Abstract
Though transplantation of pancreatic islet cells has emerged as a promising treatment for Type 1 diabetes its clinical application remains limited due to a number of limitations including both pathogenic innate and adaptive immune responses. We report here on a novel type of multifunctional cytoprotective material applied to coat living pancreatic islets. The coating utilizes hydrogen-bonded interactions of a natural polyphenol (tannic acid) with poly(N-vinylpyrrolidone) deposited on the islet surface via non-ionic layer-by-layer assembly. We demonstrate that the coating is conformal over the surface of mammalian islets including those derived from rat, non-human primate (NHP), and human. In contrast to unmodified controls, the coated islets maintain their viability and β-cell functionality for at least 96 hours in vitro. We also determine that the coating demonstrates immunomodulatory cytoprotective properties suppressing pro-inflammatory cytokine synthesis in stimulated bone marrow-derived macrophages and diabetogenic BDC-2.5 T cells. The coating material combines high chemical stability under physiologically relevant conditions with capability of suppressing cytokine synthesis, crucial parameters for prolonged islet integrity, viability, and function in vivo. Our study offers new opportunities in the area of advanced multifunctional materials to be used for a cell-based transplantation therapy.
Keywords: Cytoprotective Coating, Hydrogen-Bonding, Layer-by-Layer, Antioxidant, Polyphenol, Immunomodulatory
1. Introduction
Type 1 diabetes is a chronic autoimmune disease representing a major health care problem worldwide.[1] The disease is caused by islet-reactive immune T cells that destroy insulin-producing pancreatic β-cells. Transplantation of insulin-producing pancreatic islets (cell clusters) has been recognized as a promising approach for treating diabetes.[2,3] However, despite this opportunity, the clinical application of this approach remains limited. One of the challenges includes the isolation, viability, and functionality of islets in vitro. In the pancreas, endocrine cells of the islet clusters are separated from exocrine cells by a discontinuous mantle of collagen fibers defining their respective basement membrane.[4] The individual islets are of 50–200 μm in diameter and consist of individual cells with the insulin-producing β-cells among the main secretory cells of islets.[4] During collagenase digestion of the pancreas for isolation of islets, disruption of the islet mantle results in heterogeneous islet preparations exhibiting various morphologies (fragmentation, fusion) under routine tissue culture conditions.[5] Attenuation of islet viability and/or functionality accompanies these morphological changes.[5] In addition, transplantation requires immunosuppression strategies to protect the donor islets from the recipient’s immune responses and prevent transplant rejection.[6] Despite the fact that a range of immunosuppressive drugs have been developed for transplant patients, [7] the chronic use of these agents can have significant side effects, including deleterious effects on glucose homeostasis and β-cell function as well as opportunistic infections in the recipient.[8] These issues have inspired the development of a number of strategies to modulate immunogenic reactions and stabilize islet morphology and functionality, both in vitro and following transplantation in vivo. [9,10]
Two major approaches have been introduced such as microencapsulation of the islet cells and modification of islet cell surfaces.[11,12,13] Microencapsulation typically involves embedding islets in solid matrices, allowing for the creation of a semi-permeable microenvironment around islets, capable of immune-modulation without compromising mass and oxygen transfer. [14,15] For that, the islets are usually entrapped as clusters in high-viscous alginate droplets stabilized with divalent ions of barium or calcium.[16] However, microencapsulation methods (emulsification, discontinuous gradient density centrifugation, and interfacial polymerization) result in entrapping of islet clusters in thick, 5–50 μm, polymer coatings.[17,18,19,20] Such microencapsulated clusters often result in islet cell necrosis and do not permit regulation of the permeability properties of the coatings for modulation of cellular immune reactions caused by pro-inflammatory cytokines.[21,22] Similarly, recently developed polyethylene glycol hydrogels demonstrate facile control over porosity but the formed microbeads are large and present a barrier for rapid transport of oxygen, nutrients, and therapeutic factors.[15] Thus, new methods for the encapsulation of islets which do not increase the diameter of the transplant are required. In this respect, modification of islet surfaces would be a powerful tool that can provide an artificial nurturing environment and preserve islet viability and function.[6,23,24]
Islet surface modification traditionally has involved covalent conjugation to islet cell surfaces. Recently, it has been suggested that polyethylene glycol (PEG) grafted onto a collagen matrix of isolated islets could inhibit pathways leading to islet graft rejection by reducing antibody/complement-mediated cytotoxicity.[25] Activated PEGs were reacted with an amine group of the membrane proteins on the islet surface[26] and were used for immobilization of bioactive substances, such as albumin, to mask the surface antigen.[27] Using a bifunctional PEG linker, recombinant thrombomodulin was chemically attached to the surface of living islets in a two-step process with the purpose of creating a localized anti-inflammatory environment and reducing islet thrombogenicity.[28] Various amphiphilic polymers, such as PEG-conjugated phospholipid and polyvinyl alcohol carrying long alkyl chains were used to modify islet surface through hydrophobic interactions.[29,30,31] However, these technologies are limited to the introduction of specific functional small molecules that are compatible with cell physiology.[32,33] Moreover, covalent immobilization does not permit long-term stability due to fast degradation of the conjugation bonds.[29,34] Another important issue with this approach is the preservation of the islet integrity since loss of functionality directly correlates with islet morphology and was observed when islets dissociated or fused in suspension culture. [35]
Layer-by-layer (LbL) assembly can be applied as an alternative technique for islet surface modification.[36,37] The technique is based on alternating LbL deposition of water soluble polymers on surfaces from aqueous solutions which results in nano-thin coatings of controllable thickness and composition.[38,39] The ultrathin conformal coating affords a real-time response to various stimuli. Further, the opportunity to modify the protective ultrathin shell with other agents (e.g., biological response modifiers) capable of a subsequent slowly triggered release can be achieved.[40] By selecting specific polyelectrolytes, a defined molecular cut-off of the coating is possible, as is inhibitor binding to prevent graft rejection, macrophage attack, or antibody recognition.[41] Current LbL studies mostly focus on electrostatically-bound systems in which positively and negatively charged polymers are used for the assembly. Despite the significant promise of the LbL strategy for islet modification, the main drawback of the approach is cytotoxicity of the polycationic compounds.[42,43] Although the cytotoxic effect of poly(L-lysine) (PLL) and poly(ethylene imine) can be reduced by varying polycation concentration, exposure time, and introducing PEG grafts, polycation-based systems are still challenging for islet modification due to a narrow window of the cytocompatible graft-copolymers.[44,45]
In this regard, a hydrogen-bonded LbL approach suggests new opportunities for cytocompatible protective strategies.[46,47] The LbL assembly driven by hydrogen bonds has emerged as a powerful technique allowing inclusion of polymers which carry no charge. [48,49,50,51] Hydrogen-bonded multilayer films based on tannic acid (TA) have been recently found to be stable at physiological conditions. [52,53] TA is a natural polyphenol, which has long been known for its antioxidant, and antibacterial properties.[54,55] The ability of polyphenols to scavenge free-radicals[56] and inhibit radical-induced oxidation of adjacent molecules[57,58] can be beneficial for anti-oxidative activities of TA-containing protective coatings. These features are particularly important since most inflammatory processes are associated with oxidative stress[59,60] initiated by production of reactive oxygen species (ROS). ROS can function as signaling molecules in many cell types.[61] Activation of macrophages and T cells by oxidative stress results in the secretion of pro-inflammatory cytokines and contribute significantly to the progression of immune system activation and inflammation.[62] Traditionally, immunomodulatory compounds have been directly injected into tissues.[63] There are only few studies with active compounds immobilized around islet surfaces.[64,65] However, the studies are limited to covalent conjugation to islet surface or copolymerization into gels on the islet surfaces,[66] both of which require complicated synthetic procedures performed in the presence of islets which might compromise islet cell functionality.
Here, we report on a novel type of cytoprotective coating designed through hydrogen-bonded interactions of a natural polyphenol (TA) with poly(N-vinylpyrrolidone) (PVPON) deposited via LbL assembly. The approach utilizes advantages of LbL technique, but unlike current strategies allows for the material capable of modulating adaptive immune responses, crucial for cell enhanced viability and prolonged function during transplantation. In contrast to the currently available approaches based on LbL, our protocol (a) involves non-cationic non-toxic compounds; (b) the compounds can be modified “off-line” and later used for direct deposition on islet surface. We demonstrate that the coatings are conformal over various types of islets including those derived from rat, non-human primate (NHP), and human. All coated islets maintained their viability and β-cell functional capacity for at least 96 hours in vitro. The coatings also demonstrated newly acquired immunomodulatory effects by suppressing Th1 pro-inflammatory cytokine synthesis, the feature critical for islet transplantation.[67,68,69]
2. Results and Discussion
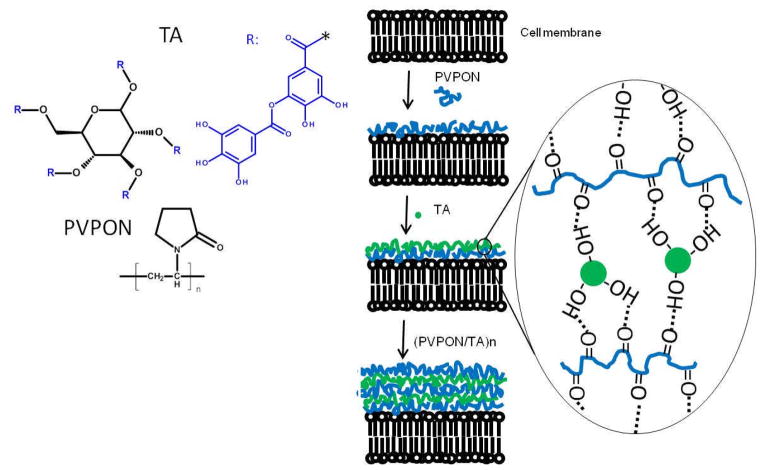
Conformal coating of living islets with the hydrogen-bonded (PVPON/TA)n multilayer film, where n denotes the number of deposited bilayers, has been established as schematically illustrated in Scheme 1. First, a layer of non-ionic PVPON was adsorbed onto the surfaces of islets followed by adsorption of TA. After each deposited layer, islets were collected by centrifugation and washed with media solution (see Experimental). Alternating polymer deposition onto islets was continued until the desired number of layers was formed. Our results with rat islets demonstrate the uniformity and integrity of a (PVPON/TA)4PVPON film, as observed with confocal microscopy (Figure 1a). Importantly, the multilayer growth was possible without priming the islet surfaces with any polycationic polymer which has been shown to be detrimental for islet viability.[70]
Scheme 1.
Left panel: Chemical structures of tannic acid (TA) and poly(N-vinyl-pyrrolidone). Right panel: Islet encapsulation in a hydrogen-bonded (PVPON/TA)n multilayer using the layer-by-layer assembly. The first PVPON layer was deposited on the islet surfaces through hydrogen-bonded interactions between collagen and PVPON followed by assembly of the TA layer. The layers of PVPON and TA were assembled stepwise until the (PVPON/TA)n multilayer was formed.
Fig. 1.
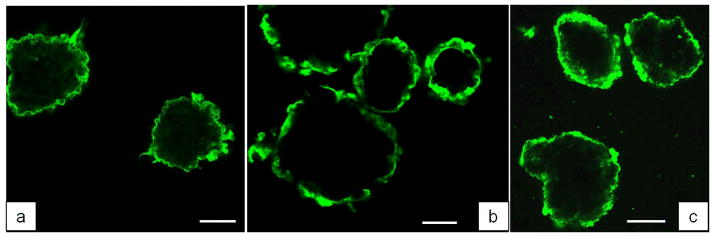
Confocal microscopy images of rat (a), NHP (b), and human (c) islets coated with (PVPON/TA)4PVPON multilayer conformal coatings. Fluorescently labeled PVPON* was assembled in the last bilayer of the coating. The scale bars are 100 μm (a), 50 μm (b), and 100 μm (c).
Direct adsorption of PVPON was achieved through non-covalent hydrogen-bonded interactions between the collagen and/or proteins on the islet surfaces and PVPON.[26] Specifically, the pyrrolidone rings in PVPON contain a proton accepting carbonyl group while collagen contains carbonyl moieties and N-H groups (amide bonds) and hydroxyl groups as side groups which are responsible for formation of hydrogen bonds.[71] These interactions have been confirmed earlier using FT-IR and DSC techniques and viscosity measurements.[72] The TA layer was formed on the PVPON-coated islet surfaces through hydrogen-bonding interactions of the hydroxyl groups on TA and carbonyl groups of PVPON.[52] For fluorescent visualization of the (PVPON/TA) coating using confocal microscopy, we synthesized PVPON copolymer containing 5% of amine-bearing units using a free radical copolymerization (Figure S1, Supporting Information). The PVPON copolymer has been labeled with Alexa Fluor® 488 carboxylic acid succinimidyl ester fluorescent dye to produce fluorescently tagged PVPON* which was adsorbed in the outermost bilayer. We have demonstrated that PVPON can be fluorescently labeled through an introduction of reactive functional groups, primary amines, along the PVPON polymer backbone followed by their reaction with the fluorescent dye. Consequently, PVPON can be easily modified with various required functionalities “off-line” (e.g. through PEGylation), and later used for direct deposition on cell surfaces. To provide evidence that the first PVPON layer was adsorbed to the islet surface, islets were coated with one PVPON* layer (Figure S2, Supporting Information). The fluorescent polymer was allowed to adsorb on the islet surfaces for 3 min followed by triple rinsing of the islets with PBS to remove unbound or loosely bound polymer chains from coated-islet suspension and the islets were imaged using confocal microscopy. As can be seen from Figure S2, evenly distributed fluorescence from the islet surfaces can be observed confirming the deposition of the PVPON* layer which can further promote (PVPON/TA)n multilayer construction.
To establish conditions for homogeneous cytocompatible coating of the islets, a series of islet coating studies were performed. We found that PVPON homopolymer with an average molecular weight of 1,300,000 g mol−1 and TA with concentrations in the range of 0.3–1.0 mg mL−1 for PVPON and 0.3–0.5 mg mL−1 could be used. We also found that TA, when deposited as the first layer on the islet surfaces instead of PVPON, also provided the successful hydrogen-bonded (TA/PVPON) multilayer coating. Indeed, polyphenols can bind to proteins present at the cell membranes through formation of multiple hydrogen bonds between phenolic hydroxyl groups of TA and the carbonyl functionalities of the protein peptide bonds.[73] A high binding ability of TA to the cell surface proteins is due to eight TA galloyl groups that promote strong hydrophobic and hydrogen-bonding interactions with cell surface proteins.[74] We found no effect of the first layer on cell viability, which indicated that those strong interactions between TA and cell membranes were not harmful to the islets (Figures S3, S4, Supporting information). Similar results on cytocompatibility of the (TA/PVPON) coating with TA as the first layer were previously found; and the viability of encapsulated yeast cells of 94% was reported [75,76]
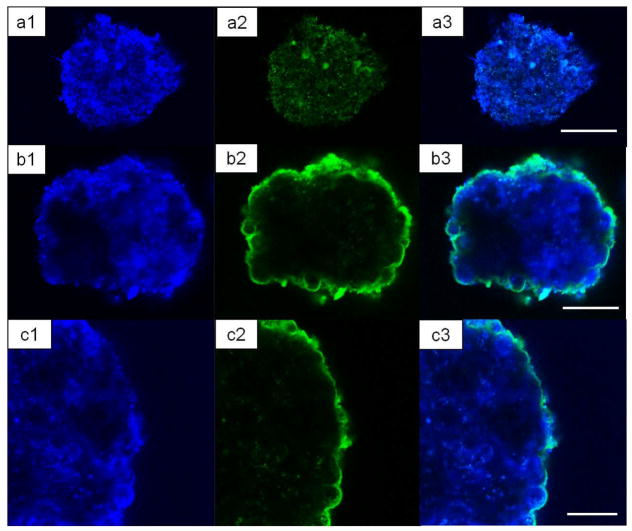
We then explored whether pancreatic islets from different species can be coated through hydrogen-bonded based LbL of (PVPON/TA). Islet preparation from human cadaveric donors, non-human primates (NHP), and rats were isolated by the Islet Resource Facility of the UAB Comprehensive Diabetes Center (IRF-UCDC) using standard protocols. As shown in Figures 1b, 1c, both NHP (b) and human (c) islets were effectively coated with (PVPON/TA)4PVPON film. The background fluorescence seen in the images is due to autofluorescence from Miami Medium #1 used as solvent for polymers (Figure S5). Figure 2 shows confocal images of uncoated (control) and (PVPON/TA)6PVPON-coated NHP islets cultured in Miami Medium #1A. The blue channel images (Figures 2a1, 2b1, 2c1, see Experimental) in both cases are due to the autofluorescence from the islets themselves presumably from the media aminoacid components, while in the green channel (see Experimental) the fluorescence from the coating containing labeled PVPON* is clearly seen (Figures 2b2, 2c2). A higher magnification CLSM image of the coated islet in Figure 2c2 demonstrates the conformal (PVPON/TA)6PVPON film on the islet surface.
Figure 2.
Confocal microscopy images of control non-coated (a1, a2, a3) and (PVPON/TA)6PVPON–coated (b1, b2, b3) non-human primate islets. (c1, c2, c3) CLSM images of the coated islets with higher magnification demonstrating the conformal coating. The left and middle images are taken using 405 nm (‘blue’ channel) and 488 nm (‘green’ channel) laser sources and emission was collected through bandpass filters for wavelengths between 410–506 nm and 494–543 nm, respectively. The right panels are their combined images. The scale bars are 50 μm (a3), 40 μm (b3), and 30 μm (c3).
The hydrogen-bonded (PVPON/TA)n film growth was investigated on flat substrates for polymer deposition from Miami Medium#1 by spectroscopic ellipsometry. The ellipsometry data revealed almost a linear growth profile with 4.2±0.4 nm bilayer thickness in dry state (Figure S6, Supporting information). The value is almost two times higher than that earlier reported for (PVPON/TA) films assembled at pH=7.5 from low salt buffer solutions.[52] However, increase in the bilayer thicknesses for the (PVPON/TA) film was observed for high salt conditions which is the case for this study when the multilayer formation was performed in Miami Medium #1 (ionic strength > 0.1 M). The thickness of the (PVPON/TA)4PVPON films in the hydrated state was explored using in situ spectroscopic ellipsometry. The data reveals almost 60% thickness increase due to hydration when the films were immersed in PBS solution at pH 7.4 for 1 hour (Figure S6, Supporting information).
The presence of the (PVPON/TA)4PVPON coating was further confirmed with transmission electron microscopy (TEM). Figure 3 compares the outer surfaces of unmodified and modified NHP islets (Figures 3a and 3b, respectively). The TEM images demonstrate the presence of the conformal coating on the surface of coated islets. The (PVPON/TA)4PVPON film thickness obtained from the TEM analysis resulted in 34±8 nm. This value corresponds to 7 nm per bilayer and correlates well with the ellipsometry data obtained on the planar surfaces (Figure S6).
Figure 3.
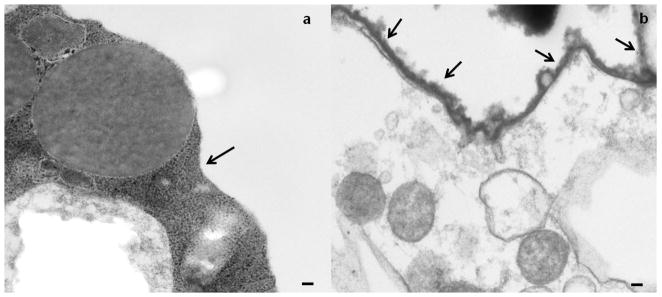
TEM images of non-coated (a) and (PVPON/TA)4PVPON-coated (b) NHP islets. The arrows point to the edges of the islet. The scale bars are 100 nm in both images.
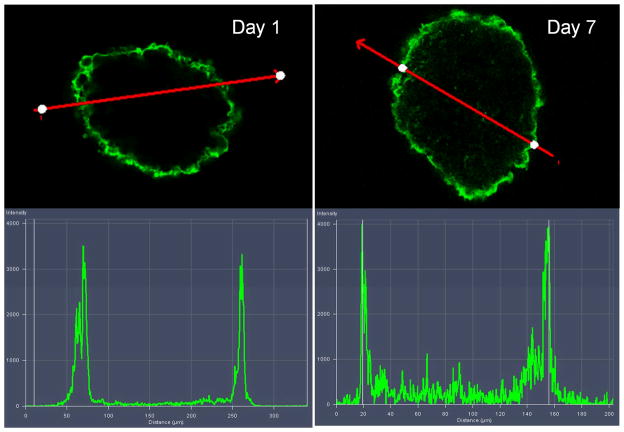
An important issue relates to the coating stability over the anticipated lifetime of the islet graft. To examine the stability of the film under in vitro conditions, islets were coated with (PVPON/TA)4PVPON film with a fluorescent layer of PVPON* deposited on top of the coated islets and cultured in vitro in Miami Medium #1A. The islets were harvested at various days and analyzed using confocal microscopy. Confocal microscopy images in Figure 4 revealed conformal coating, demonstrating that the (PVPON/TA)4PVPON film persisted on the islet surfaces for at least 7 days afterwards. No change in fluorescence intensity of the coated surfaces was found on Day 1 (day of coating) and Day 7 indicating stability of the coating for 7 days (intensity profiles in Figure 4). A slight increase in intensity observed inside the islet on Day 7 can be explained by the autofluorescence from the Miami Medium #1A (Figure S5) as well as by the autofluorescence related to cells metabolism.77 Three-dimensional reconstruction of confocal microscope images in Figure 5 demonstrate evenly distributed fluorescence over the surface of the (PVPON/TA)4PVPON- coated islets on the day of coating and after being in culture for 7 days.
Figure 4.
Top: Confocal microscopy images of (PVPON/TA)4PVPON-coated NHP islets on the day of coating (Day 1) and after being in culture for 7 days (Day 7) in Miami Medium #1A at 25°C. Bottom: Fluorescence intensity profiles from the (PVPON/TA)4PVPON-coated NHP islets cultured in Miami Medium #1A on Day 1 and Day 7.
Figure 5.
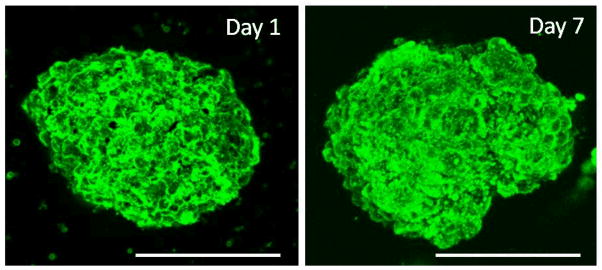
3D reconstruction of confocal microscopy images of (PVPON/TA)4PVPON-coated NHP islets on the day of coating (Day 1) and after being in culture for 7 days (Day 7) in Miami Medium #1A at 25°C. Scale bars are 150 μm.
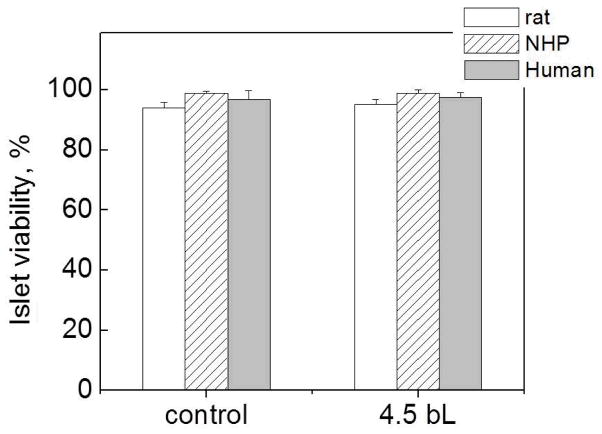
Importantly, although different types of mammalian islets contain similar types of cells, the islet architectures are different.[78] For instance, insulin-producing β-cells of rodent islets form an inner core of the islet with other secretory cells on the periphery, while in human islets β-cells are interspersed with other cells more on the islet periphery.[78] Due to these morphological differences, it was crucial to check if the presented surface modification affects the viability of different types of mammalian islets, both rodent, which are mainly used as research models, and human. We explored the viability of rat, NHP and human islets after coating using the FDA/PI viability assay. FDA is cleaved in viable cells and releases a green fluorescent molecule, while a red fluorescent molecule, PI, can enter only non-viable cells. Based on that, we analyzed the viability of islets before and after coating. In addition, in this work we adopted a simple centrifugation method (3 min deposition time at 2000 rpm) for the islet coating protocol. This method prevents loss of islets during polymer deposition unlike previously reported filtration-based methods.[24,45] Figure 6 confirms that the viability of islets coated with the (PVPON/TA)4PVPON protective film was not reduced by the centrifugation method. The viability results for coated islets were statistically indistinguishable from those for uncoated islets regardless of species type. The result also indicates cytocompatibility of the non-ionic PVPON and TA assembled on surfaces of the islets using hydrogen-bonded LbL assembly method. No change in the viability of coated islets was found after 4-day culture in Miami Medium #1 (Figures S4c and S4d).
Figure 6.
Viability of non-coated (control) and (PVPON/TA)4PVPON-coated rat (blank), NHP (dashed), and human (filled) islets assayed on the day of coating (within 6 hours after film deposition).
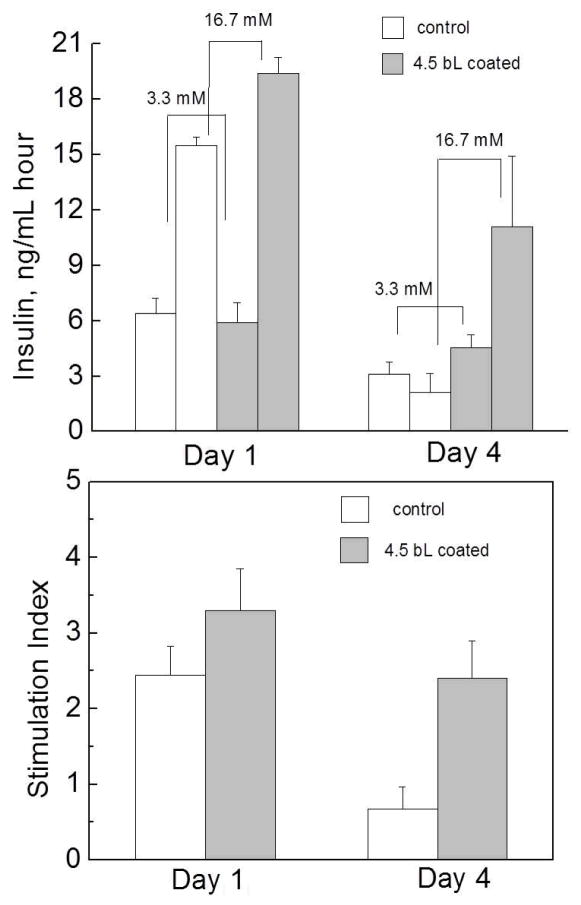
The preservation of appropriate islet functional capacity is critical for successful development of a protective strategy. There exists a possibility when the protective coating, while not adversely influencing cell viability, may hamper transport of hormones, e.g., insulin, across the film reducing islet function.[79] To evaluate functionality of the modified islets, we compared the insulin release in static incubation by non-coated and (PVPON/TA)4PVPON-coated islets as a function of time in response to variations in glucose concentration. The glucose concentration of 3.3 mM refers to a low glucose (basal glucose) level, while 16.7 mM glucose concentration is referred to as a high glucose level. As shown in Figure 7, after 24 hour culture in vitro (Day 1), both control (non-modified) and coated islets exhibited similar insulin secretion level in response to the basal glucose solution, while the insulin response to the high glucose was higher for (PVPON/TA)4PVPON-coated islets. A significant difference in islet functionality was observed after 96 hours in vitro. While islets coated with the hydrogen-bonded (PVPON/TA)4PVPON film preserved their functional capacity, a significant decrease in stimulation index for the untreated islets was observed (Figure 7). Such a protective effect of the film can be attributed along with the cytocompatibility of the coating to the ability of the film to retain islet morphological integrity in suspension culture (e.g., less fragmentation and fusion).
Figure 7.
Top: Glucose-stimulated insulin release from control (non-coated) and (PVPON/TA)4PVPON-coated rat islets in response to an increase in glucose concentration from 3.3 mM to 16.7 mM after 24 hours (Day 1) and after 72 hours (Day 4) in vitro in Miami Medium 1A at 25°C. Bottom: Stimulation index of the islets on Day1 and Day 4.
We explored the immunomodulatory effect of the PVPON/TA film based on the idea of a high biological activity of TA.[54,55,57] The immunomodulatory properties of the coating were studied by investigating the effect of (PVPON/TA)4 hollow shells on three types of pro-inflammatory cytokines synthesized in stimulated bone marrow-derived macrophages and diabetogenic BDC-2.5 T cells. The shells of (PVPON/TA)4 were prepared as hollow replicas of 4-μm silica particles coated with (PVPON/TA)4 multilayers under the conditions used for islet coating (see Experimental). CLSM and SEM images of the shells are presented as Figures S6, S7 in Supporting Information). First, the effect of the shells on synthesis of lymphocyte maturation factor IL-12p70 was tested (Figure 8A). For that, 108 of (PVPON/TA)4 shells (counted per 200 μL DMEM) were co-cultured in the presence of bone marrow-derived macrophages stimulated with 100 ng mL−1 of liposaccharide (LPS). The results were compared with IL-12p70 synthesis in a shell-free environment. Presence and absence of LPS and/or shells in culturing media are denoted as ‘+’ and ‘−‘, respectively (Figure 8A). As seen from Figure 8A, no IL-12p70 is produced in the absence of LPS in shell-free media. Adding LPS into shell-free media results in synthesis of 14±2 pg/mL of IL-12p70. In drastic difference, a 10-fold decrease in the IL-12p70 synthesis is observed in the presence of (PVPON/TA)4 shells. The concentration of synthesized IL-12p70 was found to be inversely proportional to the amount of shells in the culture media. A decrease in the amount of shells from 1×108 to 0.5×108 leads to the increase in IL-12p70 cytokine concentration from 0.6±0.2 to 4.0±0.6 pg/mL (Figure 8A). Note that 108 shells do not support any cytokine synthesis in LPS-free media indicating non-immunogenicity of (PVPON/TA).
Figure 8.
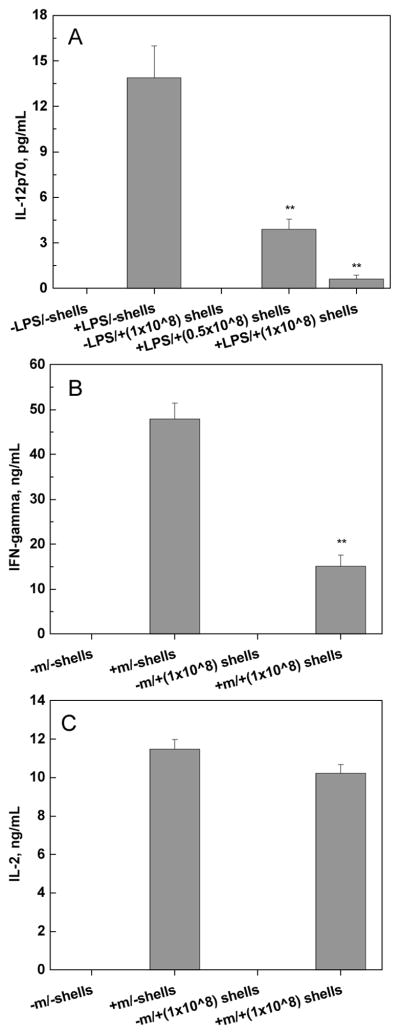
Effect of (PVPON/TA)4 shells on Th1 pro-inflammatory cytokine synthesis in stimulated bone marrow-derived macrophages and diabetogenic BDC-2.5 T cells. NOD bone marrow-derived macrophages stimulated with 100 ng mL−1 LPS in the presence of (PVPON/TA)4 shells for 24 hours exhibited a decrease in IL-12p70 synthesis (A). BDC-2.5 T cells (5×105) stimulated with 1 μM BDC-2.5 mimotope in the presence or absence of 108-103 (PVPON/TA)4 shells for 96 hours exhibited a decrease in IFN-γ (B), but no change in IL-2 synthesis (C). Results are representative of 3 independent experiments performed in triplicate. ** indicates p < 0.05.versus the control group.
The (PVPON/TA) shells were then examined for synthesis of interferon-gamma (IFN-γ and interleukin-2 (IL-2) cytokines, indicative of T cell adaptive immune activation and proliferative capacity, respectively. The syntheses of cytokines were studied by using diabetogenic BDC-2.5 T cells (Figures 8B and 8C). The BDC-2.5 T cells, also known as autoreactive CD4+ T cells, can rapidly transfer Type 1 diabetes into susceptible mice by synthesizing pro-inflammatory T helper type 1 (Th1) cytokines involved in β-cell destruction and macrophage recruitment.[67,68,69] To examine synthesis of IFN- γ and IL-2 cytokines, BDC-2.5 splenocytes were stimulated with their cognate BDC-2.5 mimotope antigenic peptides in the presence or absence of (PVPON/TA)4 shells (Figures 8B and 8C). No IFN-γ and IL-2 cytokines are produced in the mimitope-free media in the absence and presence of shells which correlates well with the results observed in the case of IL-12p70 cytokines in LPS-free media (Figure 8A). We found that synthesis of mimotope-stimulated IFN-γ was suppressed in the presence of shells, similar to the LPS-stimulated IL-12p70 synthesis. In this case, three-fold decrease in IFN-γ concentration is observed in the presence of 108 shells as compared to shell-free media (Figure 8B). In contrast, no significant change in IL-2 synthesis is found under the same conditions (Figure 8C).
Our results on immunomodulatory properties of the hydrogen-bonded coatings indicate that the shells themselves are not immunogenic, as they do not induce synthesis of the cytokines in the absence of stimulators. Importantly, the shells drastically suppress synthesis of IL-12p70 (ten-fold) and IFN-γ (four-fold) in stimulated macrophages and diabetogenic BDC-2.5 T cells, that recognize chromogranin A, an insulin secretory granule,[80,81] respectively. IL-12p70 cytokines were found to produce IFN-γ by promoting formation of T helper type 1 (Th1) effector cells from naive CD4+ T cells.[82–86] Since IFN-γ can mediate islet graft rejection,[87,88] transplanted islets with (PVPON/TA) coating should significantly decrease risk of the islet rejection because of the suppressed syntheses of IL-12p70 and IFN-γ discussed above. At the same time, the coating does not affect synthesis of IL-2, thus, is not decreasing the T cell proliferation unlike other approaches for the increase of immunoprotective properties of the coating when immunosuppressive molecules promote T-cell apoptosis.[89] Indeed, suppression of the T cell proliferation would be undesirable since a non-specific suppression of the immune system can result in serious chronic side effects including increased risk of infection.[8] Further experiments elucidating the mechanism of the observed suppression of the cytokine synthesis are currently underway.
3. Conclusions
This study demonstrates that non-ionic hydrogen-bonded LbL technology can be applied for coating individual, living pancreatic islets. The protocol can be used as a more effective approach as opposed to ionic LbL-based method. We found that the hydrogen-bonded method developed here allows for conformal coating of the islet surface under physiological conditions in a rapid and efficient manner without interfering with the viability and function of the insulin-producing β-cells. Based on our results we conclude that the coating is (a) conformal with uniform coverage over the whole islet surface; (b) stable for at least 7 days in vitro; (c) non-toxic and non-immunogenic; (d) has no effect on islet viability and function; (e) can be easily modified with functional molecules, for example, a fluorescent Alexa 488 carboxylic acid succinimidyl ester; (f) can be applied to islets derived from rat, NHP, and human; and (g) can suppress synthesis of the pro-inflammatory cytokines involved in modulation of both innate and adaptive immune responses in vivo. A decrease in Th1 cytokine responses was observed when antigen-stimulated BDC-2.5 T cells and LPS-stimulated bone marrow-derived macrophages were co-treated with (PVPON/TA)4 hollow multilayer shells, suggesting the efficacy of this encapsulation strategy to provide physical islet protection and prevent efficient autoreactive T cell responses. The proposed materials are adaptable for further functionalization and can be coupled with various active reagents (e.g. catalytic antioxidants, insulinotropic and immunoisolation moieties) thus opening new avenues in the area of advanced islet transplant materials.
4. Experimental
Materials
Poly(N-vinyl-pyrrolidone) (PVPON), (average Mw 1 300 000 g mol−1), tannic acid (TA) (Mw 1700 g mol−1), mono- and dibasic sodium phosphate, N-vinyl-pyrrolidone (VPON), N-(tert-butoxycarbonyl-aminopropyl)methacrylamide (tBOC), was from Polysciences, Inc. Alexa Fluor® 488 carboxylic acid succinimidyl ester (Ex/Em=488/510 nm) was purchased from Invitrogen. Ultrapure (Siemens) filtered water with a resistivity of 18.2 MΩ cm was used for preparation of buffered solutions. Initiator, 2, 2′-Azobis(2-methylpropionitrile) (AIBN), was purchased from Sigma-Aldrich and re-crystallized from methanol at 30° C before use. Miami Medium 1 and 1A were from Mediatech, Inc. BDC-2.5 mimotope peptide (EKAHRPIWARMDAKK) was synthesized by Proimmune. Silica microparticles of 4 μm in diameter were purchased from Polysciences Inc.
Islet isolation
Islets were isolated from Lewis rats (males, weighing 250–300g, Harland Laboratories, Indianapolis, IN, USA), cadaveric human donors, and Rhesus Macaques (NHP) by collagenase digestion.[90] After digestion, islets were isolated by discontinuous density gradient (Ficoll) purification and individually hand-picked under a dissection microscope. All islet samples were cultured at 37°C in an atmosphere of 95% air and 5% CO2 in Miami Medium #1A (Mediatech, Indianapolis, IN, USA) supplemented with 10% FCS and ciprofloxacin (1.0 μg mL−1).
Conformal coating of islets with hydrogen-bonded (PVPON/TA)n multilayer film
Islet coating was performed at 25°C. Before deposition of (PVPON/TA)n multilayer coating, where n denotes the number of deposited bilayers, islets were pelleted in 1.5 mL Eppendorf centrifuge tubes and washed two times with rinsing solutions of Miami Medium #1. PVPON was allowed to adsorb first onto islet surfaces from 1 mg mL−1 solution (Miami Medium #1, pH=7.4) for 3 min followed by the deposition of TA layer from 0.3 mg mL−1 solution (Miami Medium #1, pH=7.4) for 3 min. After each deposited layer, islets were collected by centrifugation for 2 min at 2000 rpm and washed two times with the rinsing solution (Miami Medium #1). Alternating coating of islets with the polymers was continued until the desired number of layers was achieved. To visualize the (PVPON/TA)n coating, confocal laser scanning microscopy (CLSM) was employed. Fluorescently labeled PVPON* (*Alexa Fluor® 488 carboxylic acid succinimidyl ester, see Supporting Information for details of labeling) was used during the deposition of the outermost bilayer. All solutions were filter-sterilized with polystyrene non-pyrogenic membrane systems (0.22 μm pore size) (Corning) before use.
Confocal Laser Scanning Microscopy (CLSM)
Presence of the (PVPON/TA)n conformal multilayer coating on islets was confirmed with confocal microscopy. A well of a chambered coverglass (Lab-Tek, Electron Microscopy Sciences) was filled with suspension of coated islets. Confocal images of non-coated (control) and coated islets were obtained with a LSM Zeiss 710 inverted confocal microscope equipped with 10x and 20x objective lenses (Zeiss, Germany). Before imaging, islets were rinsed several times with PBS to reduce auto-fluorescence from the media. For visualization of (PVPON/TA)nPVPON* films surrounding the islets, the fluorescence excitation was carried out through a 405 nm (‘blue’ channel) and 488 nm (‘green’ channel) laser sources and emission was collected through bandpass filters for wavelengths between 410–506 nm and 494–543 nm, respectively.
Transmission Electron Microscopy (TEM)
TEM imaging of control and (PVPON/TA)-coated islets was performed using a FEI-Tecnai T12 Spirit TWIN 20–120 kV electron miscroscope with AMT digital camera. Sample fixation and staining were done on the 3d day after islet coating with 2% formaldehyde and 1% osmium tetroxide in PBS buffer (0.1 M). Islets were dehydrated with ethanol (50–100%) and suspended in LR White™ resin system (100% ethanol, 1:1 v/v) (Electron Microscopy Sciences).
Islet Viability Assay
Viability of the non-coated and coated islets was assessed immediately following coating (within 6 hours after film deposition) and after 4 days in vitro (Miami Medium #1A, 25°C). To evaluate the viability of individual islets coated with the hydrogen-bonded (PVPON/TA) films, control or coated islets were stained with propidium iodide (PI) and fluoresceine diacetate (FDA) according to established protocols. [91] A FDA stock solution was prepared by dissolving FDA (10 mg) into acetone (2 mL). The FDA stock solution was stored at −20°C. For viability test, 10 μL of FDA stock solution were diluted with 990 μL of phosphate buffered saline (PBS). PI (1 mg mL−1, Invitrogen) was prepared each time to be used immediately, as 50 μL of solution were diluted with 450 μL of PBS. For viability staining each sample was immersed in a mixture of 2 mL PBS, 10 μL of diluted PI, and 20 μL of diluted FDA. Stained islets were imaged with a Nikon TE 2000-S fluorescence microscope equipped with a Nikon high pressure mercury arc lamp. Fluorescein that de-acetylated from FDA through non-specific esterases in the cytoplasm of living cells was observed under a green fluorescent filter, while PI stained nucleic acids of dead cells under red fluorescent filter. Optical micrographs were converted to binary images comprised of red and green pixels and analyzed using Image J software to quantify the number of pixels corresponding to live (green) and dead (red) cells. Viability is calculated as the percentage of total pixels that are green and an average viability is determined by performing this analysis on 20–25 images of individual islets.
Glucose stimulated insulin secretion (GSIS)
Functional capacity of the coated islets in comparison to uncoated islets was assessed through GSIS in 1 and 4 days after coating. All samples were pre-incubated in low glucose Krebs-Ringer bicarbonate buffer (low glucose KRB) (25 mM HEPES, 115 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, 0.1% bovine serum albumin, 3 mM D-glucose, pH 7.4) to eliminate residual insulin. Subsequently, samples were transferred to 1 mL low glucose KRB for 1 hour, followed by incubation in 1 mL high glucose KRB (20 mM D-glucose) for 1 hour. At the end of each incubation, conditioned media was collected and insulin was measured by the ELISA (Mercodia). The stimulation index (insulin release at high/low glucose concentration) was determined.
Preparation of hollow (PVPON/TA)4 shells
Hollow hydrogen-bonded shells (capsules) were prepared by coating 4-μm silica particles with (PVPON/TA)4 film followed by particle dissolution using the method established previously.53 Specifically, 1.5 mL of 10% poly(ethylene imine)-coated silica particle suspension was pelleted in a 1.5 mL Eppendorf centrifuge tube and washed two times with rinsing solutions of Miami Medium #1. TA was allowed to adsorb onto particle surfaces from 1 mg mL−1 solution (Miami Medium #1, pH=7.4) for 3 min followed by the deposition of PVPON layer from 0.3 mg mL−1 solution (Miami Medium #1, pH=7.4) for 3 min. After each deposited layer, particles were centrifuged for 2 min at 2000 rpm and washed two times with the rinsing Miami Medium #1 solution. Alternating coating of particles with the polymers was continued until the desired number of layers was achieved. Silica cores were dissolved in 8% hydrofluoric acid and the hollow capsule solution was dialyzed in de-ionized water at pH=7.4 (adjusted with 0.1 M NaOH) in the dark for 4 days.
Animals
NOD.BDC-2.5 TCR transgenic mice were bred and housed under specific pathogen-free conditions at the Research Support Building at the University of Alabama at Birmingham. Female mice at 8 to 12 weeks of age were used in all experiments.
Primary Recall Assays and Cytokine Measurements by ELISA
NOD.BDC-2.5 splenocyte single cell suspensions (5×105 cells) were seeded in a 96-well flat bottom plate with 0.1 μM or 1 μM BDC-2.5 mimotope in the presence or absence of 108–103 (PVPON/TA)4 shells in 200 μL total volume of Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 10 mM HEPES buffer, 4 mM L-glutamine, 2X non-essential amino acids, 1 mM sodium pyruvate, 61.5 μM 2-mercaptoethanol, and 100 μg mL−1 Gentamicin (Invitrogen, Carlsbad, CA) (complete DMEM). After incubation at 37°C in a 5% CO2 humid air chamber for 2, 3, or 4 days, supernatants were collected to examine cytokine synthesis. IFN-γ and IL-2 production was measured using antibody pairs from BD Pharmingen (San Diego, CA) as described previously.[92] IL-12p70 was detected with a DuoSet ELISA kit from R&D Systems (R&D Systems, Minneapolis, MN). ELISA plates were read on a BioTek Synergy2 microplate reader (BioTek, Winooski, VT) and analyzed using Gen5 v.1.10 software (BioTek, Winooski, VT).
Isolation and stimulation of mouse bone marrow-derived macrophages
NOD bone marrow-derived macrophages were cultured as described previously[93] and plated on 24-well tissue culture plates at 106 cells well−1. Macrophages were stimulated with 100 ng mL−1 of LPS from E. coli (055:B5) (Sigma Aldrich) in the presence or absence of (PVPON/TA)4 shells. Supernatants were collected at 24 hours to measure the synthesis of IL-12p70 by ELISA as described.[92]
Statistical Analysis
Data were analyzed using GraphPad Prism Version 5.0 statistical software. Determination of the difference between mean values for each experimental group was assessed using the 2-tailed Student’s t test, with p < 0.05 considered significant. All experiments were performed at least three separate times with data obtained in triplicate wells in each experiment.
Supplementary Material
Acknowledgments
The project was supported in part by the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health (Award#P30EB011319) (E.K.), the American Diabetes Association Junior Faculty Award (1-09-JF-54) (H.T.), and the Islet Resource Facility of UAB Comprehensive Diabetes Center (UAB IRF-CDC) (NIH Award#P60DK79636) (A.T.). The art-work included in TOC was created in part by Inessa Stanishevskaya.
Footnotes
Supporting Information. Synthesis of PVPON*, FDA/PI viability tests on rat, and human islets, ellipsometry data for coating growth and thickness, confocal and SEM images of the hollow shells, and CLSM on islets coated with a single layer of PVPON*. This material is available free of charge online from Wiley InterScience.
Contributor Information
Dr. Veronika Kozlovskaya, Department of Chemistry, University of Alabama at Birmingham, Birmingham, AL 35294 (USA)
Oleksandra Zavgorodnya, Department of Chemistry, University of Alabama at Birmingham, Birmingham, AL 35294 (USA).
Yi Chen, Department of Chemistry, University of Alabama at Birmingham, Birmingham, AL 35294 (USA).
Kristin Ellis, Department of Microbiology, University of Alabama at Birmingham, Birmingham, AL 35294 (USA).
Prof. Hubert M. Tse, Department of Microbiology, University of Alabama at Birmingham, Birmingham, AL 35294 (USA)
Dr. Wanxing Cui, Department of Surgery, Division of Transplantation, the University of Alabama at Birmingham, Birmingham, AL 35294 (USA)
Prof. J. Anthony Thompson, Department of Surgery, Division of Transplantation, the University of Alabama at Birmingham, Birmingham, AL 35294 (USA)
Prof. Eugenia Kharlampieva, Email: ekharlam@uab.edu, Department of Chemistry, University of Alabama at Birmingham, Birmingham, AL 35294 (USA)
References
- 1.Tierney LM, McPhee SJ, Papadakis MA. Current medical Diagnosis & Treatment. International Ed. New York: Large Medical Books/McGraw-Hill; 2002. pp. 1203–1205. [Google Scholar]
- 2.Meloche RM. World J Gastroenterol. 2007;13:6347. doi: 10.3748/wjg.v13.i47.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson RP. N Engl J Med. 2000;343:289. doi: 10.1056/NEJM200007273430409. [DOI] [PubMed] [Google Scholar]
- 4.Stendahl JC, Kaufman DB, Stupp SI. Cell Transplant. 2009;18:1. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paraskevas S, Maysinger D, Wang R, Duduid WP, Rosenberg L. Pancreas. 2000;20:270. doi: 10.1097/00006676-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Ricordi C, Strom TB. Nat Rev Immunol. 2004;4:259. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 7.Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE. Transplantation. 2002;74:1252. doi: 10.1097/00007890-200211150-00010. [DOI] [PubMed] [Google Scholar]
- 8.Narang AS, Mahato RI. Pharmacol Rev. 2006;58:194. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 9.Chandy T, Mooradian DL, Rao GHR. Artif Organs. 1999;23:894. doi: 10.1046/j.1525-1594.1999.06244.x. [DOI] [PubMed] [Google Scholar]
- 10.Abalovich A, Jatimliansky C, Diegex E, Arias M, Altamirano A, Amorena C, Martinez B, Nacucchio M. Transplant Proc. 2001;33:1977. doi: 10.1016/s0041-1345(01)01918-2. [DOI] [PubMed] [Google Scholar]
- 11.De Vos P, Van Hoogmoed CG, Van Zanten J, Netter S, Strubbe JH, Busscher HJ. Biomaterials. 2003;24:305. doi: 10.1016/s0142-9612(02)00319-8. [DOI] [PubMed] [Google Scholar]
- 12.Panza JL, Wagner WR, Rilo HL, Rao RH, Beckman EJ, Russell AJ. Biomaterials. 2000;21:1155. doi: 10.1016/s0142-9612(99)00283-5. [DOI] [PubMed] [Google Scholar]
- 13.Opara EC, Mirmalek-Sani SH, Khanna O, Moya ML, Brey EM. J Investigative Medicine. 2010;58:831. doi: 10.231/JIM.0b013e3181ed3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. Islet Tissue Eng. 2007;13:589. doi: 10.1089/ten.2006.0183. [DOI] [PubMed] [Google Scholar]
- 15.Weber LM, Cheung CY, Anseth KS. Cell Transplant. 2007;16:1049. doi: 10.3727/000000007783472336. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann U, Thurmer F, Jork A, Weber M, Mimietz S, Hillgärtner M, Brunnenmeier F, Zimmermann H, Westphal I, Fuhr G, Nöth U, Haase A, Steinert A, Hendrich C. Ann N Y Acad Sci. 2001;944:199. doi: 10.1111/j.1749-6632.2001.tb03833.x. [DOI] [PubMed] [Google Scholar]
- 17.Wyman JL, Kizilel S, Skarbek R, Zhao X, Connors M, Dillmore WS, Murphy WL, Mrksich M, Nagel SR, Garfinkel MR. Small. 2007;3:683. doi: 10.1002/smll.200600231. [DOI] [PubMed] [Google Scholar]
- 18.Sefton MF, May MH, Lahooti S, Babensee JE. J Control Release. 2000;65:173. doi: 10.1016/s0168-3659(99)00234-5. [DOI] [PubMed] [Google Scholar]
- 19.Calafiore R, Basta G, Luca G, Boselli C, Bufalari A, Cassarani MP, Giustozzi GM, Brunetti P. Ann NY Acad Sci. 1999;875:219. doi: 10.1111/j.1749-6632.1999.tb08506.x. [DOI] [PubMed] [Google Scholar]
- 20.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. Biotechnol Bioeng. 1998;57:655. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.O’Sullivan ES, Johnson AS, Omer A, Hollister-Lock J, Bonner-Weir S, Colton CK, Weir GS. Diabetologia. 2010;53:937. doi: 10.1007/s00125-009-1653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui W, Barr G, Faucher KM, Sun XL, Safley SA, Weber CJ, Chaikof EL. Transplant Proc. 2004;36:1206. doi: 10.1016/j.transproceed.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 23.Wilson JT, Chaikof EL. Adv Drug Delivery Rev. 2008;60:124. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim D-J, Antipenko SV, Anderson JM, Jaimes KF, Viera L, Stephen BR, Bryant SMJ, Yancey BD, Cui W, Thompson JA, Corbett JA, Jun H-W. Tissue Eng A. 2011;17:399. doi: 10.1089/ten.tea.2010.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie D, Smyth C, Eckstein C, Bilbao G, Mays J, Eckhoff D, Contreras J. Biomaterials. 2005;26:403. doi: 10.1016/j.biomaterials.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 26.Lee DY, Nam JH, Byun Y. Biomaterials. 2007;28:1957. doi: 10.1016/j.biomaterials.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Contreras JL, Xie D, Mays J, Smyth CA, Eckstein C, Rahemtulla FG, Young CJ, Thompson AJ, Bilbao G, Curiel DT, Eckhoff DE. Surgery. 2004;136:537. doi: 10.1016/j.surg.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Stabler CL, Sun X-L, Cui W, Wilson TW, Haller CA, Chaikof EL. Bioconjug Chem. 2007;18:1713. doi: 10.1021/bc7002814. [DOI] [PubMed] [Google Scholar]
- 29.Teramura Y, Kaneda Y, Iwata H. Biomaterials. 2007;28:1957. doi: 10.1016/j.biomaterials.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 30.Teramura Y, Iwata H. Transplantation. 2009;88:624. doi: 10.1097/TP.0b013e3181b230ac. [DOI] [PubMed] [Google Scholar]
- 31.Totani T, Teramura Y, Iwata H. Biomaterials. 2008;29:2878. doi: 10.1016/j.biomaterials.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Rabuka D, Forstner MB, Groves JT, Bertozzi CR. J Am Chem Soc. 2008;130:5947. doi: 10.1021/ja710644g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulick MG, Forstner MB, Groves JT, Bertozzi CR. Proc Natl Acad Sci USA. 2007;104:20332. doi: 10.1073/pnas.0710139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemoto N, Teramura Y, Iwata H. Bioconjug Chem. 2011;22:673. doi: 10.1021/bc100453r. [DOI] [PubMed] [Google Scholar]
- 35.Paraskevas S, Duduid WP, Maysinger D, Feldman L, Agapitos D, Rosenberg L. Transplant Proc. 1997;29:750. doi: 10.1016/s0041-1345(96)00452-6. [DOI] [PubMed] [Google Scholar]
- 36.Krol S, Guerra S, Grupillo M, Diasporo A, Gliozzi A, Marchetti P. Nano Lett. 2006;6:1933. doi: 10.1021/nl061049r. [DOI] [PubMed] [Google Scholar]
- 37.Wilson JT, Cui W, Chaikof EL. Nano Lett. 2008;8:1940. doi: 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kharlampieva E, Sukhishvili SA. J Macromol Sci, Part C – Polymer Reviews. 2006;46:377. [Google Scholar]
- 39.Tang Z, Wang Y, Podsiadlo P, Kotov NA. Adv Mater. 2006;18:3203. [Google Scholar]
- 40.Chluba J, Voegel JC, Decher G, Erbacher P, Schaaf P, Ogier J. Biomacromolecules. 2001;2:800. doi: 10.1021/bm015529i. [DOI] [PubMed] [Google Scholar]
- 41.Kim TG, Park TG. Tissue Eng. 2006;12:221. doi: 10.1089/ten.2006.12.221. [DOI] [PubMed] [Google Scholar]
- 42.Teramura Y, Kaneda Y, Totani T, Iwata H. Biomaterials. 2008;29:1345. doi: 10.1016/j.biomaterials.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 43.Choksakulnimitr S, Masuda S, Tokuda H, Takakura Y, Hashida M. J Control Release. 1995;34:233. [Google Scholar]
- 44.Wilson JT, Krishnamurthy VR, Cui W, Qu Z, Chaikof EL. J Am Chem Soc. 2009;131:18228. doi: 10.1021/ja908887v. [DOI] [PubMed] [Google Scholar]
- 45.Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J, Song Y, Tsukruk VV, Chaikof EL. J Am Chem Soc. 2011;133:7054. doi: 10.1021/ja110926s. [DOI] [PubMed] [Google Scholar]
- 46.Yang SY, Rubner MF. J Am Chem Soc. 2002;124:2100. doi: 10.1021/ja017681y. [DOI] [PubMed] [Google Scholar]
- 47.Yang SY, Mendelsohn JD, Rubner MF. Biomacromolecules. 2003;4:987. doi: 10.1021/bm034035d. [DOI] [PubMed] [Google Scholar]
- 48.Kharlampieva E, Kozlovskaya V, Sukhishvili SA. Adv Mater. 2009;21:1. [Google Scholar]
- 49.Lutkenhaus JL, Hammond PT. Soft Matter. 2007;3:804. doi: 10.1039/b701203a. [DOI] [PubMed] [Google Scholar]
- 50.DeLongchamp D, Hammond P. Langmuir. 2004;20:5403. doi: 10.1021/la049777m. [DOI] [PubMed] [Google Scholar]
- 51.Quinn JF, Caruso F. Langmuir. 2004;20:20. doi: 10.1021/la0360310. [DOI] [PubMed] [Google Scholar]
- 52.Erel-Unal I, Sukhishvili SA. Macromolecules. 2008;41:3962. [Google Scholar]
- 53.Kozlovskaya V, Kharlampieva E, Drachuk I, Cheng D, Tsukruk VV. Soft Matter. 2010;6:3596. [Google Scholar]
- 54.Riedl KM, Hagerman AE. J Agric and Food Chem. 2001;49:4917. doi: 10.1021/jf010683h. [DOI] [PubMed] [Google Scholar]
- 55.Lopes GKB, Schulman HM, Hermes-Lima M. Biochem et Biophys Acta. 1999;1472:142. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 56.Shutava TG, Balkundi SS, Vangala P, Steffan JJ, Bigelow RL, Cardelli JA, O’Neal DP, Lvov YM. ACS Nano. 2009;3:1877. doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- 57.Shutava TG, Agabekov VE, Lvov YM. Russ J Gen Chem. 2007;77:1494. [Google Scholar]
- 58.Shutava TG, Balkundi SS, Lvov YM. J Coll Interf Sci. 2009;330:276. doi: 10.1016/j.jcis.2008.10.082. [DOI] [PubMed] [Google Scholar]
- 59.Toyokuni S. Pathol Int. 1999;49:91. doi: 10.1046/j.1440-1827.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- 60.McCord JM. Am J Med. 2000;108:652. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 61.Lander HM, Milbank AJ, Tauras JM, Hajjar DP, Hempstead BL, Schwartz GD, Kraemer RT, Mirza UA, Chait BT, Burk SC, Quilliam LA. Nature. 1996;381:380. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 62.Rincon M, Flavell RA, Davis RA. Free Radic Biol Med. 2000;28:1328. doi: 10.1016/s0891-5849(00)00219-7. [DOI] [PubMed] [Google Scholar]
- 63.Batinic-Haberle I, Spasojevic I, Tse HM, Tovmasyan A, Rajic Z, StClair DK, Vujaskovic Z, Dewhirst MW, Piganelli JD. Amino Acids. doi: 10.1007/s00726-010-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hume PS, Anseth KS. J Biomed Mater Res A. 2011;99:29. doi: 10.1002/jbm.a.33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheung CY, McCartney SJ, Anseth KS. Adv Func Mater. 2008;18:3119. [Google Scholar]
- 66.Lin CC, Metters AT, Anseth KS. Biomaterials. 2009;30:4907. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cantor J, Haskins K. J Immunol. 2007;179:5760. doi: 10.4049/jimmunol.179.9.5760. [DOI] [PubMed] [Google Scholar]
- 68.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Proc Natl Acad Sci USA. 1989;86:8000. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. Diabetes. 1988;37:1444. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 70.Hong S, Leorueil PR, Janus EK, Peters JL, Kober MM, Islam MT, Orr BG, Baker JR, Banaszak Holl MM. Bioconjug Chem. 2006;17:728. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 71.Perumal S, Antipova O, Orgel JPRO. Proc Nat Acad Sci USA. 2008;105:2824. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sionkowska A. Eur Polym J. 2003;39:2135. [Google Scholar]
- 73.Van Buren JP, Robinson WB. J Agr Food Chem. 1969;17:772. [Google Scholar]
- 74.Soares S, Mateus N, de Freitas V. J Agric Food Chem. 2007;55:6726. doi: 10.1021/jf070905x. [DOI] [PubMed] [Google Scholar]
- 75.Kozlovskaya V, Harbaugh S, Drachuk I, Shchepelina O, Kelley-Loughnane N, Stone M, Tsukruk VV. Soft Matter. 2011;7:2364. [Google Scholar]
- 76.Carter JL, Drachuk I, Harbaugh S, Kelley-Loughnane N, Stone M, Tsukruk VV. Macromol Biosci. 2011;11:1244. doi: 10.1002/mabi.201100129. [DOI] [PubMed] [Google Scholar]
- 77.Richards-Kortum R, Sevick-Muraca E. Annu Rev Phys Chem. 1996;47:555. doi: 10.1146/annurev.physchem.47.1.555. [DOI] [PubMed] [Google Scholar]
- 78.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. Proc Nat Acad Sci USA. 2006;103:2334. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avgoustiniatos ES, Colton CK. Ann NY Acad Sci. 1997;831:146. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 80.Haskins K, Wegmann D. Diabetes. 1996;45:1299. doi: 10.2337/diab.45.10.1299. [DOI] [PubMed] [Google Scholar]
- 81.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K. Nat Immunol. 2010;11:225. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Jr, Murphy KM. J Exp Med. 1995;181:1755. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Annu Rev Immunol. 2000;18:451. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 84.Robinson DS, O’Garra A. Immunity. 2002;16:755. doi: 10.1016/s1074-7613(02)00331-x. [DOI] [PubMed] [Google Scholar]
- 85.Trembleau S, Penna G, Bosi E, Mortara A, Gately MK, Adorini L. J Exp Med. 1995;181:817. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trinchieri G. Annu Rev Immunol. 1995;13:251. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 87.Rabinovitch A, Suarez-Pinzon WL, Sorensen O. J Autoimmun. 1996;9:645. doi: 10.1006/jaut.1996.0084. [DOI] [PubMed] [Google Scholar]
- 88.Suarez-Pinzon W, Rajotte RV, Mosmann TR, Rabinovitch A. Diabetes. 1996;45:1350. doi: 10.2337/diab.45.10.1350. [DOI] [PubMed] [Google Scholar]
- 89.Cheung CY, Anseth KS. Bioconjug Chem. 2006;17:1036. doi: 10.1021/bc060023o. [DOI] [PubMed] [Google Scholar]
- 90.Badet L, Titus TT, McShane P, Chang LW, Song ZS, Ferguson DJP, Gray DWR. Transplantation. 2001;72:1867. doi: 10.1097/00007890-200112270-00002. [DOI] [PubMed] [Google Scholar]
- 91.Kizilel S, Scavone A, Liu X, Nothias JM, Ostrega D, Witkowski P, Millis M. Tissue Eng A. 2010;16:2217. doi: 10.1089/ten.TEA.2009.0640. [DOI] [PubMed] [Google Scholar]
- 92.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. J Immunol. 2007;178:908. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- 93.Tse HM, Josephy SI, Chan ED, Fouts D, Cooper AM. J Immunol. 2002;168:825. doi: 10.4049/jimmunol.168.2.825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.