Abstract
Adiponectin is a predominantly anti-inflammatory protein produced by adipose tissue with possible signalling activity in the lung. It is increasingly associated with inflammatory pulmonary diseases, such as asthma and chronic obstructive pulmonary disease (COPD), and in critical illness. Although mouse studies indicate causative associations between adiponectin and asthma and COPD, the human literature in this regard is inconclusive. Some, but not all, studies demonstrate that serum adiponectin concentrations are inversely associated with asthma prevalence among premenopausal women and peripubertal girls. On the other hand, serum adiponectin concentrations are associated with lower asthma severity among boys but greater severity among men. Further, case-control studies demonstrate higher systemic and airway adiponectin concentrations in primarily male COPD patients than controls. Systemic adiponectin is positively associated with lung function in healthy adults but inversely associated in studies of male subjects with COPD. Murine and human studies further show contradictory associations of systemic adiponectin with critical illness. Higher premorbid systemic adiponectin concentrations are associated with improved survival from sepsis in mice. On the other hand, higher systemic adiponectin concentrations on day 1 of critical illness are associated with lower survival in critically ill patients with respiratory failure. In the absence of adequate longitudinal data, it is not possible to determine whether the adiponectin derangements are the consequence or the cause of the disease studied. Future research will determine whether modulation of adiponectin, independent of BMI, may be helpful in the prevention or treatment of asthma, COPD or critical illness.
Keywords: Asthma, COPD, Sepsis, Respiratory Failure, Adiponectin, Lung function
Introduction
Adipose tissue produces over 50 proteins called adipokines that act as signalling molecules with effects on a wide array of bodily processes. Adiponectin, one such adipokine, plays an important role in the regulation of diabetes mellitus and atherosclerotic cardiovascular disease [1]. The possible role for adiponectin in inflammatory pulmonary diseases, such as asthma and chronic obstructive pulmonary disease (COPD), and in critical illness has been the subject of recent investigations.
Despite being produced by adipose tissue, systemic adiponectin concentrations are inversely correlated with body mass index (BMI) [2, 3]. One explanation for this surprising finding is that adipose tissue in the obese experiences localized hypoxia that inhibits its expression of adiponectin [4]. The hypoxia-induced necrosis of adipocytes attracts activated macrophages that collect to form functional syncytia surrounding the necrotizing adipocytes [5]. These syncytia produce tumor necrosis factor - alpha (TNF-α) and interleukin (IL)-6 which may inhibit the local production of adiponectin by the adipose tissue in a paracrine fashion [6].
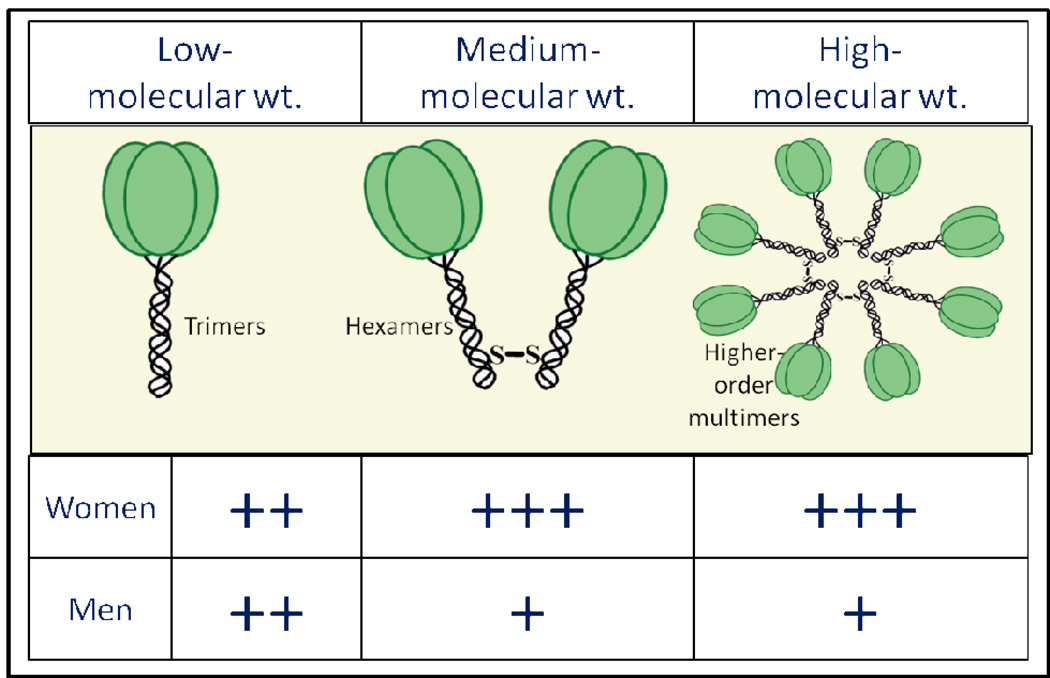
Circulating adiponectin includes three distinct isoforms – low molecular weight (trimers); medium-molecular weight (hexamers) and high-molecular weight (HMW or higher order multimers), as shown in Figure 1. Three monomers of adiponectin form a trimer. Trimers linked by a disulfide bond form a hexamer. Several linked hexamers and trimers constitute the higher multimeric form. Systemic concentrations of the HMW isoform are disproportionately reduced in obesity. Thus, the proportion of HMW isoform to total adiponectin is lower among obese individuals than healthy controls. Interestingly, there is also a marked sexual dimorphism of the distribution of adiponectin isoforms in humans [7]. Compared to men, women have higher circulating concentrations of total adiponectin and higher proportions of its HMW isoform, despite greater levels of overall adiposity [8, 9]. It is believed that testosterone production during puberty in men lowers their systemic adiponectin concentrations, particularly the HMW isoform [8]. The testosterone role is supported by castration experiments in mice that result in an increase of total adiponectin, particularly of the HMW isoform [10]. On the other hand, postmenopausal women have higher circulating concentrations of total adiponectin and higher proportions of its HMW isoform than premenopausal women, with a negative association of circulating concentrations of total and HMW adiponectin with female sex steroids (estradiol and progesterone) among women [11]. In vitro experiments offer supporting evidence that estrogen decreases adiponectin expression in adipocytes [12]. Further, the various isoforms may vary in their potency of effect [13]. For instance, the HMW isoform is the most biologically active form of adiponectin in regulating insulin resistance [14]. It is unclear however whether the HMW isoform has a specific role in pulmonary diseases and critical illness.
Figure 1.
Schematic representation of the sexual dimorphism of the absolute concentrations of the circulating adiponectin isoforms. Compared to men, women have higher absolute concentrations of circulating total adiponectin and all its isoforms. When the isoforms are expressed as a proportion of the total, women have higher proportions of high and medium molecular-weight isoforms but a lower proportion of the low molecular-weight isoform than men. The figure summarizes the data published previously by Peake et al. [9]
Most research indicates that the primary effect of adiponectin on inflammatory processes is to inhibit pro-inflammatory mediators (TNF-α, IL-6, endothelial adhesion molecules ICAM-1 and VCAM-1, and nuclear factor-κB) [15–19] as well as promote anti-inflammatory mediators (IL-10 and IL-1 receptor antagonist) [19–21]. In vitro studies indicate that adiponectin can also bind bacterial lipopolysaccharides [22] providing additional down-regulation of inflammation in infectious states. Despite these observations across multiple studies, adiponectin has been found to paradoxically exhibit pro-inflammatory effects under certain conditions [23, 24].
Both adiponectin and its multiple receptors (AdipoR1, AdipoR2, T-cadherin, and calreticulin) are expressed on various cell types in the lung [25–28]. In addition, adiponectin is transported from blood into the alveolar lining fluid via the T-cadherin molecule on the endothelium [25]. It is therefore possible that the lung is a target organ for adiponectin signaling and consequently, adiponectin derangements may be associated with diseases of the lung.
Adiponectin and Asthma
Although mouse studies show a causative association [29, 30], the human literature regarding the association between adiponectin and asthma is limited and contradictory.
Mouse studies
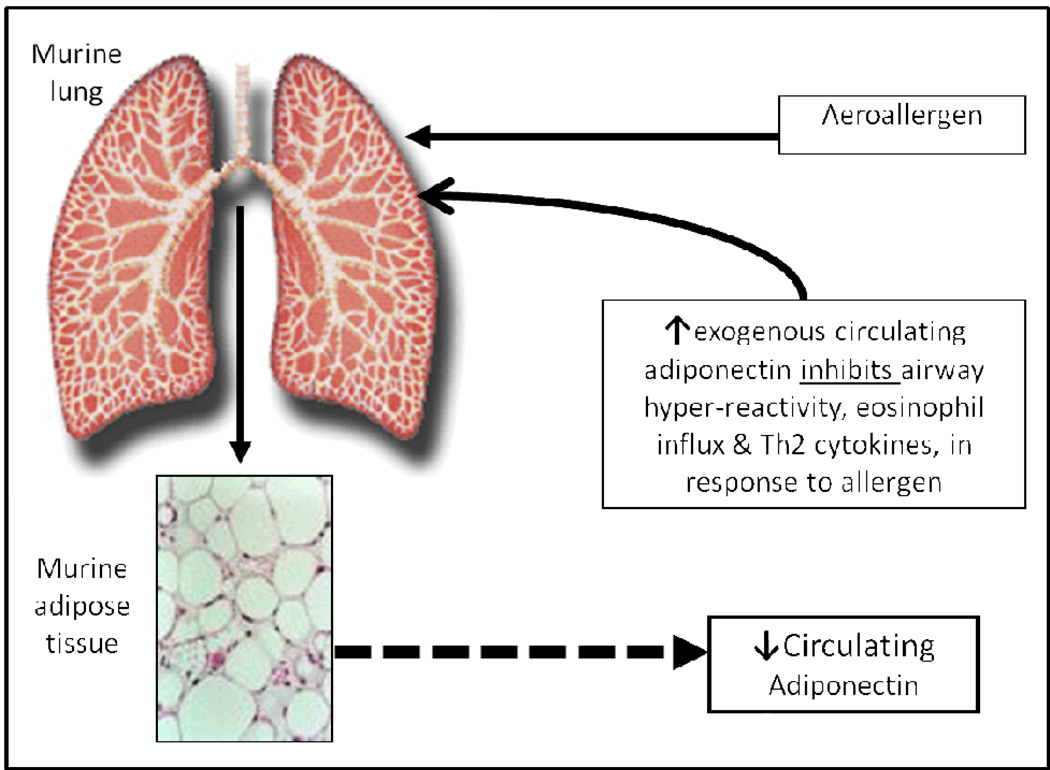
Allergen bronchoprovocation of sensitized BALB/cJ mice reduces both the adiponectin production from adipose tissue as well as the pulmonary expression of adiponectin receptor mRNA [29]. On the other hand, exogenous adiponectin infusion attenuates allergic airway inflammation and airway hyperresponsiveness in the same mouse model [29]. These findings are supported by a separate model of genetically adiponectin-deficient mice that demonstrates greater allergic airway inflammation in response to allergen bronchoprovocation than wild-type mice [30].
The adiponectin-asthma relationship in mice is bidirectional (Figure 2), whereby allergen inhalation affects serum adiponectin concentrations and exogenous adiponectin administration affects asthma. In humans with mild atopic asthma, bronchoprovocation from inhalational allergen challenge does not acutely affect serum adiponectin concentrations – thus suggesting that what is true in mice may not be entirely true in humans [31].
Figure 2.
A schematic representation of the bidirectional association between adiponectin and asthma, based upon the murine research by Shore et al. [29]. Figure as originally published in Sood et al. (2011) Serum Adiponectin is Associated with Adverse Outcomes of Asthma in Men but Not in Women Front Pharmacol 2:55. doi: 10.3389/fphar.2011.00055 [23].
Human Studies
Current human data regarding the independent association between serum adiponectin and asthma prevalence or severity remain inconclusive, although there may be more consistency in the association observed among children as compared to adults. Furthermore, the obesity-asthma association does not appear to be explained by serum adiponectin alone [32], implying multiplicity of mechanistic pathways for the obesity asthma association.
Asthma prevalence
Some, but not all, studies demonstrate that serum adiponectin concentrations are inversely associated with asthma prevalence among premenopausal women and peripubertal girls. Studies suggesting such subgroup effects however have not confirmed them with statistically significant interactions. Nevertheless, the modification of physiological effect of adiponectin by female sex hormones is tantalizing. The studies are further complicated because of their use of varying definitions for the diagnosis of asthma and its severity.
One U.S.-based cross-sectional study showed a protective association between serum adiponectin concentrations and odds for clinically diagnosed asthma in premenopausal women, independent of BMI [32]. An unpublished longitudinal study involving the same cohort further showed that the inverse association between serum adiponectin and risk of incident asthma in women was stronger among current smokers [23]. In that study, low serum adiponectin was also found to be more important than BMI in predicting the risk of incident asthma among women [23]. These findings were however not confirmed for similarly defined asthma by either Sutherland et al. in a population-based birth cohort of approximately 1,000 young adult New Zealanders [33] or by Jartti et al. [34] in sequential cross-sectional studies of a Finnish cohort of children and adults. Surprisingly, men in the Sutherland study demonstrated a positive association with bronchodilator responsiveness, an asthma marker, while simultaneously showing an inverse association with exhaled nitric oxide, a different asthma marker associated with airway inflammation [33]. Several small case-control studies have shown no difference after adjusting for obesity in either serum or bronchoalveolar lavage fluid adiponectin concentrations between adult asthmatics and controls [35–37]. On the other hand, among children, Nagel et al. demonstrated a protective association between serum adiponectin and odds for asthma in peripubertal girls, independent of BMI; this effect was stronger in nonatopic girls [38]. These results again could not be confirmed by a Korean study of similarly-aged children but mostly boys [39].
Asthma Severity
Although data are again limited and contradictory, the majority of evidence indicates that serum adiponectin concentrations are associated with lower asthma severity among boys and paradoxically with higher asthma severity among men.
In a large community-based cross-sectional study of American adults, Sood et al. showed that higher serum adiponectin concentrations were independently associated with adverse clinical outcomes of asthma (such as asthma-related symptoms, medications and disease activity) among men but not women. This is the only study in the literature that confirms the presence of statistically significant sex-specific interactions for adiponectin in asthma [23]. On the other hand, in a small clinic-based case-control study of American adults, Holguin et al. showed no association between concentrations of serum or bronchoalveolar lavage fluid adiponectin and of lung inflammatory biomarkers [37].
Studies of asthma severity among children demonstrate a clearer association with adiponectin than adults. Serum adiponectin concentrations were associated with less severe exercise-induced bronchoconstriction in a study of pre-pubertal asthmatic children, mostly boys, after adjusting for BMI [40]. Serum adiponectin concentrations were also associated with fewer maximum asthma symptom days, fewer exacerbations, and higher FEV1/FVC ratio in a study including 14-year old boys with moderate to severe asthma [41]. Similarly, serum adiponectin concentrations were positively correlated with FEF25–75% in another study of prepubertal and peripubertal children, mostly boys [39]. While it is possible that the conflicting findings between men and boys are due to significant methodological differences between the studies, testosterone-related changes in total adiponectin and adiponectin isoform distribution (towards a lower proportion of HMW isoform) in men compared to boys may also play a role [8, 42].
Longitudinal and interventional studies examining the effect of change in serum adiponectin on asthma severity are limited. A recent study found that a one-year weight reduction interdisciplinary intervention resulted in an increase in lung function and serum total adiponectin among post-pubertal obese adolescents with and without asthma as well as improved asthma severity in the asthma subgroup [43]. More importantly, the increase in serum adiponectin predicted improved lung function among both subgroups, independent of age and sex. One limitation of this multivariable analysis was that the predictive effect of change in serum adiponectin was not examined independent of the associated change in BMI.
To summarize, although adiponectin and its receptors are expressed in human airway cells, the adiponectin-asthma association in humans is currently controversial. Certain studies demonstrate that serum adiponectin concentrations are inversely associated with asthma prevalence among premenopausal women and peripubertal girls. On the other hand, serum adiponectin concentrations are associated with lower asthma severity among boys but greater asthma severity among men. It is possible that pro-inflammatory effects of adiponectin dominate under certain physiologic conditions and anti-inflammatory effects under others. It is also possible that the balance between pro-inflammatory systemic adipokines (primarily related to leptin [44–47]) and anti-inflammatory systemic adipokines (primarily related to adiponectin) is more important than the individual adipokines, in relation to asthma. Given the nascent nature of the field of research, it is not known whether attempts towards systemic or local modulation of adiponectin, independent of BMI, may be effective in asthma prevention or treatment.
Adiponectin and COPD
There is evidence supporting a causative association between adiponectin and COPD in mice but the human literature in this area is limited and clear conclusions cannot be drawn.
Mouse studies
Genetically-induced adiponectin deficient mice (APN−/−) demonstrate greater expression of TNF-α and matrix metalloproteinases in their alveolar macrophages and abnormal alveolarization, resembling an emphysema-like phenotype [48, 49]. These changes are reversible following adiponectin supplementation, supporting an anti-inflammatory role for adiponectin [49]. The latter is further supported by the greater levels of extrapulmonary inflammation, vascular endothelial dysfunction, and comorbidities (such as cachexia and osteoporosis) in APN−/− mice than wild-type mice [49].
Intranasal elastase instillation among wild-type mice, while causing emphysema, has dramatically opposite effects on systemic and local adiponectin responses. While it reduces plasma adiponectin concentrations, it increases bronchoalveolar lavage fluid adiponectin and adiponectin receptor expression on lung macrophages and epithelial cells [49]. Chronic tobacco smoke exposure in wild-type mice has similar effects on bronchoalveolar lavage fluid adiponectin [28]. Thus, as lung tissue of wild-type mice is exposed to emphysema-promoting agents, the enhanced local levels of adiponectin and of adiponectin receptor expression likely lead to greatly magnified signaling of adiponectin-dependent pathways in the lung, ostensibly to fight inflammation. However, the picture appears to be more complex than that. For instance, when genetically-induced adiponectin deficient mice (APN−/−) mice are exposed to tobacco smoke, these mice do not demonstrate a further increase in lung inflammation and air space enlargement, as would be expected, but instead show a lesser degree of abnormality than similarly exposed wild-type mice [50]. Why adiponectin has pro-inflammatory effects under certain exposure situations and anti-inflammatory effects under others is uncertain and needs to be investigated.
Human Studies
Currently, the human data on the association between adiponectin and COPD prevalence or severity remain inconclusive and suggest both pro-inflammatory and anti-inflammatory effects of adiponectin in different population subgroups.
COPD prevalence
Systemic adiponectin is positively associated with lung function in healthy adults but inversely associated in subjects with COPD. Further, men with COPD have higher systemic and local adiponectin concentrations than controls.
Thyagarajan et al. showed a positive longitudinal association between serum adiponectin and spirometric lung function in a large community-based study of young healthy adults, independent of sex, obesity and smoking [51]. Interestingly, the authors hypothesized that systemic adiponectin may affect lung growth during early adulthood rather than lung function decline in later life. The attenuation of this association after adjustment for insulin resistance and systemic inflammation suggests that these covariates are on a causal pathway linking adiponectin and lung function. The longitudinal findings by Thyagarajan et al. are opposed by a small case-control study that showed high plasma adiponectin concentrations to be associated with worse lung function in COPD [52], as discussed in the next section.
There is limited data, primarily clinic or hospital-based case-control or cross-sectional studies, that examine the predictive effect of serum adiponectin on risk for COPD, independent of BMI. It must be cautioned that in the absence of longitudinal or interventional studies, the direction of the adiponectin-COPD association cannot be conclusively established. Nevertheless, unlike the Nakanishi’s mouse model that associated hypoadiponectinemia with emphysema-like changes [49], three small human studies have demonstrated that serum adiponectin concentrations in male COPD patients were higher than those in controls [52–54]. Another study showed that levels of bronchoalveolar lavage adiponectin and adiponectin expression in airway epithelial cells in subjects with emphysema were greater than healthy (disproportionately female) non-smoking controls [28]. Interestingly, in contrast to subjects with emphysema who had increased levels of bronchoalveolar lavage adiponectin, current smokers without COPD had reduced levels of bronchoalveolar lavage adiponectin [55]. The molecular mechanism by which tobacco smoke exposure may down-regulate adiponectin expression and the development of COPD may up-regulate adiponectin expression is unknown.
COPD severity
Systemic adiponectin is associated with greater disease severity among men with COPD and has not been studied among women with COPD.
One small case-control study showed plasma adiponectin to be associated with worse spirometric parameters in a BMI-adjusted analysis [52] while two others showed no correlation [53, 54]. In a subgroup of COPD patients with elevated serum TNF-α concentrations, serum adiponectin concentrations were positively associated with serum TNF-α concentrations and inversely associated with lung volume parameters in an unadjusted analyses [54].
Acute COPD exacerbation
Systemic adiponectin concentrations rise during acute COPD exacerbations and return to baseline several days to weeks later [56]. This profile may reflect the body’s compensatory mechanisms to fight the early exuberant pro-inflammatory stimuli in acute COPD exacerbations but is opposite in direction to the profile observed in mechanically ventilated critically ill patients with acute respiratory failure [57–59], as discussed below.
To summarize, case-control studies demonstrate higher systemic and airway adiponectin concentrations among primarily male COPD patients than controls. However, in the absence of longitudinal studies, it is unclear whether the elevated concentrations are the cause or the consequence of COPD. Systemic adiponectin is positively associated with lung function in healthy adults but inversely associated in subjects with COPD. Systemic adiponectin is associated with greater COPD severity in men and has not been studied among women with COPD. The state of the literature is such that it is not even possible to hypothesize whether modulation of adiponectin, independent of BMI, may be helpful or harmful in COPD prevention and treatment in future studies.
Adiponectin and Non-small Cell Lung Cancer
The role of hypoadiponectinemia as a poor prognostic factor in non-small cell lung cancer is not well established. While one study showed lower serum adiponectin concentrations in advanced cancer, as compared to limited stage disease [60], another study did not show any association in multivariable analyses [61].
Adiponectin and Critical Illness
There are limited studies examining the role for adiponectin in critical illness with often contradictory results between murine and human studies. Adiponectin has largely anti-inflammatory effects in both the mouse model of polymicrobial sepsis [62, 63] and critically ill humans with respiratory failure [57, 64]. Walkey et al. observed that systemic adiponectin was positively correlated with systemic IL-10 (another anti-inflammatory cytokine) but not correlated with systemic pro-inflammatory cytokines in critically ill patients with respiratory failure [59].
Mouse studies
Adiponectin has anti-inflammatory effects in a mouse model of polymicrobial sepsis. Thus, APN −/− mice produced increased pro-inflammatory cytokines in response to sepsis, as compared to wild-type mice [63]. Further, higher premorbid systemic adiponectin concentrations were associated with improved survival from sepsis in mice [63]. This conclusion was observed with two sets of experiments. First, wild-type mice showed improved survival from sepsis than APN −/− mice. Next, wild-type mice treated with rosiglitazone (to increase their premorbid systemic adiponectin concentrations) showed improved survival from subsequent sepsis than untreated wild-type controls or treated APN −/− controls (that were unable to raise their premorbid systemic adiponectin concentrations with rosiglitazone). It is possible that the pharmacologic elevation of premorbid systemic adiponectin concentrations in wild-type mice may help downregulate or ‘fine-tune’ the subsequent exuberant pro-inflammatory response of early sepsis, improving their survival. If this hypothesis was tested to be true in humans, it may become possible to prevent sepsis-related mortality with premorbid use of thiazolidinedione drugs.
Human Studies
Systemic adiponectin concentrations in humans fall during the acute phase of various critical illnesses and rise again with convalescence [57–59]. Interestingly, this profile is different from that of COPD exacerbations [56], as discussed previously. The early phase of critical illness is a net pro-inflammatory state with high systemic TNF-α and IL-6 concentrations inhibiting adiponectin production. On the other hand, anti-inflammatory mechanisms are salient during the recovery phase with reduced systemic concentrations of TNF-α and IL-6 resulting in a corresponding bounce-back in systemic adiponectin concentrations. Consistent with this paradigm, serum adiponectin is associated with lower C-reactive protein levels on day 7 but not day 3 of critical illness [57].
Walkey et al.’s observational cross-sectional study of a large heterogeneous group of mechanically ventilated critically ill patients (29% with either pneumonia or sepsis) showed results opposite to those of Uji et al.’s murine sepsis study [64]. Walkey et al. found that higher systemic adiponectin concentrations on day 1 of critical illness were associated with lower, not higher, survival after adjustment for confounders such as BMI and APACHE II score [64]. In fact, the area under the receiver operating curve for day 1 adiponectin in predicting 28-day survival was higher i.e. more predictive than that for all other predictors studied, including APACHE II score. Of course, premorbid concentrations of adiponectin were not available in this observational study and thus, one does not know whether an initial expected drop in adiponectin occurred during the early phase of critical illness. The authors speculate that the higher day 1 adiponectin concentrations of non-survivors may in fact represent a dysfunctional response to the stress of early critical illness – suggesting that an early drop in adiponectin and subsequent return to baseline may in fact be beneficial for survival in the critically ill. Consistent with this hypothesis, nonsurvivors in this study showed an attenuated increase in adiponectin between Day 1 and Day 6 (when adiponectin may be expected to return close to its premorbid concentrations).
To summarize, murine and human studies show differing associations of systemic adiponectin with critical illness. We do not know whether this represents the obvious differences in mouse vs. human species; sepsis vs. respiratory failure as the inciting condition; or incomplete data from human studies. In the absence of longitudinal data from critically ill patients that includes premorbid concentrations, it is not possible to determine whether the association between higher adiponectin concentrations on day 1 and lower survival is causal or simply a biomarker for that outcome.
Adiponectin and Novel Therapeutic Agents
Although it is still currently unclear whether modulation of systemic adiponectin or its signaling pathways has any therapeutic benefit in pulmonary or critical illnesses, it may serve as a novel therapeutic or preventative tool for these illnesses in the future. One obvious pharmaceutical treatment would be the exogenous administration of adiponectin by inhalational or intravenous route. Although this has been tried in mouse models [29], problems to be overcome prior to human administration include establishing what the biologically active molecule is, what role post-translational modifications have upon the function and associated difficulties in generating biologically active molecules on a large scale.
Several existing drug classes that affect systemic adiponectin concentrations may be easier to administer than adiponectin itself. The most important drug class in this regard is the thiazolidinediones or TZDs such as pioglitazone and troglitazone. TZDs are synthetic ligands of the peroxisome proliferator-activated receptors, specifically of the gamma type (PPAR-γ), that increase adiponectin mRNA in adipocytes, resulting in increased production and secretion of adiponectin [65]. Sulfonylureas such as glimepiride, glibenclamide, and gliclazide stimulate the adiponectin production albeit through a different mechanism than the TZDs, namely an antagonistic interaction with protein kinase A activity [66]. Fibrates, such as fenofibrate, increase systemic adiponectin concentrations by enhanced PPAR-γ activity [67–69]. Angiotensin converting enzyme inhibitors such as ramipiril and angiotensin receptor blockers such as telmisartan, valsartan and candesartan have also been shown to increase systemic adiponectin concentrations [70–72]. The proposed mechanisms include adipocyte differentiation [73] and PPAR activation [74]. Calcium channel blockers such as amlodipine and efonidipine [75] and a central-acting anti-hypertensive agent rilmenidine [76] also increase systemic adiponectin concentrations whereas statins such as simvastatin have variable effects [77]. On the other hand, the opposite effect has been noted with valproic acid. A study examining valproic acid in mice found that this medication inhibits adiponectin gene expression in a dose-dependent manner [78].
In addition to pharmaceutical agents, nutritional interventions may help regulate systemic adiponectin concentrations. Results in animal models demonstrate that the consumption of diets rich in polyunsaturated fatty acids and supplementation with omega-3 and eicosapentaenoic acid increase adiponectin gene expression and plasma concentrations [79]. In humans, the consumption of a healthy and Mediterranean diet is positively associated with adiponectin concentrations, although the mechanisms are not fully understood [79]. As the literature on adiponectin’s role in a variety of disease states expands and matures, these interventions may become useful tools in modulating adiponectin concentrations for future therapeutic benefit.
Limitations of the Literature
Being a nascent field of research, the associations between adiponectin and lung diseases/critical illness currently suffer from many critical gaps in the literature. There is generally a lack of adequately powered longitudinal and weight-intervention studies; inadequate adjustment for confounding effect of obesity; limited studies of sputum or bronchoalveolar lavage fluid adiponectin; and no examination of adiponectin isoforms. Further, women with COPD have not been adequately studied – a subgroup of COPD that may indeed show the most interesting findings. Adiponectin is both positively and inversely associated with lung function, depending upon the population subgroup studied. It is therefore possible that adiponectin has both anti-inflammatory effects in the lungs of some subjects and pro-inflammatory in others.
Human studies of adiponectin and critical illness are similarly limited by small-sized single-center studies; enrollment of heterogeneous groups of critically ill patients; and inadequate control of potential covariates, such as glycemic control, BMI, and other inflammatory mediators. More importantly, adiponectin may exert varying degrees of influence at various time points in critical illness. Thus, future longitudinal studies will need to measure concentrations of adiponectin at multiple time points in the disease course and ideally, starting from the premorbid state. Both upstream regulators of adiponectin expression and downstream targets of adiponectin will need to be better studied to determine the mechanistic bases for these associations.
To summarize, there is developing literature to suggest a potential role for adiponectin in inflammatory pulmonary conditions such as asthma and COPD as well as critical illnesses such as respiratory failure with and without sepsis. Future research will determine whether pharmacological modulation of adiponectin, independent of BMI, may be helpful in the prevention or treatment of these conditions in targeted populations.
Acknowledgements
The authors would like to acknowledge the assistance provided by Mark Schuyler, M.D. and Nour Ali Assad, M.D. at the University of New Mexico, Albuquerque, NM, USA in proof-reading and critiquing the article.
NIH Source of Funding: This work was supported from funding by the National Institutes of Health (K23 HL 094531-01 and CTSA 1ULRR031977-01 for AS). The sponsor played no role in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
ABBREVIATIONS
- COPD
Chronic obstructive pulmonary disease
- IL
Interleukin
- TNF
Tumor Necrosis Factor
- BMI
Body mass index
- APN −/−
Genetically-induced adiponectin deficient mice
- FEV1/FVC
Ratio of forced expiratory volume in one second to forced vital capacity
- FEF25–75%
Maximum mid-expiratory flow
- HMW
High-molecular weight
- APACHE
Acute Physiology and Chronic Health Evaluation
- mRNA
Messenger ribonucleic acid
- PPAR
Peroxisome proliferator-activated receptor
- TZD
thiazolidinedione
Footnotes
COI Disclosure:
Pablo Garcia, M.D. states that there is no personal or financial support or involvement with organization(s) with financial interest in the subject matter or any other actual or potential conflict of interest. Pablo Garcia, M.D. also declares that he has materially participated in the article preparation including review of data, writing and editing of manuscript and creation of figures and has approved the final article.
Akshay Sood, M.D., M.P.H. states that there is no personal or financial support or involvement with organization(s) with financial interest in the subject matter or any other actual or potential conflict of interest. Akshay Sood, M.D., M.P.H. also declares that he has materially participated in the article preparation including review of data, writing and editing of manuscript and creation of figures and has approved the final article.
REFERENCES
- 1.Koenig W, Khuseyinova N, Baumert J, Meisinger C, Lowel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: results from the 18-year follow-up of a large cohort from southern Germany. Journal of the American College of Cardiology. 2006;48(7):1369–1377. doi: 10.1016/j.jacc.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 2.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 3.Steffes MW, Gross MD, Schreiner PJ, Yu X, Hilner JE, Gingerich R, Jacobs DR., Jr Serum adiponectin in young adults--interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol. 2004;14(7):492–498. doi: 10.1016/j.annepidem.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293(4):E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 5.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285(3):E527–E533. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 7.Sood A. Sex differences: implications for the obesity-asthma association. Exerc Sport Sci Rev. 2011;39(1):48–56. doi: 10.1097/JES.0b013e318201f0c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen KK, Frystyk J, Wolthers OD, Heuck C, Flyvbjerg A. Gender differences of oligomers and total adiponectin during puberty: a cross-sectional study of 859 Danish school children. The Journal of clinical endocrinology and metabolism. 2007;92(5):1857–1862. doi: 10.1210/jc.2006-2310. [DOI] [PubMed] [Google Scholar]
- 9.Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. European journal of endocrinology / European Federation of Endocrine Societies. 2005;153(3):409–417. doi: 10.1530/eje.1.01978. [DOI] [PubMed] [Google Scholar]
- 10.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51(9):2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 11.Leung KC, Xu A, Craig ME, Martin A, Lam KS, O'Sullivan AJ. Adiponectin isoform distribution in women--relationship to female sex steroids and insulin sensitivity. Metabolism. 2009;58(2):239–245. doi: 10.1016/j.metabol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52(2):268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 13.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. The Journal of biological chemistry. 2003;278(41):40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 14.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279(13):12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100(25):2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 16.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314(2):415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 17.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1220–R1225. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 18.Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40(1):177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 19.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316(3):924–929. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 20.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109(17):2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 21.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323(2):630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 22.Peake PW, Shen Y, Campbell LV, Charlesworth JA. Human adiponectin binds to bacterial lipopolysaccharide. Biochem Biophys Res Commun. 2006;341(1):108–115. doi: 10.1016/j.bbrc.2005.12.162. [DOI] [PubMed] [Google Scholar]
- 23.Sood A, Dominic E, Qualls C, Steffes MW, Thyagarajan B, Smith LJ, Lewis CE, Jacobs DR., Jr Serum Adiponectin is Associated with Adverse Outcomes of Asthma in Men but Not in Women. Front Pharmacol. 2011;2:55. doi: 10.3389/fphar.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehling A, Schaffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Scholmerich J, Neumann E, Muller-Ladner U. The potential of adiponectin in driving arthritis. J Immunol. 2006;176(7):4468–4478. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 25.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(28):10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117(2):375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 28.Miller M, Cho JY, Pham A, Ramsdell J, Broide DH. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol. 2009;182(1):684–691. doi: 10.4049/jimmunol.182.1.684. [DOI] [PubMed] [Google Scholar]
- 29.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118(2):389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41(4):397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sood A, Qualls C, Seagrave J, Stidley C, Archibeque T, Berwick M, Schuyler M. Effect of specific allergen inhalation on serum adiponectin in human asthma. Chest. 2009;135(2):287–294. doi: 10.1378/chest.08-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood A, Cui X, Qualls C, Beckett WS, Gross MD, Steffes MW, Smith LJ, Jacobs DR., Jr Association between asthma and serum adiponectin concentration in women. Thorax. 2008;63(10):877–882. doi: 10.1136/thx.2007.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland TJ, Sears MR, McLachlan CR, Poulton R, Hancox RJ. Leptin, adiponectin, and asthma: findings from a population-based cohort study. Ann Allergy Asthma Immunol. 2009;103(2):101–107. doi: 10.1016/S1081-1206(10)60161-5. [DOI] [PubMed] [Google Scholar]
- 34.Jartti T, Saarikoski L, Jartti L, Lisinen I, Jula A, Huupponen R, Viikari J, Raitakari OT. Obesity, adipokines and asthma. Allergy. 2009;64(5):770–777. doi: 10.1111/j.1398-9995.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 35.Dixon AE, Johnson SE, Griffes LV, Raymond DM, Ramdeo R, Soloveichik A, Suratt BT, Cohen RI. Relationship of adipokines with immune response and lung function in obese asthmatic and non-asthmatic women. J Asthma. 2011;48(8):811–817. doi: 10.3109/02770903.2011.613507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang AS, Kim TH, Park JS, Kim KU, Uh ST, Seo KH, Kim YH, Lim GI, Park CS. Association of serum leptin and adiponectin with obesity in asthmatics. J Asthma. 2009;46(1):59–63. doi: 10.1080/02770900802444203. [DOI] [PubMed] [Google Scholar]
- 37.Holguin F, Rojas M, Brown LA, Fitzpatrick AM. Airway and plasma leptin and adiponectin in lean and obese asthmatics and controls. J Asthma. 2011;48(3):217–223. doi: 10.3109/02770903.2011.555033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2009;20(1):81–88. doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim KW, Shin YH, Lee KE, Kim ES, Sohn MH, Kim KE. Relationship between adipokines and manifestations of childhood asthma. Pediatr Allergy Immunol. 2008;19(6):535–540. doi: 10.1111/j.1399-3038.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 40.Baek HS, Kim YD, Shin JH, Kim JH, Oh JW, Lee HB. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011;107(1):14–21. doi: 10.1016/j.anai.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, Szefler SJ, Sorkness CA, Morgan WJ, Teach SJ, Gan VN. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125(3):584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, Bremner WJ. Testosterone administration suppresses adiponectin levels in men. Journal of andrology. 2005;26(1):85–92. [PubMed] [Google Scholar]
- 43.da Silva PL, de Mello MT, Cheik NC, Sanches PL, Correia FA, de Piano A, Corgosinho FC, Campos RM, do Nascimento CM, Oyama LM, Tock L, Tufik S, Damaso AR. Interdisciplinary therapy improves biomarkers profile and lung function in asthmatic obese adolescents. Pediatr Pulmonol. 2012;47(1):8–17. doi: 10.1002/ppul.21502. [DOI] [PubMed] [Google Scholar]
- 44.Sood A, Ford ES, Camargo CA., Jr Association between leptin and asthma in adults. Thorax. 2006;61(4):300–305. doi: 10.1136/thx.2004.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurkan F, Atamer Y, Ece A, Kocyigit Y, Tuzun H, Mete N. Serum leptin levels in asthmatic children treated with an inhaled corticosteroid. Ann Allergy Asthma Immunol. 2004;93(3):277–280. doi: 10.1016/S1081-1206(10)61501-3. [DOI] [PubMed] [Google Scholar]
- 46.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114(2):254–259. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 47.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115(1):103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, Walsh K. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1035–L1042. doi: 10.1152/ajplung.00397.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakanishi K, Takeda Y, Tetsumoto S, Iwasaki T, Tsujino K, Kuhara H, Jin Y, Nagatomo I, Kida H, Goya S, Kijima T, Maeda N, Funahashi T, Shimomura I, Tachibana I, Kawase I. Involvement of endothelial apoptosis underlying chronic obstructive pulmonary disease-like phenotype in adiponectin-null mice: implications for therapy. American journal of respiratory and critical care medicine. 2011;183(9):1164–1175. doi: 10.1164/rccm.201007-1091OC. [DOI] [PubMed] [Google Scholar]
- 50.Miller M, Pham A, Cho JY, Rosenthal P, Broide DH. Adiponectin-deficient mice are protected against tobacco-induced inflammation and increased emphysema. Am J Physiol Lung Cell Mol Physiol. 2010;299(6):L834–L842. doi: 10.1152/ajplung.00326.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thyagarajan B, Jacobs DR, Jr, Smith LJ, Kalhan R, Gross MD, Sood A. Serum adiponectin is positively associated with lung function in young adults, independent of obesity: the CARDIA study. Respir Res. 2010;11:176. doi: 10.1186/1465-9921-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan KH, Yeung SC, Yao TJ, Ip MS, Cheung AH, Chan-Yeung MM, Mak JC. Elevated plasma adiponectin levels in patients with chronic obstructive pulmonary disease. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14(9):1193–1200. [PubMed] [Google Scholar]
- 53.Kirdar S, Serter M, Ceylan E, Sener AG, Kavak T, Karadag F. Adiponectin as a biomarker of systemic inflammatory response in smoker patients with stable and exacerbation phases of chronic obstructive pulmonary disease. Scand J Clin Lab Invest. 2009;69(2):219–224. doi: 10.1080/00365510802474400. [DOI] [PubMed] [Google Scholar]
- 54.Tomoda K, Yoshikawa M, Itoh T, Tamaki S, Fukuoka A, Komeda K, Kimura H. Elevated circulating plasma adiponectin in underweight patients with COPD. Chest. 2007;132(1):135–140. doi: 10.1378/chest.07-0227. [DOI] [PubMed] [Google Scholar]
- 55.Miller M, Cho JY, Pham A, Ramsdell J, Broide DH. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol. 2009;182(1):684–691. doi: 10.4049/jimmunol.182.1.684. [DOI] [PubMed] [Google Scholar]
- 56.Krommidas G, Kostikas K, Papatheodorou G, Koutsokera A, Gourgoulianis KI, Roussos C, Koulouris NG, Loukides S. Plasma leptin and adiponectin in COPD exacerbations: associations with inflammatory biomarkers. Respir Med. 2010;104(1):40–46. doi: 10.1016/j.rmed.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Venkatesh B, Hickman I, Nisbet J, Cohen J, Prins J. Changes in serum adiponectin concentrations in critical illness: a preliminary investigation. Crit Care. 2009;13(4):R105. doi: 10.1186/cc7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langouche L, Vander Perre S, Frystyk J, Flyvbjerg A, Hansen TK, Van den Berghe G. Adiponectin, retinol-binding protein 4, and leptin in protracted critical illness of pulmonary origin. Crit Care. 2009;13(4):R112. doi: 10.1186/cc7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walkey AJ, Rice TW, Konter J, Ouchi N, Shibata R, Walsh K, deBoisblanc BP, Summer R. Plasma adiponectin and mortality in critically ill subjects with acute respiratory failure. Crit Care Med. 2010;38(12):2329–2334. doi: 10.1097/CCM.0b013e3181fa0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petridou ET, Mitsiades N, Gialamas S, Angelopoulos M, Skalkidou A, Dessypris N, Hsi A, Lazaris N, Polyzos A, Syrigos C, Brennan AM, Tseleni-Balafouta S, Mantzoros CS. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case-control studies. Oncology. 2007;73(3–4):261–269. doi: 10.1159/000127424. [DOI] [PubMed] [Google Scholar]
- 61.Karapanagiotou EM, Tsochatzis EA, Dilana KD, Tourkantonis I, Gratsias I, Syrigos KN. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC) Lung Cancer. 2008;61(3):391–397. doi: 10.1016/j.lungcan.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 62.Uji Y, Yamamoto H, Maeda K, Tsuchihashi H, Akabori H, Shimizu T, Endo Y, Shimomura I, Tani T. Adiponectin deficiency promotes the production of inflammatory mediators while severely exacerbating hepatic injury in mice with polymicrobial sepsis. J Surg Res. 2010;161(2):301–311. doi: 10.1016/j.jss.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 63.Uji Y, Yamamoto H, Tsuchihashi H, Maeda K, Funahashi T, Shimomura I, Shimizu T, Endo Y, Tani T. Adiponectin deficiency is associated with severe polymicrobial sepsis, high inflammatory cytokine levels, and high mortality. Surgery. 2009;145(5):550–557. doi: 10.1016/j.surg.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Walkey AJ, Rice TW, Konter J, Ouchi N, Shibata R, Walsh K, deBoisblanc BP, Summer R. Plasma adiponectin and mortality in critically ill subjects with acute respiratory failure. Crit Care Med. 2010;38(12):2329–2334. doi: 10.1097/CCM.0b013e3181fa0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yilmaz MI, Sonmez A, Caglar K, Gok DE, Eyileten T, Yenicesu M, Acikel C, Bingol N, Kilic S, Oguz Y, Vural A. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist increases plasma adiponectin levels in type 2 diabetic patients with proteinuria. Endocrine. 2004;25(3):207–214. doi: 10.1385/ENDO:25:3:207. [DOI] [PubMed] [Google Scholar]
- 66.Kanda Y, Matsuda M, Tawaramoto K, Kawasaki F, Hashiramoto M, Matsuki M, Kaku K. Effects of sulfonylurea drugs on adiponectin production from 3T3-L1 adipocytes: implication of different mechanism from pioglitazone. Diabetes research and clinical practice. 2008;81(1):13–18. doi: 10.1016/j.diabres.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 67.Hiuge A, Tenenbaum A, Maeda N, Benderly M, Kumada M, Fisman EZ, Tanne D, Matas Z, Hibuse T, Fujita K, Nishizawa H, Adler Y, Motro M, Kihara S, Shimomura I, Behar S, Funahashi T. Effects of peroxisome proliferator-activated receptor ligands, bezafibrate and fenofibrate, on adiponectin level. Arterioscler Thromb Vasc Biol. 2007;27(3):635–641. doi: 10.1161/01.ATV.0000256469.06782.d5. [DOI] [PubMed] [Google Scholar]
- 68.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50(9):2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 69.Koh KK, Quon MJ, Lim S, Lee Y, Sakuma I, Lee YH, Han SH, Shin EK. Effects of fenofibrate therapy on circulating adipocytokines in patients with primary hypertriglyceridemia. Atherosclerosis. 2011;214(1):144–147. doi: 10.1016/j.atherosclerosis.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 70.Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42(1):76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- 71.Koh KK, Quon MJ, Han SH, Ahn JY, Lee Y, Shin EK. Combined therapy with ramipril and simvastatin has beneficial additive effects on tissue factor activity and prothrombin fragment 1+2 in patients with type 2 diabetes. Atherosclerosis. 2007;194(1):230–237. doi: 10.1016/j.atherosclerosis.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 72.Yilmaz MI, Sonmez A, Caglar K, Celik T, Yenicesu M, Eyileten T, Acikel C, Oguz Y, Yavuz I, Vural A. Effect of antihypertensive agents on plasma adiponectin levels in hypertensive patients with metabolic syndrome. Nephrology (Carlton) 2007;12(2):147–153. doi: 10.1111/j.1440-1797.2007.00764.x. [DOI] [PubMed] [Google Scholar]
- 73.Iwai M, Chen R, Imura Y, Horiuchi M. TAK-536, a new AT1 receptor blocker, improves glucose intolerance and adipocyte differentiation. Am J Hypertens. 2007;20(5):579–586. doi: 10.1016/j.amjhyper.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109(17):2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 75.Koh KK, Quon MJ, Lee SJ, Han SH, Ahn JY, Kim JA, Chung WJ, Lee Y, Shin EK. Efonidipine simultaneously improves blood pressure, endothelial function, and metabolic parameters in nondiabetic patients with hypertension. Diabetes care. 2007;30(6):1605–1607. doi: 10.2337/dc06-2267. [DOI] [PubMed] [Google Scholar]
- 76.Nowak L, Adamczak M, Wiecek A. Blockade of sympathetic nervous system activity by rilmenidine increases plasma adiponectin concentration in patients with essential hypertension. American journal of hypertension. 2005;18(11):1470–1475. doi: 10.1016/j.amjhyper.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 77.Koh KK, Quon MJ, Sakuma I, Lee Y, Lim S, Han SH, Shin EK. Effects of simvastatin therapy on circulating adipocytokines in patients with hypercholesterolemia. International journal of cardiology. 2011;146(3):434–437. doi: 10.1016/j.ijcard.2010.10.103. [DOI] [PubMed] [Google Scholar]
- 78.Qiao L, Schaack J, Shao J. Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology. 2006;147(2):865–874. doi: 10.1210/en.2005-1030. [DOI] [PubMed] [Google Scholar]
- 79.Reis CE, Bressan J, Alfenas RC. Effect of the diet components on adiponectin levels. Nutricion hospitalaria : organo oficial de la Sociedad Espanola de Nutricion Parenteral y Enteral. 2010;25(6):881–888. [PubMed] [Google Scholar]