Abstract
Problem
Plasma concentrations of fragment Bb (FBb) are a marker for activation of the alternative pathway of the complement system. High concentrations of FBb in maternal blood, as early as the first trimester, are associated with subsequent spontaneous preterm delivery <34 weeks of gestation. The study aim was to determine whether spontaneous preterm labor with intact membranes (PTL), intra-amniotic infection/inflammation (IAI) or labor at term are associated with alterations in circulating maternal FBb concentrations.
Method of Study
This cross-sectional study included women in the following groups: 1) non-pregnant (n=40); 2) normal pregnancy (gestational age range 20-36 6/7 weeks, n=63); 2) women at term not in labor (n=70); 3) women at term in spontaneous labor (n=59); 4) patients with an episode of PTL who delivered at term (n=62); 5) PTL without IAI who delivered preterm (n=30); and 6) PTL with IAI who delivered preterm (n=67). Maternal plasma FBb concentrations were determined by ELISA.
Results
1) Among patients with PTL, those who had a preterm delivery either with IAI (1.21 μg/ml, IQR 0.77-2.16) or without IAI (1.13 μg/ml, IQR 0.92-2.08;) had a higher median maternal plasma FBb concentration than those who delivered at term (0.86 μg/ml, IQR 0.64-1.57; p=0.007 and p=0.026, respectively); 2) there was no difference in the median plasma FBb concentration between patients with and without IAI who delivered preterm (p=0.9); 3) in contrast, spontaneous labor at term was not associated with a significant change in the maternal plasma FBb concentration (p=0.8); 4) maternal plasma concentration of FBb did not differ significantly between normal pregnant women and the non-pregnant controls (p=0.8) and were not correlated with advancing gestational age (r −0.28, p=0.8).
Conclusions
1) Preterm parturition is associated with activation of the alternative complement pathway in maternal circulation; 2) such activation is not detectable in spontaneous labor at term; 3) intra-amniotic infection/inflammation does not explain the activation of the alternative pathway of complement in preterm labor. Collectively, these observations suggest that preterm and term labor have fundamental differences in the regulation of innate immunity.
Keywords: inflammation, intra-amniotic infection, microbial invasion of the amniotic cavity, pregnancy, preterm labor, preterm delivery
INTRODUCTION
The complement system, an important component of innate immunity, plays a pivotal role in the process of recognition of foreign antigens and pathogens.1;2 Complement activation mediates the inflammatory response elicited against infection by recruitment of inflammatory and immucompetent cells, opsonization and killing of pathogens. Moreover, it has a role in regulating the adaptive limb of the immune response.2-4 Complement activation can be triggered by one of three pathways: 1) the “classical”; 2) the “lectin”; or 3) the “alternative” pathway. The classical pathway is triggered by antigen:antibodies complexes (protein-to-protein interactions) and the lectin pathway by mannose-binding lectin (protein-to-carbohydrate interactions).2 In contrast, the alternative pathway does not depend on binding of a protein to a pathogen, but can be initiated by the binding of spontaneously auto-activated C3 in the plasma [generating C3(H2O)] to pathogen surfaces.2;5 The three pathways converge to activate C3-convertase formation.1;2
Normal pregnancy is characterized by physiologic activation of the complement system in maternal circulation,6-10 which has been proposed to be a compensatory mechanism aimed to protect the host against infection.10 Moreover, several complications of pregnancy including spontaneous pregnancy losses,11-16 preeclampsia,17-27 pyelonephritis,28 fetal death,29;30 and preterm labor31-33 have been associated with a systemic maternal complement activation when compared to normal pregnant women.
Preterm parturition, the main cause of neonatal morbidity and mortality, is a syndrome34;35 in which several mechanisms of disease have been implicated in its pathophysiology. These mechanisms include intrauterine infection, utero-placental ischemia, uterine overdistension, abnormal allogeneic recognition, allergic-like reaction, cervical disease, as well as endocrine disorders.35 Importantly, increased activation of components of the complement system in maternal blood (i.e., C3a and C5a)31 and the amniotic fluid (i.e., C336 and fragment Bb37) has been reported in patients with preterm labor and intact membranes.
Fragment Bb, a marker for activation of the alternative complement pathway, has been recently reported to be in increased concentrations in maternal circulation in the first half of pregnancy in women who subsequently had a spontaneous preterm delivery before 34 weeks of gestation.33 However, no data exist regarding fragment Bb concentrations during normal pregnancies and whether there is an association between intra-amniotic infection/inflammation and alterations in fragment Bb concentrations in maternal circulation. Thus, the purpose of this study was to determine whether normal pregnancy, spontaneous preterm labor with intact membranes (PTL), intra-amniotic infection/inflammation (IAI) or labor at term are associated with alterations in circulating maternal plasma fragment Bb concentrations.
MATERIALS AND METHODS
Study groups and inclusion criteria
A retrospective cross-sectional study was conducted including women in the following groups: 1) non-pregnant women (n=40); 2) women with a normal pregnancy (gestational age range 20-36 6/7 weeks, n=63); 3) normal pregnant women at term not in labor (n = 70); 4) normal pregnant women at term with spontaneous labor (n=59); 5) women with an episode of spontaneous preterm labor and intact membranes who delivered at term (n=62); 6) PTL without IAI who delivered preterm (<37 weeks gestation, n=30); and 7) PTL with IAI (n=67). Women with multiple pregnancies or fetuses with chromosomal and/or congenital anomalies were excluded.
Samples and data were retrieved from our bank of biological samples and clinical databases. Many of these samples have previously been used to study the biology of inflammation, hemostasis and angiogenesis regulation in normal pregnant women and those with pregnancy complications.
All participants provided written informed consent prior to the collection of maternal blood. The collection of maternal blood and its use for research purposes was approved by the Institutional Review Boards of the Sotero del Rio Hospital (Chile), Wayne State University (Detroit, Michigan, USA) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS, Bethesda, Maryland, USA).
Clinical Definitions
Women with a normal pregnancy were defined as those without medical, obstetrical, or surgical complications at the time of the study, and who subsequently delivered at term (≥37 weeks of gestation) an appropriate-for-gestational age infant38;39 without neonatal complications. Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes associated with cervical changes before 37 completed weeks of gestation that required hospitalization. Intra-amniotic infection was defined as a positive amniotic fluid culture for aerobic/anaerobic bacteria, genital mycoplasmas and/or yeast. Intra-amniotic inflammation was diagnosed in the presence of an amniotic fluid interleukin (IL)-6 concentration ≥2.6 ng/mL.40
Sample collection and determination of fragment Bb concentration in maternal plasma
Among patients with spontaneous PTL and intact membranes, amniotic fluid samples were obtained by transabdominal amniocentesis performed for evaluation of microbial status of the amniotic cavity. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. An amniotic fluid white blood cell count, glucose concentration, and Gram-stain were also performed shortly after collection as previously described,41-43 and the results of these tests were used for clinical management. Amniotic fluid not required for clinical assessment was centrifuged for 10 min at 4°C and the supernatant was aliquoted and stored at −70°C until IL-6 analysis. Amniotic fluid IL-6 concentrations were used only for research purposes.
Maternal blood samples were collected within 48 hours before or after the amniocentesis into Vacutainer® tubes (BD, Franklin Lakes, NJ, USA). The samples were then centrifuged at 1300xg for 10 minutes at 4°C and the obtained plasma was stored at −70°C until assayed. Maternal plasma concentration of human fragment Bb was determined by sensitive enzyme-linked immunoassays (Quidel Corporation, San Diego, CA, USA). The fragment Bb immunoassay was validated for human plasma in our laboratory, prior to the conduction of this study. Immunoassays were carried out according to the manufacturer’s recommendations. The calculated inter- and intra-assay coefficients of variation for fragment Bb immunoassays in our laboratory were 3.36% and 2.62%, respectively, and the sensitivity was 0.015 μg/mL.
Statistical analysis
Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normality distribution of the data. Since maternal plasma fragment Bb concentrations were not normally distributed, Kruskal-Wallis test with post-hoc analysis by Mann-Whitney U test were used for comparisons of continuous variables. Comparisons of proportions were performed using Fisher’s exact test. Correlations between fragment Bb concentrations gestational age at blood sample collection and pre-pregnancy body mass index (BMI) were examined using Spearman’s rank correlation test. Logistic regression analysis was applied to determine the association between maternal plasma concentration of fragment Bb (μg/mL) and preterm delivery while adjusting for the following variables: maternal age (years), pre-pregnancy BMI (kg/m2), smoking status, and African-American ethnic origin. A p-value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 14 (SPSS Inc., Chicago, IL, USA).
RESULTS
Fragment Bb was detected in all plasma samples included in this study (n=391). Demographic and clinical characteristics of women with a normal pregnancy (including women at term not in labor and with spontaneous labor) and of patients with spontaneous PTL and intact membranes are displayed in Table I and Table II, respectively. Women at term in labor were younger and had a lower median BMI than those at term not in labor. The median gestational age at blood sample collection and the median gestational age at delivery were significantly higher in women at term in labor than those not in labor; however, these differences (5 and 2 days, respectively) are not of clinical significance (Table I). Patients with PTL with IAI had a higher median BMI and a lower median gestational age at blood sample collection than the other two groups of patients with PTL (Table II).
Table I.
Demographic and clinical characteristics of women with a normal pregnancy and those at term, with and without spontaneous labor.
|
|
||||
|---|---|---|---|---|
| Normal Pregnancy (n=63) | Term not in labor (n=70) | Term in labor (n=59) | P * | |
| Maternal age (years) | 23 (20-27) | 27 (22-30) | 24 (21-28) | 0.01 |
| Ethnic origin | ||||
| African-American | 79.4 (50) | 78.6 (55) | 86.4 (51) | NS |
| Caucasian | 11.1 (7) | 11.4 (8) | 3.4 (2) | |
| Hispanic | 3.2 (2) | 7.1 (5) | 6.8 (4) | |
| Asian and other | 6.3 (4) | 2.9 (2) | 3.4 (2) | |
| Smokers (%) | 19.4 (12/62) | 15.7 (11) | 25.4 (15) | NS |
| Pre-pregnancy BMI (kg/m2) | 23.8 (20.7-27.9) | 29.1 (24.3-34.8) | 23.5 (20.7-29.2) | 0.001 |
| GA at blood sample collection (weeks) | 30.9 (26.6-33.3) | 39.1 (38.9-39.6) | 40 (39.1-40.4) | <0.001 |
| GA at delivery (weeks) | 39.6 (38.6-40.6) | 39.3 (39-40) | 40 (39.1-40.4) | 0.01 |
| Neonatal birthweight (gr) | 3320 (3000-3629) | 3365 (3200-3632) | 3330 (3080-3590) | NS |
| Sample storage time (years) | 8.0 (6.9-8.1) | 7.2 (6.8-8.1) | 6.7 (6.6-8.1) | NS |
Values expressed as median (interquartile range) or percentage (number) BMI: body mass index; GA: gestational age; NS: not significant.
between term not in labor and term in labor
Table II.
Demographic and clinical characteristics of patients presenting with spontaneous preterm labor (PTL) and intact membranes.
|
|
||||||
|---|---|---|---|---|---|---|
| PTL without IAI, Term delivery (n=62) |
pa | PTL without IAI, Preterm delivery (n=30) |
pb | PTL with IAI, Preterm delivery (n=67) |
pc | |
| Maternal age (years)† | 22 (19-26.2) | NS | 23 (18.7-27.5) | NS | 22 (19-27) | NS |
| Ethnic origin | ||||||
| African-American | 50 (31) | 0.003 | 83.3 (25) | 0.03 | 62.7 (42) | NS |
| Caucasian | 4.8(3) | 6.7 (2) | 13.4 (9) | |||
| Hispanic | 43.5 (27) | 10 (3) | 22.4(15) | |||
| Asian and other | 1.6(1) | 0 (0) | 1.5(1) | |||
| Smokers (%) | 29.1 (16/55) | NS | 23.3 (7) | NS | 37.8 (17/45) | NS |
| Pre-pregnancy BMI (kg/m2)† | 21.4 (19.5-25) | NS | 22.5 (21.5-25.1) | NS | 24.5 (21.2-30.2) | 0.01 |
| GA at blood sample collection (weeks)† | 31.5 (29.4-33.1) | NS | 31 (26-32.5) | NS | 27.4 (24.9-32.4) | <0.001 |
| GA at delivery (weeks)†† | 38.7(38-39.5) | <0.001 | 34.5 (31.1-35.3) | <0.001 | 28.1 (25.2-32.9) | <0.001 |
| Neonatal birthweight (grams)‡ | 3143 (2870-3410) | <0.001 | 2100 (1569-2470) | 0.001 | 1100 (709-1960) | <0.001 |
| Sample storage time (years)† | 7.6 (6.6-8.7) | NS | 7.3 (7.0-8.1) | NS | 7.5 (6.8-8.6) | NS |
Values expressed as median (interquartile range) or percentage (number) PTL: preterm labor; IAI: intra-amniotic infection/inflammation; BMI: body mass index; GA: gestational age; NS: not significant.
comparison between PTL without IAI, term delivery and PTL without IAI, preterm delivery
comparison between PTL without IAI, preterm delivery and PTL with IAI, preterm delivery
comparison between PTL without IAI, preterm delivery and PTL with IAI, preterm delivery.
Kruskal-Wallis, p=NS,
Kruskal-Wallis, p<0.05,
Kruskal-Wallis, p=0.001
Maternal plasma fragment Bb concentration in normal pregnancies
The median maternal plasma concentration of fragment Bb was not significantly different in non-pregnant and normal pregnant women [0.85 μg/ml, interquartile range (IQR) 0.64-1.12 vs. 0.81 μg/ml, IQR 0.72-0.97, respectively; p=0.8, Figure 1]. Moreover, spontaneous labor at term was not associated with a significant change in the median maternal plasma concentration of fragment Bb (term in labor: 0.72 μg/ml, IQR 0.63-0.88 vs. term not in labor: 0.75 μg/ml, IQR 0.65-0.83; p= 0.8, Figure 2). There was no correlation between maternal plasma fragment Bb concentrations and gestational age at blood sample collection (r= −0.28, p=0.8), as well as with pre-pregnancy BMI (r=0.04, p=0.8). In addition, the median maternal plasma fragment Bb concentration did not differ significantly between African-American and non African-American women (0.83 μg/ml, IQR 0.72-0.98 vs. 0.78 μg/ml, IQR 0.73-0.82, respectively; p= 0.4) and between women who smoked and those who were not (0.78 μg/ml, IQR 0.66-0.92 vs. 0.80 μg/ml, IQR 0.73-0.97, respectively; p= 0.6).
Figure 1. Maternal plasma concentration of fragment Bb in non-pregnant versus normal pregnant women.

The median maternal plasma concentration of fragment Bb did not differ significantly between non-pregnant women and those with a normal pregnancy [0.85 μg/mL, interquartile range (IQR) 0.64-1.12 vs. 0.81 μg/mL, IQR 0.61-0.97, respectively; p=0.8].
Figure 2. Maternal plasma concentration of fragment Bb in pregnant women at term, with and without spontaneous labor.
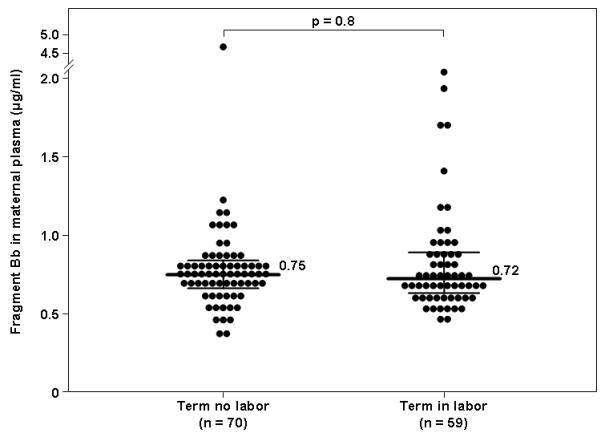
The median maternal plasma concentration of fragment Bb did not differ significantly between women at term in spontaneous labor and those not in labor (0.75 μg/mL, interquartile range (IQR) 0.66-0.83 vs. 0.72 μg/mL, IQR 0.63-0.88, respectively; p=0.8].
Maternal plasma fragment Bb concentration in women with spontaneous preterm labor and intact membranes
The median maternal plasma fragment Bb concentrations differed significantly between the three PTL study groups (Kruskal-Wallis, p=0.01). Patients with an episode of PTL with intact membranes which led to a preterm delivery, either with IAI (1.21 μg/ml, IQR 0.77-2.16) or without IAI (1.13 μg/ml, IQR 0.92-2.08), had a higher median maternal plasma fragment Bb concentration than those with an episode of PTL who delivered at term (0.86 μg/mL, IQR 0.64-1.57; p=0.007 and p=0.026, respectively) and than those with a normal pregnancy (p<0.001 for both comparisons). (Figure 3). Among patients with PTL who delivered preterm, the median maternal plasma fragment Bb concentration did not differ significantly between women with and without IAI (p=0.9, Figure 3). The median maternal plasma FBb concentration of patients with an episode of PTL who delivered at term was not significantly different from that of normal pregnant women (p=0.5, Figure 3)
Figure 3. Comparison of the median maternal plasma concentration of fragment Bb in women with a normal pregnancy and those with spontaneous preterm labor (PTL).

The median maternal plasma fragment Bb concentration was higher in patients with PTL who delivered preterm, either with IAI (1.21 μg/ml, IQR 0.77-2.16 vs. 0.86 μg/mL, IQR 0.64-1.57; p=0.007) or without IAI (1.13 μg/mL, IQR 0.92-2.08; p=0.026), than those with an episode of PTL who delivered at term and than those with a normal pregnancy (p<0.001 for both comparisons).
In order to examine the association between maternal plasma fragment Bb concentrations, preterm delivery, and possible confounding factors, a multivariable logistic regression analysis was performed adjusting for maternal age, pre-pregnancy BMI, smoking status and African-American ethnic origin. The final regression model demonstrated that both increased fragment Bb concentrations in maternal plasma (OR 1.79, 95% CI 1.12-2.85; p=0.01) and higher pre-pregnancy BMI (OR 1.09, 95% CI 1.01-1.18) were significantly and independently associated with preterm delivery among patients with an episode of PTL.
DISCUSSION
Principal findings of this study
1) Preterm parturition, with or without intra-amniotic infection/inflammation, is associated with a higher median maternal plasma fragment Bb concentration than patients with an episode of PTL who delivered at term; 2) In contrast, spontaneous labor at term was not associated with a significant change in the median maternal plasma concentration of fragment Bb; 3) normal pregnancy is not associated with increased maternal plasma concentrations compared to non-pregnant women; and 4) maternal plasma fragment Bb concentrations does not change significantly with advancing gestation.
The alternative complement pathway
The complement system, an effector arm of the innate immune system, plays a pivotal role in the host defense against infections. In addition, the complement system mediates the inflammatory response elicited against infection, and participates in activating the adaptive immune system.2-4 Activation of complement cascade through either of the three complement pathways converges at the point of C3 convertase formation,1;2 and the latter cleaves C3 to C3a and C3b. C3b participates in the formation of the C5 convertase, which cleaves C5 to C5a and C5b. C3a and C5a, termed anaphylatoxins, are pleiotropic inflammatory mediators.2
In contrast to the classical pathway, which is initiated by the binding of C1q to antigen-antibody complexes and to the lectin pathway which is initiated by the binding of mannose-binding lectin (MBL) to sugars present on the bacterial cell wall, the alternative pathway is capable of spontaneous auto-activation through a slow rate hydrolysis of C3, generating C3(H2O).5 The latter can covalently attach to permissive surface and bind factor B, which is cleaved by factor D to become the active protease, fragment Bb.44 The active convertase, C3(H2O)Bb complex, can cleave additional native C3 molecules, generating C3b which in turn can associates with factor B to generate more C3-convertase,2 creating an amplification loop. Thus, increased production of fragment Bb can represent activation of the alternative pathway.
Inappropriate activation of the complement system has been implicated in the pathophysiology of numerous disorders such as systemic lupus erythematosus,45 rheumatoid arthritis,46;47 and stroke.48;49 The alternative complement pathway has been specifically suggested to have a role in the pathophysiology of several diseases such as age-related macular degeneration,4;50-53 rheumatoid arthritis, lupus nephritis, and anti-phospholipid syndrome.4
The complement in normal and complicated pregnancies
Physiologic activation of complement in maternal circulation during normal pregnancy6-10 has been proposed to be a compensatory mechanism aimed to protect the host against infection.10 Nevertheless, excessive systemic maternal complement activation has been linked to pregnancy complications such as spontaneous pregnancy losses,11-16 preeclampsia,17-27 pyelonephritis,28 fetal death,29;30 and preterm birth.31-33
A specific association between systemic maternal activation of the alternative pathway and pregnancy complications was first suggested for spontaneous fetal loss.12;54-56 Subsequently, the alternative pathway has been implicated in other obstetrical complications. Lynch et al.27 were the first to report an association between elevated maternal plasma fragment Bb concentrations (≥90th percentile of their entire cohort) in early pregnancy (< 20 weeks of gestation) and an increased risk for later development of preeclampsia. A subsequent report by the same group33 demonstrated an association between high maternal plasma concentrations of fragment Bb (≥75th percentile) in early pregnancy and spontaneous preterm birth < 34 weeks. Consistent with this report, our group37 has recently reported an association between increased amniotic fluid concentration of fragment Bb and intra-amniotic infection/inflammation in patients in preterm labor. This has been suggested to represent alternative pathway activation as part of the fetal innate immune response57;58 to intra-amniotic infection. In the present study we intended to further examine the behavior of fragment Bb in the maternal compartment during normal pregnancy and whether an excessive activation of the alternative pathway, as is in amniotic fluid, exist also in the maternal compartment in women with preterm labor and in the presence of IAI.
Fragment Bb in maternal circulation during a normal pregnancy
The present study reports, for the first time, data regarding fragment Bb concentrations in maternal circulation during normal pregnancy, and its correlation with advancing gestational age and labor at term. In contrast to other complement activation products (i.e., C3a, C4a and C5a),10 the concentrations of fragment Bb, an activation component of the alternative pathway, were comparable in non-pregnant and normal pregnant women. This finding is in agreement with the study by Lynch et al,33 who reported in their cohort a mean maternal plasma fragment Bb concentration of 0.7 μg/mL in the first half of pregnancy which was in the range of the laboratory normal reference for non-pregnant controls. The present study extends this observation by demonstrating that the median fragment Bb concentration did not change with advancing gestation. Collectively, these results suggest that normal pregnancy is not characterized by physiologic activation of the alternative pathway of the complement system.
In addition, we demonstrated that maternal plasma fragment Bb concentrations were not significantly different between women at term with or without spontaneous labor. Spontaneous labor at term is regarded as an inflammatory process.59-75 This view is supported by the association between term parturition and infiltration of inflammatory cells in the cervix,59;60;70 myometrium,68;70 and chorioamniotic membranes.69;70 Moreover, labor at term is accompanied by increased production of proinflammatory cytokines71;74 (i.e. IL-1ß,61;63;65;70 IL-6,64;65;70 TNF-α,63;65 and IL-862;64;66;70) and chemokines71;74 (i.e. GRO-α,67 G-CSF,64 MCP-172;73;75). Nevertheless, the present study suggests that term parturition does not involve activation of the alternative complement pathway in maternal circulation.
Fragment Bb in maternal circulation is increased in preterm parturition with or without intra-amniotic infection/inflammation but not in term labor
The finding that preterm parturition is associated with an increased concentration of fragment Bb in maternal circulation is in agreement with the results reported by Lynch et al.33 These investigators found significantly higher fragment Bb concentrations early in pregnancy in women who will subsequently have a spontaneous preterm birth < 34 weeks, but not among those who had a late spontaneous preterm birth, compared to women who delivered at term. The authors proposed that elevated fragment Bb concentration from early pregnancy may represent a subclinical phase of an inflammatory state or infection.
The present study further demonstrates that in the maternal circulation there were no differences in the concentrations of fragment Bb between women with preterm labor and preterm delivery, with or without IAI. These findings were different from those previously reported in amniotic fluid in which fragment Bb concentrations are higher in women with IAI.37 These observations, together with the finding that an episode of PTL that did not result in a preterm delivery was not associated with higher fragment Bb concentrations, may imply that activation of the alternative complement pathway in maternal circulation relates to the process of preterm delivery itself, regardless of its etiology. In other words, it is possible that activation of the alternative pathway is part of the pathologic common terminal pathway of preterm labor that can be triggered through a variety of stimuli. However, the cross-sectional nature of this study does not allow determining causality or temporal relationship between increased maternal concentration of fragment Bb and preterm delivery.
Other possible explanations can be raised for the association between high fragment Bb concentrations in maternal circulation and preterm delivery, regardless of the presence of IAI. Several other etiologic factors that had been implicated in the pathophysiology of preterm delivery may be accompanied by activation of the alternative pathway. One such factor is systemic maternal inflammation for which there is a growing body of evidence to support the relationship with preterm parturition: 1) systemic administration of microbial products76-78 or IL-179 to pregnant mice can result in preterm delivery; 2) treating mice exposed to IL-1 with IL-1 receptor antagonist abrogates parturition;80 3) systemic infections81-94 and chronic inflammatory states,95-103 have been associated with preterm parturition. Indeed, the alternative complement pathway has been suggested to play a role in the pathophysiology of several inflammatory-related diseases such as rheumatoid arthritis, lupus nephritis, anti-phospholipid syndrome, asthma4 and age-related macular degeneration.50-53 Furthermore, stress which is associated with increased risk of spontaneous preterm delivery,104-106 has been shown in healthy volunteers to lead to perturbation of the complement system homeostasis107-109 (including complement factors of the alternative pathway109) and this was suggested as a potential mechanism for stress-induced inflammatory activation in individuals with inflammatory disorders.109
CONCLUSIONS
The present study demonstrates that preterm parturition is associated with activation of complement through the alternative pathway as reflected by the increased fragment Bb concentration only in women with PTL who delivered preterm. Such activation is not detectable in spontaneous labor at term. Intra-amniotic infection/inflammation does not explain the alternative complement pathway activation of preterm labor. Collectively, these observations suggest that the pathologic preterm parturition and the physiologic term labor have fundamental differences in regulation of innate immunity.
Acknowledgment
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. The authors would like to thank Dr. Jane E. Salmon (Hospital for Special Surgery, Weill Medical College, Cornell University, New York, NY) for the thoughtful and valuable advice during the conduction of this work.
Reference List
- (1).Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- (2).Murphy K, Travers P, Walport M. Innate immunity. In: Murphy K, Travers P, Walport M, editors. Janeway’s Immunobiology. Seventh ed. Garland Science; New York: 2008. pp. 39–103. [Google Scholar]
- (3).Holers VM. The complement system as a therapeutic target in autoimmunity. Clin Immunol. 2003;107:140–151. doi: 10.1016/s1521-6616(03)00034-2. [DOI] [PubMed] [Google Scholar]
- (4).Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- (5).Muller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–47. 321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- (6).Kitzmiller JL, Stoneburner L, Yelenosky PF, Lucas WE. Serum complement in normal pregnancy and pre-eclampsia. Am J Obstet Gynecol. 1973;117:312–315. doi: 10.1016/0002-9378(73)90031-8. [DOI] [PubMed] [Google Scholar]
- (7).Baines MG, Millar KG, Mills P. Studies of complement levels in normal human pregnancy. Obstet Gynecol. 1974;43:806–810. [PubMed] [Google Scholar]
- (8).Tedder RS, Nelson M, Eisen V. Effects on serum complement of normal and pre-eclamptic pregnancy and of oral contraceptives. Br J Exp Pathol. 1975;56:389–395. [PMC free article] [PubMed] [Google Scholar]
- (9).Schena FP, Manno C, Selvaggi L, Loverro G, Bettocchi S, Bonomo L. Behaviour of immune complexes and the complement system in normal pregnancy and pre-eclampsia. J Clin Lab Immunol. 1982;7:21–26. [PubMed] [Google Scholar]
- (10).Richani K, Soto E, Romero R, et al. Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med. 2005;17:239–245. doi: 10.1080/14767050500072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Tichenor JR, Bledsoe LB, Opsahl MS, Cunningham DS. Activation of complement in humans with a first-trimester pregnancy loss. Gynecol Obstet Invest. 1995;39:79–82. doi: 10.1159/000292384. [DOI] [PubMed] [Google Scholar]
- (12).Mellor AL, Sivakumar J, Chandler P, et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- (13).Holers VM, Girardi G, Mo L, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Caucheteux SM, C Langevin, Ojcius DM. At the innate frontiers between mother and fetus: linking abortion with complement activation. Immunity. 2003;18:169–172. doi: 10.1016/s1074-7613(03)00028-1. [DOI] [PubMed] [Google Scholar]
- (15).Girardi G, Salmon JB. The role of complement in pregnancy and fetal loss. Autoimmunity. 2003;36:19–26. doi: 10.1080/0891693031000067322. [DOI] [PubMed] [Google Scholar]
- (16).Girardi G. Complement inhibition keeps mothers calm and avoids fetal rejection. Immunol Invest. 2008;37:645–659. doi: 10.1080/08820130802191615. [DOI] [PubMed] [Google Scholar]
- (17).Haeger M, Bengtson A, Karlsson K, Heideman M. Complement activation and anaphylatoxin (C3a and C5a) formation in preeclampsia and by amniotic fluid. Obstet Gynecol. 1989;73:551–556. [PubMed] [Google Scholar]
- (18).Haeger M, Unander M, Bengtsson A. Complement activation in relation to development of preeclampsia. Obstet Gynecol. 1991;78:46–49. [PubMed] [Google Scholar]
- (19).Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 1992;79:19–26. [PubMed] [Google Scholar]
- (20).Haeger M. The role of complement in pregnancy-induced hypertensive disease. Int J Gynaecol Obstet. 1993;43:113–127. doi: 10.1016/0020-7292(93)90318-q. [DOI] [PubMed] [Google Scholar]
- (21).de Messias-Reason IJ, Aleixo V, de FH, Nisihara RM, Mocelin V, Urbanetz A. Complement activation in Brazilian patients with preeclampsia. J Investig Allergol Clin Immunol. 2000;10:209–214. [PubMed] [Google Scholar]
- (22).Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, et al. Preeclampsia and SGA differ in the maternal plasma complememt split products profile. J Soc Gynecol Investig. 2005;12:148A. Ref Type: Abstract. [Google Scholar]
- (23).Sziller I, Babula O, Hupuczi P, et al. Mannose-binding lectin (MBL) codon 54 gene polymorphism protects against development of pre-eclampsia, HELLP syndrome and pre-eclampsia-associated intrauterine growth restriction. Mol Hum Reprod. 2007;13:281–285. doi: 10.1093/molehr/gam003. [DOI] [PubMed] [Google Scholar]
- (24).van de Geijn FE, Dolhain RJ, van RW, Hazes JM, de Groot CJ. Mannose-binding lectin genotypes and pre-eclampsia: a case-control study. Hum Immunol. 2007;68:888–893. doi: 10.1016/j.humimm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- (25).Wang CC, Yim KW, Poon TC, et al. Innate immune response by ficolin binding in apoptotic placenta is associated with the clinical syndrome of preeclampsia. Clin Chem. 2007;53:42–52. doi: 10.1373/clinchem.2007.074401. [DOI] [PubMed] [Google Scholar]
- (26).Than NG, Romero R, Erez O, et al. A role for mannose-binding lectin, a component of the innate immune system in pre-eclampsia. Am J Reprod Immunol. 2008;60:333–345. doi: 10.1111/j.1600-0897.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lynch AM, Murphy JR, Byers T, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198:385–389. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Soto E, Richani K, Romero R, et al. Increased concentration of the complement split product C5a in acute pyelonephritis during pregnancy. J Matern Fetal Neonatal Med. 2005;17:247–252. doi: 10.1080/14767050500072805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Richani K, Romero R, Soto E, et al. Unexplained intrauterine fetal death is accompanied by activation of complement. J Perinat Med. 2005;33:296–305. doi: 10.1515/JPM.2005.052. [DOI] [PubMed] [Google Scholar]
- (30).Girardi G. Guilty as charged: all available evidence implicates complement’s role in fetal demise. Am J Reprod Immunol. 2008;59:183–192. doi: 10.1111/j.1600-0897.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- (31).Soto E, Romero R, Richani K, et al. Anaphylatoxins in preterm and term labor. J Perinat Med. 2005;33:306–313. doi: 10.1515/JPM.2005.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).van de Geijn FE, Dolhain RJ, van RW, Willemsen SP, Hazes JM, de Groot CJ. Mannose-binding lectin genotypes are associated with shorter gestational age. An evolutionary advantage of low MBL production genotypes? Mol Immunol. 2008;45:1514–1518. doi: 10.1016/j.molimm.2007.08.021. [DOI] [PubMed] [Google Scholar]
- (33).Lynch AM, Gibbs RS, Murphy JR, et al. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2008;199:354–358. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. 414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- (35).Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. 17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Elimian A, Figueroa R, Canterino J, Verma U, guero-Rosenfeld M, Tejani N. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet Gynecol. 1998;92:72–76. doi: 10.1016/s0029-7844(98)00123-9. [DOI] [PubMed] [Google Scholar]
- (37).Vaisbuch E, Romero R, Erez O, et al. Fragment Bb in amniotic fluid: evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2009;22:905–916. doi: 10.1080/14767050902994663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Alexander GR, Kogan M, Martin J, Papiernik E. What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clin Obstet Gynecol. 1998;41:114–125. doi: 10.1097/00003081-199803000-00017. [DOI] [PubMed] [Google Scholar]
- (39).Gonzalez RP, Gomez RM, Castro RS, et al. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- (40).Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- (41).Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- (42).Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- (43).Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- (44).Gotze O, Muller-Eberhard HJ. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971;134:90s–108s. [PubMed] [Google Scholar]
- (45).Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res. 2002;4(Suppl 3):S279–S293. doi: 10.1186/ar586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Nakagawa K, Sakiyama H, Tsuchida T, et al. Complement C1s activation in degenerating articular cartilage of rheumatoid arthritis patients: immunohistochemical studies with an active form specific antibody. Ann Rheum Dis. 1999;58:175–181. doi: 10.1136/ard.58.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Neumann E, Barnum SR, Tarner IH, et al. Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum. 2002;46:934–945. doi: 10.1002/art.10183. [DOI] [PubMed] [Google Scholar]
- (48).del Zoppo GJ. In stroke, complement will get you nowhere. Nat Med. 1999;5:995–996. doi: 10.1038/12431. [DOI] [PubMed] [Google Scholar]
- (49).Huang J, Kim LJ, Mealey R, et al. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285:595–599. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- (50).Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- (51).Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- (52).Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma Complement Components and Activation Fragments are Associated with Age-Related Macular Degeneration Genotypes and Phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818–5827. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- (55).Mao D, Wu X, Deppong C, et al. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity. 2003;19:813–822. doi: 10.1016/s1074-7613(03)00321-2. [DOI] [PubMed] [Google Scholar]
- (56).Girardi G, Berman J, Redecha P, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- (58).Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- (59).Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273–281. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- (60).Liggins GC. Cervical ripening as an inflammatory reaction. Edinburgh, Churchill Livingstone: 1981. [Google Scholar]
- (61).Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- (62).Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–820. doi: 10.1016/0002-9378(91)90422-n. [DOI] [PubMed] [Google Scholar]
- (63).Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–1587. doi: 10.1016/0002-9378(92)91636-o. [DOI] [PubMed] [Google Scholar]
- (64).Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine. 1993;5:81–88. doi: 10.1016/1043-4666(93)90027-3. [DOI] [PubMed] [Google Scholar]
- (65).Opsjln SL, Wathen NC, Tingulstad S, et al. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993;169:397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- (66).Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- (67).Cohen J, Ghezzi F, Romero R, et al. GRO alpha in the fetomaternal and amniotic fluid compartments during pregnancy and parturition. Am J Reprod Immunol. 1996;35:23–29. doi: 10.1111/j.1600-0897.1996.tb00004.x. [DOI] [PubMed] [Google Scholar]
- (68).Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- (69).Keski-Nisula L, Aalto ML, Katila ML, Kirkinen P. Intrauterine inflammation at term: a histopathologic study. Hum Pathol. 2000;31:841–846. doi: 10.1053/hupa.2000.8449. [DOI] [PubMed] [Google Scholar]
- (70).Osman I, Young A, Ledingham MA, et al. Leukocyte density and proinflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- (71).Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–46. S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- (72).Esplin MS, Romero R, Chaiworapongsa T, et al. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. J Matern Fetal Neonatal Med. 2003;14:51–56. doi: 10.1080/jmf.14.1.51.56. [DOI] [PubMed] [Google Scholar]
- (73).Esplin MS, Peltier MR, Hamblin S, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26:661–671. doi: 10.1016/j.placenta.2004.09.012. [DOI] [PubMed] [Google Scholar]
- (74).Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol. 2008;181:1470–1479. doi: 10.4049/jimmunol.181.2.1470. [DOI] [PubMed] [Google Scholar]
- (76).Fidel PL, Jr., Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- (77).Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol. 1995;172:1598–1603. doi: 10.1016/0002-9378(95)90503-0. [DOI] [PubMed] [Google Scholar]
- (78).Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol. 1996;174:754–759. doi: 10.1016/s0002-9378(96)70460-x. [DOI] [PubMed] [Google Scholar]
- (79).Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- (80).Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol. 1992;167:1041–1045. doi: 10.1016/s0002-9378(12)80035-4. [DOI] [PubMed] [Google Scholar]
- (81).Gilles HM, Lawson JB, Sibelas M, Voller A, Allan N. Malaria, anaemia and pregnancy. Ann Trop Med Parasitol. 1969;63:245–263. doi: 10.1080/00034983.1969.11686625. [DOI] [PubMed] [Google Scholar]
- (82).Patrick MJ. Influence of maternal renal infection on the foetus and infant. Arch Dis Child. 1967;42:208–213. doi: 10.1136/adc.42.222.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Wren BG. Subclinical renal infection and prematurity. Med J Aust. 1969;2:596–600. doi: 10.5694/j.1326-5377.1969.tb107290.x. [DOI] [PubMed] [Google Scholar]
- (84).Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: A clinical review. Obstet Gynecol. 1973;42:112–117. [PubMed] [Google Scholar]
- (85).Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun. 1999;67:5958–5966. doi: 10.1128/iai.67.11.5958-5966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Benedetti TJ, Valle R, Ledger WJ. Antepartum pneumonia in pregnancy. Am J Obstet Gynecol. 1982;144:413–417. doi: 10.1016/0002-9378(82)90246-0. [DOI] [PubMed] [Google Scholar]
- (87).Madinger NE, Greenspoon JS, Ellrodt AG. Pneumonia during pregnancy: has modern technology improved maternal and fetal outcome? Am J Obstet Gynecol. 1989;161:657–662. doi: 10.1016/0002-9378(89)90373-6. [DOI] [PubMed] [Google Scholar]
- (88).Munn MB, Groome LJ, Atterbury JL, Baker SL, Hoff C. Pneumonia as a complication of pregnancy. J Matern Fetal Med. 1999;8:151–154. doi: 10.1002/(SICI)1520-6661(199907/08)8:4<151::AID-MFM2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- (89).Offenbacher S, Beck JD, Lieff S, Slade G. Role of periodontitis in systemic health: spontaneous preterm birth. J Dent Educ. 1998;62:852–858. [PubMed] [Google Scholar]
- (90).Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: results of a prospective study. J Am Dent Assoc. 2001;132:875–880. doi: 10.14219/jada.archive.2001.0299. [DOI] [PubMed] [Google Scholar]
- (91).Offenbacher S, Lieff S, Boggess KA, et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001;6:164–174. doi: 10.1902/annals.2001.6.1.164. [DOI] [PubMed] [Google Scholar]
- (92).Madianos PN, Lieff S, Murtha AP, et al. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol. 2001;6:175–182. doi: 10.1902/annals.2001.6.1.175. [DOI] [PubMed] [Google Scholar]
- (93).Goepfert AR, Jeffcoat MK, Andrews WW, et al. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet Gynecol. 2004;104:777–783. doi: 10.1097/01.AOG.0000139836.47777.6d. [DOI] [PubMed] [Google Scholar]
- (94).Jarjoura K, Devine PC, Perez-Delboy A, Herrera-Abreu M, D’Alton M, Papapanou PN. Markers of periodontal infection and preterm birth. Am J Obstet Gynecol. 2005;192:513–519. doi: 10.1016/j.ajog.2004.07.018. [DOI] [PubMed] [Google Scholar]
- (95).Johnson MJ, Petri M, Witter FR, Repke JT. Evaluation of preterm delivery in a systemic lupus erythematosus pregnancy clinic. Obstet Gynecol. 1995;86:396–399. doi: 10.1016/0029-7844(95)00186-U. [DOI] [PubMed] [Google Scholar]
- (96).Le HD, Wechsler B, Vauthier-Brouzes D, et al. Outcome of planned pregnancies in systemic lupus erythematosus: a prospective study on 62 pregnancies. Br J Rheumatol. 1997;36:772–777. doi: 10.1093/rheumatology/36.7.772. [DOI] [PubMed] [Google Scholar]
- (97).Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am J Gastroenterol. 2002;97:641–648. doi: 10.1111/j.1572-0241.2002.05543.x. [DOI] [PubMed] [Google Scholar]
- (98).Norgard B, Fonager K, Pedersen L, Jacobsen BA, Sorensen HT. Birth outcome in women exposed to 5-aminosalicylic acid during pregnancy: a Danish cohort study. Gut. 2003;52:243–247. doi: 10.1136/gut.52.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Kallen B, Rydhstroem H, Aberg A. Asthma during pregnancy--a population based study. Eur J Epidemiol. 2000;16:167–171. doi: 10.1023/a:1007678404911. [DOI] [PubMed] [Google Scholar]
- (100).Dombrowski MP, Schatz M, Wise R, et al. Asthma during pregnancy. Obstet Gynecol. 2004;103:5–12. doi: 10.1097/01.AOG.0000103994.75162.16. [DOI] [PubMed] [Google Scholar]
- (101).Adams MM, Sarno AP, Harlass FE, Rawlings JS, Read JA. Risk factors for preterm delivery in a healthy cohort. Epidemiology. 1995;6:525–532. doi: 10.1097/00001648-199509000-00011. [DOI] [PubMed] [Google Scholar]
- (102).Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of Body Mass Index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007;7:168. doi: 10.1186/1471-2458-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Nohr EA, Bech BH, Vaeth M, Rasmussen KM, Henriksen TB, Olsen J. Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2007;21:5–14. doi: 10.1111/j.1365-3016.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- (104).Copper RL, Goldenberg RL, Das A, et al. The preterm prediction study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175:1286–1292. doi: 10.1016/s0002-9378(96)70042-x. [DOI] [PubMed] [Google Scholar]
- (105).Farley TA, Mason K, Rice J, Habel JD, Scribner R, Cohen DA. The relationship between the neighbourhood environment and adverse birth outcomes. Paediatr Perinat Epidemiol. 2006;20:188–200. doi: 10.1111/j.1365-3016.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- (106).Lobel M, Dunkel-Schetter C, Scrimshaw SC. Prenatal maternal stress and prematurity: a prospective study of socioeconomically disadvantaged women. Health Psychol. 1992;11:32–40. doi: 10.1037//0278-6133.11.1.32. [DOI] [PubMed] [Google Scholar]
- (107).Endresen IM, Relling GB, Tonder O, Myking O, Walther BT, Ursin H. Brief uncontrollable stress and psychological parameters influence human plasma concentrations of IgM and complement component C3. Behav Med. 1991;17:167–176. doi: 10.1080/08964289.1991.9935168. [DOI] [PubMed] [Google Scholar]
- (108).Maes M, Hendriks D, Van GA, et al. Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendocrinology. 1997;22:397–409. doi: 10.1016/s0306-4530(97)00042-5. [DOI] [PubMed] [Google Scholar]
- (109).Burns VE, Edwards KM, Ring C, Drayson M, Carroll D. Complement cascade activation after an acute psychological stress task. Psychosom Med. 2008;70:387–396. doi: 10.1097/PSY.0b013e31816ded22. [DOI] [PubMed] [Google Scholar]


