian virus 40 DNA replication. J Biol Chem. 1989 Feb 15;264(5):2801–2809. [PubMed] [Google Scholar]