Abstract
Udenafil is a potent novel phosphodiesterase-5 inhibitor approved for use in Korea. Udenafil has unique properties, with a T max of 1.0–1.5 h and a T 1/2 of 11–13 h (a relatively rapid onset and a long duration of action). Therefore, both on-demand and once-daily use of udenafil have been reported. Udenafil’s efficacy and tolerability have been evaluated in several studies, and recent and continuing studies have demonstrated udenafil’s promise in both dosing regimens. Presently, tadalafil is the only FDA-approved drug for daily dosing, but udenafil can be used as a once-daily dose for erectile dysfunction patients who cannot tolerate tadalafil due to phosphodiesterase subtype selectivity. Udenafil as an on-demand or once-daily dose is effective and tolerable, but more studies are needed in patients of other ethnicities and with comorbid conditions such as diabetes mellitus, hypertension, and benign prostate hyperplasia.
Keywords: Erectile dysfuction, Once-daily dosing, Phosphodiesterase type 5, Udenafil
Introduction
Erectile dysfunction (ED) is defined as the inability to achieve and maintain a sufficient erection to permit satisfactory intercourse [Montorsi et al. 2010]. Numerous strategies have been used to overcome ED. Therapies for ED include intracavernosal injection, vacuum erection devices, intraurethral suppositories, penile prosthesis surgery and oral phosphodiesterase-5 (PDE5) inhibitors [Dinsmore and Evans, 1999]. Oral PDE5-inhibitor medications have revolutionized the treatment of ED. Men prefer oral medications as the first-line therapeutic option in the absence of a specific contraindication to their use [Ding et al. 2012].
There are currently four PDE5 inhibitors (sildenafil, vardenafil, tadalafil, and avanafil) approved worldwide for the treatment of male erectile dysfunction, with two other agents (udenafil and mirodenafil) currently approved only in Korea [Bell and Palmer, 2011]. The choice of PDE5 inhibitor for each patient should be determined after physician and patient discuss the characteristics of different drugs and the individual patient’s sexual habits, preferences, and expectations [Hatzimouratidis et al. 2010]. There are two types of treatment usage of PDE5 inhibitors according to their pharmacological characteristics. On-demand treatment of ED with PDE5 inhibitors allows the patient to have intercourse within 1 hour, but can remove spontaneity from sexual activity and be burdensome to patients and their partners [Hanson-Divers et al. 1998]. Once-daily dosing of a PDE5 inhibitor is an alternative for couples that prefer spontaneous sexual activities.
A new oral selective PDE5 inhibitor, udenafil (Zydena, Dong-A, Seoul, Korea), has recently been developed for the treatment of ED. Udenafil is a novel pyrazolopyrimidinone compound developed by Dong-A Pharmaceutical Co., Ltd (Seoul, Korea) for the treatment of ED which has the same mechanism of action as sildenafil [Kim et al. 2008]. Udenafil is rapidly absorbed, reaching peak plasma concentrations at 0.8–1.3 h, then declining monoexponentially with a terminal half-life (T 1/2) between 7.3 and 12.1 hours, giving it the unique pharmacokinetics of both relatively rapid onset and long duration [Salem et al. 2006]. Thus, both on-demand treatment and once-daily dosing have been reported in the literature. The purpose of this review is to evaluate the efficacy and tolerability of udenafil for patients with ED according to the currently available literature.
Chemistry and physiology
Sexual stimulation induces local release of nitric oxide (NO) by nerve endings within the corpora cavernosa. NO then activates guanylate cyclase, increasing levels of guanosine 3′, 5′-cyclic monophosphate (cGMP) [Lue, 2000]. Increased cGMP leads to cellular hyperpolarization and decreased cytoplasmic calcium, which in turn stimulates smooth muscle relaxation [Dean and Lue, 2005]. PDE5 predominates in the corpus cavernosum and catalyzes the breakdown of cGMP. PDE5 inhibitors therefore maintain penile erection by blocking the breakdown of cGMP [Dean and Lue, 2005].
The chemical designation of udenafil is 5-[2-propyloxy-5-(1-methyl-2-pyrollidiny lethylamidosulphonyl) phenhyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo(4,3-d)-pyrimidin-7-one (Zydena®; Dong-A Pharmaceutical Company, Seoul, Korea) [Kim et al. 2008]. Udenafil is a newly developed PDE5 inhibitor that is both potent and selective and has a similar molecular structure to sildenafil citrate (Viagra®) (Figure 1). An in vitro study of udenafil has shown it to be comparable to sildenafil (Viagra®) in selectivity to PDE5 [Doh et al. 2002]. Udenafil has also been shown in preclinical studies to have potent erectogenic properties in rats and rabbits with a broad safety margin [Salem et al. 2006]. In addition, unlike tadalafil, udenafil does not inhibit PDE11 [Doh et al. 2002; Salem et al. 2006].
Figure 1.
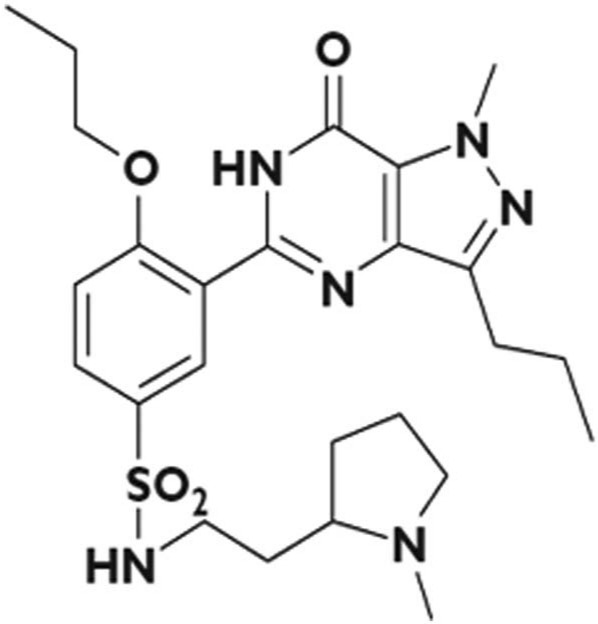
Chemical structure of udenafil.
Pharmacokinetics: phase I study
In 2008, Kim and colleagues published the safety, tolerability, and pharmacokinetics of udenafil [Kim et al. 2008]. This was a double-blind, randomized, placebo-controlled, dose-rising, parallel-group, single- and multiple-dose study in healthy Korean subjects. In the single-dose study, subjects were randomly assigned to one of five dosage groups (25, 50, 100, 200, or 300 mg). The multiple-dose study was performed after the single-dose study. Subjects were randomly assigned to one of two dose groups (100 or 200 mg). Based on this clinical phase I trial in healthy male subjects, udenafil was found to be rapidly absorbed, reaching maximum plasma concentrations (C max) at 0.8–1.3 h, then declining monoexponentially with a T 1/2 between 7.3 and 12.1 h in the single-dose group. This half-life is significantly longer than that of sildenafil or vardenafil, but shorter than that of tadalafil [Corona et al. 2011]. Onset of action was comparable with sildenafil and vardenafil, but significantly shorter than tadalafil. Its pharmacokinetic profile shows the unique clinical properties of both relatively rapid onset and long duration of action. In the multiple-dose study, the mean concentration–time profiles showed that udenafil had reached steady state by day five. This was confirmed by using a paired t-test between the dose-normalized concentration on day five and that on day six. The concentration–time profiles on day seven were comparable to those of day one. Pharmacokinetic parameters exhibited similar individual values for C max and T max between day one and day seven.
In the single-dose study, the common adverse events (AEs) which were potentially related to udenafil were headache, penile erection, and facial flushing. In the multiple-dose study, there were seven moderate AEs. The common AEs potentially related to udenafil were headache, penile erection, facial flushing, and febrile sensation. There were no AEs of ‘moderate’ or ‘severe’ intensity in the single-dose study, and no AEs of ‘severe’ intensity in the multiple-dose study. In the multiple-dose study, a color discrimination abnormality was detected which affected the ‘blue–green’ region of the spectrum. However, this abnormality spontaneously resolved upon successive testing (1.5 h after the fourth dose). There were no clinically significant findings in the routine laboratory parameters, including platelet aggregation test, bleeding time, or semen analysis. In addition, vital signs taken in a sitting posture demonstrated no clinically significant changes in arterial blood pressure (BP) or total pulse rate between the udenafil group and placebo group.
Clinical efficacy and tolerability
Phase II study
Zhao and colleagues reported a randomized, double-blind, placebo-controlled, fix-dosed clinical trial involving 237 patients with ED [Zhao et al. 2011]. The subjects were assigned randomly to receive placebo or udenafil (25, 50, or 75 mg once-daily dosing [lower doses compared with the 100 or 200 mg on-demand doses]) with an interval of 24 hours. They were then asked to complete the International Index of Erectile Function (IIEF), the Sexual Encounter Profile (SEP) diary, and the Global Assessment Questionnaire (GAQ) during the study. Primary outcomes were changes from baseline of the IIEF erectile function domain (EFD) scores. The secondary outcome parameters were SEP question two (‘Were you able to insert your penis into your partner’s vagina?’) and question three (‘Did your erection last long enough for you to complete intercourse with ejaculation?’), the shift to normal rate (EFD≥26), and the response to the GAQ (‘Has the treatment you have been taking during the study improved your erections?’). Compared with placebo, patients who took 50 or 75 mg of udenafil had a significantly improved IIEF-EFD score. However, no significant difference existed in the udenafil (25 mg) versus placebo groups. Similar results were observed when comparing questions two and three in the SEP diary and the GAQ.
With regard to AEs and safety, udenafil was well tolerated in general. Most AEs were mild or moderate in severity. The most commonly reported treatment-related AE was flushing. No clinically significant changes in laboratory tests, ECGs, or BP were observed in the udenafil groups.
Phase III studies
The efficacy and safety of udenafil for treating men with ED was definitively demonstrated by Paick and colleagues in 2008 in a phase III, multicenter, randomized, double-blind, placebo-controlled, fixed-dose, parallel-group, fixed-dose comparison [Paick et al. 2008]. In this study, 167 patients with ED of diverse origin and severity were randomized to take placebo or udenafil at fixed doses of 100 or 200 mg as needed for 12 weeks. After 12 weeks of treatment, the patients treated with udenafil showed significantly greater change from baseline in the IIEF-EFD score compared with placebo. The rates of successful penetration (SEP Q2) were 53.4% for the placebo, 88.8% for the 100 mg, and 92.4% for the 200 mg udenafil groups. The rates of successful maintenance of erection (SEP Q3) were 70.1% in the 100 mg udenafil group and 75.7% in the 200 mg udenafil group, compared with 15.4% for the placebo group. Furthermore, the proportion of ‘yes’ responses to GAQ was 81.5% in the 100 mg udenafil group and 88.5% in the 200 mg udenafil group, which were both significantly higher than the 25.9% of the placebo group. Treatment-related AEs were generally mild to moderate, with facial flushing and headache being the most common.
In 2010, Park and colleagues demonstrated the efficacy of udenafil in treating ED for up to 12 hours after dosing [Park et al. 2010]. This was a randomized, double-blind, placebo-controlled, parallel-group, fixed-dose, multicenter study. A total of 104 men with ED of broad etiology and severity were randomized to take placebo or 100 mg udenafil. Participants were requested to attempt sexual intercourse at 12 hours after udenafil or placebo dosing during a 4-week treatment period. The primary efficacy variable was SEP Q3 and the secondary efficacy measures were SEP Q2. Additional secondary efficacy measures included changes from baseline in IIEF-EFD. The rates of successful maintenance of erection (SEP Q3) were 54.7% with udenafil compared with 28.3% for the placebo. For SEP Q2, the mean per-patient proportions of successful penetration attempts were 73.2% for the placebo group and 82.3% for the udenafil group. In addition, significant change was observed from baseline in the IIEF-EFD in the udenafil group (placebo, −0.58 + 0.67 versus udenafil, 4.40 + 0.84; p < 0.0001). In this study, most AEs were mild or moderate in severity and commonly reported treatment-related AEs included stomach discomfort, flushing, headache, and nasal congestion. There were no serious AEs during the study and the follow-up period. Table 1 shows the efficacy of the udenafil in phase II and III studies.
Table 1.
Efficacy of udenafil in phase II and III studies.
| Reference, Study design | Dosage, usage | Intervention (n) | Change from IIEF domain score | SEP Q2, % Change from baseline percentage | SEP Q3, % Change from baseline percentage | Response to GAQ, % | Shift to normal rate (EFD>26), % |
|---|---|---|---|---|---|---|---|
| Zhao et al. [2011], Multicenter randomized double-blind, placebo-controlled phase II trial | 25 mg, once-daily dose | 59 | Subgroup analysis | 22.10 | 42.09* | 69.5* | 30.5* |
| 50 mg, once-daily dose | 60 | Subgroup analysis | 27.9* | 51.41* | 75* | 40* | |
| 75 mg, once-daily dose | 59 | Subgroup analysis | 39.11* | 73.5* | 88.1* | 44.1* | |
| Paick et al. [2008], Multicenter, double-blind, placebo-controlled, fixed-dose, parallel group phase III trial | 100 mg, on demand | 56 | 7.52 ± 0.87 | 31.18* | 52.94* | 81.5* | 35.2* |
| 200 mg, on demand | 54 | 9.93 ± 0.94* | 28.97* | 66.44* | 88.5* | 48.1* | |
| Park et al. [2010], Multicenter randomized, double-blind, placebo-controlled, parallel-group, fixed dose phase III trial | 100 mg, once 12 hours before sexual intercourse | 53 | 4.40 ± 0.84* | 7.27 | 35.63%* | 82.27 | 24.5* |
IIEF, International Index of Erectile Function; SEP, Sexual Encounter Profile diary; GAQ, Global Assessment Questionnaire; EFD, erectile function domain
Statistically significant p < 0.01.
ED with frequent comorbid conditions
ED with diabetes mellitus
Moon and colleagues demonstrated the safety and efficacy of udenafil for the treatment of ED in a larger number of patients with diabetes mellitus (DM) in 2011 [Moon et al. 2011]. A placebo-controlled, randomized, double-blind, double-dummy, parallel-group, fixed-dose, multicenter study was conducted in 174 ED patients with DM.
The primary efficacy parameter was a change from baseline in the IIEF-EFD at 12 weeks. Secondary efficacy parameters were IIEF Q3 and Q4, SEP Q2 and Q3, rate of achieving normal erectile function (EFD ≥ 26), and response to GAQ. IIEF-EFD scores at baseline were significantly improved in both 100 and 200 mg udenafil groups, with improvements by ≥7 and ≥8 points, respectively, at week 12 compared with placebo. However, there was no significant dose-related difference between the udenafil 100 and 200 mg groups. For secondary efficacy variables, including the comparison of Q3 and Q4 of IIEF, SEP diary, and GAQ, statistically significant intergroup differences were also observed in the udenafil group as compared with the placebo group.
In this study, the udenafil treatment groups showed significantly higher rates of AEs compared with the placebo group, but there were no significant differences between the udenafil 100 and 200 mg groups. All AEs were mild in severity and did not require any specific action. The most frequent treatment related AEs were flushing and headache with incidences of 10% and 5% for each treatment group, respectively. Table 2 shows the efficacy of the udenafil in ED patients with comorbid conditions such as DM, hypertension (HTN) and benign prostatic hyperplasia (BPH)/lower urinary tract symptoms (LUTS).
Table 2.
Efficacy of udenafil in erectile dysfunction with comorbid conditions.
| Reference, Study design | Comorbid condition, Dosage | Intervention (n) | Change from IIEF domain score | SEP Q2, % Change from baseline percentage | SEP Q3, % Change from baseline percentage | Response to GAQ, % | Shift to normal rate (EFD >26), % |
|---|---|---|---|---|---|---|---|
| Moon et al. [2011], Multicenter, placebo-controlled, randomized, double-blind, double-dummy, parallel-grouped trial | DM, 100 mg | 55 | 7.00* | 23.84* | 45.97* | 65.5 | NR |
| DM, 200 mg | 58 | 8.21* | 31.07* | 55.56* | 83.9 | NR | |
| Paick et al. [2009]Multicenter, randomized, double-blind, placebo-controlled, fixed-dose trial | HTN 100 mg | 52 | 8.71* | 25.85* | 29.82* | 78.8* | 44.2* |
| HTN 200 mg | 55 | 10.04* | 57.88* | 71.11* | 85.2* | 54.5* | |
| Chung et al. [2009] Open, prospective non-comparative study | BPH/LUTS 100 mg | 120 | 6.37* | NR | NR | NR | NR |
IIEF, International Index of Erectile Function; SEP, Sexual Encounter Profile diary; GAQ, Global Assessment Questionnaire; EFD, erectile function domain; DM, diabetes mellitus; HTN, hypertension; BPH, benign prostatic hyperplasia; LUTS, lower urinary tract symptoms; NR, not reported
Statistically significant p < 0.01.
ED with HTN
In 2009, Paick and colleagues published a report regarding the safety and efficacy of udenafil in ED patients with HTN [Paick et al. 2009]. A multicenter, randomized, double-blind, placebo-controlled, fix-dosed clinical trial was conducted among 165 ED patients receiving antihypertensive medications.
After 12 weeks of treatment, changes in the mean IIEF-EFD score were 22.9 ± 6.2 in the 100 mg and 24.3 ± 6.5 in the 200 mg udenafil groups, compared with 18.0 ± 7.4 in the placebo group. Udenafil significantly improved all tested secondary efficacy parameters, including the comparison of Q3 and Q4 of IIEF, SEP diary, and GAQ.
With regard to BP and heart rate (HR), significant reduction of diastolic blood pressure (DBP) was noted in both sitting and standing positions after the placebo treatment. However, no patient experienced any symptoms related to BP change. HR in the 100 mg udenafil group was elevated, with mean HR in the sitting position significantly changed from 71.6 to 74.5 bpm after 12 weeks of treatment. However, udenafil treatment did not result in laboratory examination or electrocardiogram. Headache and flushing were the most common treatment-related AEs, which were transient and mild to moderate in severity. No parameters of efficacy and safety were affected among the subsets, which were stratified according to either the number of antihypertensive medication received or previous experience of PDE5 inhibitor treatment. In this study, two patients stopped medication due to AEs such as moderate conjunctival hyperemia (one case) and mild headache and facial flushing (one case), which spontaneously resolved after discontinuation of udenafil.
ED with BPH
In 2009, Chung and colleagues reported an open, prospective, noncomparative study conducted in two urology centers in Korea [Chung et al. 2009]. A total of 120 patients with BPH and ED were enrolled in their study. α-blocker therapy for BPH and 100 mg udenafil for ED were co-administered for 8 weeks. The primary pharmacodynamic endpoint was mean change of BP and HR. Clinical efficacy was evaluated every 4 weeks by changes in the international prostatic symptom score (IPSS) and the international index of ED (IIEF-5). BP and HR were not significantly changed, whereas IPSS and IIEF-5 scores were significantly improved compared with baseline (IPSS 14.3–11.5, IIEF-5 11.95–18.32, p < 0.05). All AEs were mild, and the common AEs were hot flushes, headaches, and a sensation of fever. In addition, co-administration of udenafil and an α-blocker did not result in severe hypotension or syncope. Table 3 summarizes the incidence of udenafil-related AEs in phase II and III studies and ED patients with comorbid conditions.
Table 3.
Incidence of udenafil-related adverse events.
| Reference, Study type | Dosage, usage | Comorbid condition, visit | Patients with ≥1 event, N (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Any AE | SAE | Flushing | Headache | Dyspepsia | Visual disturbance | |||
| Zhao et al. [2011], Phase II trial | 25 mg, once-daily dose | 8.5 | NR | 1.7 | NR | NR | 1.7 | |
| 50 mg, once-daily dose | 23.7 | NR | 8.3 | 1.7 | NR | NR | ||
| 75 mg, once-daily dose | 22.0 | NR | 6.8 | 3.4 | NR | NR | ||
| Paick et al. [2008], Phase III trial | 100 mg, on demand | 19.3 | 0 | 10.5 | 1.8 | 0 | 0 | |
| 200 mg, on demand | 37.5 | 0 | 23.2 | 8.9 | 0 | 0 | ||
| Park et al. [2010], Phase III trial | 100 mg, once 12 hours before sexual intercourse | 11.3 | 0 | 1.89 | 1.89 | 3.77 | NR | |
| Moon et al. [2011] | 100 mg, on demand | DM | 15.8 | 0 | 10 | 5 | NR | NR |
| 200 mg | 22.4 | 0 | 10 | 5 | NR | NR | ||
| Paick et al. [2009] | 100 mg, on demand | HTN | NR | NR | 5.7 | 1.9 | 1.9 | NR |
| 200 mg, on demand | NR | NR | 5.3 | 8.8 | 5.3 | NR | ||
| Chung et al. [2009] | 100 mg, on demand | BPH/LUTS Visit at 4 weeks | 23.3 | 0 | 14.2 | 5.0 | 1.67 | 0 |
AE, adverse event; SAE, serious adverse event; NR, not reported; DM, diabetes mellitus; HTN, hypertension; BPH, benign prostatic hyperplasia; LUTS, lower urinary tract symptoms.
Comments
Since sildenafil was first introduced for the treatment of ED, PDE5 inhibitors have been used as the first-line treatment for ED. Currently, four PDE5 inhibitors, (sildenafil, vardenafil, tadalafil and avanafil) are approved worldwide and two agents (udenafil and mirodenafil) are approved in Korea only [Bell and Palmer, 2011].
Each drug appeals to different patients, and a drug should be chosen based on its individual merits. Sildenafil and vardenafil have relatively short onset times, while tadalafil has a longer duration [Corona et al. 2011]. PDE5 inhibitors which have shorter onset times are better for patients who desire on-demand treatment, whereas PDE5 inhibitors which have longer durations might be more suited to once-daily treatment. Although PDE5 inhibitors radically improved the symptoms of ED and are currently first line of treatment for ED, on-demand treatment of ED with PDE5 inhibitor is not ideal for patients with ED because there is no spontaneity in sexual activity [Hanson-Divers et al. 1998]. In this respect, once-daily dosing of PDE5 inhibitor is a good alternative for couples that do not want scheduled, but rather more natural, sexual activities [Corona et al. 2011].
Tadalafil has recently been approved for once-daily treatment, and is a promising treatment due to its long duration of action. In addition, recent clinical trials have reported that daily dosing of PDE5 inhibitors shows more effective treatment results compared to on-demand dosing [Fusco et al. 2010]. According to preclinical studies, the pharmacokinetic profile of udenafil includes a T max of 1.0–1.5 h and a T 1/2 of 11–13 h. This relatively rapid onset and long duration of action are unique clinical properties [Kim et al. 2008]. Owing to this relatively longer duration, once-daily udenafil dosing has been studied previously [Zhao et al. 2011]. In 2011, Zhao and colleagues published a study examining the efficacy and safety of once-daily dosing of udenafil in the treatment of ED [Zhao et al. 2011]. The subjects were treated with udenafil (25, 50, or 75 mg) or placebo once daily for 12 weeks and then asked to complete IIEF, SEP diary, and GAQ during the study. Compared with placebo, patients who took 50 or 75 mg of udenafil had a significantly improved IIEF-EFD score. Similar results were observed when comparing Q2 and Q3 in the SEP diary and GAQ. Currently, tadalafil (5 mg) is the only drug currently approved for daily administration to treat ED. However, tadalafil has a lower selectivity to PDE11 compared with other PDE5 inhibitors such as sildenafil, vardenafil, and udenafil. In isoenzyme selectivity, udenafil (selectivity ratio: 96) showed much higher PDE11 selectivity compared with tadalafil (selectivity ratio: 7.1) [Kouvelas et al. 2009]. This shortcoming of tadalafil could be an advantage for udenafil in the market. In recent meta-analysis, the efficacy of on-demand treatment showed comparable efficacy with sildenafil and vardenafil [Ding et al. 2012]. In addition, Zhao and colleagues reported similar changes in IIEF-EFD scores and in the rate of response to SEP Q2 and Q3 compared with once-daily dosing of tadalafil for 12 weeks [Zhao et al. 2011]. In their study, udenafil treatment also improved the mean sexual desire domain score at 12 weeks. Taking these results together, udenafil seems to be an ideal drug, due to short onset time and long duration. However, udenafil has a longer onset time compared with vardenafil and a shorter duration compared with tadalafil. When patients choose a PDE5 inhibitor, many factors must be taken into consideration, including age, frequency of sexual activity, food and alcohol intake, and partner habits [Corona et al. 2011]. Corona and colleagues suggested that a long-acting PDE5 inhibitor should be considered for elderly men who frequently have sexual intercourse in the morning after waking up and patients who anticipate frequent sexual intercourse [Corona et al. 2011]. Zhao and colleagues reported that the efficacy of udenafil with once-daily dosing is similar to tadalafil, and has more PDE11 selectivity [Zhao et al. 2011]. If some patients wish to receive a long-acting PDE5 inhibitor, but experience side effects with tadalafil, udenafil might be a more appropriate option.
In patients with DM, erectile function is affected by various etiologies including vascular, neurogenic, drug-induced, and psychogenic factors, and male patients with DM have been reported to experience more severe ED symptoms [Moon et al. 2011]. Moon and colleagues reported that IIEF-EFD scores at baseline were significantly improved in both udenafil 100 mg and 200 mg groups, with improvements by ≥7 and ≥8 points, respectively, at week 12 compared with placebo [Moon et al. 2011]. With respect to GAQ score, 83.9% in udenafil 200 mg group answered ‘yes’. This result was comparable to 73.6% in the group taking sildenafil and 64% in the group taking 20 mg tadalafil. In addition, they found that the percentage of subjects with an improvement in IIEF-EFD to normal levels (score ≥26) is very important. The percentage of subjects with an improvement in erectile function to normal level at week 12 were 38.2% in the udenafil 100 mg and 44.8% in the 200 mg group, compared with 3.6% in the placebo group. The researchers reported that this result indicated statistically significant improvements following udenafil treatment. Vardi and Nini reported that sildenafil improved the IIEF-EFD score by an average 7.83 points [Vardi and Nini, 2007]. Tadalafil and vardenafil resulted in an average of 3.39 and 3.93 points of improvement, respectively [Vardi and Nini, 2007]. Ding and colleagues reported that udenafil improved the IIEF-EFD score by an average of 4.06 points in their meta-analysis, results that were comparable with tadalafil and vardenafil [Ding et al. 2012].
Patients with HTN frequently suffer from ED, and it has been reported that 52–68% men with HTN also have ED [Burchardt et al. 2000; Chew et al. 2000]. Paick et al. reported that patients taking udenafil improved in the rate of response to SEP Q2 and Q3 by 29.8% and 71.1%, respectively [Paick et al. 2009]. This result was comparable with or a greater improvement than with other PDE5 inhibitors (for SEP Q2 and Q3, tadalafil, 34% and 45%; vardenafil, 33% and 49%, respectively). With respect to GAQ, udenafil showed acceptable improvement when compared with other PDE5 inhibitors (udenafil versus placebo, 85.2% versus 41.2; tadalafil versus placebo, 87% versus 33%; vardenafil versus placebo, 80% versus 40%, respectively). However, treatment response was not different between the 100 and 200 mg groups. This result could be due to insufficient power to detect differences between doses. For hypertensive patients with ED, Shabsigh and colleagues reported that vardenafil’s efficacy increased an average of 8.9 points in the IIEF erectile function at week 12 compared with placebo in meta-analysis of 2427 patients [Shabsigh et al. 2007]. Ding and colleagues reported that udenafil treatment resulted in an average increase of 5.58 points [Ding et al. 2012]. In patients with HTN and ED, additional studies will need to be conducted with a larger number of patients to obtain more objective results for udenafil.
ED, BPH, and LUTS increase with age and can decrease quality of life for aging men [Braun et al. 2000; Hoesl et al. 2005]. Recent studies revealed that PDE5 inhibitors could improve LUTS because there may be a common pathophysiological mechanism between BPH and ED [Carson, 2006; Giuliano, 2008]. In a recent meta-analysis, the use of PDE5 inhibitors alone significantly improved IIEF score (+5.5; p < 0.0001) and IPSS score (–2.8; p < 0.0001) but not the maximum flow rate at the end of the study as compared with placebo [Gacci et al. 2012]. Co-administration of an α-blocker and PDE5 inhibitors improved the IIEF score (+3.6; p < 0.0001), IPSS score (–1.8; p = 0.05), and Q max (+1.5; p < 0.0001) at the end of the study compared with α-blockers alone. Chung and colleagues reported the efficacy of udenafil in men with ED with BPH/LUTS [Chung et al. 2009]. In their report, BP and HR were not significantly changed, whereas IPSS and IIEF-5 scores were significantly improved compared with baseline (IPSS –2.8, IIEF-5 +6.37, p < 0.05). However, their study had several limitations, which included lack of a placebo control group and intermittent udenafil administration design. Additional randomized clinical trials will be needed to clarify the definite effect of udenafil in ED for BPH/LUTS patients.
With respect to udenafil-related AEs, AEs were dose-dependent across treatments in a phase I trial [Kim et al. 2008]. However, no AEs were severe in nature, and all spontaneously resolved without any treatment. In general, udenafil was well tolerated in the healthy subjects, and no serious AEs occurred in the phase I trial. In the studies reviewed here, the most common AEs were flushing and headache. Headache and facial flushing were frequently reported for other PDE5 inhibitors, which are pharmacologically associated with vasodilation. Recently, Tsertsvadze and colleagues reported a meta-analysis of sildenafil, including 49 randomized clinical trials [Tsertsvadze et al. 2009]. In their review, the most frequently reported AEs were headache, flushing, dyspepsia, and visual disturbances compared with placebo-treated men. This indicated that the AEs of udenafil are more comparable to sildenafil than tadalafil [Ding et al. 2012]. However, this vasodilatory effect of udenafil did not seem to result in hemodynamic problems [Chung et al. 2009; Kim et al. 2008; Paick et al. 2009]. In a phase I study, ABP and TPR between the udenafil and placebo groups exhibited little difference, indicating there were no clinically significant changes. In addition, co-administration of a HTN drug and udenafil did not increase vasodilatory symptoms (headache, flushing) or the occurrence of significant hypotensive symptoms (dizziness, faintness, vertigo) [Paick et al. 2009].
To the best of the authors’ knowledge, very few post-marketing side effects were reported as associated with udenafil. There was a case report of a visual field defect associated with udenafil. Kim and Kim reported the first literature case of anterior ischemic optic neuropathy (AION) associated with udenafil [Kim and Kim, 2012]. Although the disc swelling spontaneously resolved without any medication, the patient had to discontinue the use of udenafil for 1 month. Previously, several cases reported sildenafil-associated AION and a recurrent visual field defect and ischemic optic neuropathy associated with tadalafil rechallenge. However, this was the first AION report related to udenafil [Bollinger and Lee, 2005; Cunningham and Smith, 2001]. HTN, high cholesterol, smoking, and decreased cup-to-disc ratio are known risk factors for AION [Hayreh et al. 1994]. Therefore, if patients present with an acute onset visual field defect after using PDE5 inhibitors, including udenafil, medication should be discontinued and ophthalmology consult obtained [Kim and Kim, 2012].
There are several limitations in most reports related to udenafil. Although udenafil seems to be generally well tolerated, most studies have been done in Korean populations. Further studies are needed that investigate the effects in other ethnicities. In addition, few studies include comorbid conditions such as DM, HTN, and BPH when comparing udenafil with sildenafil or tadalafil. Reliable conclusions cannot be reached through these few studies. This study also has a methodological limitation. Owing to a lack of randomized and comparative studies with sildenafil, the reference PDE5 inhibitor drug, this study was dependent only on the review of the currently available studies. With regard to once-daily dosing, tadalafil 5 mg is the only drug currently approved for daily administration for ED treatment. In the same way, there is no comparative trial between tadalafil 5 mg and udenafil 50mg. To more fairly investigate the udenafil, randomized comparative studies between drugs are needed.
Conclusions
The literature reviewed here indicates that udenafil seems to be safe and well tolerated in general ED patients in Korea. In addition, udenafil seems to be an ideal PDE5 inhibitor, with both short onset time and long duration. However, udenafil has a longer onset time compared with vardenafil and a shorter duration compared with tadalafil. Patients who want to choose a long-acting PDE5 inhibitor, but experience side effects with tadalafil, should consider udenafil. If udenafil continues to perform well among the available PDE5 inhibitors, more studies will be needed, not only in the Korean population, but also among other ethnicities, in order to validate udenafil’s efficacy and AEs.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Conflict of interest statement
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Sung Gu Kang, Department of Urology, Korea University School of Medicine.
Je Jong Kim, Department of Urology, Korea University School of Medicine, 126-1, Anam-dong 5-ga, Sungbuk-gu, Seoul 136-705, Republic of Korea.
References
- Bell A., Palmer M. (2011) Novel phosphodiesterase type 5 modulators: a patent survey (2008–2010). Expert Opin Therapeut Patents 21: 1631–1641 [DOI] [PubMed] [Google Scholar]
- Bollinger K., Lee M. (2005) Recurrent visual field defect and ischemic optic neuropathy associated with tadalafil rechallenge. Arch Ophthalmol 123: 400–401 [DOI] [PubMed] [Google Scholar]
- Braun M., Wassmer G., Klotz T., Reifenrath B., Mathers M., Engelmann U. (2000) Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey’. Int J Impotence Res 12: 305–311 [DOI] [PubMed] [Google Scholar]
- Burchardt M., Burchardt T., Baer L., Kiss A., Pawar R., Shabsigh A., et al. (2000) Hypertension is associated with severe erectile dysfunction. J Urol 164: 1188–1191 [PubMed] [Google Scholar]
- Carson C. (2006) Combination of phosphodiesterase-5 inhibitors and alpha-blockers in patients with benign prostatic hyperplasia: treatments of lower urinary tract symptoms, erectile dysfunction, or both? BJU Int 97(Suppl. 2): 39–43; discussion 44–35. [DOI] [PubMed] [Google Scholar]
- Chew K., Earle C., Stuckey B., Jamrozik K., Keogh E. (2000) Erectile dysfunction in general medicine practice: prevalence and clinical correlates. Int J Impotence Res 12: 41–45 [DOI] [PubMed] [Google Scholar]
- Chung B., Lee J., Lee S., Yoo S., Lee S., Oh C. (2009) Safety and efficacy of the simultaneous administration of udenafil and an alpha-blocker in men with erectile dysfunction concomitant with BPH/LUTS. Int J Impotence Res 21: 122–128 [DOI] [PubMed] [Google Scholar]
- Corona G., Mondaini N., Ungar A., Razzoli E., Rossi A., Fusco F. (2011) Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: the proper drug for the proper patient; J Sexual Med 8: 3418–3432 [DOI] [PubMed] [Google Scholar]
- Cunningham A., Smith K. (2001) Anterior ischemic optic neuropathy associated with Viagra. J Neuro-ophthalmol 21: 22–25 [DOI] [PubMed] [Google Scholar]
- Dean R., Lue T. (2005) Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin N Amer 32: 379–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Du W., Wang H., Zhang L., Wang Z., Du C., et al. (2012) Efficacy and safety of udenafil for erectile dysfunction: a meta-analysis of randomized controlled trials. Urology: 80: 134–139 [DOI] [PubMed] [Google Scholar]
- Dinsmore W., Evans C. (1999) ABC of sexual health: erectile dysfunction. BMJ 318: 387–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doh H., Shin C., Son M., Ko J., Yoo M., Kim S., et al. (2002) Mechanism of erectogenic effect of the selective phosphodiesterase type 5 inhibitor, DA-8159. Arch Pharm Res 25: 873–878 [DOI] [PubMed] [Google Scholar]
- Fusco F., Razzoli E., Imbimbo C., Rossi A., Verze P., Mirone V. (2010) A new era in the treatment of erectile dysfunction: chronic phosphodiesterase type 5 inhibition. BJU Int 105: 1634–1639 [DOI] [PubMed] [Google Scholar]
- Gacci M., Corona G., Salvi M., Vignozzi L., McVary K., Kaplan S., et al. (2012) A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with alpha-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol 61: 994–1003 [DOI] [PubMed] [Google Scholar]
- Giuliano F. (2008) Lower urinary tract symptoms and sexual dysfunction: a common approach. BJU Int 101(Suppl. 3): 22–26 [DOI] [PubMed] [Google Scholar]
- Hanson-Divers C., Jackson S., Lue T., Crawford S., Rosen R. (1998) Health outcomes variables important to patients in the treatment of erectile dysfunction. J Urol 159: 1541–1547 [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K., Amar E., Eardley I., Giuliano F., Hatzichristou D., Montorsi F., et al. (2010) Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol 57: 804–814 [DOI] [PubMed] [Google Scholar]
- Hayreh S., Joos K., Podhajsky P., Long C. (1994) Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Amer J Ophthalmol 118: 766–780 [DOI] [PubMed] [Google Scholar]
- Hoesl C., Woll E., Burkart M., Altwein J. (2005) Erectile dysfunction (ED) is prevalent, bothersome and underdiagnosed in patients consulting urologists for benign prostatic syndrome (BPS). Eur Urol 47: 511–517 [DOI] [PubMed] [Google Scholar]
- Kim B., Lim H., Chung J., Kim J., Lim K., Sohn D., et al. (2008) Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young Korean subjects. Br J Clin Pharmacol 65: 848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Kim D. (2012) Anterior ischemic optic neuropathy associated with udenafil. Korean J Ophthalmol 26: 235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvelas D., Goulas A., Papazisis G., Sardeli C., Pourzitaki C. (2009) PDE5 inhibitors: in vitro and in vivo pharmacological profile. Curr Pharm Des 15: 3464–3475 [DOI] [PubMed] [Google Scholar]
- Lue T. (2000) Erectile dysfunction. N Engl J Med 342: 1802–1813 [DOI] [PubMed] [Google Scholar]
- Montorsi F., Adaikan G., Becher E., Giuliano F., Khoury S., Lue T., et al. (2010) Summary of the recommendations on sexual dysfunctions in men. J Sexual Med 7: 3572–3588 [DOI] [PubMed] [Google Scholar]
- Moon D.G., Yang D., Lee C., Ahn T., Min K., Park K., et al. (2011) A therapeutic confirmatory study to assess the safety and efficacy of Zydena (udenafil) for the treatment of erectile dysfunction in male patients with diabetes mellitus. J Sexual Med 8: 2048–2061 [DOI] [PubMed] [Google Scholar]
- Paick J., Kim S., Park Y., Hyun J., Park N., Lee S., et al. (2009) The efficacy and safety of udenafil [Zydena] for the treatment of erectile dysfunction in hypertensive men taking concomitant antihypertensive agents. J Sexual Med 6(11): 3166–3176 [DOI] [PubMed] [Google Scholar]
- Paick J., Kim S., Yang D., Kim J., Lee S., Ahn T., et al. (2008) The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. J Sexual Med 5: 946–953 [DOI] [PubMed] [Google Scholar]
- Park H.J., Park J.K, Park K., Min K., Park N.C. (2010) Efficacy of udenafil for the treatment of erectile dysfunction up to 12 hours after dosing: a randomized placebo-controlled trial. J Sexual Med 7: 2209–2216 [DOI] [PubMed] [Google Scholar]
- Salem E., Kendirci M., Hellstrom W. (2006) Udenafil, a long-acting PDE5 inhibitor for erectile dysfunction. Curr Opin Investigational Drugs 7: 661–669 [PubMed] [Google Scholar]
- Shabsigh R., Duval S., Shah M., Regan T., Juhasz M., Veltry L.G. (2007) Efficacy of vardenafil for the treatment of erectile dysfunction in men with hypertension: a meta-analysis of clinical trial data. Curr Med Res Opin 23: 2453–2460 [DOI] [PubMed] [Google Scholar]
- Tsertsvadze A., Yazdi F., Fink H., MacDonald R., Wilt T., Bella A., et al. (2009) Oral sildenafil citrate (Viagra) for erectile dysfunction: a systematic review and meta-analysis of harms. Urology 74: 831–836 e838. [DOI] [PubMed] [Google Scholar]
- Vardi M., Nini A. (2007) Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus. Cochrane Database Systematic Rev 1: CD002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Kim S., Yang D., Kim J., Park N., Lee S., et al. (2011) Efficacy and safety of once-daily dosing of udenafil in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol 60: 380–387 [DOI] [PubMed] [Google Scholar]


