Abstract
Obesity has been associated with multiple chronic pain disorders, including migraine. We hypothesized that diet-induced obesity would be associated with a reduced threshold for thermal nociception in the trigeminal system. In this study, we sought to examine the effect of diet-induced obesity on facial pain behavior. Mice of two different strains were fed high-fat or regular diet and tested using a well-established operant facial pain assay. We found that the effects of diet on behavior in this assay were strain and reward dependent. Obesity prone C57BL/6J mice fed high-fat diet display lower number of licks of a caloric, palatable reward (33% sweetened condensed milk or 30% sucrose) than control mice. This occurred at all temperatures, in both sexes, and was evident even before the onset of obesity. This diminished reward-seeking behavior was not observed in obesity resistant SKH1E mice. These findings suggest that diet and strain interact to modulate reward-seeking behavior. Furthermore, we observed a difference between diet groups in operant behavior with caloric, palatable rewards, but not with a non-caloric neutral reward (water). Importantly, we found no effect of diet-induced obesity on acute thermal nociception in the absence of inflammation or injury. This indicates that thermal sensation in the face is not affected by obesity-associated peripheral neuropathy as it occurs when studying pain behaviors in the rodent hindpaw. Future studies using this model may reveal whether obesity facilitates the development of chronic pain after injury or inflammation.
Keywords: trigeminal, operant, pain, reward, obesity, high fat diet
1. Introduction
Obesity has been associated with multiple chronic pain disorders (Hitt et al., 2007, Wright et al., 2010, Stone and Broderick, 2012). The mechanisms that link obesity to chronic pain conditions are not known and may vary depending on the specific pain condition being examined. These pain conditions are not limited to weight bearing structures like the lower back or knee joints. Several population-based studies have found that obesity is associated with higher frequency and severity of migraine attacks (Bigal et al., 2006, Bigal and Lipton, 2006, Bigal et al., 2007b, Winter et al., 2009). While a number of mechanisms explaining this relationship have been proposed (Bigal et al., 2007a, Bigal and Lipton, 2008, Peterlin et al., 2010, Recober and Goadsby, 2010), these hypotheses have not been tested.
Animal studies can provide important information regarding the role of molecular mediators shared by migraine and obesity. Ideally, such studies would include behavioral end points that can be measured in the same animal multiple times, before and after pharmacological or molecular manipulation (Olesen and Jansen-Olesen, 2012). While most studies have examined sensation in the rodent hindpaw, this approach has some limitations. Importantly, diabetic peripheral neuropathy is typically present in models of obesity and results in abnormal sensation in the extremities. Furthermore, head pain may be distinct from pain in other parts of the body, so it is important to specifically examine the trigeminal system in the context of obesity. The measurement of “headache” in rodents remains elusive, but cutaneous allodynia occurs in most migraineurs (Lipton et al., 2008, Schwedt et al., 2011) and is frequently used to study headache-related pain in rodent models (Oshinsky and Gomonchareonsiri, 2007, Edelmayer et al., 2009, Stucky et al., 2011).
Neubert and colleagues have developed and validated an operant assay to measure cutaneous thermal sensitivity of the face in rodents (Neubert et al., 2005, Neubert et al., 2008). In this assay, the animal chooses between experiencing a painful facial stimulus in order to obtain a reward or to avoid the painful stimulation forgoing the reward. Thus, higher order processing of nociception is directly evaluated. This may provide advantage over the use of spinally-driven withdrawal measures that have been questioned for their translatability to the human condition (Vierck et al., 2008, Mogil, 2009). This assay represents a valid, objective, and automated method to assess trigeminal nociception, which is otherwise challenging in mice. However, this operant pain assay uses a highly palatable and calorically rich substance (sweetened condensed milk) as reward (Nolan et al., 2011a), which needs to be tested and validated in the context of dietary manipulation and obesity. Both diet and obesity can modulate sensitivity to appetitive reward (Kenny, 2011). Leknes and colleagues have shown that relief from pain, a non-appetitive reward, engages much of the same circuitry as responses to appetitive reward (Leknes et al., 2011). Moreover, it has been suggested that the effect of pain on the reward-aversion circuitry may contribute to the development of chronic pain (Becker et al., 2012). Therefore, the effect of diet and obesity on the reward circuitry is likely to have implications for pain processing and assessment of nociception.
In this study, we sought to determine what effect diet-induced obesity has on trigeminal nociception in mice. Additionally, we wanted to establish the behavioral profile of mice with diet-induced obesity in this operant facial pain assay. Our findings suggest that the reward circuitry should be considered when studying the interaction between obesity and pain.
2. Experimental Procedures
2.1 Animals
We used male and female C57BL/6J (C57) mice purchased from The Jackson Laboratory (Bar Harbor, ME) or bred in our colony from breeders purchased from The Jackson Laboratory. Male SKH1-E (SK) mice were purchased from Charles River (Charleston, SC). After weaning, mice were placed either on a high-fat diet (HFD) (45% or 60% fat, Research Diets® product numbers D12451 or D12492, respectively) or regular diet (RD) (Purina rodent chow). A cohort of C57 mice underwent training and testing between 20 and 30 weeks of age (starting after 16 weeks on HFD or RD). Another cohort of C57 mice underwent training and testing between 6 and 12 weeks of age (starting after 2 weeks on HFD or RD), before the development of significant weight gain. SK mice were only tested between 20 and 30 weeks of age because it became evident early on that their weight gain on high fat diet was slower and less pronounced than C57. Mice were weighed every other week. Animals were maintained on a 12:12 hour light dark cycle, with food and water available ad libitum. All procedures were approved by the Animal Care and Use Committee of University of Iowa and performed in accordance with the standards set by the National Institutes of Health.
2.2 Operant Facial Pain Assay
2.2.1 Assay description
The operant facial pain assay relies on a reward-conflict paradigm in which the animal must tolerate thermal stimulation of the face in order to obtain a liquid reward. In this case, the reward is 33% sweetened condensed milk (Casa Solana, Sysco Corp.). The concentration of fat in the dilute solution is 3g/45mL. Training and testing of operant pain-related behaviors were performed as previously described (Neubert et al., 2008). Mice were always acclimated to the behavioral testing room for one hour prior to each training or testing session. Mice were trained to lick the reward bottle while placing their face against the stimulus thermodes, set to a neutral (32 or 37°C) temperature. Training takes approximately 10 sessions over 2 weeks. At least 24 hours prior to testing, C57 mice were lightly anesthetized with inhaled isoflurane (2%, in air) and their facial fur between the vibrissal pad and the angle of the mandible was removed bilaterally using either depilatory cream or clippers. This was done with care to spare the vibrissae. SK mice do not require facial fur removal. Mice were fasted overnight (18±3 hrs) with access to water prior to each testing session to motivate reward acquisition. Fasting was limited to a maximum of three days per week, with at least one day of recovery between sessions.
2.2.2 Assessment of diet effects on reward-seeking behavior
To evaluate motivation for the reward solution in the absence of facial stimulation, we tested C57 mice with free access to the reward bottle. This was done by placing the reward bottle tube all the way in the chamber so that mice did not have to touch the thermode to access the reward solution. This was repeated using 30% sucrose as a reward solution, which has been used previously in this assay (Nolan et al., 2011a) and may produce a different behavioral profile from the one obtained with the fatty milk reward.
2.2.3 Modification of the assay to use water as reward
To avoid the possible confounding factor of using a palatable caloric reward in our obesity model, we tested water as the reward after water deprivation. A cohort of C57 mice was trained as described above, but with overnight water deprivation, instead of fasting, prior to each training and testing session.
2.2.4 Outcome measures
For each 20-minute testing session, the number of licks at the reward bottle, the number of facial contacts with the stimulus, and the total duration of facial contact with the stimulus were determined using a custom-written routine in Labview (v.8, Texas Instruments, Inc.). Additionally, the average duration of each facial contact (“duration/contact”) was calculated by dividing the total duration by the number of facial contacts made with the stimulus. Outcome measures were collected and summarized offline following data acquisition. All mice underwent two testing sessions at each temperature, in a random order, and the two measurements were then averaged.
2.3 Statistical Analysis
We used two-way Analysis of Variance (ANOVA) to assess weights differences between diet groups. We used repeated measures two-way ANOVAs with between-subject effects for operant facial behaviors. In some occasions, the number of animals tested at each temperature was different and two-way ANOVA was used. All statistical comparisons were followed by Bonferroni post-hoc tests where applicable. A summary of all statistical analyses comparing diet, sex, or time on diet is presented in Table 1. Graph Pad Prism (v.5) was used and p < 0.05 was considered significant.
Table 1.
Summary of statistical comparisons for between subject effects, temperature effects, and their interaction for diet groups depicted in Figures 2, 4, and 5.
| Summary of Repeated-Measures two-way ANOVA Statistics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group that Comparison is made in | Comparison Between Subjects | Between Subjects Effect | Temperature Effect | Interaction | ||||
| F | p | F | p | F | p | |||
| Fig. 2A | C57 males SK males |
Diet Diet |
5.62 0.2196 |
0.0307* 0.6404 |
14.67 26.63 |
<0.0001* <0.0001* |
0.09058 0.09866 |
0.9136 0.9062 |
| Fig. 2B | C57 males SK males |
Diet Diet |
4.539 0.02191 |
0.049* 0. 8827 |
4.421 10.91 |
0.0202* < 0.0001* |
0.02884 0.6101 |
0.9716 0. 5459 |
| Fig. 4A | HFD RD |
Sex Sex |
0.1485 1.273 |
0.705 0.2719 |
10.86 29.89 |
0.0003* < 0.0001* |
0.7005 1.405 |
0.5038 0.2566 |
| Fig. 4B | HFD RD |
Sex Sex |
4.525 3.649 |
0.0493* 0.0699 |
10.58 6.081 |
0.0003* 0.0048* |
4.621 0.01185 |
0.0173* 0.9882 |
| Fig. 5A | HFD C57 RD C57 |
Diet length Diet length |
0.2397 0.01419 |
0.6276 0.9058 |
23.15 61.97 |
<0.0001* <0.0001* |
0.8313 0.9159 |
0.4399 0.4047 |
| Fig. 5B | HFD C57 RD C57 |
Diet length Diet length |
8.938 2.193 |
0.0052* 0.1471 |
15.5 19.65 |
<0.0001* <0.0001* |
0.7179 1.967 |
0.4915 0.1471 |
| Fig. 5A | 16 weeks 2 weeks |
Diet Diet |
14.12 39.28 |
0.0006* < 0.0001* |
36.67 49 |
<0.0001* <0.0001* |
2.561 9.134 |
0.0837 0.0003* |
| Fig. 5B | 16 weeks 2 weeks |
Diet Diet |
11.08 18.9 |
0.0019* 0.0001* |
9.665 32.52 |
0.0002* < 0.0001* |
1.154 4.522 |
0.3208 0.0146* |
indicates a significant difference, p <0.05. Note that there is a significant effect of temperature in all comparisons.
3. Results
3.1 C57 mice gain more weight on a high-fat diet than SK mice
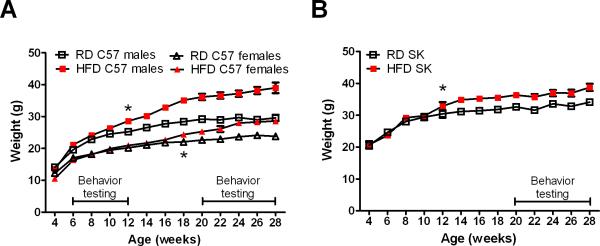
We sought to determine the effects of diet-induced obesity on trigeminal nociception in C57 and SK mice. We chose these strains because they had been previously characterized in this operant facial pain assay (Neubert et al., 2008). All mice gained weight from 4 to 10 weeks of age as part of normal growth, which is indicated by a lack of difference between the diet groups at those time points (Fig. 1). Significant diet-induced weight gain was seen in all males starting at 12 weeks of age, and in C57 females starting at 18 weeks of age (Fig. 1). However, by the end of the study, C57 males on HFD weighed 10g more than their RD counterparts, while C57 females and SK males on HFD only weighed 5g more than their respective control groups (Fig. 1).
Figure 1.
Effect of high-fat diet (HFD) on weight gain in C57 male and female (A) and SK male mice (B). The line in the lower right of the graphs in A and B corresponds to the time when training and behavioral testing was conducted. * indicates the first time a significant difference (p<0.05) occurs between HFD and RD.
3.2 Obesity prone C57 mice and obesity resistant SK mice have normal facial thermal nociception but they display different behavior in the operant assay
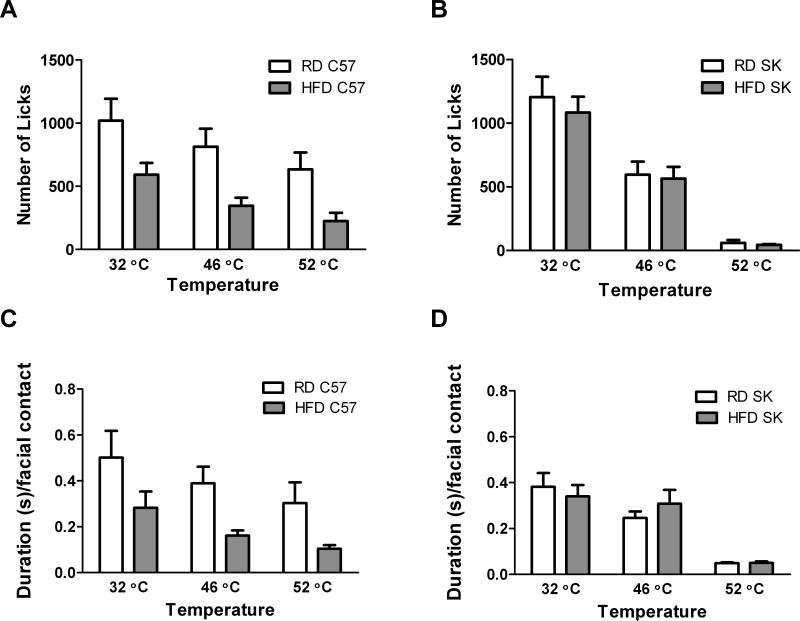
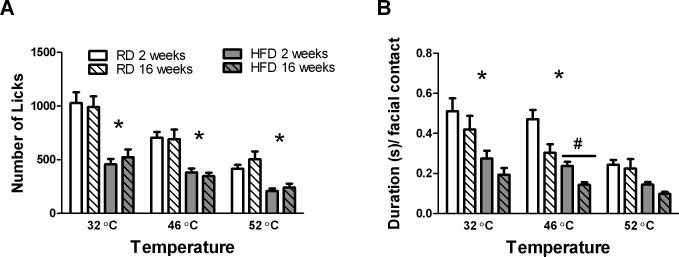
Along with this different pattern of weight gain, we also observed different effects of the diet on operant behavior in the two strains (Fig. 2) (Table 1). For C57 mice fed HFD for 16 weeks, diet had a significant effect on the number of licks (F1,16 = 5.62, p < 0.05) and duration per facial contact (F1,16 = 4.539, p < 0.05) in the operant assay (Fig 2A, C). These mice exhibited lower number of licks and duration per facial contact than the RD group at all temperatures, even with a neutral stimulus (32 °C), although Bonferroni post-tests did not reach statistical significance (Fig. 2A, C). In contrast, for SK mice we found no effect of diet on number of licks or duration per facial contact (Fig. 2B, D). Importantly, both strains in either diet group displayed the expected decrease in number of licks and duration per facial contact in response to increasing temperature, indicating that facial thermal sensation is intact regardless of strain and diet (Fig 2).
Figure 2.
Effect of diet on number of licks and duration per facial contact in male C57 and SK mice. HFD had a significant effect on the number of licks in C57 males (A), but not SK males (B). There was also a significant effect of diet on the duration per facial contact in C57 males (C), but not SK males (D). However, post-hoc tests did not reveal a statistically significant effect of diet at each temperature for C57 males. Sample sizes were as follows: for C57 males n = 7 on HFD and n= 11 on RD, at all temperatures. For SK males n=18 per diet at 32°C and 46°C, and n=6 per diet at 52°C.
We tested another cohort of C57 mice (n=20 per diet group) purchased directly from The Jackson Laboratories after 16 weeks on diet (60% HFD instead of 45% HFD) and found similar results. The number of licks at 32 °C was significantly lower in the HFD group (365± 37) compared with the RD group (1417±79) (p<0.001). We observed the expected decrease in number of licks at 52 °C, but the difference remained between the HFD and the RD groups (101±16 vs 522±65, respectively) (p<0.001). To assess thermal nociception, while accounting for the difference observed at neutral temperature, we calculated the percent change from 32 °C to 52 °C for each diet group. We found that duration per facial contact at 52 °C decreased in all diet groups similarly (56±6% in the RD group, 65±5% in the 45% HFD group, and 61±5% in the 60% HFD group) (p>0.05). This confirmed that facial thermal nociception is normal in high-fat diet-induced obesity in mice. However, we wanted to ensure that difference in reward-seeking behavior observed in C57 mice on HFD was not interfering with our assessment of nociception in the operant assay. To that end, we modified the assay by substituting the caloric reward (33% sweetened condensed milk in fasted mice) for a non-caloric reward (water in water-deprived mice).
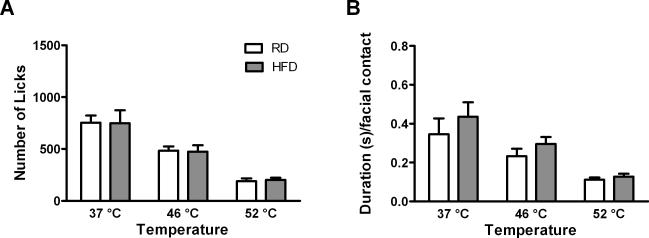
For the studies using water as reward, we used a new cohort of C57 mice directly purchased from The Jackson Laboratories after 16 weeks on diet (60% HFD). We found that the number of licks and duration per facial contact were similar in C57 on HFD and RD (Fig. 3). There was no significant effect of diet on the number of licks or duration per facial contact. However, there was still a significant effect of temperature on the number of licks (F1, 38=17.98, p<0.0001) (Fig. 3A) and duration per facial contact (F1,38=9.151, p<0.0005) (Fig. 3B). These results support the initial finding of normal facial thermal nociception in diet induced obesity. This experiment provides an important modification to the operant facial pain assay that may facilitate the study of chronic pain in obesity.
Figure 3.
Effect of diet on number of licks (A) and duration per facial contact (B) in C57 males when water is the reward. When water was used as a reward, we no longer observed a significant diet effect. There was a significant effect of temperature on the number of licks and on the duration per facial contact. Sample sizes were as follows: for testing at 37 and 46°C, n = 16 on RD and n = 23 on HFD. For testing at 52°C, n = 8 on RD and n = 15 on HFD.
Taken together, these results demonstrate that acute thermal nociception in the face is not affected by high-fat diet-induced obesity. Furthermore, our findings suggest that differences in behavior observed at all temperatures are a consequence of reduced reward-seeking behavior in the HFD mice rather than an increased sensitivity to thermal stimuli as we originally hypothesized. To investigate the unexpected HFD effect on C57 mice behavior in the operant assay, we performed additional experiments to addressed possible influences of sex, duration of high-fat diet, and type of reward on this behavior.
3.3 High-fat diet decreases operant assay outcomes equally in male and female C57 mice
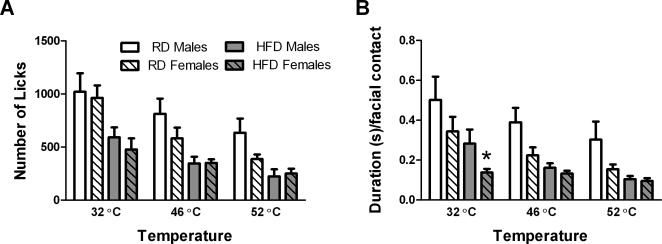
Since trigeminal pain disorders affect females predominantly, we compared operant facial pain behavior in male and female C57 mice (Fig. 4). The number of licks was similar in male and female C57 mice in each diet group (Fig. 4A). Duration per facial contact was also similar in male and female C57 mice at 46 and 52 °C in each diet group. We only observed a statistically significant effect of sex at neutral temperature (32 °C) in the high fat diet group (F1, 16=4.525, p=0.0493) (Fig. 4B).
Figure 4.
Effect of sex on number of licks (A) and duration per facial contact (B) in HFD or RD C57 mice after 16 weeks on the diet. There was no effect of sex on the number of licks in C57 mice (A). There was only an effect of sex on the duration per facial contact at 32°C in HFD C57 mice (B). * p<0.05 between sexes at the indicated temperature and diet group. Sample sizes were as follows: RD Males n = 11, RD Females n = 12, HFD Males n = 7, and HFD Females n = 11.
3.4 High-fat diet decreases operant assay outcomes in C57 mice before they develop significant weight gain
Next we wanted to determine whether the differences in reward-seeking behavior between the HFD and RD groups were a consequence of the high-fat diet or the obese state. To investigate this, we tested C57 mice starting 2 weeks after they had been placed on HFD, before they developed significant weight gain (see Fig. 1: “behavioral testing” between 6-12 weeks of age). As we observed in the 16-weeks on diet cohort, there was practically no difference between male and female C57 after 2 weeks on diet. The only statistically significant sex difference was in the number of licks at neutral temperature in both HFD (F1, 16= 4.611, p=0.0474) and RD (F1, 14=4.642, p=0.0491) mice. Otherwise, male and female C57 mice after 2 weeks on either diet displayed similar number of licks at 46 and 52 °C, and similar duration per facial contact at all three temperatures. Thus, we decided it would be reasonable to pool the sexes for further analysis to minimize the number of animals used.
Overall, we found that C57 mice had very similar behavior whether they had been on HFD for 2 or 16 weeks (Fig. 5). After only 2 weeks on the diet, C57 HFD mice displayed lower number of licks (F1,32 = 39.28, p<0.0001) and duration per contact (F1,32 = 18.9, p=0.0001) than their RD counterparts (Fig.5). Furthermore, the number of licks was similar after 2 or 16 weeks on diet at all temperatures tested (Fig. 5A). The only significant difference detected between 2 and 16 weeks on diet was observed for duration per contact within the HFD group at 46°C (F1, 34=8.938, p=0.0052), but not at 32 and 52 °C (Fig. 5B). These findings suggest that HFD may be sufficient to cause a decrease in reward-seeking behavior in C57 mice even before the development of obesity.
Figure 5.
Effect of diet on number of licks (A) and duration per facial contact (B) after 2 or 16 weeks on the diet. There was no effect of time on diet, but a significant effect of diet on the number of licks at all temperatures tested (A). There was only a significant effect of time on diet on the duration per facial contact in the high fat diet group at 46°C, and a significant effect of diet on duration per facial contact at 32 and 46°C after either 2 or 16 weeks on the diet (B). # indicates a significant difference, p<0.05, between mice on HFD for 2 or 16 weeks. * indicates a significant difference, p<0.05, between diets after either 2 or 16 weeks on diet at the indicated temperatures. Sample sizes were as follows: C57 mice on RD for 16 weeks n = 23, mice on RD for 2 weeks n = 16, mice on HFD for 16 weeks n = 18, and mice on HFD for 2 weeks n = 18.
3.5 High-fat diet-related effects persist despite free access to reward and use of a fat-free rewarding solution
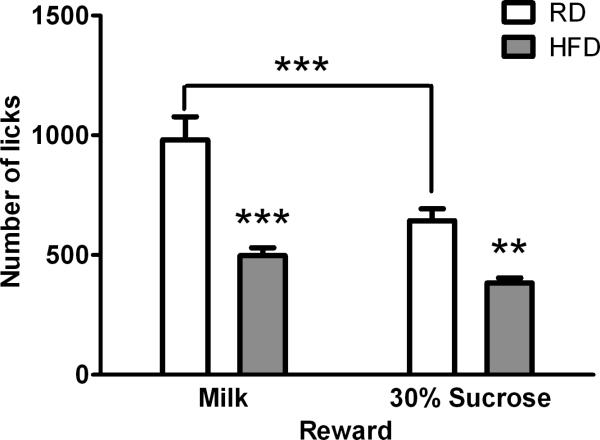
We reasoned that diet-induced obesity may reduce willingness to consume the palatable reward in the presence of a physical obstacle (i.e. the thermodes) and offer a plausible explanation for the lower number of licks and duration per facial contact exhibited by C57 mice on HFD. To test this hypothesis, we introduced the reward bottle between the facial bars allowing free access to the reward without having to press the face against the bars. Again, we observed lower number of licks in the HFD group (F1, 14=35.68, p<0.0001), whether we used sweetened condensed milk or 30% sucrose (Fig. 6). There was also a significant effect of reward solution (F1, 14=31.96, p<0.0001) and a significant interaction between diet and reward type (F1, 14=7.764, p=0.0146). The RD group exhibited lower number of licks with 30% sucrose than sweetened condensed milk, but the HFD group did not show a difference between the two rewarding solutions. Overall, the lower number of licks in the HFD group compared with the RD group suggests reduced motivation for these rewards, even when the physical obstacles are removed.
Figure 6.
Effect of diet on motivation towards reward solutions (milk or 30% sucrose) in the absence of facial stimulation. The number of licks for either reward were significantly lower in the HFD mice ( n = 9) than the RD mice (n =7). RD mice also exhibited a significantly lower number of licks with 30% sucrose than milk reward. Data from these mice (2 weeks on diet) are also depicted in Fig.5. **p<0.01 and ***p<0.001
4. Discussion
Our results suggest that high-fat diet-induced obesity in mice is not associated with changes in thermal nociception in the face. To our knowledge, this is the first study investigating trigeminal pain behavior in obese mice. Previous studies assessing thermal or mechanical nociception in rodent models of obesity have been limited to the hindpaw or tail in a variety of obesity models. In many cases, thermal insensitivity in the hindpaw has been attributed to advanced diabetic neuropathy that can be concomitant with obesity (Zhang et al., 1994, Vareniuk et al., 2007, Sugimoto et al., 2008, Davidson et al., 2009). Our findings suggest that, unlike nerves in the extremities, the trigeminal system does not seem to be affected by diabetic neuropathy. This is in agreement with human data showing that trigeminal sensation is spared in patients with diabetic neuropathy (Arap et al., 2010).
Mice provide an important tool for genetic and pharmacological investigation of pathways that may be involved in the interaction between obesity and pain. However, the study of head pain in rodents is challenging. Although some have successfully assessed mechanical allodynia in the trigeminal distribution in rats (Oshinsky and Gomonchareonsiri, 2007, Edelmayer et al., 2009, Liverman et al., 2009, Wieseler et al., 2012), very few studies have been published using mice (Krzyzanowska et al., 2011, Yasuda et al., 2012), underscoring the difficulty in translating traditional methods from rats to mice. The operant assay we used represents an objective, reproducible, automatized method to assess thermal nociception in the face of both rats and mice (Neubert et al., 2005, Neubert et al., 2008, Nolan et al., 2011b). We indicate here that modifying this assay to use water as reward provides a useful tool to study the interaction between obesity and trigeminal nociception. Our findings indicate that diet-induced obesity does not affect acute thermal nociception in the face. We cannot exclude the possibility of abnormal mechanical sensitivity in our model of diet-induced obesity. This assay can also be modified to evaluate mechanical stimuli (Nolan et al., 2011b), which we may explore in the future.
While obesity seems to have no effect on acute thermal pain thresholds, it may have effects on the trigeminal system by facilitating the transition from acute to chronic pain. A large longitudinal population study has shown that obesity is associated with a 5-fold higher risk to transition from episodic to chronic headaches (Scher et al., 2003). Furthermore, Bigal et al. found that obese migraineurs have more severe and frequent attacks than non-obese migraine sufferers (Bigal et al., 2007b). The mechanisms underlying this process remain unknown, but several hypotheses have been proposed (Bigal et al., 2007a, Peterlin et al., 2010, Recober and Goadsby, 2010). Increasing evidence supports the role of adipose tissue as a dynamic endocrine organ that secretes a number of factors that contribute to systemic inflammation (Vachharajani and Granger, 2009). This could influence the activation threshold of the trigeminal system. We have recently shown that diet-induced obesity in mice enhances nociceptive activation of the trigeminal system in response to a very low dose of capsaicin (Rossi et al., 2012). In future studies, we will investigate how diet-induced obesity may interact with inflammation or injury to affect trigeminal nociception.
In addition to normal trigeminal nociception, we also observed an interesting negative effect of the diet on reward-seeking behavior in obesity-prone C57 mice. This effect was not observed in obesity-resistant SK mice despite being fed the same diet for 16 weeks. To our knowledge, this is the first study to examine the effect of high fat diet on weight and behavior in SKH1-E hairless mice. Their inefficient weight gain on a high fat diet may be related to increased brown fat thermogenesis, which is associated with reduced weight gain (Fisler et al., 1987), but other mechanisms may be involved. This strain of mice may provide insight into the mechanisms of weight regulation that could be of interest to the obesity field.
As for the diminished reward-seeking behavior exhibited by C57 mice, we found that this was present in both sexes, even before significant weight gain had occurred. Additionally, this effect was observed for sweetened condensed milk and 30% sucrose. In agreement with our findings, others have shown decreased intake of rewarding solutions (corn oil or sucrose) in rodent models of obesity using different paradigms (Shin et al., 2011, Johnson, 2012). Moreover, in traditional operant experiments, high-fat diet is associated with reduced conditioned response (i.e. lever pressing) to palatable rewards (Davis et al., 2008, Shin et al., 2011, Finger et al., 2012). In a progressive ratio design, the number of times that the animal has to press the lever to obtain a reward increases within the testing session. Eventually, the effort required to press the lever will outweigh the reward's incentive. The number of times that the animal presses the lever to obtain the last reward is referred to as the break point. This measurement is thought to reflect the reward's strength (Hodos, 1961). High-fat diet and obesity-propensity are also associated with reduced break points in a progressive ratio schedule of operant reinforcement, suggesting that the rewarding properties of palatable food are not as strong for obese animals (Davis et al., 2008, Shin et al., 2011, Finger et al., 2012). Collectively, this information suggests that high-fat diet coupled with a genetic predisposition for obesity diminishes motivation for palatable food rewards.
When we switched to water as reward after overnight water deprivation, we no longer observed a difference in number of licks between the two diet groups. Interestingly, this was due primarily to a change in the RD group, while the HFD group had similar behavior in response to water and sweet rewarding solution. The higher number of licks exhibited by RD mice when a sweet reward was used suggests that sweet solutions are more motivating than water. This supports the idea that caloric, palatable substances enhance performance in this assay (Nolan et al., 2011a), at least under normal dietary conditions. These findings may have implications for potential dietary effects on the interaction between feeding and higher order nociceptive processing. Mason and Foo have demonstrated that feeding-related analgesia produced by palatable foods is mediated by the medullary raphe magnus in rats (Foo and Mason, 2009). It would be interesting to determine what effects dietary manipulation might have on this process.
The interaction among diet, propensity for weight gain, and reward circuitry has implications for pain research in the setting of obesity. Both chronic pain (Borsook et al., 2007, Becker et al., 2012) and obesity (Kenny, 2011) have been associated with diminished reward sensitivity and changes in reward-related structures. Several groups have found that high-fat diet-induced obesity alters the rewarding properties of non-foods (Blendy et al., 2005, Wellman et al., 2007, Davis et al., 2008, Thanos et al., 2010, Morales et al., 2012). For example, conditioned place preference induced by drugs of addiction (Blendy et al., 2005, Davis et al., 2008, Thanos et al., 2010, Morales et al., 2012) and lever pressing for drug stimulation (Wellman et al., 2007) are attenuated in obese rodents. This may have implications for the effects that obesity may exert on the rewarding aspects of pain relief. It is possible that obesity, through its actions on the reward circuitry, could promote a pain-facilitating state. Further work will be needed to determine the degree to which changes in the reward system may contribute to the association between obesity and pain.
This study sets the stage for future research that will investigate possible obesity-related effects on different pain conditions. By establishing that trigeminal thermal nociception is not affected by diet induced obesity in the uninjured mouse, we provide a model to study whether obesity facilitates the development of chronic pain after injury.
5. Conclusions
We have demonstrated that diet-induced obesity does not appear to have an effect on basal nociception in the trigeminal system. However, reward seeking behavior was reduced in C57 mice that are prone to developing obesity, even before significant weight gain occurred. Our findings suggest that the water modified trigeminal pain assay may be used to examine the interaction between obesity and nociception in mice. The impact of diet and genetic background on reward sensitivity may also have implications for the exacerbation of chronic pain conditions by obesity. Understanding these relationships may help us develop better therapies to lessen the impact of obesity and chronic pain.
Diet-induced obesity does not alter nociception in the face of uninjured mice
Diet-induced obesity reduces reward-seeking behavior in obesity prone C57 mice
This reduced motivation occurs in both sexes even before the onset of obesity
This reduced motivation is only displayed for palatable caloric rewards, not water
Control mice respond more to palatable caloric rewards than water after deprivation
Acknowledgements
We thank Drs. Donna L. Hammond and Andrew F. Russo for their helpful advice. This work was supported by the National Institutes of Health (National Institute of Neurological Disorders and Stroke) [K08 –NS066087]; the University of Iowa Clinical and Translational Science Award [UL1 RR024979]; and the University of Iowa Pain Research Program Training Grant [T32NS045549].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arap A, Siqueira SRDT, Silva CB, Teixeira MJ, Siqueira JTT. Trigeminal pain and quantitative sensory testing in painful peripheral diabetic neuropathy. Archives of Oral Biology. 2010;55:486–493. doi: 10.1016/j.archoralbio.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Becker S, Gandhi W, Schweinhardt P. Cerebral interactions of pain and reward and their relevance for chronic pain. Neuroscience Letters. 2012;520:182–187. doi: 10.1016/j.neulet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Liberman JN, Lipton RB. Obesity and migraine. Neurology. 2006;66:545–550. doi: 10.1212/01.wnl.0000197218.05284.82. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. Obesity is a risk factor for transformed migraine but not chronic tension-type headache. Neurology. 2006;67:252–257. doi: 10.1212/01.wnl.0000225052.35019.f9. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB. Concepts and Mechanisms of Migraine Chronification. Headache: The Journal of Head and Face Pain. 2008;48:7–15. doi: 10.1111/j.1526-4610.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Lipton RB, Holland PR, Goadsby PJ. Obesity, migraine and chronic migraine: possible mechanisms of interaction. Neurology. 2007a;68:1851–1861. doi: 10.1212/01.wnl.0000262045.11646.b1. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Tsang A, Loder E, Serrano D, Reed ML, Lipton RB, Prevalence ftAM, Prevention Advisory Group Body Mass Index and Episodic Headaches: A Population-Based Study. Arch Intern Med. 2007b;167:1964–1970. doi: 10.1001/archinte.167.18.1964. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Strasser A, Walters CL, Perkins KA, Patterson F, Berkowitz R, Lerman C. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology. 2005;180:306–315. doi: 10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: Implications for psychiatric disorders. European Journal of Pain. 2007;11:7–7. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Davidson E, Coppey L, Lu B, Arballo V, Calcutt NA, Gerard C, Yorek M. The Roles of Streptozotocin Neurotoxicity and Neutral Endopeptidase in Murine Experimental Diabetic Neuropathy. Experimental Diabetes Research 2009. 2009 doi: 10.1155/2009/431980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behavioral neuroscience. 2008;122:1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmayer RM, Vanderah TW, Majuta L, Zhang E-T, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Annals of Neurology. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger B, Dinan T, Cryan J. Diet-induced obesity blunts the behavioural effects of ghrelin: studies in a mouse-progressive ratio task. Psychopharmacology. 2012;220:173–181. doi: 10.1007/s00213-011-2468-0. [DOI] [PubMed] [Google Scholar]
- Fisler JS, Lupien JR, Wood RD, Bray GA, Schemmel RA. Brown fat thermogenesis in a rat model of dietary obesity. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1987;253:R756–R762. doi: 10.1152/ajpregu.1987.253.5.R756. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Analgesia Accompanying Food Consumption Requires Ingestion of Hedonic Foods. The Journal of Neuroscience. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of Obesity and Pain in a General Population: Results from the Southern Pain Prevalence Study. The Journal of Pain. 2007;8:430–436. doi: 10.1016/j.jpain.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive Ratio as a Measure of Reward Strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Johnson AW. Dietary manipulations influence sucrose acceptance in diet induced obese mice. Appetite. 2012;58:215–221. doi: 10.1016/j.appet.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kenny Paul J. Reward Mechanisms in Obesity: New Insights and Future Directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzanowska A, Pittolo S, Cabrerizo M, Sánchez-López J, Krishnasamy S, Venero C, Avendaño C. Assessing nociceptive sensitivity in mouse models of inflammatory and neuropathic trigeminal pain. Journal of Neuroscience Methods. 2011;201:46–54. doi: 10.1016/j.jneumeth.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a Reward: Hedonic and Neural Responses to Safety from Pain. PLoS ONE. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, Serrano D, Stewart WF. Cutaneous allodynia in the migraine population. Annals of Neurology. 2008;63:148–158. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman C, Brown J, Sandhir R, Klein R, McCarson K, Berman N. Oestrogen Increases Nociception Through ERK Activation in the Trigeminal Ganglion: Evidence for a Peripheral Mechanism of Allodynia. Cephalalgia. 2009;29:520–531. doi: 10.1111/j.1468-2982.2008.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Morales L, Del Olmo N, Valladolid-Acebes I, Fole A, Cano V, Merino B, Stucchi P, Ruggieri D, López L, Alguacil LF, Ruiz-Gayo M. Shift of Circadian Feeding Pattern by High-Fat Diets Is Coincident with Reward Deficits in Obese Mice. PLoS ONE. 2012;7:e36139. doi: 10.1371/journal.pone.0036139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, King C, Malphurs W, Wong F, Weaver JP, Jenkins AC, Rossi HL, Caudle RM. Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior. Molecular Pain. 2008;4 doi: 10.1186/1744-8069-4-43. doi:10.1186/1744-8069-1184-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Nolan TA, Caudle RM, Neubert JK. Effect of caloric and non-caloric sweet reward solutions on thermal facial operant conditioning. Behavioural Brain Research. 2011a;216:723–725. doi: 10.1016/j.bbr.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TA, Hester J, Bokrand-Donatelli Y, Caudle RM, Neubert JK. Adaptation of a novel operant orofacial testing system to characterize both mechanical and thermal pain. Behavioural Brain Research. 2011b;217:477–480. doi: 10.1016/j.bbr.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Jansen-Olesen I. Towards a reliable animal model of migraine. Cephalalgia. 2012 doi: 10.1177/0333102412441719. [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, Gomonchareonsiri S. Episodic Dural Stimulation in Awake Rats: A Model for Recurrent Headache. Headache: The Journal of Head and Face Pain. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin BL, Rapoport AM, Kurth T. Migraine and Obesity: Epidemiology, Mechanisms, and Implications. Headache: The Journal of Head and Face Pain. 2010;50:631–648. doi: 10.1111/j.1526-4610.2009.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Goadsby PJ. Calcitonin gene-related peptide: a molecular link between obesity and migraine? Drug New Perspect. 2010;23:112. doi: 10.1358/dnp.2010.23.2.1475909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi HL, Luu AKS, DeVilbiss JL, Recober A. Obesity increases nociceptive activation of the trigeminal system. European Journal of Pain. 2012 doi: 10.1002/j.1532-2149.2012.00230.x. [epub ahead of print] Oct 16. 10.1002/j.1532-2149.2012.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher AI, Stewart WF, Ricci JA, Lipton RB. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain. 2003;106:81–89. doi: 10.1016/s0304-3959(03)00293-8. [DOI] [PubMed] [Google Scholar]
- Schwedt TJ, Krauss MJ, Frey K, Gereau RW. Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia. 2011;31:6–12. doi: 10.1177/0333102410365108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AC, Townsend RL, Patterson LM, Berthoud H-R. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;301:R1267–R1280. doi: 10.1152/ajpregu.00314.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Broderick JE. Obesity and Pain Are Associated in the United States. Obesity. 2012 doi: 10.1038/oby.2011.397. [DOI] [PubMed] [Google Scholar]
- Stucky NL, Gregory E, Winter MK, He Y-Y, Hamilton ES, McCarson KE, Berman NEJ. Sex Differences in Behavior and Expression of CGRP-Related Genes in a Rodent Model of Chronic Migraine. Headache: The Journal of Head and Face Pain. 2011;51:674–692. doi: 10.1111/j.1526-4610.2011.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Rashid IB, Kojima K, Shoji M, Tanabe J, Tamasawa N, Suda T, Yasujima M. Time course of pain sensation in rat models of insulin resistance, type 2 diabetes, and exogenous hyperinsulinaemia. Diabetes/Metabolism Research and Reviews. 2008;24:642–650. doi: 10.1002/dmrr.903. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Kim R, Cho J, Michaelides M, Anderson BJ, Primeaux SD, Bray GA, Wang G-J, Robinson JK, Volkow ND. Obesity-resistant S5B rats showed greater cocaine conditioned place preference than the obesity-prone OM rats. Physiology & Behavior. 2010;101:713–718. doi: 10.1016/j.physbeh.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachharajani V, Granger DN. Adipose tissue: A motor for the inflammation associated with obesity. IUBMB Life. 2009;61:424–430. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareniuk I, Pavlov IA, Drel VR, Lyzogubov VV, Ilnytska O, Bell SR, Tibrewala J, Groves JT, Obrosova IG. Nitrosative stress and peripheral diabetic neuropathy in leptin-deficient (ob/ob) mice. Experimental Neurology. 2007;205:425–436. doi: 10.1016/j.expneurol.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: Are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Nation JR, Davis KW. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharmacology Biochemistry and Behavior. 2007;88:89–93. doi: 10.1016/j.pbb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieseler J, Sprunger D, Ellis A, Maier SF, Watkins LR. Indwelling Supradural Catheters for Induction of Facial Allodynia: Surgical Procedures, Application of Inflammatory Stimuli, and Behavioral Testing. In: Luo ZD, editor. Pain Research. Vol. 851. Humana Press; 2012. pp. 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A, Berger K, Buring J, Kurth T. Body Mass Index, Migraine, Migraine Frequency and Migraine Features in Women. Cephalalgia. 2009;29:269–278. doi: 10.1111/j.1468-2982.2008.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright LJ, Schur E, Noonan C, Ahumada S, Buchwald D, Afari N. Chronic Pain, Overweight, and Obesity: Findings from a Community-Based Twin Registry. The Journal of Pain. 2010;11:628–635. doi: 10.1016/j.jpain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Shinoda M, Kiyomoto M, Honda K, Suzuki A, Tamagawa T, Kaji K, Kimoto S, Iwata K. P2X3 receptor mediates ectopic mechanical allodynia with inflamed lower lip in mice. Neuroscience Letters. 2012;528:67–72. doi: 10.1016/j.neulet.2012.08.067. [DOI] [PubMed] [Google Scholar]
- Zhang T, Reid K, Acuff CG, Jin CB, Rockhold RW. Cardiovascular and analgesic effects of a highly palatable diet in spontaneously hypertensive and Wistar-Kyoto rats. Pharmacology Biochemistry and Behavior. 1994;48:57–61. doi: 10.1016/0091-3057(94)90498-7. [DOI] [PubMed] [Google Scholar]