Abstract
In this study, we employed a murine D5 melanoma model to study the effects of local tumor irradiation on the therapeutic efficacy of adoptive T cell therapy. Tumor irradiation was delivered in 5 daily fractions (8.5 Gy) to s.c. tumors on days 7-11 after tumor inoculation. After the last radiation dose, activated tumor-draining lymph node cells were transferred i.v. followed by i.p. IL-2 administration. Tumor irradiation alone had no significant effect on tumor growth; however it synergistically enhanced the therapeutic efficacy of T cell therapy. For 2 days following tumor irradiation there was a significant reduction in T, B cells and CD11c+ dendritic cells in both the tumor microenvironment and the systemic lymphoid compartments. By days 4-6 after irradiation, the relative reduction in the number of Treg cells within the tumor and the systemic compartments was greater than the reduction in conventional T cells. Furthermore, the suppressive function of the Tregs was significantly impaired in irradiated versus untreated mice. Using effector T cells derived from congenic mice, we found that local tumor irradiation resulted in increased proliferation of donor T cells within the tumor and the systemic lymphoid compartments. Radiation was associated with increased expression of the effector cytokines IFN-γ and TNF-α by donor and host CD4+ and CD8+ T cells. Altogether, our data indicate that local tumor irradiation has a distinct modulatory effect on Tregs and can enhance systemic antitumor immunity associated with adoptive T cell therapy.
Keywords: T regulatory cells, adoptive immunotherapy, radiation therapy
Introduction
Radiotherapy is a major modality in cancer therapy due to its direct tumoricidal effects. Over the last 25 years, there have been various reports describing the potential role of ionizing radiation in modulating the immune response to cancer. North et al. first described that whole-body non-lethal irradiation was necessary for the successful therapy of established subcutaneous murine tumors with the adoptive transfer of immune T cells.1 Immune splenocytes were generated by immunizing mice with an admixture of tumor cells plus the bacterial adjuvant Corynebacterium parvum. In that report, radiation was found to eliminate host suppressor T cells, and in subsequent reports this group found that the suppressor T cells responsible for preventing successful tumor regression after adoptive immunotherapy were CD4+ T cells.2 Using a similar model, we also found that whole-body irradiation was necessary for the successful adoptive immunotherapy of subcutaneous tumors; but not for visceral tumors in the lung or liver.3 This suggested that host suppression had a different impact on the antitumor activity of adoptive transferred immune cells based upon the location of the tumor. In that study, immune splenocytes were obtained from mice vaccinated in a similar manner to that reported by North1 and used fresh after harvesting. More current methods of generating effector T cells involve in vitro activation and expansion procedures to generate adequate quantities of cells for clinical applications.
Using in vitro activated tumor-infiltrating lymphocytes (TIL) for adoptive immunotherapy, Cameron et al. reported the synergistic effect of whole-body and local irradiation in the treatment of macrometastatic liver metastases in mediating tumor regression.4 In that report, local tumor irradiation was delivered only to half of the liver to assess whether suppressor cells present in the unirradiated half of the liver would abrogate the antitumor activity of the TIL cells. They did not find evidence of a suppressor cell and concluded that the radiation had a direct antitumor effect resulting in the synergy with TIL therapy.
Rosenberg and co-workers have pioneered the use of TIL therapy in conjunction with the administration of nonmyeloblative preparative regimens consisting of chemotherapy with or without total body irradiation (TBI) in the treatment of patients with advanced melanoma.5 Significant objective response rates were seen with 20 of 93 (22%) patients achieving complete tumor regression, with 19 being durable beyond 3 years. The addition of TBI increases the lymphodepletion that occurs with the chemotherapy regimen and may enhance adoptive T cell therapy by augmenting innate immunity6 depressing suppressor cells7,8 and allowing increased access to homeostatic cytokines by eliminating competing host immune cells.9 Although TBI can enhance adoptive T cell therapy, the increased intensity of lymphodepletion can be associated with significant clinical toxicities such as sepsis, renal insufficiency, interstitial pneumonitis, veno-occlusive liver disease and secondary solid and hematologic malignancies.10
In this report, we investigated the immune modulatory effects of local tumor irradiation on the treatment of established tumors in conjunction with adoptive T cell therapy. Tumor irradiation as a conventional treatment modality is not associated with the toxicities observed with TBI. We have extensive experience with the adoptive transfer of T effector cells derived from tumor-draining lymph nodes (TDLN). Utilizing various in vitro activation procedures we have reported on the efficacy of these cells in adoptive immunotherapy models.11-16 We have also utilized these techniques to generate effector T cells from vaccine-primed lymph nodes for clinical use.17-19 We chose to use the poorly immunogenic D5 melanoma tumor to investigate the effects of local tumor irradiation on host Treg cells and the host immune response in the setting of adoptive T cell therapy.
Materials and Methods
Mice
Female C57BL/6 (B6) and B6.PL-Thy1a/CyJ (CD90.1) mice were purchased from Charles River and Jackson Laboratory (Bar Harbor, ME), respectively. Mice were maintained in specific pathogen-free conditions and were used for experiments at 6-8 weeks of age. Recognized principles of laboratory animals care (NIH publication No. 85-23, revised 1985) were followed, and the University of Michigan Laboratory of Animal Medicine approved all animal protocols.
Tumor cells
D5 melanoma is a poorly immunogenic subclone of the B16 tumor of spontaneous origin in the C57BL/6 strain.13 D5-G6 is a D5 clone, transduced to express murine granulocyte macrophage colony-stimulating factor established by our laboratory.14 Tumor cells were cultured in complete medium (CM), which consisted of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 0.1mM nonessential amino acids, 1mM sodium pyruvate, 2mM fresh L-glutamine, 100μg/ml streptomycin, 100 units/ml penicillin, 50μg/ml gentamicin, 0.5μg/ml Fungizone (all from Life Technologies, Inc., Carlsbad, CA) and 0.05mM 2-mercaptoethanol (Sigma, St. Louis, MO).
In vivo D5 tumor models
Mice were inoculated subcutaneously (s.c.) in the right flank with 1.5 × 106 D5 cells to establish tumors.
Monitoring antitumor responses
Subcutaneous tumors were measured every other day in the largest perpendicular diameters using calipers fitted with a Vernier scale. Tumor size was recorded as tumor area (in mm2). Data are reported as the average tumor area ± SEM of 6 or more mice per group. Mice were euthanized when they have reached a moribund state as defined by the University Committee on Use and Care of Animals policy for end-stage illness and humane endpoints. Mouse survival was followed and recorded as the percentage of surviving animals over time (in days) after tumor inoculation. The median survival time (MST) of each experimental group was calculated and reported as mean ± SEM of 8 or more mice per group. The percent increase in MST was calculated as (MSTexperimental – MSTcontrol)/MSTcontrol × 100% for each treatment group relative to untreated control mice.
Tumor irradiation
Local tumor irradiation was delivered in 5 consecutive daily fractions of 8.5 Gy, starting 7 days after tumor inoculation, when mean tumor size reached 25 mm2. Radiation was administered using a Philips RT250 (Kimtron Medical, Woodbury, CT) at a dose rate of approximately 1.4 Gy/min by the University of Michigan Comprehensive Cancer Center Experimental Irradiation Core. Dosimetry was carried out using an ionization chamber connected to an electrometer system that is directly traceable to a National Institute of Standards and Technology calibration. For tumor irradiation, animals were placed in a restraint device and positioned such that the apex of each flank tumor was at the center of a 2-cm aperture in the secondary collimator, with the rest of the mouse shielded from radiation.
Generation of T cells for adoptive transfer
Mice were inoculated s.c. in bilateral flanks with 1 × 106 D5-G6 cells. On day 9, inguinal lymph nodes (tumor draining lymph nodes, TDLN) were harvested. TDLN cells (1 × 106/ml) were activated in 6-well culture plates with immobilized anti-CD3 and anti-CD28 monoclonal antibodies (BD Biosciences, San Diego, CA) for 2 days as previously described.20 Culture plates were pre-coated with 1ug/ml of each mAb in PBS (3 ml/well) overnight at 4°C. After activation, cells (0.1 × 106/ml) were expanded in CM containing 60 IU/ml human recombinant IL-2 (Novartis, East Hanover, NJ) for 3 days. Activated and expanded TDLN cells (30 × 106) were injected intravenously (i.v.) to D5 tumor-bearing mice 11 days after tumor inoculation. Following adoptive transfer (AT), mice received IL-2 intraperitoneally (i.p.), 40,000 IU bid, for a total of 8 doses. For each treatment group, there were 8 mice.
Fluorescence activated cell sorter (FACS) analysis
Blood samples were drawn via the lateral tail vein. Spleens were harvested and disrupted to single cell suspensions. Erythrocytes in blood and spleens were depleted. Tumors were harvested and disrupted; and tumor-infiltrating cells were isolated by density gradient centrifugation using double Ficoll (Ficoll-Paque, GE Healthcare, Piscataway,NJ).
All antibodies for flow cytometry were purchased from BD PharMingen (San Jose, CA); except antibodies targeting Foxp3 which were purchased from eBioscience (San Diego, CA). Foxp3 staining was performed using a commercial kit (eBioscience) according to the manufacturer’s directions. Flow cytometry was performed on an LSRII (BD Bioscience, San Jose, CA) and data were analyzed using Diva software (BD Bioscience).
In order to quantify the cell population, immediately before analysis on the flow cytometer, a known quantity of 15-um polystyrene microbeads (Bangs Laboratories, Fishers, IN) was added to each sample. The absolute number of cells of interest in each sample was calculated using the following formula: (number of cells of interest analyzed in sample) x (number of beads added to sample) / (number of beads analyzed in sample). These numbers were then normalized to blood volume or tissue weight as previously described. 21
T cell intracellular cytokine profile analysis
T cells were stimulated for 4 hours with a leukocyte activation cocktail [ready-to-use polyclonal cell activation mixture with phorbol 12-myristate 13-acetate, ionomycin, and the protein transport inhibitor BD GolgiPlug™ (brefeldin A, BD PharMingen)]. Cells were first stained for surface antigens using anti-CD45, anti-CD90.1, anti-CD90.2, anti-CD4, and anti-CD8 monoclonal antibodies. Then, cells were fixed and permeabilized with Fixation/Permeabilization solution (eBioscience) and stained intracellularly with antibodies targeting mouse IFN-γ and TNF-α (BD PharMingen). Samples were acquired on an LSR II and data were analyzed with DIVA software.22
Regulatory T cell function assay
CD4+ T cells were isolated from spleens of irradiated or untreated tumor bearing mice by StemSep Mouse CD4+ T cell enrichment kit (13052, Stemcell Technology, Vancouver, Canada). CD4+CD25high Tregs were sorted after anti-CD25-FITC (clone 7D4, BD Pharmingen) staining using a FACSAria (BD Bioscience). Cell populations were >95% pure by post-sort analysis. CD11c+ dendritic cells (DCs) were isolated by EasySep§ Mouse CD11c Positive Selection Kit (18758, Stemcell Technology). Conventional CD4+ T cells from healthy mice were used as responders. Tregs and responders were admixed at different ratios and then co-cultured for 3 days in the presence of 3 ug/ml anti-CD3 and CD11c+ DCs as a source of antigen presenting cells (APCs). T cell proliferation was measured by radioactive thymidine incorporation as described previously.23,24
Immune analysis of transferred and host T cells
To determine the effects of local tumor irradiation on transferred and host T cells, TDLN cells from CD90.1+ C57BL/6 mice were transferred to tumor bearing CD90.2+ mice. Spleens, flank tumors and TDLNs were harvested 6 and 10 days after adoptive transfer. Single-cell suspensions were stained first for surface CD45, CD90.1, CD90.2, CD4, and CD8; then for intracellular IFNγ and TNF-α as described above. Donor T cells were gated on CD45+CD90.1+, and recipient T cells were gated on CD45+CD90.2+.
Statistical Analysis
Data were evaluated by unpaired t test (2 cohorts) or one-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference test for multiple comparisons (>2 cohorts).
Results
Tumor irradiation enhances the efficacy of adoptive T cell therapy
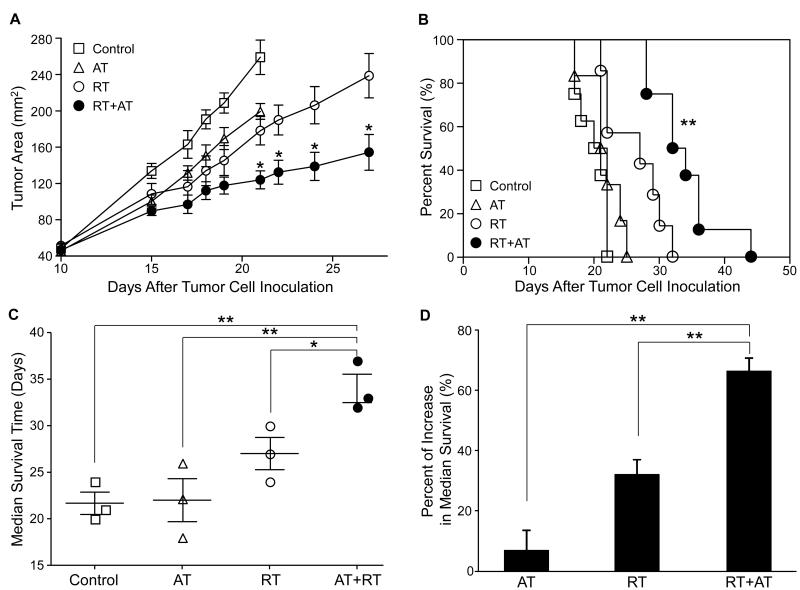
To investigate the effects of tumor irradiation on the therapeutic efficacy of adoptive T cell therapy, we established a melanoma-bearing mouse model with local tumor radiation and systemic T cell therapy (Supplementary Fig. 1). B6 mice were inoculated s.c. in the mid-flank with D5 cells on day 0. Mice were treated with either local radiation therapy (RT) on day 7 to 11, adoptively transferred TDLN cells on day 11 (AT), or local radiation combined with T cell therapy (RT+AT). As expected, adoptive T cell therapy or local tumor radiation therapy resulted in marginal tumor regression (Fig 1A) which was not significant. However, administration of RT before AT, significantly inhibited tumor growth compared to all other control groups (Fig. 1A, p < 0.05). Importantly, we also observed that RT plus AT significantly prolonged mouse survival compared to other treatments and the untreated control group (Fig. 1B, p < 0.01). Median survival times (MST) derived from three independent experiments revealed that RT plus AT resulted in significantly prolonged MST compared to RT or AT alone (Fig. 1C, p < 0.01). This interaction was also observed when the percentage of increased MST compared to control mice was derived showing that RT plus AT was significantly superior to RT or AT alone (Fig 1D, p < 0.01). Furthermore, the increase in MST from RT and AT is greater than the sum of the increase in MST for each therapy, indicating a synergistic effect between the two treatments. Thus, tumor irradiation synergistically enhances the therapeutic efficacy of adoptive T cell therapy.
Figure 1. Local radiation therapy enhances the therapeutic efficacy of T cell therapy.
B6 mice were inoculated s.c. in the flank with 1.5 × 106 D5 cells on day 0. Mice were treated with either local radiation therapy (RT) on day 7 to 11, adoptively transferred TDLN cells on day 11 (AT), or local radiation combined with T cell therapy (RT+AT). A: Tumor size in mice was measured every other day and data are reported as the average tumor area (mm2) ± SEM of 6 or more mice per group. B: Mice survival was recorded as the percentage of surviving animals over time after tumor inoculation. One of 3 representative experiments is shown. C: The median survival time (MST) of each experimental group from 3 independent experiments was calculated and reported as mean ± SEM of 8 or more mice per group. D: The percent increase in MST was calculated as (MSTexperimental – MSTcontrol)/MSTcontrol x 100% for each treatment group relative to untreated control mice. * p < 0.05, ** p < 0.01, for RT+AT versus all other groups.
Tumor irradiation induces lymphocyte depletion
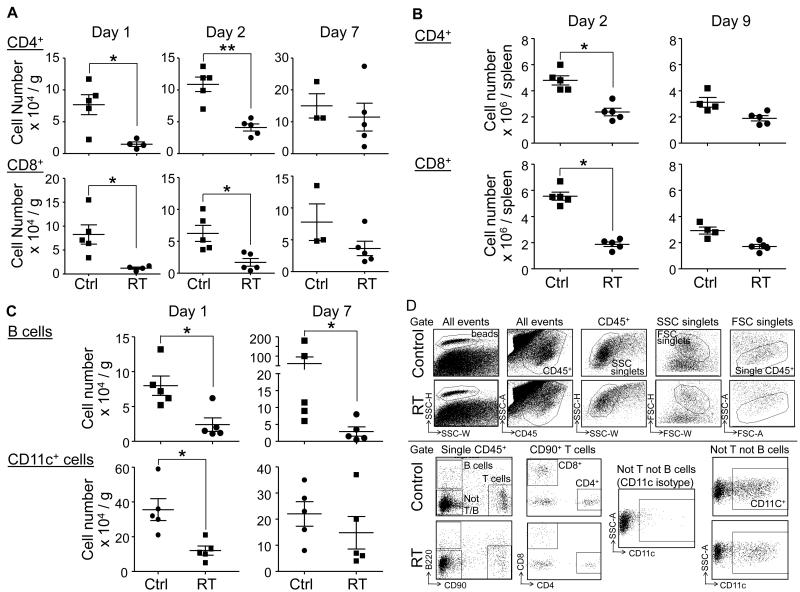
We next examined the potential effects of local tumor irradiation on the host immune cell components. After the last tumor irradiation dose, we analyzed the changes in the major immune cell subsets in the tumor, spleen and the blood of treated mice over a period of 10 days. We found that 1-2 days after local tumor irradiation, the number of CD4+ and CD8+ T cells was significantly reduced in the tumor (Fig. 2A) and the spleen (Fig. 2B). B cells and CD11c+ dendritic cells in the tumor microenvironment were also significantly reduced (Fig. 2C), with the B cell reduction persisting out to day 7. Similar results were observed in the peripheral blood (data not shown). The gating strategy and dot plots for the immune cell subsets is depicted in Fig 2D. Our data indicates that local tumor irradiation induces systemic and tumor microenvironment lymphocyte depletion.
Figure 2. Local radiation induces systemic and tumor microenvironment lymphocyte depletion.
1.5 × 106 D5 cells were inoculated s.c. in the flank of B6 mice. 7 days after inoculation, established D5 tumors were irradiated or left untreated. Tumors and spleens were harvested over a period of 10 days after the last radiation treatment for analysis. The number of immune cells was quantified by flow cytometry using polystyrene microbeads. A and B: The number of CD4+ and CD8+ T cells in the tumor environment (panel A) and in the spleen (panel B).C: The number of B cells and CD11c+ dendritic cells in the tumor microenvironment. Each experimental group comprised 5 mice. Each data point represents an individual mouse; bars depict mean ± SEM. Representative data from one experiment is shown; similar results were observed in 3 independent experiments; * p < 0.05; ** p < 0.01, for RT versus control. D: T, B and CD11c+ dendritic cells were identified as CD45+B220−CD90+; CD45+CD90−B220+; and CD45+CD90−B220−CD11c+, respectively. Representative dot plots of these cell populations from control and irradiated mice (RT) are shown when the same amount of beads were collected.
Tumor irradiation reduces the number and impairs the function of regulatory T cells
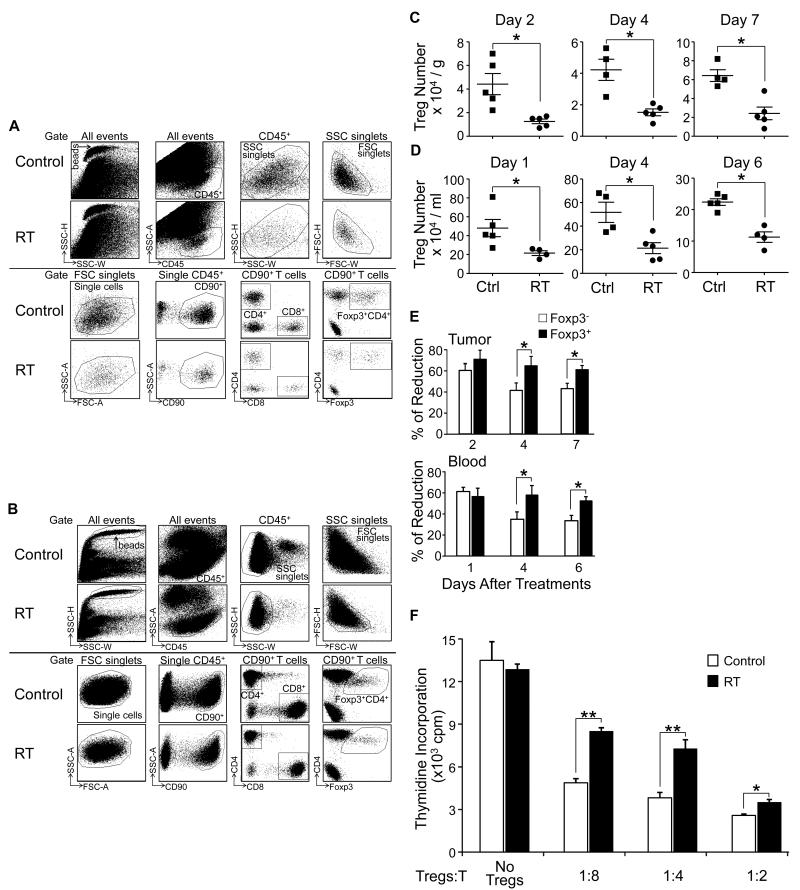
After determining that local tumor irradiation induces lymphocyte depletion, we further analyzed the effects of tumor irradiation on the regulatory T cell compartment. Tumor, blood and spleens were harvested at different intervals after local tumor irradiation. The gating strategy and dot plots for Treg (Foxp3+CD4+) cells from the tumor and blood are depicted in Fig 3A and 3B, respectively. Tregs were found to be reduced by more than 50% in the tumor microenvironment (Fig. 3C) and blood (Fig 3D) of mice that received radiotherapy compared to controls and persisted out to day 7 (p < 0.05). This reduction was also observed in the spleen (data not shown). We also compared the magnitude of the reduction in Treg numbers with the reduction in conventional T cells. One to 2 days after tumor irradiation, the percentage of reduction was similar for Tregs and conventional T cells (Fig. 3E). However, 4 to 7 days after radiation, the percentage of Treg reduction was significantly higher than that of conventional T cells in both the peripheral blood and in the tumor microenvironment (Fig 3E; p < 0.05). This suggests that Tregs recover from radiation more slowly compared to conventional T cells.In order to study the effects of in vivo tumor irradiation on the function of Tregs, we sorted Treg cells from the spleens of tumor bearing mice the day after radiation was completed, and examined their suppressive activity using a standard Treg functional assay as described in the Methods section. Thymidine incorporation experiments demonstrated that Tregs from both irradiated and untreated mice inhibited T cell proliferation in a dose-dependent manner. Interestingly though, at identical ratios between Tregs and T cells, thymidine incorporation was significantly higher in T cells co-cultured with Tregs derived from irradiated mice compared to Tregs from untreated mice (Fig. 3F). This indicates that tumor irradiation impairs Treg cell functionality.
Figure 3. Local tumor irradiation reduces the number and impairs the function of regulatory T cells.
B6 mice were inoculated s.c. in the flank with 1.5 × 106 D5 cells; and 7 days later, mice with established tumor received no treatment (Ctrl) or local radiation therapy (RT). Tumors and blood samples were collected after the last dose of RT for analysis. The number of Tregs was quantified by flow cytometry using microbeads. A and B: The gating strategy is shown using representative dot plots of Tregs in tumor (panel A) and blood (panel B) from control and irradiated mice when the same amount of beads were collected. Tregs are identified as CD45+CD90+CD4+Foxp3+. C and D: The number of Tregs in the tumor (panel C), and blood (panel D). The number of cells was normalized to tissue weight or blood volume. Each data point represents an individual mouse; bars depict the mean ± SEM, * p < 0.05.E: Percent reduction of Tregs (Foxp3+CD4+) versus conventional T cells (Foxp3−CD4+) in tumor and blood after radiation, * p < 0.05. F: The suppressive function of Tregs after tumor radiation. CD4+CD25high Tregs were sorted from the spleen of irradiated and untreated tumor bearing mice, then co-cultured with healthy CD4+ T cells at the indicated ratios for 3 days in the presence of anti-CD3 and CD11c+ cells from healthy mice. T cell proliferation was measured by radioactive thymidine incorporation; * p < 0.05, ** p < 0.01. Similar results were obtained in a replicate experiment.
Tumor irradiation enhances proliferation and function of adoptively transferred T cells and host T cells
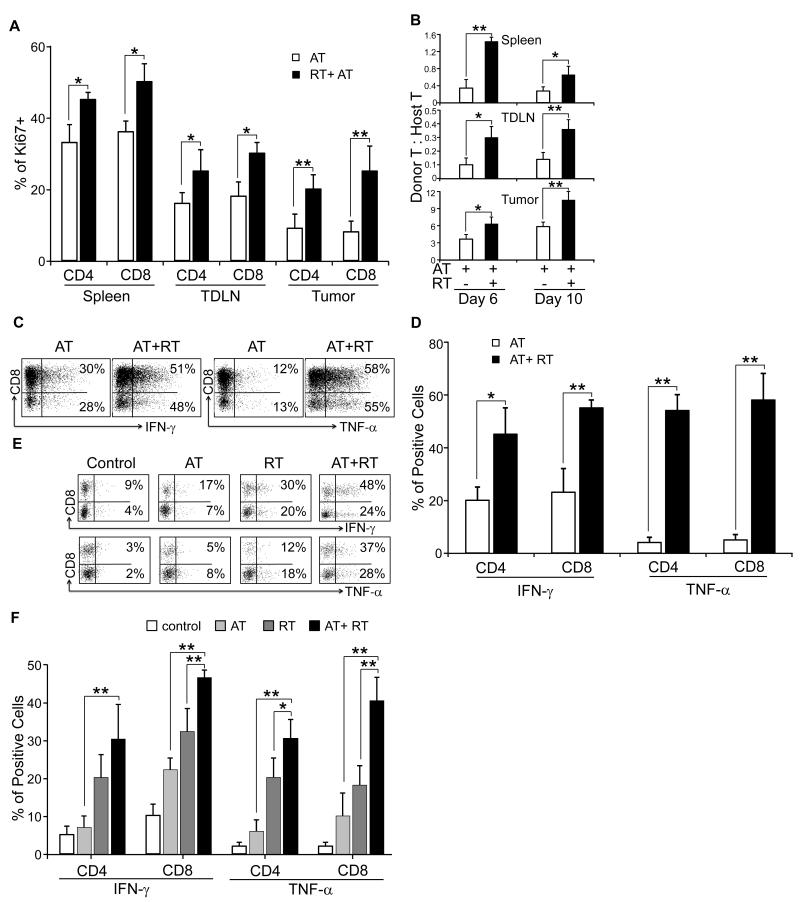
We further assessed the effects of tumor irradiation on the proliferation and survival of adoptively transferred T cells in the recipient mice. To this end, CD90.1+ T cells were adoptively transferred to tumor bearing CD90.2+ mice with or without radiation therapy. 1.5×106 D5 cells were inoculated s.c. in the flanks of CD90.2+ B6 mice. Mice were treated either with daily local irradiation at 8.5 Gy between day 7 and 11 (RT); or adoptively transferred 30 × 106 ex-vivo activated TDLN cells on day 11 (AT); or combined local radiation and T cell therapy (AT+RT). Following T cell transfer, mice received 40,000 IU IL-2 by i.p. bid, for a total of 8 doses. Spleens, tumors and TDLNs were harvested 6 and 10 days after adoptive transfer. T cell proliferation was measured by Ki67 expression using flow cytometry analysis as described in the Methods section. Day 6 after adoptive transfer, there were significantly higher levels of Ki67 expressing CD90.1+CD4+ and CD90.1+CD8+ T cells in the tumor, TDLN, and spleens of irradiated versus untreated mice (Fig. 4A, p < 0.05, AT vs. AT+RT for spleen and TDLN; p < 0.01 for tumor). These findings were also observed 10 days after AT (data not shown). Furthermore, the proliferation of host CD90.2+ T cells was not significantly altered by radiation (data not shown). As a result, the ratio between donor CD90.1+ T cells and host CD90.2+ T cells was increased in the spleen, tumor and TDLN of irradiated mice versus untreated mice 6 and 10 days after adoptive transfer (Fig. 4B). These results suggest that tumor irradiation prior to AT enhances the proliferation of the transferred T cells, and promotes their persistence in the tumor microenvironment.
Figure 4. Local radiation enhances proliferation and effector function of adoptively transferred and host T cells.
1.5×106 D5 cells were inoculated s.c. in the flank of CD90.2 B6 mice. Mice were treated either with daily tumor irradiation between D7 and 11 (RT); or adoptively transferred effector cells on day 11 (AT); or combined radiation and T cell therapy (AT+RT). The effector cells were derived from CD90.1+ B6 mice. A: Proliferation of donor T cells in the tumor, spleen and TDLN was monitored by measuring Ki67 expression 6 days after T cell adoptive transfer. Donor T cells were gated on CD45+CD90.1+. Tumor bearing mice that were not irradiated prior to T cell therapy were used as controls; * p < 0.05, ** p < 0.01, for RT+AT versus AT. B: Donor to host T cell ratios 6 and 10 days after adoptive transfer. Donor and recipient T cells were gated on CD45+CD90.1+ and CD45+CD90.2+, respectively. Tumor bearing mice that were not irradiated prior to T cell therapy were used as controls; * p < 0.05, ** p < 0.01, for RT+AT versus AT. C and D: Expression of the effector cytokines IFN-γ and TNF-α of donor T cells. Cells were recovered from tumors 10 days after T cell adoptive transfer; and cytokines were evaluated by flow cytometry. Tumor bearing mice that were not irradiated prior to T cell therapy were used as controls. Representative flow histograms (panel C) were gated on CD45+CD90.1+ for donor cells. Percent of cytokine-producing donor CD4+ and CD8+ T cells were expressed as percent of positive cells ± SEM (panel D); * p < 0.05, ** p < 0.01, for RT+AT versus AT. E and F: Expression of the effector cytokines of host T cells. Cells were collected from tumors 10 days after T cell adoptive transfer, and cytokines were measured by flow cytometry. Representative flow histograms were gated on CD45+CD90.2+ for host T cells (panel E), indicating expression of effector cytokines after AT, RT, AT+RT or no treatment (control). Percent of CD4+ and CD8+ host T cells producing effector cytokines were expressed as percent of positive cells ± SEM (panel F); * p < 0.05, ** p < 0.01, for RT+AT versus RT or AT alone. At least 6 mice were in each experiment group. Spleens, tumors or TDLN from 2 animals were pooled for analysis. Similar results were obtained from a replicate experiment
We further evaluated the effector function of the transferred T cells. Using the model described above, the expression of IFN-γ and TNF-α was measured by flow cytometry. Transferred T cells were gated on CD45+CD90.1+. The cytokine expression of cells from the tumor environment was compared between the mice received both radiation and T cell therapy RT+AT); and the mice did not receive radiation therapy prior to adoptive T cell therapy (AT). The percent of CD4+ and CD8+ donor T cells expressing these cytokines was significantly greater in the irradiated versus untreated mice 10 days after adoptive transfer (Fig 4C and D). Finally, we analyzed cytokine expression of host T cells after no treatment, AT, RT or AT+RT by flow cytometry. Host T cells were identified as CD45+CD90.2+. We found that the levels of IFN-γ and TNF-α producing host T cells were higher after AT, RT, and AT+RT compared to control mice 10 days after AT (Fig. 4E). Both RT alone and AT alone increased the percent of IFN-γ and TNF-α producing host CD4+ and CD8+ T cells (p < 0.05 versus. control). This incidence was further increased by the combined therapy (p < 0.05 RT+AT versus RT or AT alone) (Fig. 4F). Similar results for IFN-γ and TNF-α expression by transferred and host T cell were observed 6 days after T cell therapy (data not shown). Altogether, the data indicate that tumor irradiation increases the anti-tumor function of adoptively transferred T cells as well as host T cells.
Discussion
In this study, we have established an in vivo model of subcutaneous melanoma where tumor irradiation synergistically augmented the efficacy of adoptive therapy with in vitro activated T cells. We found that tumor irradiation was associated with a significant decrease in the number of Tregs in both the tumor microenvironment as well as in the systemic lymphoid compartments. This reduction in cell number was proportionally greater than what was observed for conventional T cells. Moreover, the function of Tregs was down-regulated by tumor irradiation. These findings may have contributed to the greater effector phenotype (IFN-γ and TNF-α) observed in host and donor T cells following tumor irradiation and AT.
Recently, there have been several reports regarding the effects of ionizing radiation on Tregs. Most of these reports involve whole body irradiation. Qu et al. reported that 5 Gy of whole-body irradiation to mice resulted in decreased Tregs in lymphoid compartments and these Tregs displayed an impaired function.25 In another report, 1.25 Gy of whole-body irradiation in a mouse model resulted in selective reduction of Tregs with enhancement of antitumor immunity induced by a DC/peptide vaccine.26 Wrzesinski et al. examined the increased intensity of lymphodepletion induced by TBI in the context of adoptive cell therapy and found a dose response effect on the intensity of lymphodepletion and therapeutic efficacy of adoptive T cell therapy.27 It was assumed that host T cell depletion would eliminate the endogenous cells with the potential of inhibitory activity. However, in that study, there was no direct evidence for how Treg cell numbers were changed after radiation; and functional analysis of host Tregs was not evaluated.
Few studies were available regarding the effects of local radiation on regulatory T cells.28 In a murine model, Billiard et al. reported that exposure of the abdomen of naïve mice to a single dose of 10 Gy resulted in an increased accumulation of Tregs in mesenteric lymph nodes. Utilizing a similar functional assay that we employed, these investigators found a reduction in the suppressive function of Tregs from irrradiated mice which were associated with decreased levels of Foxp3, TGF-β, and CTLA-4 mRNA.29
Our model is the first to show the effects of local tumor radiation on host Tregs in the context of adoptive T cell therapy. In our model, we employed 5 daily fractions of localized tumor irradiation with each fraction being 8.5 Gy. The regimen represents a significantly greater cumulative dose compared to the ones administered in prior reports; and we have utilized this regimen because it is more clinically relevant.
We found that local tumor irradiation also resulted in systemic lymphodepletion. This observation mimics what is seen when solid malignancies in humans are clinically treated with local radiation delivered in daily fractions.30-32 This does not approach the intensity of lymphodepletion seen with TBI given in a clinical setting where stem cells are administered for recovery.
We observed reduced Treg numbers with impaired suppressive function in the tumor environment and lymphoid organs after local tumor radiation. In vitro models of radiation and Tregs have revealed a dose response relationship of radiosensitivity with reduction in proliferation and abrogation of suppressive function.33 Another study from the same group showed that Tregs were more radiosensitive to low-dose irradiation (0.94Gy) than effector T cells.34 In our model, the number of Tregs was reduced to a similar high level compared to conventional T cells 24 hours after radiation; however, at later times the reduction in Treg numbers was significantly greater than what was observed for conventional T cells, which suggested that Tregs may recover more slowly from high dose irradiation (5 × 8.5Gy), compared to conventional T cells; rather than having different radiosensitivity.
Besides the changes in Treg numbers and function associated with tumor irradiation, we found enhanced effector phenotypic changes in donor T cells as measured by expression of IFN-γ and TNF-α cytokines as well as an increased proliferation of these cells. The latter can be ascribed to the systemic lymphodepletion that resulted from tumor irradiation. Previous reports have described the effects of lymphodepletion on homeostatic changes that occur within the host and adoptively transferred T cells.9,35 Together with the persistent reduction of Treg cells in the host, the ratio of transferred tumor-reactive T cells relative to endogenous cells with potential inhibitory activity was significantly increased by local tumor irradiation. In line with our study, Wrzesinski et al. showed that this higher ratio attributed to the increased therapeutic efficacy of adoptive T cell therapy. 27
The augmented effector function of host cells can be ascribed to several potential mechanisms besides the reduction in Tregs associated with tumor irradiation. Lugade et al. used a B16 melanoma model to show that single dose (10 Gy) or fractionated (5 × 3 Gy) doses to a tumor resulted in enhanced immune activation of T cells in TDLNs compared to non-irradiated hosts as well as increased trafficking of effector cells to irradiated tumors.36 In their study, they documented increased antigen-presenting cells within the TDLN of irradiated hosts. In a D5 melanoma model in which intratumoral injections of dendritic cells were administered, we reported that local tumor irradiation (given in a similar fashion to the present report) resulted in enhanced T cell sensitization within the TDLN and an increased influx of DC.21 It was also reported that the tumor-specific CD8+ CTL at local tumor sites and tumor draining lymph nodes, induced by local tumor irradiation, is essential to inhibit tumor growth.37 Another mechanism by which local tumor irradiation results in synergistic tumor regression with adoptive T cell therapy is inducing tumors to be more susceptible to T cell killing. Chakraborty and co-workers have reported that tumor irradiation upregulates Fas expression and can enhance Fas-dependent lysis by T cells in an adoptive T cell model as well as a vaccine model which induces specific T cell responses.38,39
Our report confirms the salutary effects of tumor irradiation in the context of adoptive T cell therapy. One of the immune modulatory effects associated with radiation was found to be a reduction in Treg cells in the lymphoid compartments as well as in the tumor microenvironment. Furthermore, radiation resulted in the down-regulation of Treg function. This phenomenon along with other potential mechanisms results in synergistic tumor regression in the setting of adoptive T cell therapy.
Supplementary Material
Figure S1. A melanoma tumor model employed to study the effect of local tumor irradiation on T cell adoptive therapy. D5 cells (1.5 × 106) were inoculated s.c. in the flanks of C57BL/6 mice. Established tumors were locally irradiated for 5 consecutive days (8.5 Gy x 5) starting 7 days after tumor cell inoculation. On day 11, 30 × 106 ex vivo activated and expanded effector T cells, were transferred by i.v. injection. Following T cell transfer, mice received 40,000 IU IL-2 by i.p. bid, for a total of 8 doses.
Acknowledgments
This work was partly supported by the National Insitutes of Health (RO1 CA082529 and T32 CA009672) and the Gillson Longenbough Foundation.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.North RJ. Gamma-irradiation facilitates the expression of adoptive immunity against established tumors by eliminating suppressor T cells. Cancer Immunol Immunother. 1984;16:175–81. doi: 10.1007/BF00205425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awwad M, North RJ. Radiosensitive barrier to T-cell-mediated adoptive immunotherapy of established tumors. Cancer Res. 1990;50:2228–33. [PubMed] [Google Scholar]
- 3.Chang AE, Shu SY, Chou T, Lafreniere R, Rosenberg SA. Differences in the effects of host suppression on the adoptive immunotherapy of subcutaneous and visceral tumors. Cancer Res. 1986;46:3426–30. [PubMed] [Google Scholar]
- 4.Cameron RB, Spiess PJ, Rosenberg SA. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med. 1990;171:249–63. doi: 10.1084/jem.171.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulos CM, Kaiser A, Wrzesinski C, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–9. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–46. [PMC free article] [PubMed] [Google Scholar]
- 9.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nat Clin Pract Oncol. 2006;3:668–81. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu SY, Chou T, Sakai K. Lymphocytes generated by in vivo priming and in vitro sensitization demonstrate therapeutic efficacy against a murine tumor that lacks apparent immunogenicity. J Immunol. 1989;143:740–8. [PubMed] [Google Scholar]
- 12.Aruga A, Aruga E, Tanigawa K, Bishop DK, Sondak VK, Chang AE. Type 1 versus type 2 cytokine release by Vbeta T cell subpopulations determines in vivo antitumor reactivity: IL-10 mediates a suppressive role. J Immunol. 1997;159:664–73. [PubMed] [Google Scholar]
- 13.Arca MJ, Krauss JC, Aruga A, Cameron MJ, Shu S, Chang AE. Therapeutic efficacy of T cells derived from lymph nodes draining a poorly immunogenic tumor transduced to secrete granulocyte-macrophage colony-stimulating factor. Cancer Gene Ther. 1996;3:39–47. [PubMed] [Google Scholar]
- 14.Arca MJ, Krauss JC, Strome SE, Cameron MJ, Chang AE. Diverse manifestations of tumorigenicity and immunogenicity displayed by the poorly immunogenic B16-BL6 melanoma transduced with cytokine genes. Cancer Immunol Immunother. 1996;42:237–45. doi: 10.1007/s002620050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito F, Carr A, Svensson H, Yu J, Chang AE, Li Q. Antitumor reactivity of anti-CD3/anti-CD28 bead-activated lymphoid cells: implications for cell therapy in a murine model. J Immunother. 2003;26:222–33. doi: 10.1097/00002371-200305000-00006. (1997) [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Furman SA, Bradford CR, Chang AE. Expanded tumor-reactive CD4+ T-cell responses to human cancers induced by secondary anti-CD3/anti-CD28 activation. Clin Cancer Res. 1999;5:461–9. [PubMed] [Google Scholar]
- 17.Chang AE, Aruga A, Cameron MJ, et al. Adoptive immunotherapy with vaccine-primed lymph node cells secondarily activated with anti-CD3 and interleukin-2. J Clin Oncol. 1997;15:796–807. doi: 10.1200/JCO.1997.15.2.796. [DOI] [PubMed] [Google Scholar]
- 18.Chang AE, Li Q, Jiang G, Sayre DM, Braun TM, Redman BG. Phase II trial of autologous tumor vaccination, anti-CD3-activated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. J Clin Oncol. 2003;21:884–90. doi: 10.1200/JCO.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Chang AE, Li Q, Bishop DK, Normolle DP, Redman BD, Nickoloff BJ. Immunogenetic therapy of human melanoma utilizing autologous tumor cells transduced to secrete granulocyte-macrophage colony-stimulating factor. Hum Gene Ther. 2000;11:839–50. doi: 10.1089/10430340050015455. [DOI] [PubMed] [Google Scholar]
- 20.Teitz-Tennenbaum S, Li Q, Davis MA, et al. Radiotherapy combined with intratumoral dendritic cell vaccination enhances the therapeutic efficacy of adoptive T-cell transfer. J Immunother. 2009;32:602–12. doi: 10.1097/CJI.0b013e3181a95165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teitz-Tennenbaum S, Li Q, Okuyama R, et al. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother. 2008;31:345–58. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei S, Shreiner AB, Takeshita N, Chen L, Zou W, Chang AE. Tumor-induced immune suppression of in vivo effector T-cell priming is mediated by the B7-H1/PD-1 axis and transforming growth factor beta. Cancer Res. 2008;68:5432–8. doi: 10.1158/0008-5472.CAN-07-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 24.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu Y, Jin S, Zhang A, et al. Gamma-ray resistance of regulatory CD4+CD25+Foxp3+ T cells in mice. Radiat Res. 2010;173:148–57. doi: 10.1667/RR0978.1. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Xiong S, Zhang L, Chu Y. Enhancement of antitumor immunity by low-dose total body irradiationis associated with selectively decreasing the proportion and number of T regulatory cells. Cell Mol Immunol. 2010;7:157–62. doi: 10.1038/cmi.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wrzesinski C, Paulos CM, Kaiser A, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kachikwu EL, Iwamoto KS, Liao YP, et al. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81:1128–35. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Billiard F, Buard V, Benderitter M, Linard C. Abdominal gamma-radiation induces an accumulation of function-impaired regulatory T cells in the small intestine. Int J Radiat Oncol Biol Phys. 2011;80:869–76. doi: 10.1016/j.ijrobp.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Wasserman J, Blomgren H, Petrini B, et al. Effect of radiation therapy and in vitro x-ray exposure on lymphocyte subpopulations and their functions. Am J Clin Oncol. 1982;5:195–208. doi: 10.1097/00000421-198204000-00069. [DOI] [PubMed] [Google Scholar]
- 31.Raben M, Walach N, Galili U, Schlesinger M. The effect of radiation therapy on lymphocyte subpopulations in cancer patients. Cancer. 1976;37:1417–21. doi: 10.1002/1097-0142(197603)37:3<1417::aid-cncr2820370324>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 32.Gray WC, Chretien PB, Suter CM, et al. Effects of radiation therapy on T-lymphocyte subpopulations in patients with head and neck cancer. Otolaryngol Head Neck Surg. 1985;93:650–60. doi: 10.1177/019459988509300515. [DOI] [PubMed] [Google Scholar]
- 33.Cao M, Cabrera R, Xu Y, Liu C, Nelson D. Gamma irradiation alters the phenotype and function of CD4+CD25+ regulatory T cells. Cell Biol Int. 2009;33:565–71. doi: 10.1016/j.cellbi.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao M, Cabrera R, Xu Y, Liu C, Nelson D. Different radiosensitivity of CD4(+)CD25(+) regulatory T cells and effector T cells to low dose gamma irradiation in vitro. Int J Radiat Biol. 2011;87:71–80. doi: 10.3109/09553002.2010.518208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang LX, Shu S, Plautz GE. Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells. Cancer Res. 2005;65:9547–54. doi: 10.1158/0008-5472.CAN-05-1175. [DOI] [PubMed] [Google Scholar]
- 36.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 37.Takeshima T, Chamoto K, Wakita D, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70:2697–706. doi: 10.1158/0008-5472.CAN-09-2982. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 39.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A melanoma tumor model employed to study the effect of local tumor irradiation on T cell adoptive therapy. D5 cells (1.5 × 106) were inoculated s.c. in the flanks of C57BL/6 mice. Established tumors were locally irradiated for 5 consecutive days (8.5 Gy x 5) starting 7 days after tumor cell inoculation. On day 11, 30 × 106 ex vivo activated and expanded effector T cells, were transferred by i.v. injection. Following T cell transfer, mice received 40,000 IU IL-2 by i.p. bid, for a total of 8 doses.