Abstract
N-heterocyclic carbenes are well known for their role in catalyzing benzoin and Stetter reactions: the generation of acyl anion equivalents from simple aldehydes to react with a variety of electrophiles. However, when an aldehyde bearing a leaving group or unsaturation adjacent to the acyl anion equivalent is subjected to an NHC, a new avenue of reactivity is unlocked, leading to a number of novel transformations which can generate highly complex products from simple starting materials, many of which are assembled through unconventional bond disconnections. The field of these new reactions - those utilizing α-reducible aldehydes to access previously unexplored catalytic intermediates – has expanded rapidly in the past eight years. This review aims to provide the reader with a historical perspective on the underlying discoveries that led to the current state of the art, a mechanistic description of these reactions, and a summary of the recent advances in this area.
Keywords: N-heterocyclic carbene, Acylation, Homoenolate, Asymmetric, Organocatalysis
1 Introduction
The catalytic generation of acyl anion equivalents from aldehydes, so-called polarity reversal or “umpolung”,[1] and the subsequent functionalization of these intermediates has become a topic of intense research in recent years. Within this field, N-heterocyclic carbenes (NHCs) have found broad use as catalysts. This rapidly emerging field of organocatalysis has been the subject of many reviews that detail the numerous efforts in this area.[2]
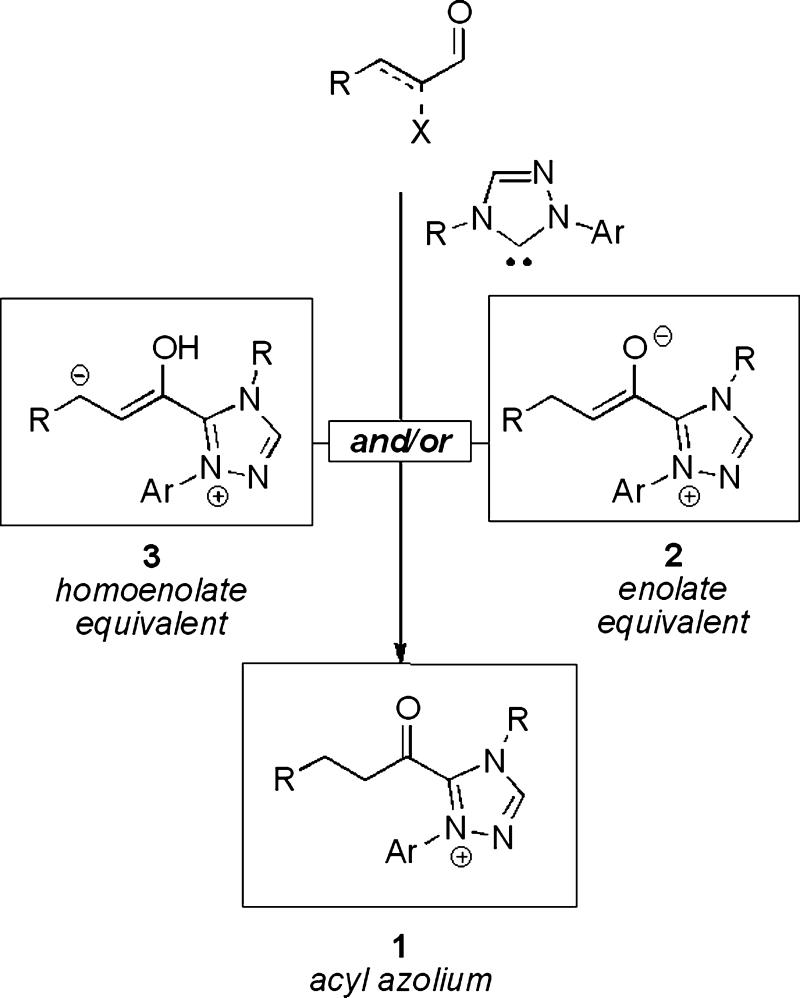
An emerging interest in reactions beyond the scope of traditional acyl anion equivalents (benzoin and Stetter-type reactions) has led to the discovery of an assortment of novel transformations that are the focus of this review. Specifically, substrates that bear α-reducible functionality, a leaving group or unsaturation adjacent to the carbonyl, can be diverted to unique reaction pathways via three distinct catalytic intermediates: electrophilic acyl azolium 1, nucleophilic enol 2, and nucleophilic homoenolate 3 (Scheme 1). This class of reactivity has been termed NHC-redox catalysis, after the concomitant reduction of α-functionality and oxidation of the aldehyde that occurs in the process.
Scheme 1.
Catalytic intermediates exploited in NHC-redox reactions.
Each of these intermediates has been exploited in new reactions to give a variety of highly functionalized and enantioenriched products from simple starting materials. A diverse array of acylated products, heterocycles and carbocycles have been made accessible by this reaction manifold, often by bond disconnections that would not have been available previously.
The purpose of this review is to provide the reader with an overview of the progress made in this area since 2004. Section 2 will provide the reader with an historical perspective into the cyanide catalyzed redox reaction and ensuing mechanistic elucidation. Section 3 will focus on the reactivity of acyl azolium 1, and application of the NHC-redox reaction as a mild acylation strategy for the synthesis of esters, amides, and carboxylic acids. Section 4 will highlight the reactivity of enol 2 and its use in the synthesis of oxygen and nitrogen-containing heterocycles. Section 5 will discuss the reacticity of homoenolate 3 and its use in the synthesis of both heterocycles and carbocycles. Lastly, the review will conclude with current challenges and limitations in this area of research.
2 Cyanide Catalyzed Reactions
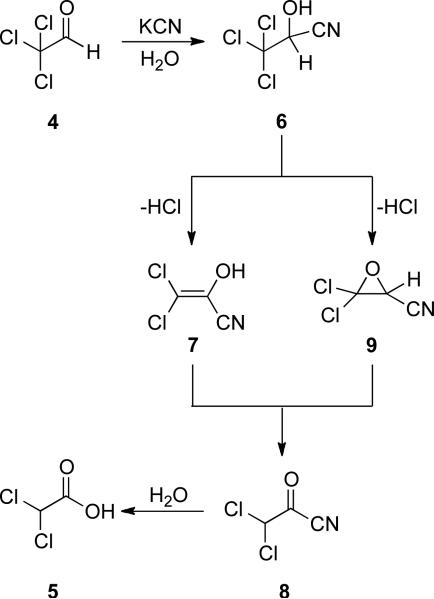
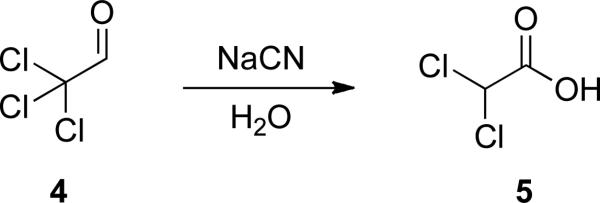
Like the related benzoin and Stetter reactions, NHC-catalyzed reactions of reducible aldehydes are rooted in cyanide catalysis. In 1873, Wallach reported the redox neutral conversion of chloral 4 to dichloroacetic acid 5 in the presence of aqueous potassium cyanide (Scheme 2).[3]
Scheme 2.
Cyanide-catalyzed redox.
The mechanism for this reaction was first proposed by Kötz in 1913.[4] Two distinct pathways were envisioned upon formation of cyanohydrin 6 (Scheme 3). Intermediate 7 can arise via elimination of HCl to generate nucleophilic enol, which can tautomerize to afford the acyl cyanide 8. On the other hand, intermediate 9 can arise from cyanohydrin 6 by nucleophilic displacement of a chloride by the alcohol to form an epoxide followed by a hydride shift to result in formation of the acyl cyanide 8. Hydrolysis of 8 affords dichloroacetic acid 5. Subsequent studies focused on validating or dismissing the proposal put forth by Kötz.
Scheme 3.
Kötz mechanistic proposal.
The ensuing mechanistic investigations spanned 5 decades and included contributions by Chattaway and Irving,[5] Rosenblum,[6] Lapworth,[7] Cram and Hammond,[8] and Katritzky.[9] However, it was the work of Nowak in 1963 which led to the currently accepted mechanism for the cyanide-catalyzed transformation of chloral to dichloroacetic acid.[10]
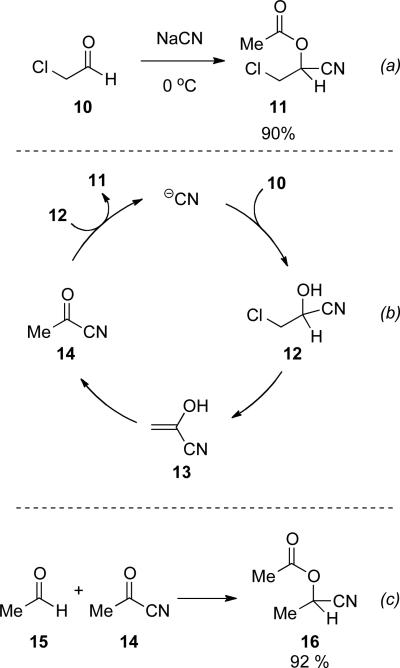
Nowak reported the conversion of chloroacetaldehyde 10 to 2-chloro-1-cyanoethyl acetate 11 in 90 % yield catalyzed by aqueous cyanide (Scheme 4, a).
Scheme 4.
Nowak's mechanistic proposal.
He proposed that formation of cyanohydrin 12 is followed by net loss of HCl to provide enol 13. Tautomerization of 13 provides acyl cyanide 14, upon which cyanohydrin 12 can condense to provide 11 (Scheme 4, b). The formation of acyl cyanide 14 as an intermediate was validated by addition of cyanoacetate to an aqueous solution of acetaldehyde 15 and sodium cyanide, which provides 1-cyanoethyl acetate 16 in 92 % yield (Scheme 4, c).
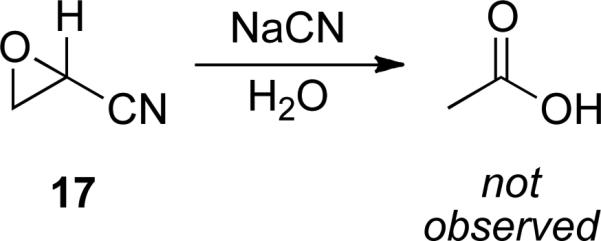
To probe the intermediacy of epoxide 9 (Scheme 3), epoxynitrile 17 was subjected to aqueous cyanide with no observation of the formation of acetic acid (Scheme 5). Therefore, epoxide 9 is an improbable intermediate in the redox process, and a mechanism via enol 7 is more likely (Scheme 4).
Scheme 5.
Possible epoxide intermediate.
3 NHC Redox Acylation
Acylations represent an important class of reactions in the synthetic chemist's tool box. However, these reactions can be problematic and often require superstoichiometric quantities of activating reagents to conduct sometimes trivial condensation reactions. The conversion of chloral to dichloroacetic acid described in Section 2 represents, in principle, complementary access to the ester, amide and carboxylic acid products furnished by such reactions. Furthermore, the application of enantioenriched NHC catalysts offers the opportunity for asymmetric catalysis. Finally, the NHC catalyzed redox acylation strategy offers an alternative closer to the ideal process in terms atom economy,[11] redox neutrality[12] and efficiency.[13]
3.1 Redox Esterification
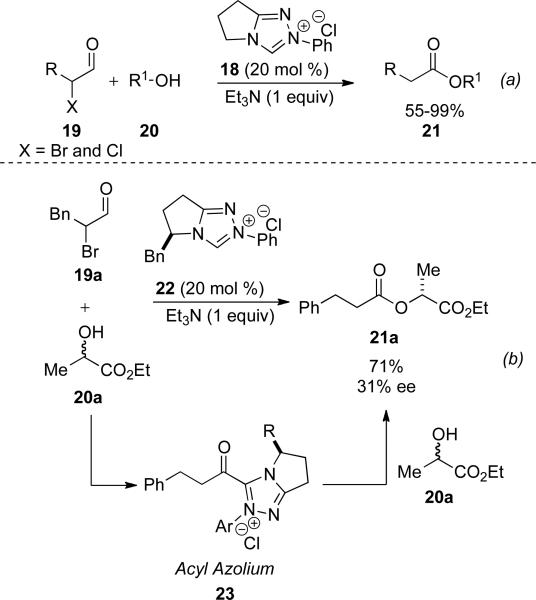
In 2004 the Rovis and Bode groups independently and concurrently reported the use of N-heterocyclic carbenes with α-reducible aldehydes and alcohols to furnish esters. α-Halo aldehydes 19 were demonstrated by Rovis as substrates with alcohols 20 as nucleophiles to provide saturated esters 21 in 55-99 % yield (Scheme 6, a).[14] Bromide was found to eliminate more readily than chloride, but both proved competent in the reaction.
Scheme 6.
Redox esterification of α-halo aldehydes.
Additionally, enantioenriched ethyl lactate (99 % ee) may also be used in the acylation process, which provides the respective product in 56 % yield and 94 % ee. Use of a chiral carbene 22 and racemic lactate 20a leads to a kinetic resolution providing the ester 21a in 71 % yield and 31 % ee suggesting that acylation occurs on the acyl azolium 23 (Scheme 6, b).
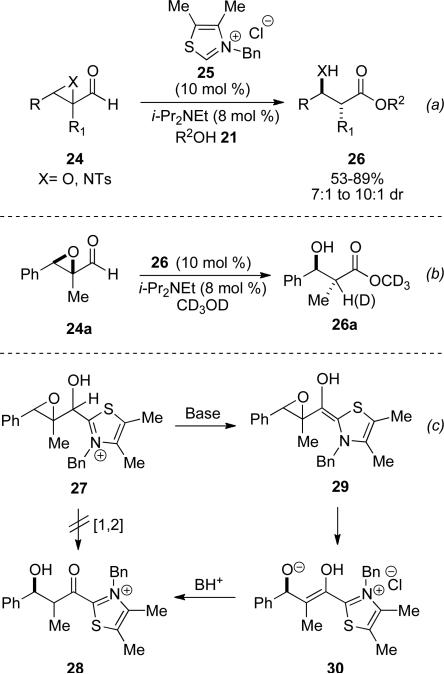
Bode and coworkers demonstrated α,β-epoxy aldehydes and α,β-aziridinyl aldehydes 24 as viable substrates in the NHC-redox acylation catalyzed by thiazolium catalyst 25 with a variety of alcohols 20 to furnish β-hydroxy esters and β-amino esters 26, respectively (Scheme 7, a).[15] Mechanistic investigation into the redox process revealed an intramolecular hydride transfer of 27 directly to the acyl azolium 28 is not operative based on subjection of substrate 24a to deuterated methanol and termination of the reaction at 50 % conversion. The product 26a is obtained with 50 % deuterium incorporation at the α-position (Scheme 7, b). Inspection of the recovered starting material also shows no deuterium incorporation at the aldehydic carbon disfavoring a hydride-shift mechanism and thus favoring an E2 mechanism, where a concerted opening of the epoxide 29 occurs to give enol 30, which can tautomerize to 28 (Scheme 7, c).
Scheme 7.
Redox esterification of α,β-epoxy aldehydes.
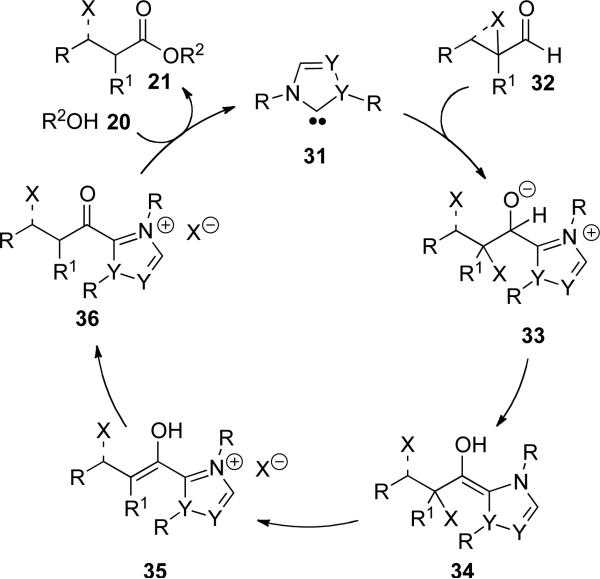
Based on the findings of Rovis and Bode, the proposed mechanism of this redox acylation starts with deprotonation of the azolium salt to generate the nucleophilic carbene 31 (Scheme 8). Addition of 31 to the α-reducible aldehyde 32 furnishes tetrahedral intermediate 33. Intermolecular proton transfer provides the acyl anion equivalent or Breslow intermediate 34,[16] followed by loss of the leaving group to generate enol 35. Tautomerization of 35 furnishes the acyl azolium 36 which is intercepted by the alcohol to provide the ester 21 and regenerate carbene 31 (Scheme 8).
Scheme 8.
Mechanism of redox esterification of α-reducible aldehydes.
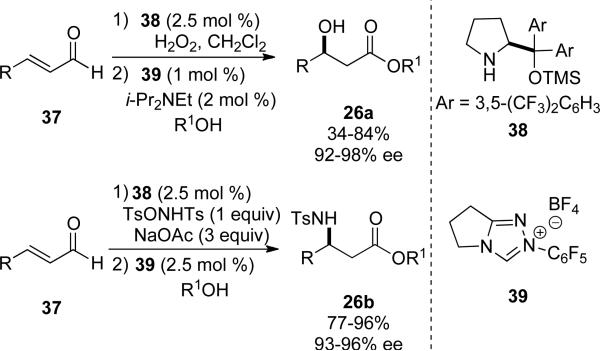
The Córdova[17] and Jørgensen[18] groups have recently reported that secondary amine catalysis coupled with NHC catalysis is a viable strategy for the synthesis of β-hydroxy and β-amino esters 24, respectively, directly from α,β-unsaturated aldehydes (Scheme 9). The complete consumption of enal 37 is necessary to provide the β-epoxy or β-aziridinyl aldehyde utilizing secondary amine 38 prior to addition of carbene precursor 39 and alcohol, providing the enantioenriched β-hydroxy esters 27a in 34-84 % yield and 92-98 % ee, and β-amino esters 27b in 77-96 % yield and 93-96 % ee (Scheme 9). Additionally, triazolium precatalyst 39 was shown to provide higher yields in the optimized one-pot procedure than the original report by Bode using thiazolium precatalyst 25 (Scheme 7).
Scheme 9.
Secondary amine and NHC catalysis.
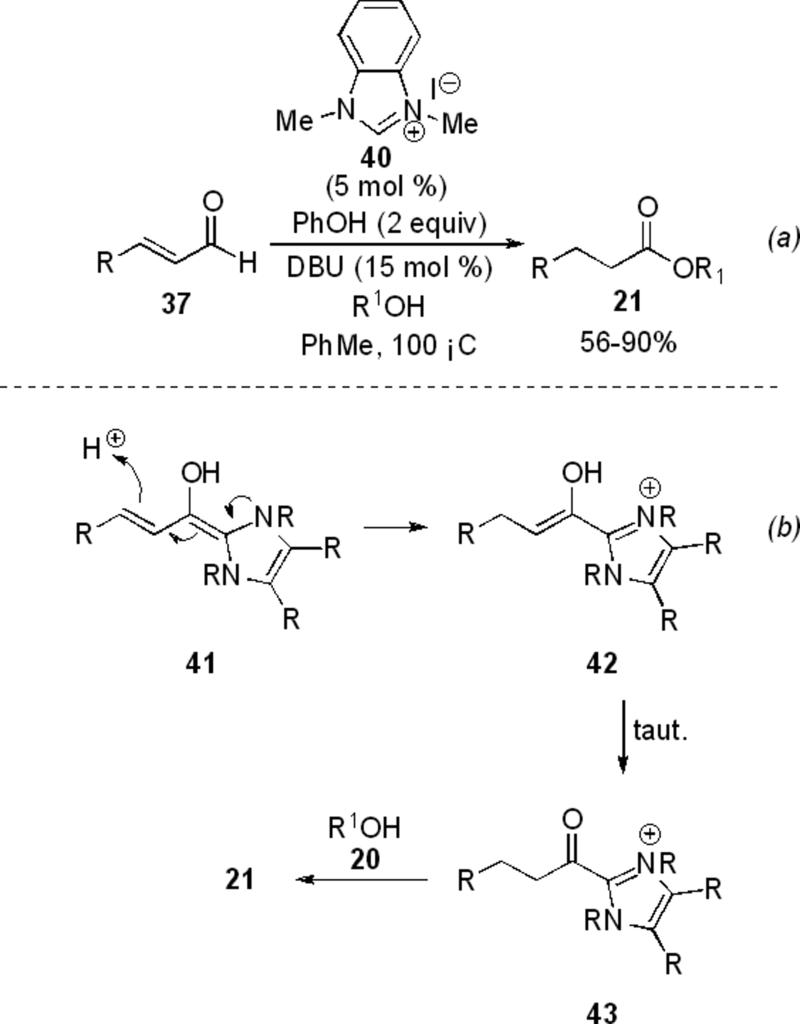
Bode and Scheidt have independently shown that α,β-unsaturated aldehydes can be converted to saturated esters in the presence of NHC and alcohols. Scheidt and coworkers observe that enals 37 can be used with benzimidazolium precursor 40 to acylate primary and secondary alcohols as well as phenols. Aromatic and aliphatic substitution on 37 is tolerated to give saturated esters 21 in 56-90 % yield (Scheme 10, a).[19] In this reaction, the extended Breslow intermediate 41 (homoenolate) is quenched with proton to give enol 42, which tautomerizes to acyl azolium 43 and reacts with the alcohol 21 to give the product (Scheme 10, b).
Scheme 10.
Acylation of alcohols by enals.
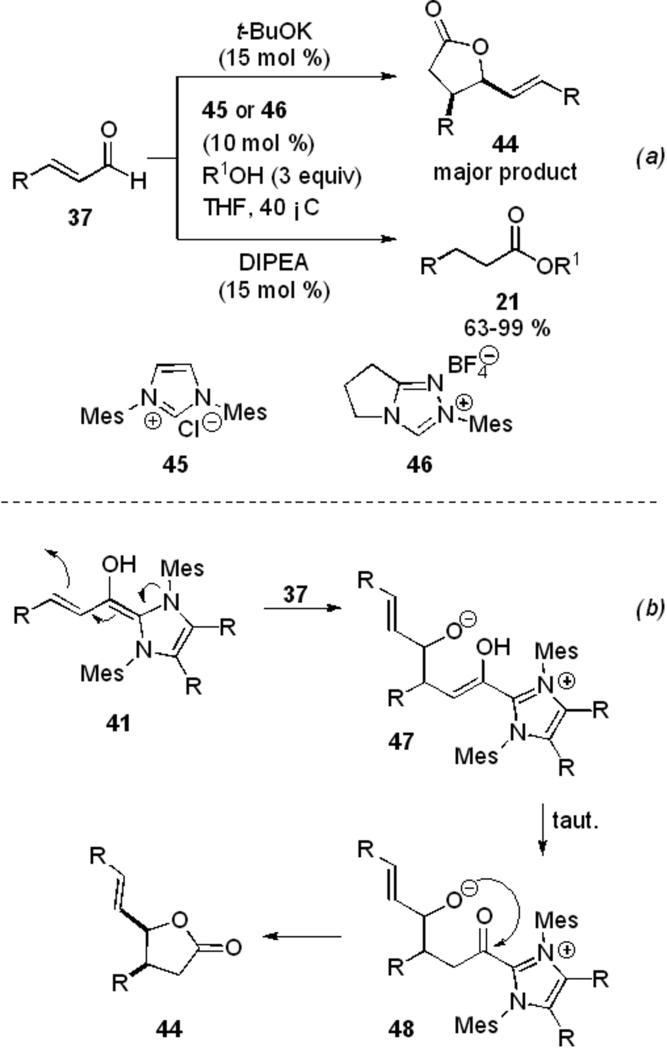
Bode and Sohn have also demonstrated that the choice of base can have a profound effect on the reaction outcome generating lactone dimer 44 (see Section 5.1) or saturated ester 21 (Scheme 11, a).[20] With either NHC precursor 45 or 46 (Scheme 11, b), the use of a strong base such as potassium tert-butoxide leads to generation of the lactone dimer 44 which arises from the nucleophilic addition of the homoenolate 41 to another equivalent of aldehyde 37 (Scheme 11, b), presumably due to the absence of an efficient proton source (pKa of t-butanol = 29.4 in DMSO). This can tautomerize to the acyl azolium 47 and trap the alkoxide intramolecularly to give the product. However, when a base whose conjugate acid is sufficiently strong is employed, such as a tertiary amine, monomeric ester 21 is obtained (pKa of i-Pr2EtNH+ = 13 in THF) from a similar pathway as above (Scheme 10, b). Phenols, primary and secondary alcohols are tolerated in the reaction to produce the saturated esters in 63-99 % yield (Scheme 11, a).
Scheme 11.
Divergent pathways leading to saturated esters and lactone dimers.
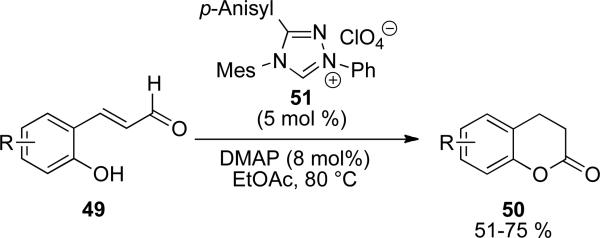
An intramolecular variant of the redox esterification of enals 49 has been reported by Zeitler to give 3,4-dihydrocoumarins 50 with precatalyst 51 (Scheme 12).[21] Additionally, it was found that exclusion of oxygen from the solvent prevents the formation of coumarin products. Substitution at all positions is tolerated on the aromatic backbone.
Scheme 12.
Intramolecular redox esterification to form dihydrocoumarins.
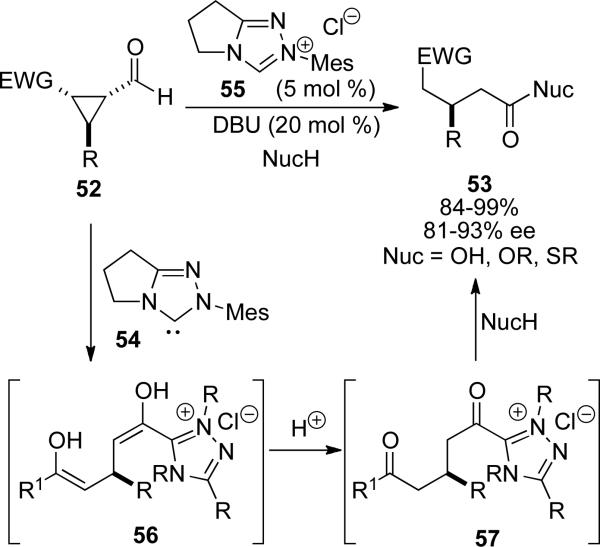
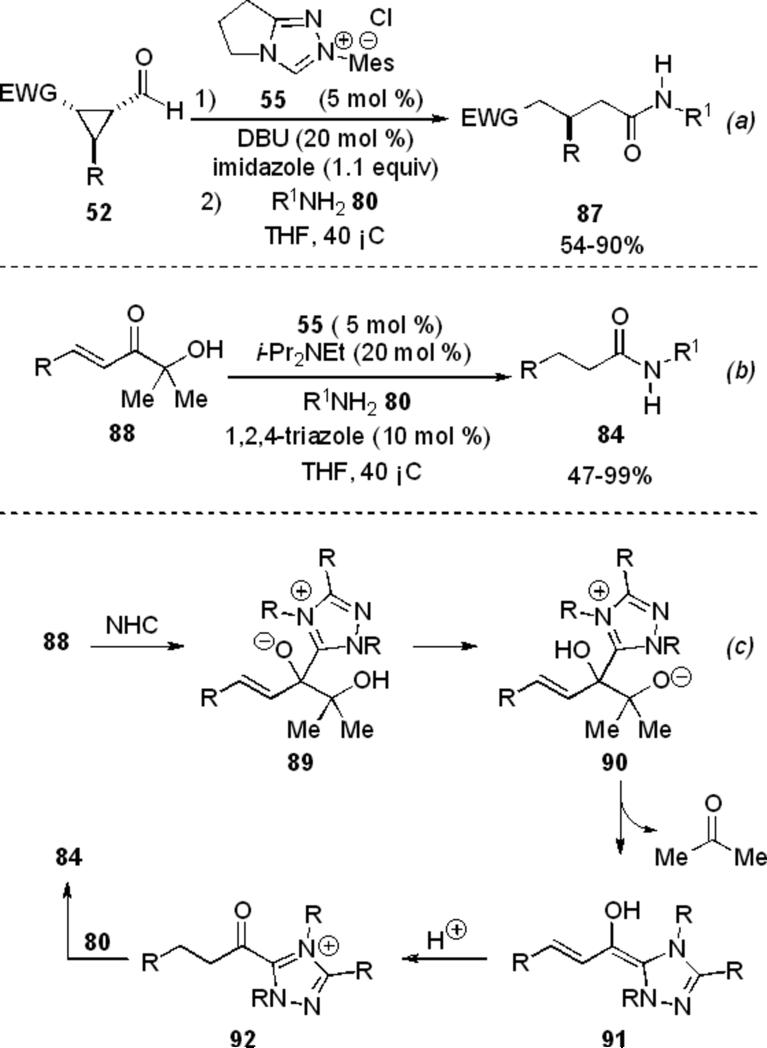
The use of formyl cyclopropanes 52 in the NHC redox esterification reaction was also developed by Bode and Sohn.[22] Active catalyst is formed from triazolium 55 and DBU, which catalyzes a C–C bond cleavage reaction in the presence of electron withdrawing substituents to provide intermediate 56 (Scheme 13). Two sequential proton transfers provide the acyl azolium 57, which is then shown to react with primary alcohols, thiols and water to provide the respective acyl derivatives 53.
Scheme 13.
Redox esterification of formylcylopropanes.
The electron withdrawing group on the cyclopropane encompasses ketones, esters, amides, and nitro groups. Mechanistic investigation into the reaction with 5 equivalents of deuterated methanol shows mono-deuterium incorporation at the β and α-carbons. Reversible formation of the acyl anion equivalent was confirmed by deuterium labeling experiments.
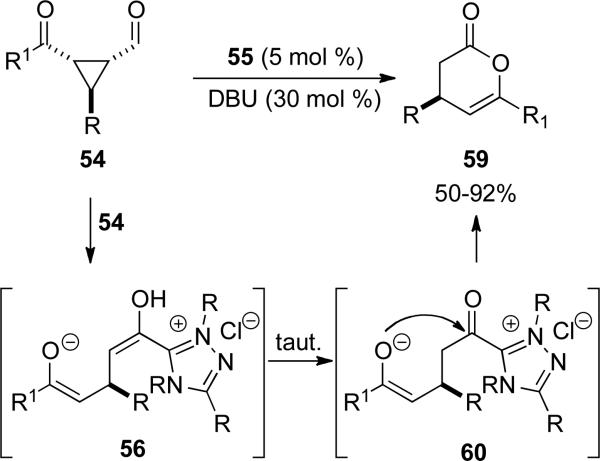
A divergent reaction pathway with formylcyclopropanes 52 leading to 3,4-dihydroxy-α-pyrone derivatives 59 was identified by You and coworkers with triazolium 55 (Scheme 14).[23] In the absence of an external nucleophile, enol 56 generated upon C-C bond cleavage tautomerizes to the acyl azolium 60 which O-acylates the enolate in an intramolecular process to provide the ring expansion product. For this transformation, the electron withdrawing group must be an aryl ketone. Aliphatic ketones give tetrahydropyrone as the major product.
Scheme 14.
Intramolecular esterification of cyclopropyl carboxaldehydes.
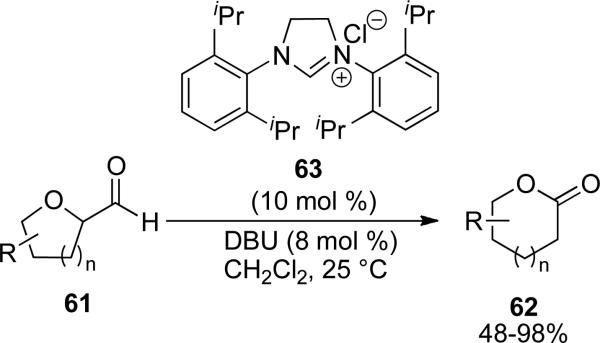
Oxacycloalkyl carboxyaldehydes 61 can also be converted to lactones 62 via an NHC catalyzed intramolecular esterification reaction. Gravel and coworkers identified partially saturated imidazolium 63 as proficient in this ring expansion process, giving lactones in 48-98 % (Scheme 15). Expansion of 4- and 5-membered oxacycloalkyl carboxaldehydes to provide 5- and 6-membered lactones proceeds efficiently, while expansion of a 6-membered oxacycloalkyl carboxaldehyde to furnish the 7-membered lactone is low yielding.[24]
Scheme 15.
NHC catalyzed synthesis of lactones.
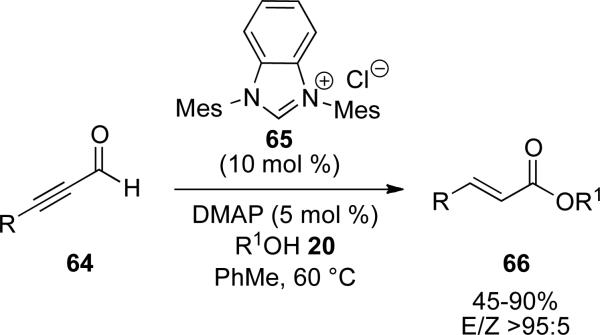
The synthesis of (E)-α,β-unsaturated esters can also be accomplished by redox esterification of propargylic aldehydes 64. Zeitler has identified benzimidazolium salt 65 to be efficient in this process in the presence of DMAP as the base to furnish enoates with high levels of E:Z selectivity (typically >95:5). A variety of alcohols participate in the reaction and aromatic, heteroaromatic, and aliphatic substituents are tolerated on the propargylic aldehyde to provide (E)-α,β-unsaturated esters 66 in 45-90 % yield (Scheme 16).[25]
Scheme 16.
Redox esterification of ynals.
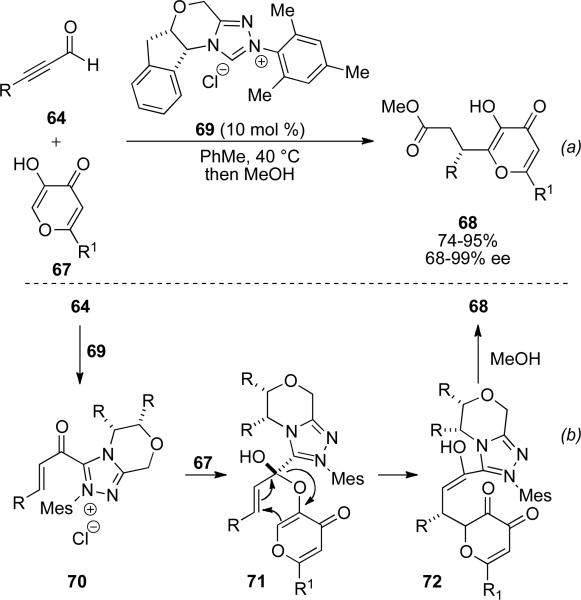
Recently, Bode and coworkers have extended the scope of the redox esterification of propargylic aldehydes 64 with enols 67, which undergo a Coates-Claisen rearrangement upon acylation of the acyl azolium to provide lactones 68 in excellent enantioselectivities with chiral NHC 69 (Scheme 19). The reaction proceeds via interception of acyl azolium 70 by the enol 67 to give tetrahedral intermediate 71 (Scheme 19, b). This then undergoes a [3,3] sigmatropic rearrangement to enol 72. Tautomerization to the acyl azolium followed by displacement with methanol gives the observed product in 74-95 % yield and 68-99 % ee (Scheme 17).[26]
Scheme 19.
Esterification of dichloroaldehydes.
Scheme 17.
NHC Coates-Claisen rearrangement.
Ynals bearing aromatic and aliphatic substituents are tolerated. Mildly acidic nucleophiles such as enols of Kojic acids, pyruvic esters and phenols are competent. It is noteworthy that the process is catalyzed without base to facilitate carbene formation from the azolium salt. It is hypothesized that the chloride anion of the azolium salt is responsible for generating trace amounts of the carbene under these conditions which catalyze the reaction.
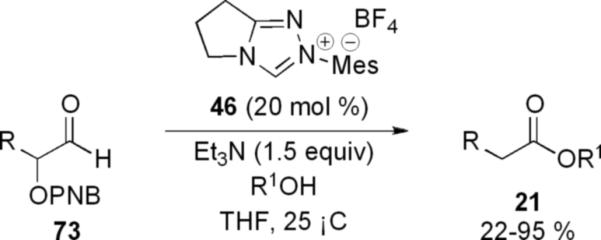
α-Aryloxy aldehydes 73 are also competent substrates in the esterification reaction. Their enhanced stability compared to that of α-halo aldehydes makes these substrates useful in the arsenal of reducible substrates available in the redox reaction. Using triazolium precatalyst 46, Smith and Ling observed that saturated esters 21 can be obtained from substrates 73 in 22-95 % yield. [27]
A para-nitro benzoyl (PNB) group was necessary to obtain optimal reactivity. Phenols, primary and secondary alcohols are viable nucleophiles with aliphatic and cinnamyl derived substrates. However, tertiary aryloxy aldehydes do not provide any product. This may be due to sterics and the necessary orbital overlap required for elimination.
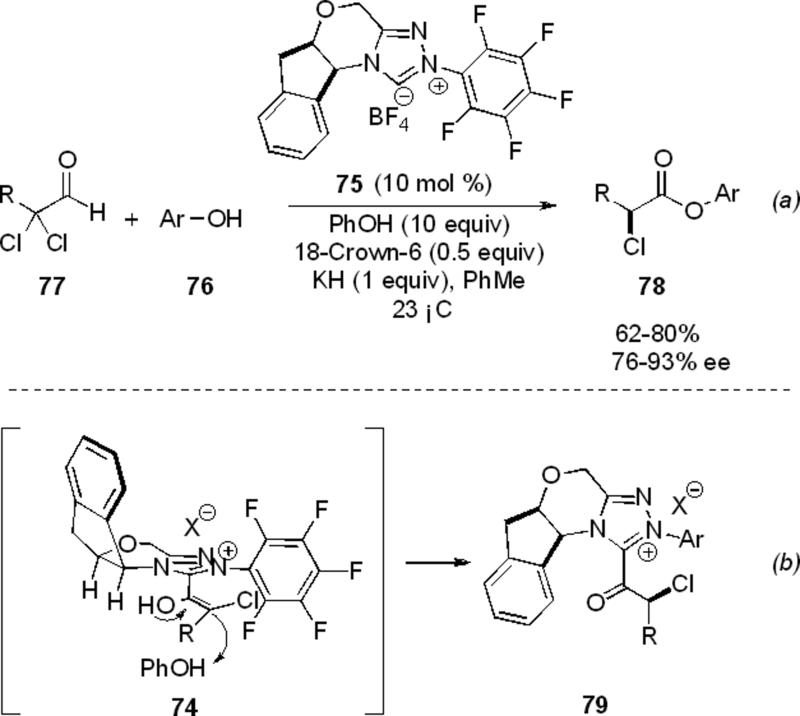
The asymmetric synthesis of enantioenriched α-chloro esters was also reported by Rovis and Reynolds promoted by a chiral NHC via an asymmetric protonation of enol intermediate 74 (Scheme 19, a).[28] The use of triazolium 75 under basic conditions with phenols 76 and α,α-dichloro aldehydes 77 provides the enantioenriched ester 78 in 62-80 % yield and 76-93 % ee (Scheme 19, b). Excess phenol is necessary to insulate the newly generated stereocenter, which is highly prone to epimerization at the acyl azolium 79. A variety of α,α-dichloro aldehydes and substituted phenols participate in the reaction to provide aryl esters; however, β-branching on the aldehyde is not tolerated.
3.2 Redox Amidation
Early on, alcohols were well established as competent nucleophiles with a variety of reducible aldehydes. However, a notable limitation of this catalytic acylation strategy was the inability of amines to participate in this mild process. Clearly, access to amides in a catalytic method would be a useful addition to the synthetic chemist's arsenal. However, the addition of an amine nucleophile directly to the acyl azolium 1 presents a greater challenge than that of oxygen nucleophiles.
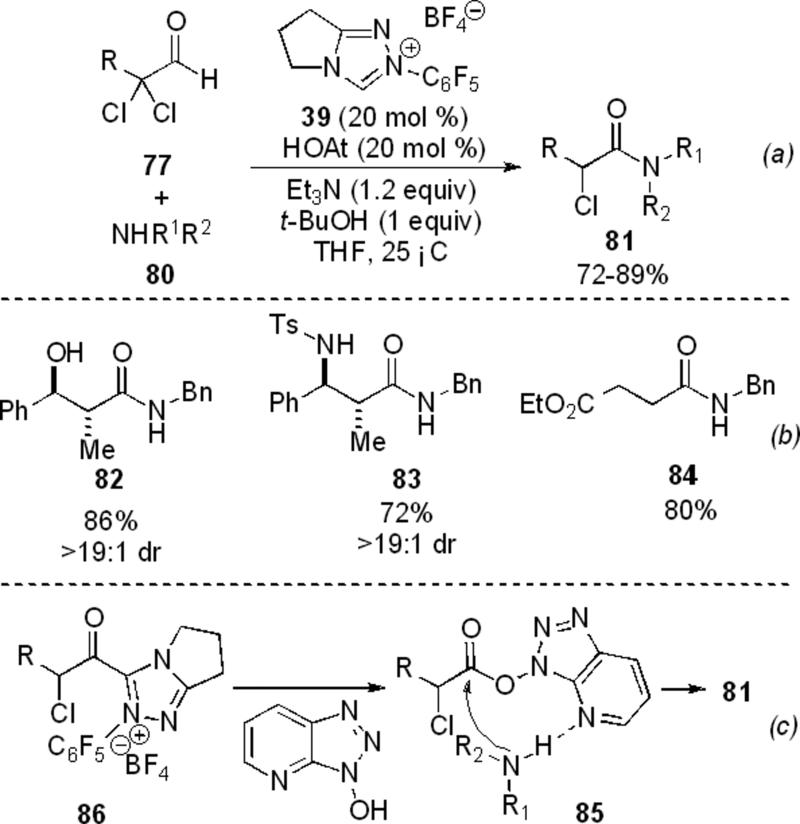
In 2007, the Rovis[29] and Bode[30] groups concurrently and independently showed that the use of acyl transfer reagents in the presence of amines and NHCs with reducible aldehydes provides amide products. The method developed by Rovis and Vora uses substoichiometric amounts of additives commonly used in peptide coupling reactions as acyl transfer reagents to give amide products from a variety of reducible aldehydes. Using α,α-dichloro aldehydes 77 a variety of aromatic and aliphatic amines 80 can be added to provide α-chloroamide products 81 in 72-89 % yields (Scheme 20, a). Epoxy and aziridinyl aldehydes were also shown to participate in the reaction to provide β-hydroxy- 82 and β-amino amides 83 with high levels of diastereoselectivity (Scheme 20, b). It was noted that non α-substituted epoxy aldehydes provide a complex mixture of products. Enals also provide saturated amides 84 in moderate yields. The reaction can be performed asymmetrically on α,α-dichloro aldehydes with chiral NHC precursor 75 and benzyl amine to provide the amide in 80 % ee. Acylation is thought to proceed via a general base catalyzed condensation on the HOAt ester 85 which is generated by HOAt intercepting the acyl azolium 86 (Scheme 20, c).
Scheme 20.
Redox amidation using HOAt as the acyl transfer reagent.
Concurrently, the Bode group identified imidazole as the acyl transfer reagent which is used in stoichiometric quantities to furnish an acyl imidazole from formylcyclopropanes 52. Subsequent addition of the amine 77 provides the desired amide product 87 in 54-90 % yield (Scheme 21, a). Further work in this area by the Bode group has revealed that α‘-hydroxy enones 88 obtained from aldehydes are also competent substrates with substoichiometric quantities of 1,2,4-triazole to provide saturated amide products 84 in 47-99 % yield (Scheme 21, b).[31] The reaction proceeds through a retro-benzoin reaction catalyzed by 55 and base to liberate acetone and give the Breslow intermediate 91 (Scheme 21, c). This then continues to the redox pathway to provide product. An example of chemoselective N-acylation over O-acylation is provided and attributed to the cocatalyst. However, one limitation to this process is that amine hydrochloride salts are not compatible.
Scheme 21.
Redox amidation using imidazole or triazole as the acyl transfer reagent.
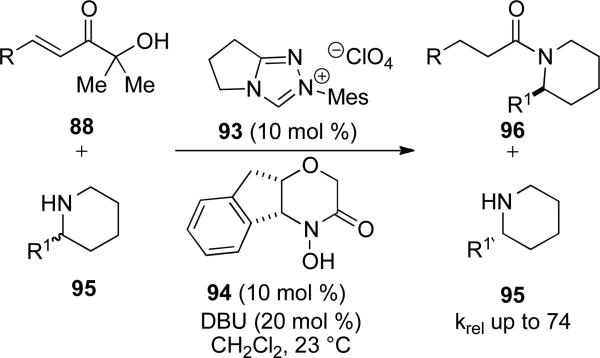
Recently, Bode has also found that a chiral acyl transfer cocatalyst can effect a kinetic resolution of secondary amines. In a similar fashion as above, α‘-hydroxy enones 88 are first converted to the corresponding Breslow intermediate by a retro benzoin reaction catalyzed by achiral NHC precursor 93; then, the redox pathway gives the corresponding acyl azolium. Chiral hydroxamic acid 94 then displaces the azolium to give a chiral acylating reagent capable of resolving secondary amines 95 with an S-value up to 74.[32]
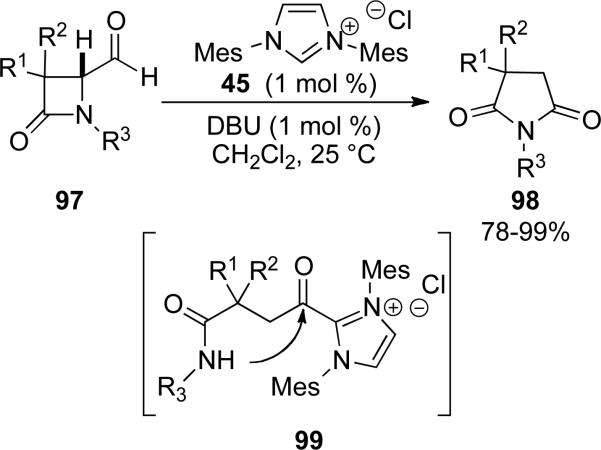
A cocatalyst does not seem to be necessary for the amide bond forming process to take place in an intramolecular reaction, based on the findings of You and Gravel. You and coworkers identified that 4-formyl β-lactams 97 were viable substrates in the presence of NHC precursor 45 to furnish succinimide derivatives 98 (Scheme 23). Redox reaction proceeds with elimination of the amide to form acyl azolium 99, which traps the amide to give the observed ring expanded product. It was found that a N-PMP or N-Mes group is necessary on the β-lactam.[33]
Scheme 23.
NHC catalyzed ring expansion of β-lactams.
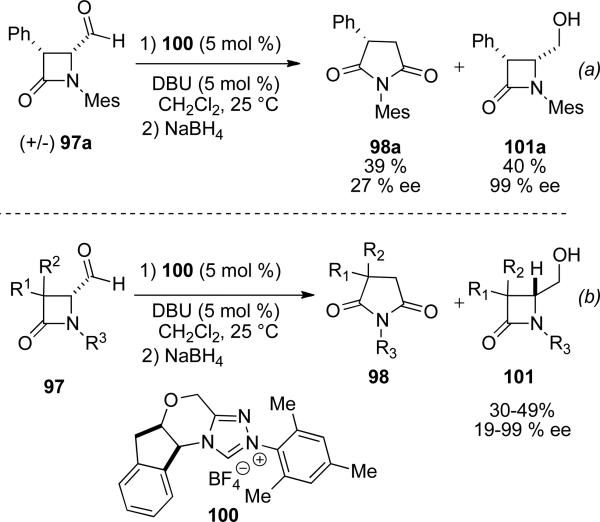
The ring expansion product is generated without epimerization of enantioenriched substrate under the reaction conditions. Additionally, subjection of rac-4-formyl-β-lactam 97a to the reaction catalyzed by chiral NHC precursor 100 provides the respective product 98a in low enantioselectivites (39 % yield, 27 % ee), while providing unreacted aldehyde in 40 % yield and 99 % ee, converted to the corresponding alcohol 101a by reductive workup (this corresponds to an S-value of 24, if one assumes that conversion is 60 %). A variety of racemic substrates were demonstrated, giving alcohol products 101 in 19-99 % ee after reductive workup (Scheme 24).[34]
Scheme 24.
Kinetic resolution of racemic β-lactam.
Gravel and coworkers have extended the scope of the NHC catalyzed ring expansion amidation reaction to provide 6-membered lactams.[35] A variety of common protecting groups on the nitrogen of 2-formylpyrrolidines 102 are tolerated to furnish the 6-membered lactams 103 in 49-100 % yields (Scheme 25). Expansion of piperidines to seven membered lactams was found to be difficult, giving less than 5 % yield.
Scheme 25.
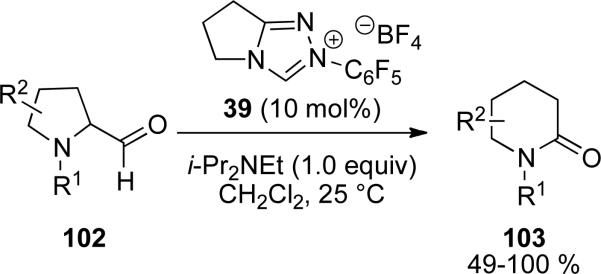
Ring expansions of 2-formyl pyrrolidines to lactams.
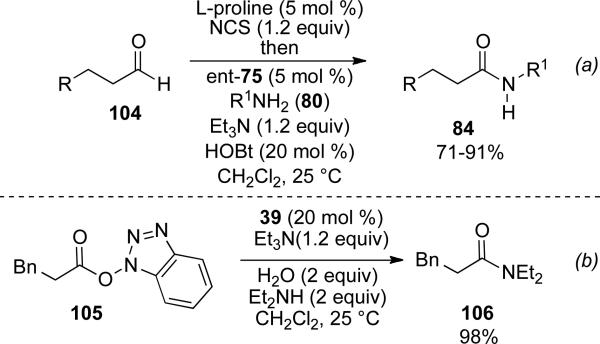
Recently, Yamada and co-workers have developed a one-pot NHC catalyzed amidation with saturated aldehydes and N-chloro succinimide (NCS).[36] The redox reaction thus takes place on the α-chloro aldehyde generated from saturated aldehyde 104 via secondary amine catalysis to provide the acyl azolium. The use of NHC precursor ent-75 and base with substoichiometric quantitites of HOBt generates the HOBt ester which is substituted by the amine to provide the saturated amide products 84 in 71-91 % yield (Scheme 26, a). Additionally, the authors report that substitution of the HOBt ester 105 occurs selectively with the amine over water to provide the amide product 106 (Scheme 26, b).
Scheme 26.
One-pot chlorination/amidation.
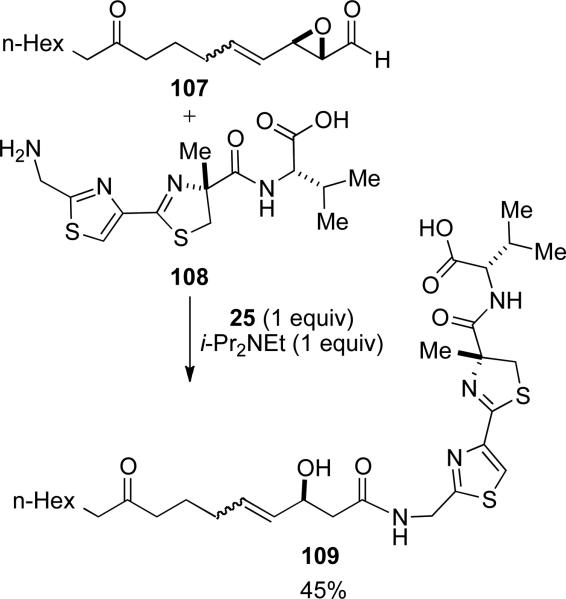
An NHC mediated intermolecular amidation was used by Forsyth and coworkers to unite two complex fragments in an effort toward the natural product largazole (Scheme 27).[37] Under substoichiometric conditions, decomposition of 107 was observed, thus necessitating the use of stoichiometric thiazolium 25. Despite the failure of the catalytic reaction, the complementarity of this acylation to traditional peptide coupling allowed the authors to carry an unprotected carboxylic acid on the amine fragment 108.
Scheme 27.
Application of NHC mediated redox acylation to the synthesis of largazole.
3.3 Redox Azidation
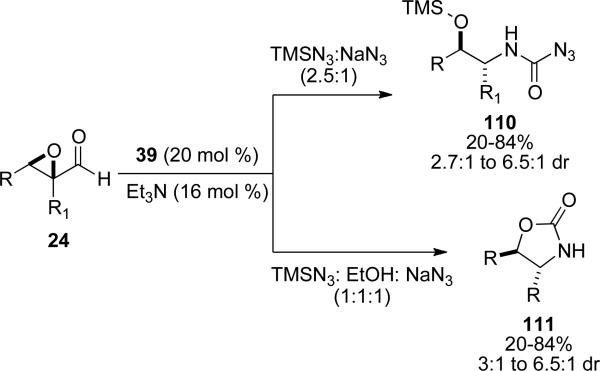
Rovis and coworkers have also reported the synthesis of carbamoyl azides and oxazolidinones with epoxy aldehydes as the reducible aldehyde with NHC precursor 39 and an azide source. Judicious choice of reaction conditions dictates product selectivity.[38] A combination of azidotrimethyl silane and sodium azide in a (2.5:1) ratio provides the carbamoyl azide 110 in 20-84 % with a dr of 2.7:1 to 6.5:1. Conditions developed with azidotrimethylsilane, sodium azide and ethanol provide the oxazolidinones 111 in 20-84 % yield and 3:1 to 6.5:1 dr (Scheme 28). The low diastereoselectivites are attributed to epimerization at the acyl azolium or acyl azide.
Scheme 28.
Azidation of epoxyaldehydes.
3.4 Redox Hydration
The use of water in the NHC catalyzed redox reaction would provide carboxylic acids in a mild and atom economical manner. Previous work inspired by interest in thiamine catalysis led to the study of stoichometrically generated acyl azoliums derived from imidazolylidene and thiazolylidene carbenes and their capacity to react with nucleophiles. Contributions from Breslow,[16b] Daigo,[39] White and Ingharam,[40] Bruice,[41] Lienhard and Owen[42] revealed that water was the most proficient nucleophile followed by alcohols and amines. Additionally, work by Bode has recently confirmed this paradox of nucleophilicity versus reactivity with triazolylidene derived acyl azoliums.[43]
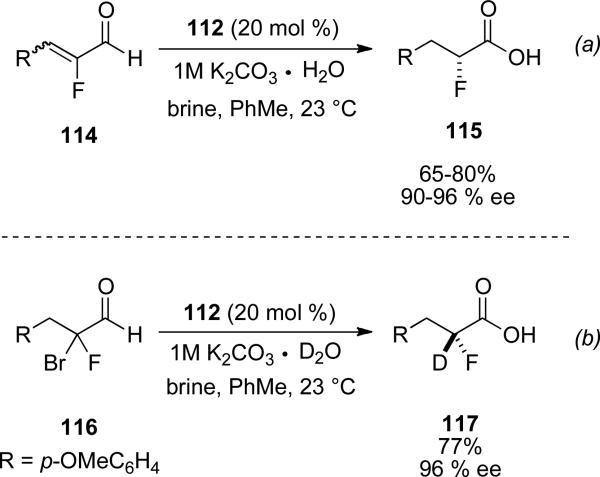
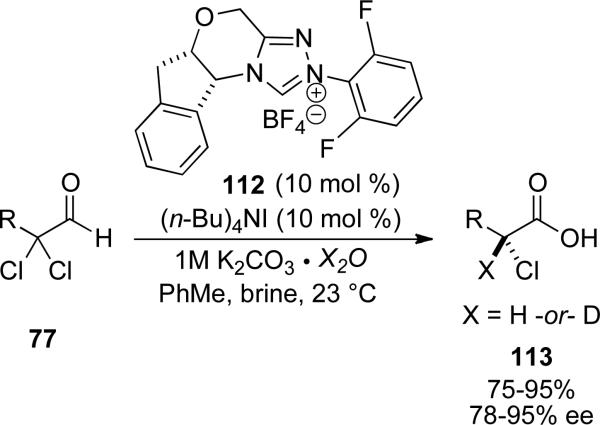
Rovis and Vora applied the propensity of water to react with the acyl azolium in the synthesis of enantioenriched α-chloro carboxylic acids and α-fluoro carboxylic acids.[44] The use of NHC 112 in conjuction with α,α-dichloro aldehydes 77 with 1M potassium carbonate, tetrabutyl ammonium iodide and brine provides the respective carboxylic acid 113 in 75-95 % yield and 78-95 % ee. Additionally, if 1M potassium carbonate in D2O is used the reaction proceeds with installation of an α-deuterium in an asymmetric manner to provide isotopically labelled carboxylic acids in simlar yield and enantioselectivity.
Enantioenriched α-fluoro carboxylic acids can also be generated by subjecting α-fluoro enals 114 with NHC 112 and 1M potassium bicarbonate in brine. Aromatic and aliphatic substitution is tolerated at the β-position of the enal to provide the respective acid 115 in 65-80 % yield and 90-96 % ee (Scheme 30, a). Isotopically labeled fluoro carboxylic acids can also be obtained by subjecting α-bromo,α-fluoro carboxaldehyde 116 to the optimized conditions to obtain the deuterated product 117 in 77 % yield and 96 % ee (Scheme 30, b).
Scheme 30.
Hydration of fluoroenals.
4 Enolate Equivalents
The NHC-redox catalysis pathway proceeds through a number of reactive catalytic intermediates. The acylation reactions described above are applications of the acyl azolium intermediate as an electrophile. However, in the process of generating the acyl azolium, two key nucleophilic intermediates are also generated: the acyl anion equivalent and the enol resulting from the elimination step. While limited at this point in time, electrophilic trapping of the enol intermediate has been demonstrated, most notably to allow for the rapid generation of heterocycles.
4.1 Synthesis of Oxygen Heterocycles
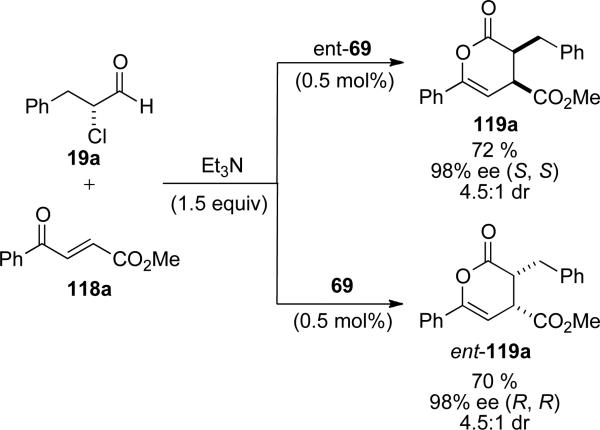
In 2006, Bode and coworkers reported the enantioselective NHC catalyzed Diels-Alder reaction of racemic α-chloro aldehyde with enones to provide 3,4,6-trisubstituted dihydropyran-2-ones.[45] The reaction is amenable to aromatic and aliphatic α-chloro aldehydes 19 and electron withdrawing aromatic and aliphatic enones 118. The reaction proceeds with 0.5 mol % catalyst loadings of NHC precursor ent-69 and provides the dihydropyran-2-ones 119 in 70-95 %, 86-99 % ee, and 3:1 to >20:1 d.r. (Scheme 31).
Scheme 31.
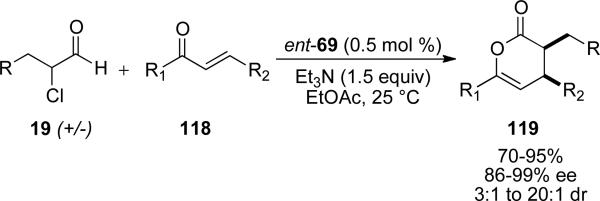
Hetero Diels-Alder reaction on the catalytically generated nucleophilic enol.
The cis-diastereoselectivity is attributed to the formation of the (Z)-enolate in the NHC redox reaction with the α-chloro aldehyde and a high preference for the (Z)-enolate to react in an endo cycloaddition with the enone. Subjection of enantioenriched α-chloro aldehyde with either antipode of chiral NHC precursor 69 provides identical ee (98 %) and d.r. (4.5:1) with an inversion of absolute stereochemistry (Scheme 32).
Scheme 32.
Catalyst control of enantioselectivity.
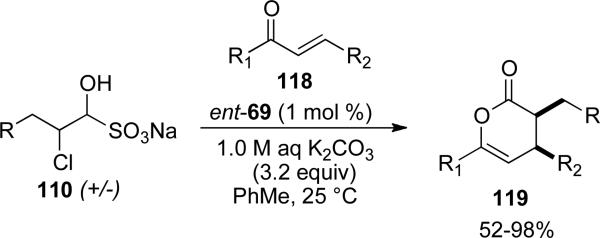
Due to the sensitivity of α-chloro aldehydes, a more process friendly alternative was developed by Bode.[46] Bisulfite salts of α-chloro aldehydes 120 are also viable substrates in the Diels-Alder reaction under biphasic conditions. An excess of K2CO3 is used to liberate the aldehyde which undergoes the redox reaction to provide the diene for the cycloaddition process.
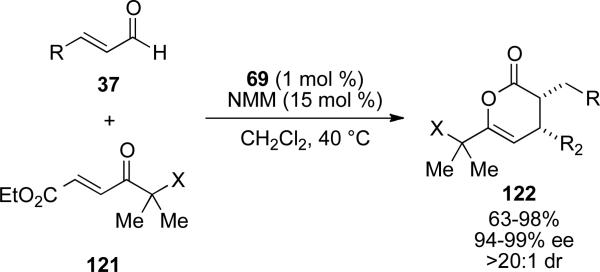
Moreover, Bode has shown that enals can also be used to generate enolate equivalents from the reaction with NHC in the presence of weak base such as N-methylmorpholine (NMM, conjugate acid pKa = 7.4).[47] The enolate equivalent then undergoes a hetero-Diels-Alder reaction with a variety of α-hydroxyenone or α-Cbz-aminoenones 121 to provide dihydropyran-2-ones 122 in 63-98 % yields, 94-99 % ee and >20:1 d.r. (Scheme 34). Additionally, preliminary computational experiments suggest that the high levels of enantioselectivity are attributed to a non-conjugated complex of azolium and the enolate olefin.
Scheme 34.
Enals as enolate precursors.
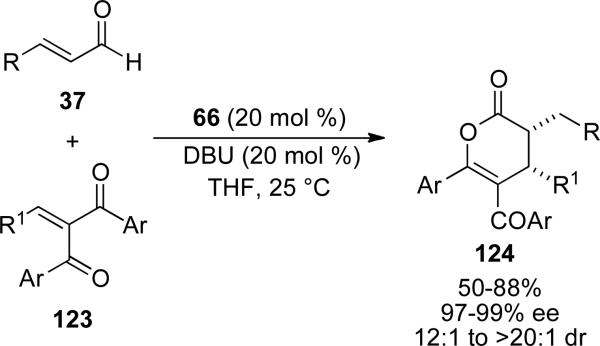
Recently, Chi and co-workers have expanded on the scope of the NHC catalyzed hetero-Diels Alder reaction of enals and enones to benzylidene β-diketone derivatives 123.[48] The scope of enals includes aromatic and hetero-aromatic which provide the products 124 in 50-88 % yield with high enantioselectivities of 97-99 % ee and dr of 12:1 to >20:1 (Scheme 35).
Scheme 35.
Hetero Diels-Alder reaction with chalcones.
Scheidt and coworkers have reported the use of α-aryloxy aldehydes for the synthesis of coumarins and β-amino amides.[49] Coumarins are prepared from a tethered α-phenoxy aldehyde 125 which provides the enolate equivalent upon reaction with the NHC generated from 126. This then adds in a conjugate fashion to the Michael acceptor to generate the acyl azolium which acylates the phenoxide to provide 3,4-dihydrocoumarins 127 in 66-91 % yield (Scheme 36).
Scheme 36.
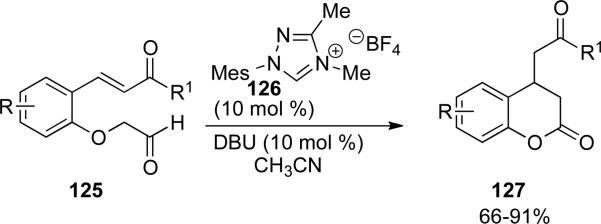
Coumarin synthesis.
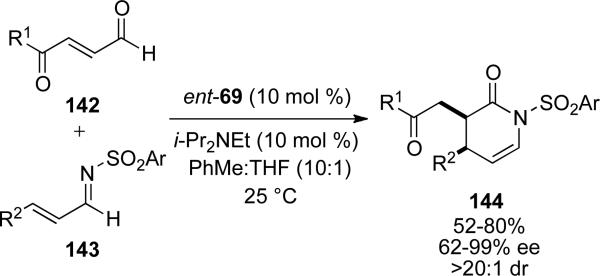
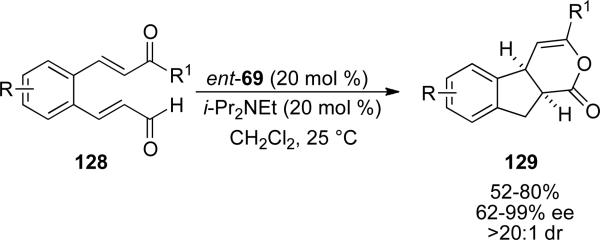
Subsequent work by Scheidt utilized a similar intramolecular Michael-redox reaction to provide fused indanes 129 from substrates 128 with chiral NHC ent-69.[50] The base was found to play a key role not only in yield but also selectivity as DBU provides the product in 6 % yield. Using DIPEA as the base, product is obtained in 50 % yield with a d.r. of >20:1. Thus, products 129 are provided in 52-80 % yield, 62-99 % ee and excellent diastereoselectivity. The tether in the reaction typically contains an aromatic backbone, although two alkyl tethered substrates are also reported.
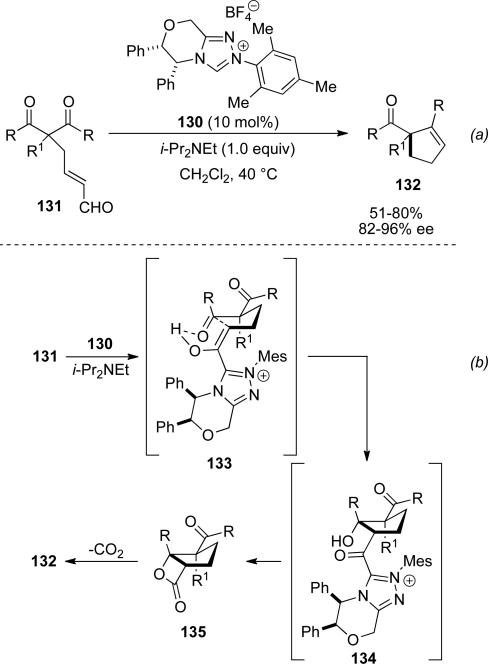
Additionally, Scheidt and coworkers have reported the synthesis of carbocycles which proceed via a fused β-lactone to undergo a spontaneous decarboxylation.[51] NHC 130 was identified as the optimal catalyst with enal 131 to give cyclopentene products 132 (Scheme 38, a). This reaction is thought to proceed through enol 133, which is stabilized by an intramolecular hydrogen bond to the ketone (Scheme 38, b). Addition of the enol to the ketone followed by displacement of the acyl azolium 134 leads to β-lactone 135. This can then decarboxylate to give the observed cyclopentene products, obtained in 51-80 % yield and 82-96 % ee. Moreover, two examples are provided in which the β-lactone 135 can be isolated without decarboxylation.
Scheme 38.
Decarboxylative cyclization to form cyclopentenes.
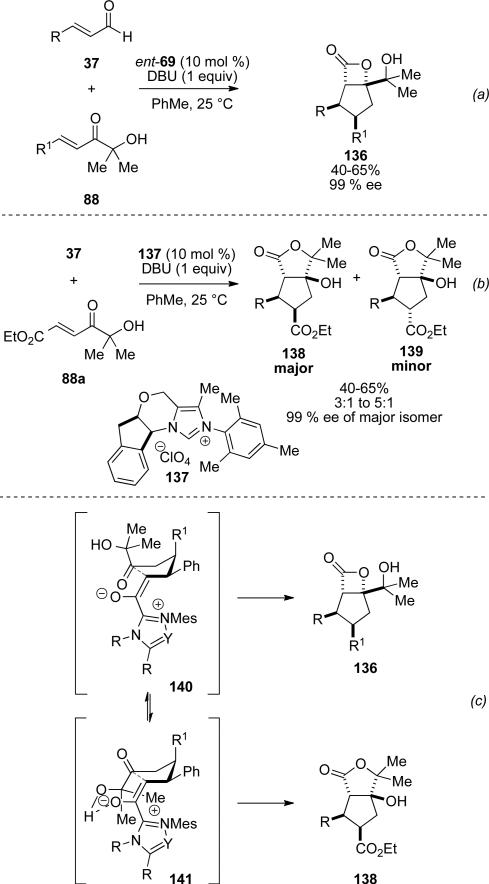
A divergent reaction pathway was identified by Bode toward the synthesis of fused β-lactones and γ-lactones governed by choice of carbene precursor.[52] It was found that use of triazolium NHC 69 provides the fused β-lactone 136 in 40-65 % yield and 99% ee Scheme 39, a). However, use of chiral imidazolium precatalyst 137 affords fused γ-lactones 138 and 139 in 40-65 % yield, 3:1 to 5:1 d.r. and 99 % ee of the major cis-diastereomer (Scheme 39, b). Selectivity is determined by whether conformer 140 closes to give the stereoisomer capable of closing to the β-lactone 136, or if a hydrogen bond from the tertiary alcohol reinforces conformer 141, which closes to the γ-lactone 138 (Scheme 39, c). The relative rates are rationalized by the steric and electronic differences between 69 and 137.
Scheme 39.
Divergent synthesis of β-lactone and γ-lactone products.
4.2 Synthesis of Nitrogen Heterocycles
In addition to α-chloroaldehydes, α,β-unsaturated aldehydes were identified by Bode and coworkers as competent precursors for enolate reactivity and applied to the synthesis of nitrogen-containing heterocycles.[53] Utilizing NHC precursor 69, protonation of the extended Breslow intermediate (or homoenolate) derived from 142 at the β-position under optimized conditions proceeds efficiently to provide the enolate equivalent (dienophile) which can participate in an inverse electron demand hetero Diels-Alder reaction with α,β-unsaturated para-methoxybenzenesulfonyl imine 143 (diene) to provide dihydropyridones 144. The enal must bear an electron withdrawing ester or ketone at the β-position, while the diene can have aromatic and aliphatic substitution providing the dihydropyridone in 52-80 % yield, 62-99 % ee and >20:1 d.r. (Scheme 40). Selectivity for the cis isomer in the reaction is rationalized by a Z-selective dienophile reacting with the diene in an endo fashion. It is hypothesized that protonation of the Breslow intermediate proceeds via a fully conjugated extended system affording the (Z)-enol exclusively, which leads to the invariably excellent diastereoselectivity.
Scheme 40.
Hetero Diels-Alder reaction to give dihydropyridones.
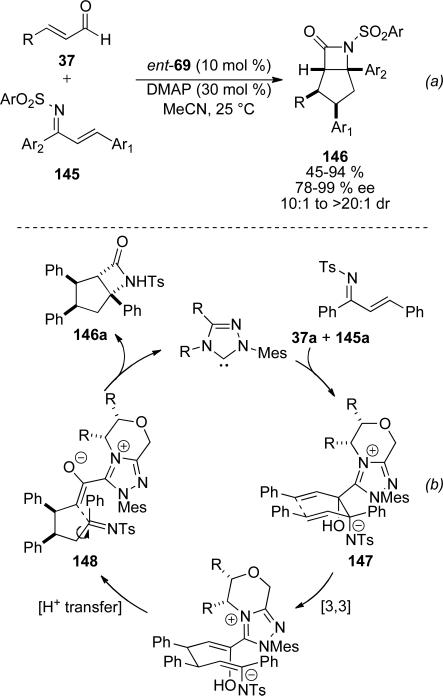
The synthesis of β-lactams is also feasible with enals and sulfonylimines 145 derived from chalcones. Bode and He identified NHC 69 to be optimal with the appropriate base to provide the respective β-lactams 146 in 45-94 %, 78-99 % ee, and as single diastereomers in most cases (Scheme 41).[54] A profound effect of the base on diasteroselectivity was identified wherein DMAP was found to be optimal. While a homoenolate pathway can be envisioned for this process, the authors rationalize the sterochemistry as arising from a concerted crossed- aza-benzoin/oxy-Cope reaction. The cis-relatioship is thought to arise from secondary orbital overlap considerations proceeding via a boat configuration 147 (Scheme 41, b). The remaining stereocenter can then equilibrate by a reversible intramolecular Mannich reaction from enolate 148 onto the sulfonylimine, whose adjacent C-C bond has free rotation. Catalyst can only be turned over when the acyl azolium and resultant amine are cis and therefore capabale to react and form β-lactam 146.
Scheme 41.
β-lactam synthesis by NHC catalysis.
4.3 Intermolecular Reactions
Subsequently, Scheidt reported the synthesis of enantioenriched β-amino amides from the NHC catalyzed redox reaction of α-aryloxy aldehydes 149 and tosyl imines 150.[55] NHC precursor 151 was identified as optimal for the reaction of α-4-nitrophenoxy-acetaldehyde and tosyl imines derived from aromatic aldehydes to provide the β-amino phenoxy ester. Once complete, the arylester is then treated with benzyl amine to provide the β-amino amide product 152 in 56-75 % yield and 88-95 % ee (Scheme 42).
Scheme 42.
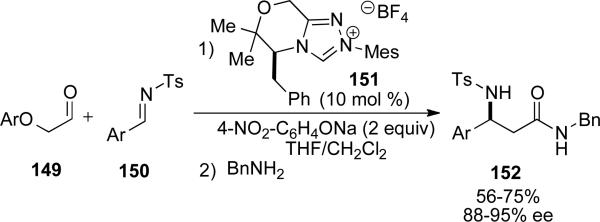
Synthesis of β-amino amides.
5 Homoenolate Equivalents
Homoenolate reactivity represents a sought after umpolung reaction manifold that has been of interest to the synthetic community. Catalytic generation of homoenolate intermediates from readily available starting materials has been achieved only recently. NHC catalysis has enabled a powerful method to access diverse heterocycles and carbocycles from α,β-unsaturated aldehydes. Typically, imidazolium and electron rich sterically encumbered N-aryl triazolium catalyst precursors have been identified as optimal catalysts for this process. Additionally, their deprotonation by strong bases to generate weak acids has been found to be crucial.
5.1 Synthesis of Oxygen Heterocycles
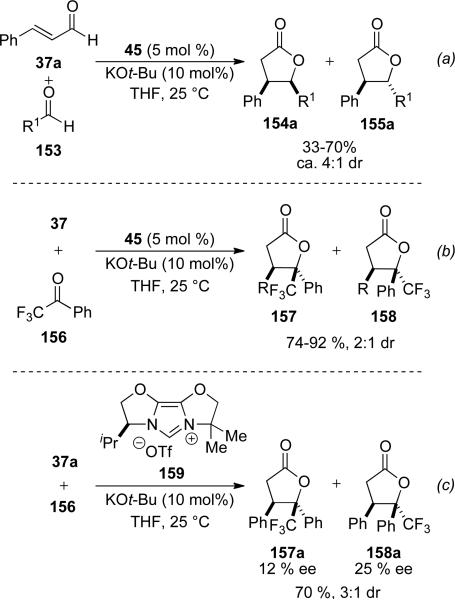
In 2004, Bode[56] and Glorius[57] independently reported the synthesis of γ-butyrolactones from the reaction of α,β-unsaturated aldehydes with aromatic aldehydes 153. NHC precatalyst 45 was identified as optimal with aromatic enals and ynenals. Aromatic aldehydes are typically the coupling partner to provide a mixture of cis to trans γ-butyrolactones (154/155) in 41-87 % yield and 3:1 to 5:1 d.r. (Scheme 43). Mechanistic insight is provided by the discovery that both cis and trans enals provide identical stereochemical outcomes. Additionally, quenching the reaction prior to completion reveals extensive isomerization of cis-enal to trans-enal. Bode has also reported that in the absence of an exogenous electrophile, homodimerization is a potential pathway.
Scheme 43.
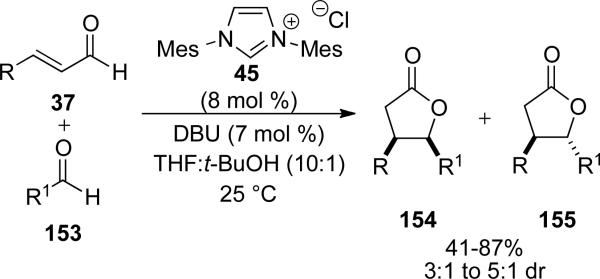
Homoenolate reactivity to form γ-butyrolactones from aryl aldehydes.
Concurrent to the report by Bode, Glorius and coworkers developed a similar process also utilizing NHC 45. With cinnamaldehyde 37a as the reducible aldehyde, a variety of aromatic aldehydes were demonstrated in this process in 32-70 % yield and 4:1 d.r. favoring the cis-isomer 154a (Scheme 44, a). Electron deficient ketones 156 gave trisubstituted lactone diastereomers 157 and 158 in 74-92 % yield and 2:1 d.r. (Scheme 44, b) Use of chiral imidazolium 159 with cinnamaldehyde 37a provides the products 157a and 158a in 70 %, 3:1 d.r. and 12 and 25 % ee, respectively (Scheme 44, c).
Scheme 44.
Homoenolate reactivity to form γ-butyrolactones from aryl aldehydes and trifluoromethyl ketones.
Recently, Scheidt and Cohen have demonstrated that a chiral Lewis acid can promote this transformation enantioselectively with an achiral NHC. Using imidazolium precatalyst 45, Taddol-based titanium complex 159 and DBU as the base, cinnamaldehyde can be dimerized to γ-butyrolactone 160 in 60 % yield, 20:1 dr, and 60 % ee (Scheme 45).[58]
Scheme 45.
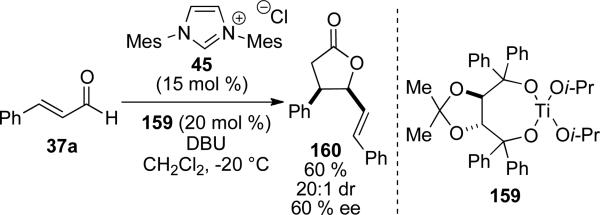
Chiral Lewis acid controlled γ-butyrolactone synthesis.
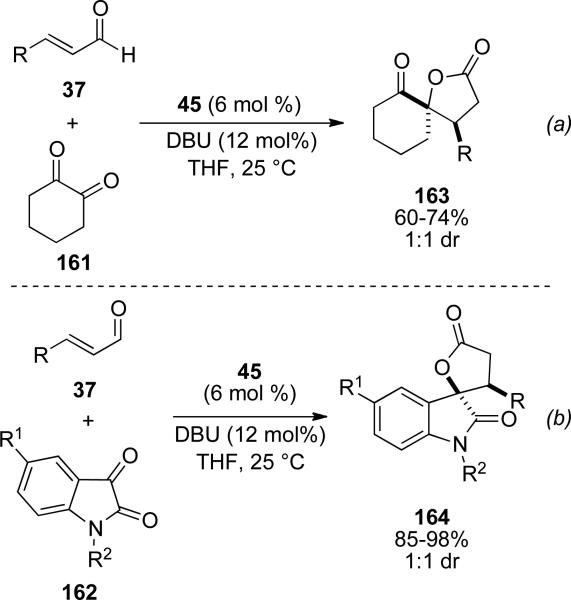
In 2006, Nair and coworkers reported the synthesis of γ-butyrolactones from 1,2-dicarbonyl derivatives facilitated by NHC 57.[59] It was identified that cyclohexane dione 161 and isatins 162 are suitable electrophiles in this process with electron rich and electron withdrawing substituents tolerated on the aromatic enals to provide the respective spiro-cyclohexanone γ-lactones 163 in 60-74 % yield (Scheme 46a), and the spiro isatin γ-lactones 164 in 85-98% yield (Scheme 46b). In both cases, a 1:1 d.r. is obtained. The scope was later extended to incorporate diaryl 1,2-diones.[60]
Scheme 46.
γ-butyrolactone synthesis from 1,2-dicarbonyl compounds.
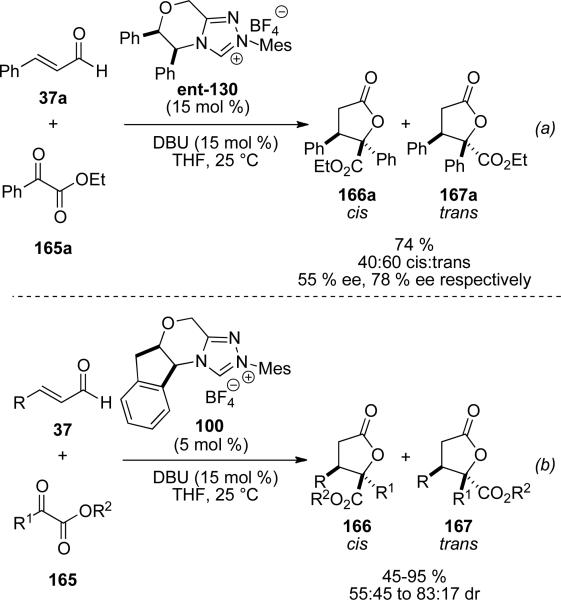
An independent discovery by You demonstrated that glyoxylate derivatives 165 can also undergo reaction with homoenolate equivalents generated from cinnamaldehyde to give γ-butyrolactones.[61] A wide study of chiral NHC precursors revealed NHC 130 as the most enantioselective catalyst giving 78 % ee of the trans isomer (Scheme 47, a). However, this catalyst also gives low diastereoselectivity (60:40 trans:cis). Optimal diastereoselectity was obtained with NHC 100, providing γ-butyrolactone diastereomers 166 and 167 in 45-95 % yield and up to 82:18 selectivity for the cis isomer (Scheme 47, b).
Scheme 47.
Homoenolate addition to glyoxylates.
Nair also discovered an [8+3] annulation of homoenolate equivalents generated by NHC 45 with tropone 168.[62] The reaction proceeds in 39-62 % yield to generate fused δ-lactones 169 (Scheme 48).
Scheme 48.
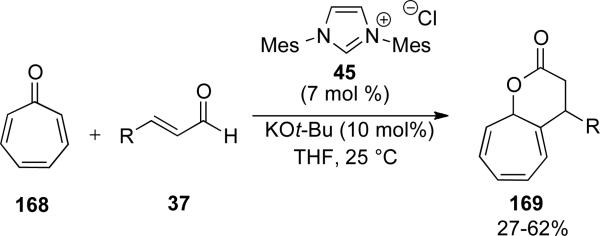
[8+3] annulation of homoenolate with tropone.
5.2 Synthesis of Nitrogen Heterocycles
Recently, a number of reports have described additions of homoenolate equivalents into nitrogen-containing electrophiles followed by displacement of the acyl azolium to give nitrogen heterocycles. This reaction presents a challenge in terms of substrate scope as the NHC itself can react with many such electrophiles. A careful balance must be struck in regard to the reactivity of the electrophile. Regardless, a number of structurally interesting products have been obtained.
The use of imines as electrophiles in this process to form γ-lactams was reported by Bode and He.[63] To overcome inhibition of NHC 46 by the imine itself, para-methoxysulfonylimines 170 were necessary as substrates. It was observed that a balance between electrophilicity and reversibility of the carbene addition was required in the design of the imine. The scope of the reaction encompasses aromatic aldehydes and aryl and heteroaryl imines, giving γ-lactams 171 in 50-73 % yield and 1.7:1 to 10:1 d.r. (Scheme 49).
Scheme 49.
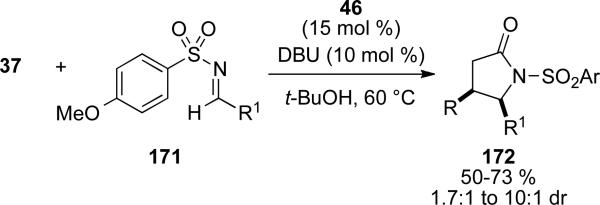
γ-lactam synthesis by homoenolate addition to sulfonylimines.
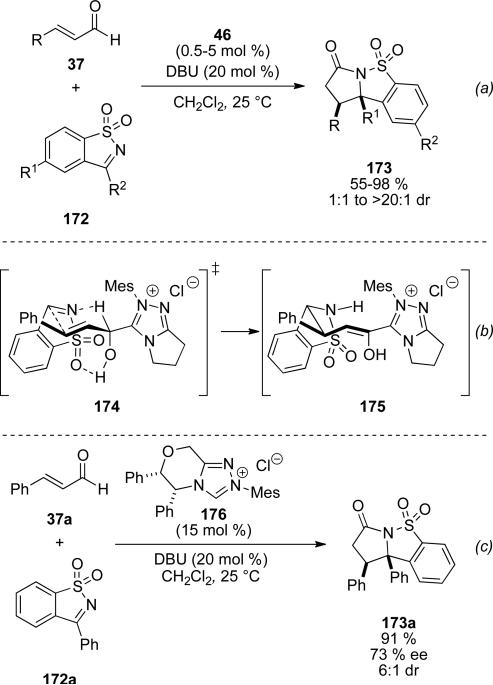
Cyclic sulfonylketimines were also identified to be superior electrophiles by Bode, in the NHC catalyzed homoenolate additions to furnish cyclic suflonyl-γ-lactams.[64] NHC precursor 46 facilitates the reaction with aliphatic and aromatic enals 37 and saccharin-derived imines 172 to give cyclic sulfonamide derivatives 173 in 55-95 % and 1:1 to >20:1 d.r. (Scheme 50, a). Cyclic sulfonyl imines undergo reversible reactions with the NHC catalyst as identified with acyclic variants. To rationalize enhanced reactivity and selectivity observed with the cyclic sulfonyl imine, the authors proposed a transition state analogous to that of the ene reaction in which a hydrogen bond to the sulfonyl oxygen in 174 facilitates proton transfer of the acyl proton to the nitrogen of the sulfonyl imine nitrogen with concomitant C-C bond formation. Tautomerization of enol 175 to the acyl azolium is followed by intramolecular trapping with the amine to give the observed product (Scheme 50, b). The asymmetric variant of this process with chiral, enantioenriched NHC precursor 176 proceeds in 91 % yield, 73 % ee and 6:1 d.r. (Scheme 50, c).
Scheme 50.
Enhanced reactivity with cyclic sulfonylketimines.
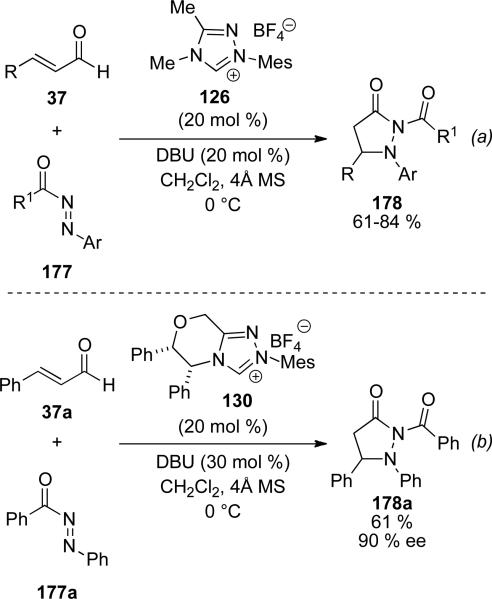
Diazines 177 have also proven suitable electrophiles for homoenolates generated by NHC precursor 126 to provide pyrazolidinones 178 in 61-84 % yield.[65] Electron-donating and electron withdrawing substitutents are tolerated on the aromatic system along with β-alkyl substituents. The diazine component is limited to electron rich aromatic substituents; electron deficient systems do not provide any product. The reaction can be rendered asymmetric with NHC 120 to provide pyrazolidinone 178a in 61 % yield and 90 % ee.
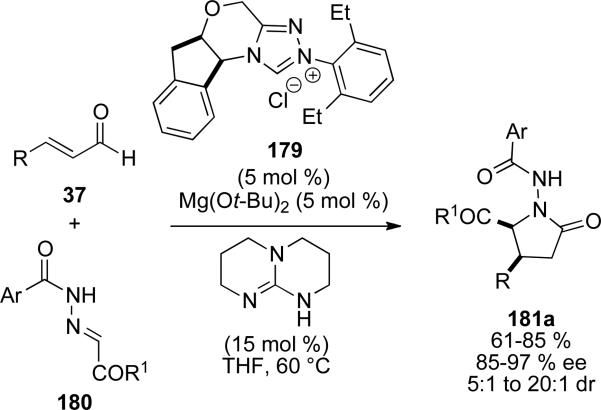
Scheidt and coworkers have reported the Lewis acid catalyzed synthesis of γ-lactams with NHC precursor 179, magnesium tert-butoxide and N-acyl hydrazones 180.[66] Enals bearing both aromatic and aliphatic substituents are tolerated to provide the γ-lactams 181 in 61-85 % yield, 85-97 % ee and 5:1 to >20:1 d.r. (Scheme 52). The authors propose that Lewis acid activation of the N-acyl hydrazone occurs with reversible binding of the carbene to the metal.
Scheme 52.
Synthesis of γ-lactams by homoenolate addition to acylhydrazones.
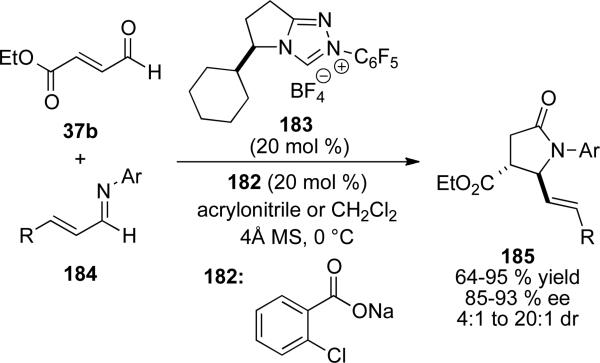
Recently, Rovis and coworkers reported an example of NHC and Brønsted acid cooperative catalysis to give γ-lactams.[67] A catalytic amount of weak base 182 generates the NHC from 183 which forms the extended Breslow intermediate from 37b. Meanwhile, the conjugate acid of 182 can activate the imine 184 for attack selectively over protonation of the homoenolate. In contrast to examples by Bode, Glorius and others which deliver cis-γ-lactones (see Section 5.1), this method furnishes trans γ-lactam products 185 selectively in 64-95 % yield, 85-93 % ee and 4:1 to 20:1 d.r. (Scheme 53).
Scheme 53.
Brønsted acid catalyzed homoenolate addition to imines generating trans-γ-lactams.
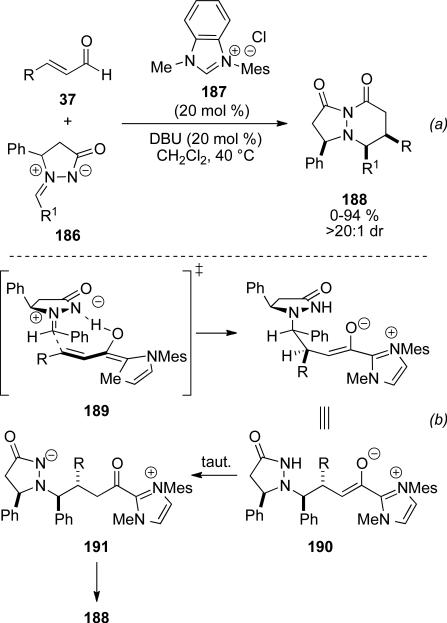
Scheidt and Chan have also identified azomethine ylides 186 as viable electrophiles with enals 37 and NHC 187 to provide pyridazinones 188 with high levels of cis-selectivity.[68] Only enals bearing electron releasing substituents participate in this process. Additionally, dienyl and β-alkyl substituents are tolerated on the enal to provide the pyridazinones in up to 94 % yield, and >20:1 d.r. in each example. (Scheme 54, a). Electron withdrawing and electron donating groups on the imine are tolerated, while 2-substituted aryl substitutents or enolizabile imines do not provide any product. The cis-diastereoselectivity is attributed to a hydrogen bond between extended Breslow intermediate 189 and the azomethine ylide 186 leading to syn-addition (Scheme 55, b). The resultant enolate 190 can tautomerize with proton transfer to acyl azolium 191, which closes to give the observed product.
Scheme 54.
Homoenolate addition to azomethine ylides.
Scheme 55.
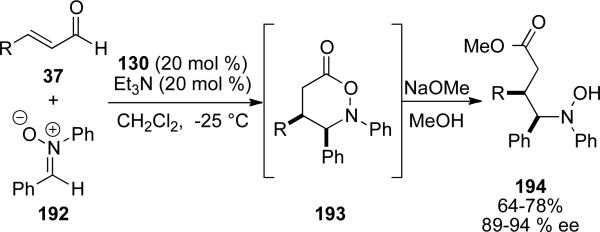
Homoenolate addition to nitrones.
Nitrones as useful electrophiles in the NHC catalyzed homoenolate reaction were demonstrated by Scheidt and coworkers.[69] NHC generated from 130 activates the enal as the homoenolate, which attacks nitrone 192 and tautomerizes to the acyl azolium. The nitrone then closes on the acyl azolium to give 193. The heterocyclic lactone generated from the formal [3+3] cycloaddition then undergoes ring opening upon treatment with alcohols to provide 194 in 64-78 % yield and 89-94 % ee (Scheme 55).
5.3 Synthesis of Carbocycles
Homoenolate intermediates have also been exploited to form carbon-carbon bonds in a number of instances. Cyclopentenes, spirocycles, and densely functionalized cyclopentanes can all be accessed through this reaction manifold.
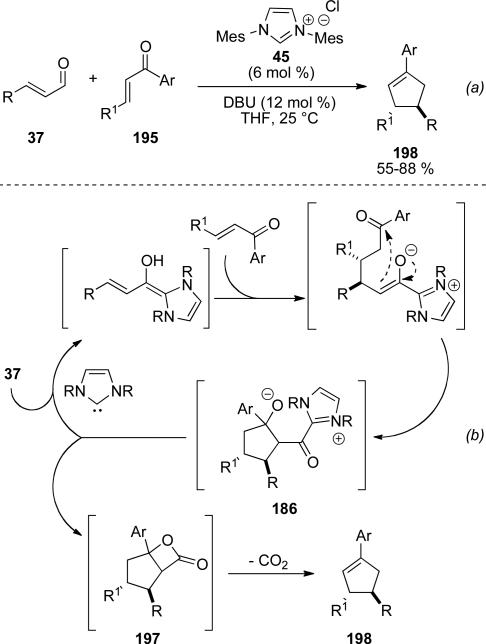
In 2006, Nair and coworkers reported an NHC catalyzed carboannulation giving cyclopentenes from cinnamaldehyde derivatives 37 and chalcones 195 in 55-88 % yield (Scheme 56, a).[70] He proposed a catalytic cycle wherein the extended Breslow intermediate adds in a conjugate fashion to the chalcone, followed by proton transfer and enolate addition to the aryl ketone to give tertiary alkoxide 196. This alkoxide can then displace the azolium to regenerate the NHC and give β-lactone 197. This can then eliminate CO2 to give the observed cyclopentene product 198 (Scheme 56, b).
Scheme 56.
Carboannulation and proposed homoenolate mechanism.
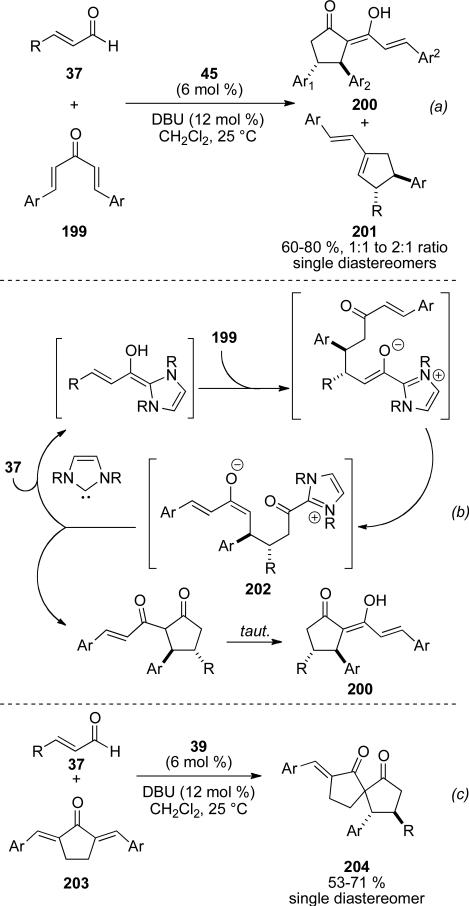
Following his initial report, Nair described the reaction of dienones 199 in this reaction manifold to give both cyclopentanone 200 and cyclopentene products 201 (Scheme 57, a).[71] In this case, intramolecular C-acylation of the acyl azolium 202 leads to the cyclopentenone (Scheme 57, b), while aldol reaction as in the original report leads to the cyclopentene (Scheme 56, b). Dibenzylidene cyclopentanones 203 can also lead to spirocycles 204 (Scheme 57, c).
Scheme 57.
Spirocyclopentane synthesis.
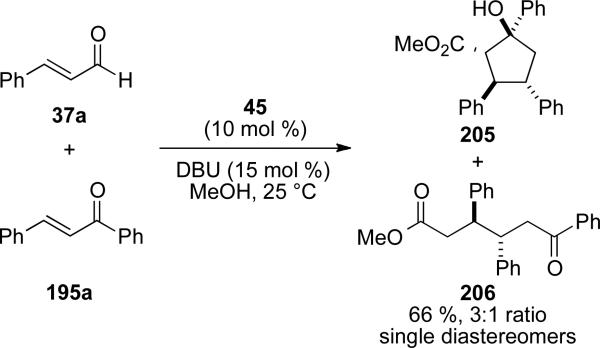
Nair also demonstrated that the intermediate acyl azolium can be intercepted by an external alcohol nucleophile to give cyclopentanol products 205 and acyclic ketoester products 206 (Scheme 58).[72] Each of the products is isolated as a single diastereomer.
Scheme 58.
Cyclopentanol and acyclic ester formation.
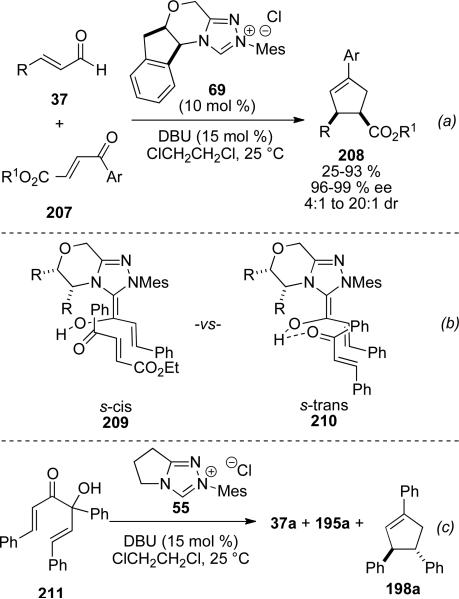
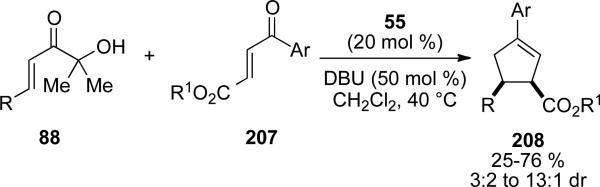
Bode and coworkers reported an enantioselective version of the carboannulation catalyzed by the NHC corresponding to chiral precursor 67. Starting from enones 207 bearing an ester at the β-position, 1,3,4-trisubstituted cyclopentenes 208 can be obtained in 26-93 % yield, 4:1 to >20:1 dr and 96-99 % ee (Scheme 59, a).[73] In contrast to Nair's observation with chalcones, cis-cyclopentene products are formed selectively. Furthermore, mechanistic studies provided evidence that this transformation does not, in fact, proceed through a homoenolate mechanism. Rather, an asymmetric cross-benzoin reaction followed by a stereospecific oxy-Cope rearrangement can give the observed products. The divergent stereoselectivity can be explained by an s-cis conformer 209 being accessible to the ester substrate leading to a boat transition state in the [3,3]-rearrangement. Chalcone substrates are locked in an s-trans conformation 210 and must proceed through a chair transition state to give trans products. Tautomerization and enolate addition to acyl azolium gives the β-lactone 197 proposed by Nair, which eliminates CO2 to the observed cis-cyclopentene product 208. This mechanistic proposal is supported by the fact that α‘-hydroxyenone 211 gives both Nair‘s observed trans product 198a and the starting materials 37a and 195a under the reaction conditions (Scheme 59, c).[74]
Scheme 59.
Asymmetric cyclopentene synthesis.
Working from the proposed reversible benzoin mechanism, Bode discovered that by using α‘-hydroxyenones 88 as substrates, he could expand the scope of the cyclopentene annulation. Substrates 88 can be prepared readily from aromatic aldehydes.
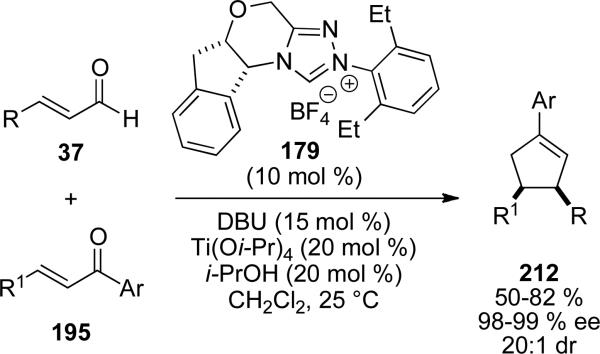
In 2010, Scheidt and coworkers demonstrated that by adding a Lewis acid to the NHC catalyzed carboannulation reaction of chalcones, high selectivity for the cis stereoisomer is obtained rather than the trans selectivity reported by Bode and Nair.[75] Using chiral NHC presursor 179, cis-cyclopentene products 212 are furnished in 50-82 % yield, 20:1 dr, and 98-99 % ee (Scheme 61).
Scheme 61.
Enantioselective cyclopentene synthesis using NHC/Lewis acis cooperative catalysis.
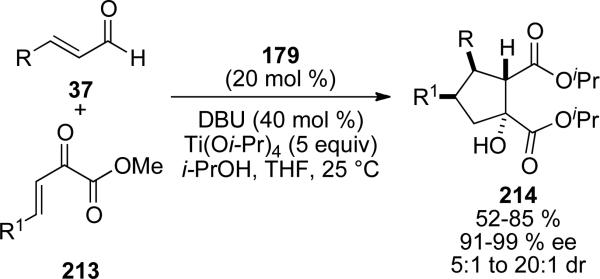
Scheidt also reported the cooperative NHC/Lewis acid catalyzed enantioselective addition of homoenolates derived from cinnamaldehydes 37 to α,β-unsaturated α‘-ketoesters 213. NHC presursor 179 with DBU in isopropanol delivers cyclopentane products 214 in 52-85 % yield, 91-99 % ee and 5:1 to 20:1 d.r. (Scheme 62).[76]
Scheme 62.
Cooperative catalysis to give enantioenriched cyclopentane products.
6 Conclusion
The field of NHC-catalyzed redox reactions has experienced tremendous growth in the eight years since work was first published in this area. This has led to the development of waste minimized acylation processes which provide acess to a valuable array of acyl derivatives. In some very recent and complementary work, Studer has demonstrated that access to acyl azoliums is also possible through oxidation of the Breslow intermediate, allowing similar reactivity without the restrictive need for an a-reducible aldehyde.[77] Additionally, enolate and homoenolate equivalents generated catalytically can lead to the formation of complex heterocycles and carbocycles under mild conditions with high diastereo- and enantioselectivities.
However, as with any method, limitations exist and have been highlighted during the previous studies. Most notably, many of these methods suffer from limited scope. In the case of acylation reactions utilizing acyl azolium intermediates 1, oxygen, sulfur, and nitrogen nucleophiles have been demonstrated as well as intramolecular addition of enolates. On the other hand, reactions of enolate equivalents 2 and homoenolate equivalents 3 are largely limited to a narrow range of substrates owing to the complexity of the catalytic cycles that can lead down many unproductive pathways. Further exploration of catalyst scaffolds is a promising avenue to overcome these limitations. Moreover, the advent of Lewis and Brønsted acid activation in the NHC-mediated redox reaction will no doubt provide reactivity with less specialized substrates and expand the current scope of products available from this promising reaction manifold.
Scheme 18.
Redox esterification of α-Aryloxy aldehydes.
Scheme 22.
Kinetic resolution of secondary amines.
Scheme 29.
Hydration of dichloroaldehydes.
Scheme 33.
Bisulfites as an alternative to chloroaldehydes.
Scheme 37.
Intramolecular Michael reaction.
Scheme 51.
Homoenolate addition to diazines.
Scheme 60.
α‘-hydroxyenone as substrates for NHC catalyzed cyclopentene synthesis.
Acknowledgements
Our work in this area has been generously supported by NIGMS (GM72586). HUV thanks Lilly for a graduate fellowship. PW thanks the National Science Foundation for a graduate research fellowship under grant number 0822211. TR thanks Amgen and Roche for unrestricted support. We thank Donald Gauthier (Merck) for a generous gift of aminoindanol.
Biography
Harit U. Vora was born in Calcutta, India and raised in Tobyhanna, PA. He received his undergraduate education at the University of Pittsburgh, where he carried out research under the guidance of Professor Paul E. Floreancig. He then joined the Medicinal Chemistry Division of Roche USA in Palo Alto, CA. He earned his doctoral degree in 2011 at Colorado State University under the guidance of Professor Tomislav Rovis and is currently a postdoctoral research associate at the Scripps Research Institute with Professor Jin-Quan Yu.
Philip Wheeler was born in Walnut Creek, CA and raised in Northern California. He received his undergraduate degree from the University of California at Santa Cruz after which he joined the Chemical Process Research and Development department at Amgen in Thousand Oaks, CA. He is currently a graduate student in the research group of Prof. Tomislav Rovis.
Tomislav Rovis was born in Zagreb in the former Yugoslavia, but was largely raised in Southern Ontario, Canada. Following his undergraduate studies at the University of Toronto, he earned his Ph.D. degree at the same institution in 1998 under the direction of Professor Mark Lautens. From 1998 to 2000, he was a NSERC postdoctoral fellow at Harvard University with Professor David A. Evans. In 2000, he began his independent career at Colorado State University, and was promoted to Associate Professor in 2005 and Professor in 2008. He currently holds the John K. Stille Chair in Chemistry.
Footnotes
Present address: Department of Chemistry, Colorado State University, Fort Collins, CO 80523, USA
References
- 1.Seebach D. Angew. Chem. Int. Ed. Engl. 1979;18:239–258. [Google Scholar]
- 2.a Zeitler K. Angew. Chem. Int. Ed. 2005;44:7506. doi: 10.1002/anie.200502617. [DOI] [PubMed] [Google Scholar]; b Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]; c Nair V, Vellalath S, Babu BP. Chem. Soc. Rev. 2008;37:2691–2698. doi: 10.1039/b719083m. [DOI] [PubMed] [Google Scholar]; d Read de Alaniz J, Rovis T. Synlett. 2009:1189–1207. doi: 10.1055/s-0029-1216654. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Phillips EM, Chan A, Scheidt KA. Aldrichimica Acta. 2009;43:55–66. [PMC free article] [PubMed] [Google Scholar]; f Moore JL, Rovis T. Top. Curr. Chem. 2010;291:77–144. doi: 10.1007/978-3-642-02815-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Vora HU, Rovis T. Aldrichimica Acta. 2010;44:3–10. [PMC free article] [PubMed] [Google Scholar]; h Nair V, Menon RS, Biju AT, Sinu CR, Paul RR, Jose A, Sreekumar V. Chem. Soc. Rev. 2011;40:5336–5346. doi: 10.1039/c1cs15139h. [DOI] [PubMed] [Google Scholar]; i Chiang P-C, Bode JW. N-Heterocyclic Carbenes. The Royal Society of Chemistry; 2011. pp. 399–435. [Google Scholar]; j Chiang P-C, Bode JW. TCI MAIL. 2011;149:2–17. [Google Scholar]; k Bode JW. Chimia. 2011;65:150–156. doi: 10.2533/chimia.2011.150. [DOI] [PubMed] [Google Scholar]; l Biju AT, Kuhl N, Glorius F. Acc. Chem. Res. 2011;44:1182–1195. doi: 10.1021/ar2000716. [DOI] [PubMed] [Google Scholar]; m Hirano K, Piel I, Glorius F. Chem. Lett. 2011;40:786–791. [Google Scholar]; n Cohen DT, Scheidt KA. Chem. Sci. 2012:53–57. doi: 10.1039/C1SC00621E. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Chiang P-C, Bode JW. Asymmetric Organocatalysis. Vol. 1. Georg Thieme Verlag; Stuttgart: 2012. pp. 639–672. [Google Scholar]
- 3.a Wallach O. Ber. Dtsch. Chem. Ges. 1873:6. [Google Scholar]; b Christmann M. Angew. Chem. Int. Ed. 2010;49:9580–9586. doi: 10.1002/anie.201003155. [DOI] [PubMed] [Google Scholar]
- 4.Kötz A. J. Prakt. Chem. 1913;88:531–522. [Google Scholar]
- 5.Chattaway FD, Irving H. J. Chem. Soc. 1929:1382. [Google Scholar]
- 6.Rosenblum C, Taverna C, Wendler NL. Chem. & Ind. 1960:718. [Google Scholar]
- 7.Cocker W, Lapworth A, Peters AT. J. Chem. Soc. 1931:1382. [Google Scholar]
- 8.Cram D, Hammond G. Organic Chemistry. McGraw-Hill; New York: 1958. [Google Scholar]
- 9.Fodor G, Katritzky AR. Chem. & Ind. 1961:718. [Google Scholar]
- 10.Nowak RM. J. Org. Chem. 1963;28:1182. [Google Scholar]
- 11.a Trost BM. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]; b Trost BM. Angew. Chem. Int. Ed. Engl. 1995;34:259–281. [Google Scholar]
- 12.Burns NZ, Baran PS, Hoffmann RW. Angew. Chem. Int. Ed. 2009;48:2854–2867. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]
- 13.Newhouse T, Baran PS, Hoffmann RW. Chem. Soc. Rev. 2009;38:3010–3021. doi: 10.1039/b821200g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds NT, Read de Alaniz J, Rovis T. J. Am. Chem. Soc. 2004;126:9518–9519. doi: 10.1021/ja046991o. [DOI] [PubMed] [Google Scholar]
- 15.Chow KY-K, Bode JW. J. Am. Chem. Soc. 2004;126:8126–8127. doi: 10.1021/ja047407e. [DOI] [PubMed] [Google Scholar]
- 16.a Breslow R. J. Am. Chem. Soc. 1958;80:3719–3726. [Google Scholar]; b Breslow R, McNelis E. J. Am. Chem. Soc. 1960;82:2394–2395. [Google Scholar]
- 17.Zhao G-L, Córdova A. Tetrahedron Lett. 2007;48:5976. [Google Scholar]
- 18.Jiang H, Gschwend B, Albrecht L, Jørgensen KA. Org. Lett. 2010;12:5052. doi: 10.1021/ol102164y. [DOI] [PubMed] [Google Scholar]
- 19.a Chan A, Scheidt KA. Org. Lett. 2005;7:905–908. doi: 10.1021/ol050100f. [DOI] [PubMed] [Google Scholar]; b Maki BE, Chan A, Scheidt KA. Synthesis. 2008:1306–1315. doi: 10.1055/s-2008-1072516. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Maki BE, Patterson EV, Cramer CJ, Scheidt KA. Org. Lett. 2009;11:3942–3945. doi: 10.1021/ol901545m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn SS, Bode JW. Org. Lett. 2005;7:3873–3876. doi: 10.1021/ol051269w. [DOI] [PubMed] [Google Scholar]
- 21.Zeitler K, Rose CA. J. Org. Chem. 2009;74:1759–1762. doi: 10.1021/jo802285r. [DOI] [PubMed] [Google Scholar]
- 22.Sohn SS, Bode JW. Angew. Chem. Int. Ed. 2006;45:6021–6024. doi: 10.1002/anie.200601919. [DOI] [PubMed] [Google Scholar]
- 23.Li G-Q, Dai L-X, You S-L. Org. Lett. 2009;11:1623–1625. doi: 10.1021/ol9002898. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Thai K, Gravel M. Org. Lett. 2009;11:891–893. doi: 10.1021/ol8029005. [DOI] [PubMed] [Google Scholar]
- 25.Zeitler K. Org. Lett. 2006;8:637–640. doi: 10.1021/ol052826h. [DOI] [PubMed] [Google Scholar]
- 26.Kaeobamrung J, Mahatthananchai J, Zheng P, Bode JW. J. Am. Chem. Soc. 2010;132:8810–8812. doi: 10.1021/ja103631u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AD, Ling KB. Chem. Commun. 2011;47:373–375. doi: 10.1039/c0cc02456b. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds NT, Rovis T. J. Am. Chem. Soc. 2005;127:16406–16407. doi: 10.1021/ja055918a. [DOI] [PubMed] [Google Scholar]
- 29.Vora HU, Rovis T. J. Am. Chem. Soc. 2007;129:13796–13797. doi: 10.1021/ja0764052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bode JW, Sohn SS. J. Am. Chem. Soc. 2007;129:13798–13799. doi: 10.1021/ja0768136. [DOI] [PubMed] [Google Scholar]
- 31.Chiang P-C, Kim Y, Bode JW. Chem. Commun. 2009:4566–4568. doi: 10.1039/b909360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binanzer M, Hsieh S-Y, Bode JW. J. Am. Chem. Soc. 2011;49:19698–19701. doi: 10.1021/ja209472h. [DOI] [PubMed] [Google Scholar]
- 33.Li G-Q, Li Y, Dai L-X, You S-L. Org. Lett. 2007;9:3519–3521. doi: 10.1021/ol0713537. [DOI] [PubMed] [Google Scholar]
- 34.Li G-Q, Li Y, Dai L-X, You S-L. Adv. Synth. Catal. 2008;350:1258–1262. [Google Scholar]
- 35.Thai K, Wang L, Dudding T, Bilodeau F, Gravel M. Org. Lett. 2010;12:5708–5711. doi: 10.1021/ol102536s. [DOI] [PubMed] [Google Scholar]
- 36.Kuwano S, Harada S, Oriez R, Yamada KI. Chem. Commun. 2012:145–147. doi: 10.1039/c1cc15539c. [DOI] [PubMed] [Google Scholar]
- 37.a Wang B, Forsyth CJ. Synthesis. 2009:2873–2880. [Google Scholar]; b Forsyth CJ, Wang B, Huang PH, Chen CS. J. Org. Chem. 2011;76:1140–1150. doi: 10.1021/jo102478x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vora HU, Moncecchi JR, Epstein O, Rovis T. J. Org. Chem. 2008;73:9727–9731. doi: 10.1021/jo8020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daigo K, Reed LJ. J. Am. Chem. Soc. 1962;84:659–662. [Google Scholar]
- 40.White FG, Ingraham LL. J. Am. Chem. Soc. 1962;84:3109–3111. [Google Scholar]
- 41.Bruice TC, Kundu NG. J. Am. Chem. Soc. 1966;88:4097–4098. [Google Scholar]
- 42.a Owen TC, Richards A. J. Am. Chem. Soc. 1987;119:2520–2521. [Google Scholar]; b Owen TC, Harris JN. J. Am. Chem. Soc. 1990;122:6136–6137. [Google Scholar]
- 43.Mahatthananchai J, Zheng P, Bode JW. Angew. Chem. Int. Ed. 2011;50:1673–1677. doi: 10.1002/anie.201005352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vora HU, Rovis T. J. Am. Chem. Soc. 2010;132:2860–2861. doi: 10.1021/ja910281s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He M, Uc GJ, Bode JW. J. Am. Chem. Soc. 2006;128:15088–15089. doi: 10.1021/ja066380r. [DOI] [PubMed] [Google Scholar]
- 46.He M, Beahm BJ, Bode JW. Org. Lett. 2008;10:3817–3820. doi: 10.1021/ol801502h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaeobamrung J, Kozlowski MC, Bode JW. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20661–20665. doi: 10.1073/pnas.1007469107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang X, Chen X, Chi RY. Org. Lett. 2011;13:4708–4711. doi: 10.1021/ol201917u. [DOI] [PubMed] [Google Scholar]
- 49.Phillips EM, Wadamoto M, Roth HS, Ott AW, Scheidt KA. Org. Lett. 2009;11:105–108. doi: 10.1021/ol802448c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips EM, Wadamoto M, Chan A, Scheidt KA. Angew. Chem. Int. Ed. 2007;46:3107–3110. doi: 10.1002/anie.200605235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.a Wadamoto M, Phillips EM, Reynolds TE, Scheidt KA. J. Am. Chem. Soc. 2007;129:10098–10099. doi: 10.1021/ja073987e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Phillips EM, Wadamoto M, Scheidt KA. Synthesis. 2009:687–690. doi: 10.1055/s-0028-1083284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaeobamrung J, Bode JW. Org. Lett. 2009;11:677–680. doi: 10.1021/ol802739d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He M, Struble JR, Bode JW. J. Am. Chem. Soc. 2006;128:8418–8420. doi: 10.1021/ja062707c. [DOI] [PubMed] [Google Scholar]
- 54.He M, Bode JW. J. Am. Chem. Soc. 2008;130:418–419. doi: 10.1021/ja0778592. [DOI] [PubMed] [Google Scholar]
- 55.Kawanaka Y, Phillips EM, Scheidt KA. J. Am. Chem. Soc. 2009;131:18028–18029. doi: 10.1021/ja9094044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sohn SS, Rosen EL, Bode JW. J. Am. Chem. Soc. 2004;126:14370–14371. doi: 10.1021/ja044714b. [DOI] [PubMed] [Google Scholar]
- 57.a Burstein C, Glorius F. Angew. Chem. Int. Ed. 2004;43:6205–6208. doi: 10.1002/anie.200461572. [DOI] [PubMed] [Google Scholar]; b Burstein C, Tschan S, Xie X, Glorius F. Synthesis. 2006:2418–2439. [Google Scholar]
- 58.Cohen DT, Scheidt KA. Chem. Sci. 2012:53–57. doi: 10.1039/C1SC00621E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nair V, Vellalath S, Poonoth M, Mohan R, Suresh E. Org. Lett. 2006;8:507–509. doi: 10.1021/ol052926n. [DOI] [PubMed] [Google Scholar]
- 60.Nair V, Vellalath S, Poonoth M, Suresh E, Viji S. Synthesis. 2007:3195–3200. [Google Scholar]
- 61.Li Y, Zhao Z-A, He H, You S-L. Adv. Synth. Catal. 2008;350:1885–1890. [Google Scholar]
- 62.Nair V, Poonoth M, Vellalath S, Suresh E, Thirumalai R. J. Org. Chem. 2006:8964–8965. doi: 10.1021/jo0615706. [DOI] [PubMed] [Google Scholar]
- 63.He M, Bode JW. Org. Lett. 2005;7:3131–3134. doi: 10.1021/ol051234w. [DOI] [PubMed] [Google Scholar]
- 64.Rommel M, Fukuzumi T, Bode JW. J. Am. Chem. Soc. 2008;130:17266–17267. doi: 10.1021/ja807937m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan A, Scheidt KA. J. Am. Chem. Soc. 2008;130:2740–2741. doi: 10.1021/ja711130p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raup DEA, Cardinal-David B, Holte D, Scheidt KA. Nat. Chem. 2010;2:766–771. doi: 10.1038/nchem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X, DiRocco DA, Rovis T. J. Am. Chem. Soc. 2011;133:12466–12469. doi: 10.1021/ja205714g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan A, Scheidt KA. J. Am. Chem. Soc. 2007;129:5334–5335. doi: 10.1021/ja0709167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phillips EM, Reynolds TE, Scheidt KA. J. Am. Chem. Soc. 2008;130:2416–2417. doi: 10.1021/ja710521m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nair V, Vellalath S, Poonoth M, Suresh E. J. Am. Chem. Soc. 2006;128:8736–8737. doi: 10.1021/ja0625677. [DOI] [PubMed] [Google Scholar]
- 71.a Nair V, Babu BP, Vellalath S, Suresh E. Chem. Commun. 2008:747–749. doi: 10.1039/b715733a. [DOI] [PubMed] [Google Scholar]; b Verma P, Patni PA, Sunoj RB. J. Org. Chem. 2011;76:5606–5613. doi: 10.1021/jo200560t. [DOI] [PubMed] [Google Scholar]
- 72.Nair V, Babu BP, Vellalath S, Varghese V, Raveendran AE, Suresh E. Org. Lett. 2009;11:2507–2510. doi: 10.1021/ol900571x. [DOI] [PubMed] [Google Scholar]
- 73.Chiang P-C, Kaeobamrung J, Bode JW. J. Am. Chem. Soc. 2007;129:3520–3521. doi: 10.1021/ja0705543. [DOI] [PubMed] [Google Scholar]
- 74.Chiang P-C, Rommel M, Bode JW. J. Am. Chem. Soc. 2009;131:8714–8718. doi: 10.1021/ja902143w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cardinal-David B, Raup DEA, Scheidt KA. J. Am. Chem. Soc. 2010:5345–5347. doi: 10.1021/ja910666n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen DT, Cardinal-David B, Scheidt KA. Angew. Chem. Int. Ed. 2011;50:1678–1682. doi: 10.1002/anie.201005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.a De Sarkar S, Studer A. Org. Lett. 2010;12:1992–1995. doi: 10.1021/ol1004643. [DOI] [PubMed] [Google Scholar]; b De Sarkar S, Grimme S, Studer A. J. Am. Chem. Soc. 2010;132:1190–1191. doi: 10.1021/ja910540j. [DOI] [PubMed] [Google Scholar]; c De Sarkar S, Biswas A, Song CH, Studer A. Synthesis. 2011:1974–1983. [Google Scholar]