Abstract
The relationship between dyslipidemia and hearing is unclear. This study was conducted to investigate whether elevated serum lipid levels impact auditory function in humans and in guinea pigs. In the human study, a cross-sectional study of 40 volunteers with dyslipidemia was conducted. Pure tone thresholds, distortion product otoacoustic emissions, and lipid profiles were analyzed. When controlled for patient age and sex, we found that elevated triglycerides were associated with reduced hearing. In the guinea pig study, a prospective study of animals fed a high-fat diet for 14 weeks was conducted. Although the high-fat diet led to a dramatic elevation in the average weight and total cholesterol in all animals (from 61 to 589 mg/dl), there were no meaningful changes in distortion product otoacoustic emission magnitudes. These results suggest that whereas chronic dyslipidemia associated with elevated triglycerides may reduce auditory function, short-term dietary changes may not.
Keywords: Cochlea, DPOAE, Hypercholesterolemia, Lipid
Dyslipidemia is a well-known factor leading to coronary artery disease and atherosclerosis, and is a leading cause of myocardial infarction, stroke, and death in the United States. However, it is unclear whether dyslipidemia is associated with hearing loss. There are two primary lipoprotein fractions constituting total serum cholesterol: low-density lipoprotein cholesterol (LDL) and high-density lipoprotein cholesterol (HDL). Serum LDL transports cholesterol from the liver, via the circulatory system, to be deposited in other organs, specifically the arteries and heart. In contrast, HDL transports cholesterol from the organs and tissues back to the liver via the circulatory system. The HDL transport system is thought to be beneficial to the cardiovascular system because it reduces the formation of cholesterol plaques in major arteries. Elevated LDL and decreased HDL characterizes coronary artery disease. In addition, an elevated serum triglyceride level is often present, indicating a high level of fat in the bloodstream.
Cholesterol is a vital component of eukaryotic cellular membranes because it stabilizes them and modulates lipid and protein translocation across the membrane. Specifically related to the cochlea, the lipid composition, fluidity, and stiffness of the outer hair cell lateral wall membrane have been shown to be important to its electromotile function and the cochlear amplifier (1–5). The lateral wall plasma membrane of the outer hair cell also seems to have less cholesterol than other cells (6). These data suggest that outer hair cell function may be particularly sensitive to dyslipidemic states. Histologic changes in the guinea pig cochlea in response to dyslipidemia have been identified in the strial marginal layer and in outer hair cells (7). Hypercholesterolemia may also decrease cochlear vascularity and cause hearing loss.
We sought to determine whether dyslipidemia is associated with decreased auditory function. Two study arms were used. First, a cross-sectional study was performed using adult humans with dyslipidemia. Second, a prospective study was conducted with normal guinea pigs fed a high-fat diet. Auditory function was assessed using pure tone thresholds and distortion product otoacoustic emissions (DPOAEs) for the humans and DPOAEs alone for the guinea pigs.
METHODS
Human Cross-Sectional Study Arm
The first 40 subjects from the Baylor College of Medicine Lipid Research Clinic were invited to participate in this study and all agreed to enter the study. None of the subjects had participated in a dyslipidemia clinical trial for the previous 6 months, nor were they currently receiving treatment for dyslipidemia. All subjects had stable lipid levels on their habitual diet. Venous blood samples were collected after a 12-hour fast and analyzed for serum total cholesterol, HDL, and triglyceride levels. LDL levels were estimated using the Friedewald equation. All samples were analyzed by Medical Research Laboratories (Highland Heights, KY) or SmithKline Beecham (Houston, TX). Hearing loss did not exclude a subject from this study, but no subject used hearing aids. A history of noise exposure was not determined at the time of testing. The Institutional Review Board of Baylor College of Medicine approved this study, and a total of 40 patients were enrolled. After enrolling in the study and getting their audiometric evaluation (see next section), all patients began therapy for their dyslipidemia.
Human Audiometry
Subjects were evaluated by audiometry within 7 days of their blood draw, most on the day of the draw. Immittance testing was used to determine normal middle ear function using the Amplaid Model 720 immittance bridge (Amplaid, Milan, Italy). All subjects had type A tympanograms with normal middle ear pressures and normal static compliance. Acoustic reflexes were present bilaterally at 1 and 2 kHz at presentation levels not exceeding 100 dB hearing level.
Pure tone thresholds were determined in a sound booth with insert earphones (Eartone 3A, AERO Auditory Systems, Indianapolis, IN) coupled with a Virtual Audiometer 320 (Virtual Corporation, Portland, OR). Thresholds were obtained at 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz using the “down 10 dB and up 5 dB” threshold measurement approach. Pure tone average thresholds for the right and left ears were obtained. These responses were then combined and averaged to yield a combined ear average pure tone threshold for each subject.
DPOAEs were measured in a double-walled sound booth using a Virtual 330 system (Virtual Corporation]. Each ear was presented with two primary signals (f1 and f2) at intensities of 55 db sound pressure level (SPL) and 45 dB SPL, with an f2/f1 ratio of 1.3 to elicit the DPOAE. The DPOAE amplitude was measured at 2f1-f2. The stimuli frequencies were adjusted to produce a DPOAE at 25 individual frequencies between 0.32 and 5.22 kHz. High-frequency DPOAEs were not measured because of equipment limitations. Each ear was measured twice and the amplitudes were averaged. The noise floor was also measured. Because of the large variability of DPOAE magnitudes between patients, we simplified the data by determining whether a DPOAE was present at each frequency and intensity setting (DPOAE amplitude greater than 3 dB above the noise floor). The number of DPOAEs present was then summed for the right and left ears and averaged to yield a combined ear average.
Guinea Pig Prospective Study Arm
Two-week-old guinea pigs weighing 116 to 217g (Harlan Sprague, Indianapolis, IN) were divided into three groups. The control cohort, Group 1 (7 male guinea pigs), was fed a standard guinea pig diet containing 4% fat (Purina guinea pig chow 5025; Purina, St. Louis, MO). The study cohorts, Groups 2 (9 male guinea pigs) and 3 (8 female guinea pigs), were fed a high-fat diet (TD 96217; Harlan Teklad, Madison, WI) containing 10% coconut oil and 1% cholesterol. The three groups of guinea pigs were followed for 14 weeks, and their serum total cholesterol was measured on Weeks 0 and 14 of the study. The animals were fasted for 12 hours before determination of the lipid levels. They were then anesthetized by intramuscular injection of ketamine (45 mg/kg) and xylazine (5 mg/kg), and blood was drawn (1.0 ml) by cardiac puncture. Total cholesterol was measured by the Baylor College of Medicine Center for Comparative Medicine Pathology Lab. In addition to cholesterol measurement, the animal’s weights were measured. The guinea pigs were housed in a quiet room in our animal care facility and were not exposed to acoustic stress.
Guinea Pig Audiometry
DPOAEs were measured as an indicator of cochlear function on Weeks 0 and 14 in anesthetized guinea pigs. One ear in each guinea pig was used for the measurements. Otoscopy was first performed to verify that the middle ear was free of fluid and that the tympanic membrane was not retracted. For each animal, the same ear was used for both measurements. Two primary signals (f1 and f2), at intensities of 45, 55, 65, and 75 dB SPL, with an f2/f1 ratio of 1.3 were delivered to the ear to elicit the DPOAE. The primary tones were produced by two speakers (Etymotic Research ER2, Elk Grove Village, IL) mounted in an earplug designed to seal the external auditory canal. The stimulus frequencies were adjusted to produce DPOAEs at 6, 8, 10, 12, 14, and 16 kHz. The DPOAE magnitude was measured using low-noise microphone (Etymotic Research ER10B) also mounted in the earplug. The noise floor was also measured. An average DPOAE was then calculated for Weeks 0 and 14 by averaging the DPOAE magnitudes over all the frequencies at each intensity level.
Statistical Analyses
Statistical analyses were performed using SPSS for Windows Standard Version 11.0.1 (SPSS, Inc., Chicago, IL). For the human study arm, multiple linear regression analysis was performed. For the guinea pig study arm, the Student’s t test was used. Statistical significance was assessed if p < 0.05.
RESULTS
Human Study Arm
Forty adult volunteer subjects, 24 men and 16 women, were included in this study (Table 1). Ages ranged from 34 to 73 years with an average age of 54 years. All subjects were hypercholesterolemic by standards of the American Heart Association. All subjects had triglyceride levels less than 400 mg/dl, making the calculation of LDL levels reliable. Pure tone thresholds were measured and averaged for the right and left ears. These values were then averaged to yield a combined ear average over all frequencies tested. By analyzing the range of values, it can be observed that the combined ear average pure tone thresholds varied from normal hearing to a moderate sensorineural hearing loss (Table 2). The number of DPOAEs present at each frequency was also averaged for the right and left ears and then combined to yield a combined ear average DPOAE for each subject tested (Table 2).
TABLE 1.
Average values, standard error of the mean, and ranges for age in years, serum total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides in milligrams per deciliter for the human subjectsa
| n | Age (yr)
|
Total cholesterol (mg/dl)
|
LDL (mg/dl)
|
HDL (mg/dl)
|
Triglycerides (mg/dl)
|
|
|---|---|---|---|---|---|---|
| Mean ± SEM (range) | Mean ± SEM (range) | Mean ± SEM (range) | Mean ± SEM (range) | Mean ± SEM (range) | ||
| All subjects | 40 | 54.9 ± 1.53 (34–73) | 290.2 ± 7.63 (222–386) | 200.9 ± 7.57 (124–326) | 47.3 ± 1.58 (32–72) | 206.3 ± 12.26 (70–341) |
| Men | 24 | 54.2 ± 2.07 (34–73) | 281.1 ± 9.59 (226–386) | 202.0 ± 10.0 (140–326) | 44.8 ± 1.78 (32–61) | 173.5 ± 14.46 (70–278) |
| Women | 16 | 56.0 ± 2.29 (44–70) | 303.9 ± 12.10 (222–370) | 199.2 ± 11.9 (124–285) | 51.2 ± 2.69 (36–72) | 255.5 ± 15.05 (116–341) |
LDL indicates low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; SEM, standard error of the mean.
TABLE 2.
Average values with standard error of the mean and ranges of the combined ear average pure tone thresholds (decibel sound pressure level) and the combined ear average distortion product otoacoustic emissions (number of positive responses over frequency levels tested)a
| n | Average pure tone thresholds
|
Average DPOAE responses (out of a maximum of 25 responses for each ear)
|
|
|---|---|---|---|
| Mean ± SEM (range) | Mean ± SEM (range) | ||
| All subjects | 40 | 24.7 ± 2.07 (4.6–55.0) | 12.9 ± 0.68 (7–22) |
| Men | 24 | 26.9 ± 2.91 (6.7–55.0) | 12.4 ± 0.90 (7–22) |
| Women | 16 | 21.4 ± 2.67 (4.6–41.3) | 13.7 ± 1.04 (9–19) |
DPOAE indicates distortion product otoacoustic emission; SEM, standard error of the mean.
Human Pure Tone Thresholds
For the statistical analysis, multiple linear regression was performed using the combined ear average pure tone threshold as the dependent variable with age, sex, HDL, LDL, and triglycerides as the predictor variables. Age, sex, and triglyceride level were all found to have a statistically significant effect on the pure tone threshold (Table 3). Higher triglycerides, older age, and male sex were all associated with higher thresholds. Importantly, LDL and HDL levels did not demonstrate statistically significant effects.
TABLE 3.
Calculated B coefficients, beta values, and p values of the individual explanatory variables on the dependent variables average pure tone thresholds and average distortion product otoacoustic emissionsa
| Average pure tone thresholds
|
Average DPOAE responses
|
|||||
|---|---|---|---|---|---|---|
| B | Beta | p | B | Beta | p | |
| Age | 0.918 | 0.678 | <0.01* | −0.289 | −0.760 | <0.01* |
| Sex | −13.727 | −0.520 | <0.01* | 3.696 | 0.468 | <0.01* |
| LDL | −0.014 | −0.052 | 0.642 | 0.003 | 0.42 | 0.738 |
| HDL | 0.170 | 0.129 | 0.290 | −0.039 | −0.101 | 0.474 |
| Triglycerides | 0.066 | 0.392 | <0.01* | −0.018 | −0.359 | <0.05* |
DPOAE indicates distortion product otoacoustic emission; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol.
An asterisk indicates statistical significance.
To verify our findings and rule out other subtle associations, we broke down our data and analyzed various subgroups. Multiple linear regression analyses were then performed separately for the dependent variables right ear average pure tone threshold and left ear average pure tone threshold using the same predictor variables of age, sex, LDL, HDL, and triglycerides. Again, age, sex, and triglyceride levels were found to be statistically significant with p values < 0.05, whereas LDL and HDL levels were not statistically significant. We then studied each frequency separately for each ear. For the right ear, age was a statistically significant predictor at all frequencies tested, sex was a statistically significant predictor between the frequencies 2 and 8 kHz, and triglyceride level was a statistically significant predictor at all frequencies except 500 Hz. For the left ear, age was a statistically significant predictor for the frequencies 1 to 8 kHz, sex was a statistically significant predictor at 3 to 6 kHz, and triglyceride level was a statistically significant predictor at 4 to 8 kHz. LDL and HDL levels were not statistically significant predictors for pure tone thresholds for any frequency in either ear.
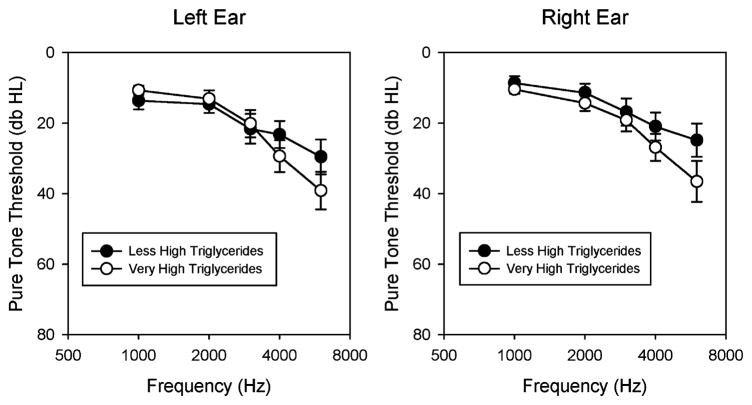
An estimate of the clinical significance of the association between triglycerides and pure tone thresholds was made by calculating age- and sex-adjusted audiograms for each of the 40 patients. This was done according to the United States Department of Labor Occupational Health and Safety guidelines (www.osha.gov, 1910.95 App F, subpart G). On the basis of these correction tables, we adjusted each patient’s thresholds by subtracting median thresholds for the appropriate age, sex, and frequency from each subject’s threshold. We then added this value to the mean thresholds of a 40-year-old man to get representative “average” thresholds. We then plotted average audiograms from the top 20 patients with “very high” triglyceride values and the bottom 20 patients with “less high” triglyceride values (Fig. 1).
FIG. 1.
Age- and sex-adjusted audiograms for the human study arm. The 20 patients with the highest triglycerides (very high: 272 ± 7 mg/dl) are compared with the 20 patients with the lower triglycerides (less high: 141 ± 11 mg/dl). Patients with very high triglycerides had slightly worse thresholds than those with less high triglycerides. This effect was more prominent in the high frequencies. Data are not shown below 1,000 Hz because the Occupational Safety and Health Administration correction tables do not include this frequency range. Data are plotted as mean ± SEM.
These simulated audiograms demonstrated that patients with very high triglycerides had slightly worse pure tone thresholds than those with less high triglycerides, and that these differences were greater at high frequencies compared with low frequencies. At 4 kHz, the differences between the two groups were approximately 6 dB; and at 6 kHz, they were approximately 12 dB. It should be noted that although this figure can be used to visualize the clinical significance of the triglyceride effect, these curves only represent corrected means from two groups of patients. In contrast, the regression analysis performed earlier is more sensitive at detecting associations between variables because patients at the extremes are not averaged with those in the middle of the range.
Human Distortion Product Otoacoustic Emissions
Multiple linear regression analysis was performed for the combined ear average DPOAE as the dependent variable with age, sex, HDL, LDL, and triglyceride level as the predictor variables. Age, sex, and triglyceride level were all found to have statistically significant effects on DPOAEs (Table 3). Higher triglycerides, older age, and male sex were all associated with a reduced number of DPOAE responses. LDL and HDL levels did not demonstrate statistically significant effects on DPOAEs.
Further analysis was undertaken using the right ear average DPOAE and the left ear average DPOAE separately using the same predictor variables: age, sex, HDL, LDL, and triglyceride level. For the right ear, age, sex, and triglyceride level were statistically significant for predicting DPOAEs. For the left ear, only age was a statistically significant predictor for predicting DPOAEs. LDL and HDL were not statistically significant predictors for the number of DPOAE responses in any of the analyses.
Guinea Pig Study Arm
All guinea pigs were healthy throughout the 14-week study period. All three groups experienced significant weight gain over the study period, which was statistically significant when analyzed by the paired Student’s t test (Table 4). The animals in Group 1 (control) had stable cholesterol levels throughout the study period. This is in contrast to the two study groups, which experienced a large increase in their serum cholesterol.
TABLE 4.
Average weights and cholesterol levels with standard error of the mean and range of valuesa
| n | Weight (g)
|
Total cholesterol (mg/dl)
|
|||||
|---|---|---|---|---|---|---|---|
| Mean ± SEM (range)
|
Mean ± SEM (range)
|
||||||
| Week 0 | Week 14 | p | Week 0 | Week 14 | p | ||
| Group 1 (male guinea pigs fed a normal diet) | 7 | 175.0 ± 11.1 (135–217) | 745.6 ± 18.0 (693 –796) | < 0.01* | 53.7 ± 5.56 (33–77) | 58.3 ± 6.87 (40–97) | 0.63 |
| Group 2 (male guinea pigs fed a high-fat diet) | 9 | 167.0 ± 5.2 (149–188) | 602.4 ± 18.4 (497 –669) | < 0.01* | 61.4 ± 3.24 (50–79) | 719.9 ± 90.39 (345 –1,042) | < 0.01* |
| Group 3 (female guinea pigs fed a high-fat diet) | 8 | 141.4 ± 8.5 (116–178) | 567.3 ± 37.2 (347 –693) | < 0.01* | 70.0 ± 10.38 (50–140) | 442.1 ± 62.98 (271 –829) | < 0.01* |
SEM indicates standard error of the mean.
p values were determined by using a paired Student’s t test. An asterisk indicates statistical significance.
Guinea Pig Distortion Product Otoacoustic Emissions
For all three groups, the average DPOAE magnitude for each intensity level (45, 55, 65, and 75 dB SPL) were compared at Weeks 0 and 14 using the paired Student’s t test. DPOAEs that were not at least 3 dB above the noise floor at each individual frequency and stimulus level were not included for comparison. For the control group (Group 1), there was no statistically significant change in the magnitude of the DPOAE at any of the intensity levels tested. Likewise, for the male and female groups (Groups 2 and 3, respectively), there were also no statistically significant changes in the magnitude of the DPOAE between Weeks 0 and 14 at any of the intensity levels tested (Table 5).
TABLE 5.
Average distortion product otoacoustic emissions at each intensity level for the three groups of animals at Weeks 0 and 14 with associated p values as determined by a paired Student’s t testa
| Average DPOAE magnitude
|
||||
|---|---|---|---|---|
| (Mean ± SEM)
| ||||
| Week 0 | Week 14 | p | ||
| Group 1 (male guinea pigs fed a normal diet) | 45 dB SPL | 6.5 ± 1.52 | 1.7 ± 2.38 | 0.12 |
| 55 dB SPL | 15.1 ± 1.57 | 9.9 ± 3.24 | 0.20 | |
| 65 dB SPL | 23.0 ± 1.64 | 17.4 ± 4.08 | 0.22 | |
| 75 dB SPL | 36.0 ± 2.13 | 31.3 ± 4.91 | 0.27 | |
| Group 2 (male guinea pigs fed a high-fat diet) | 45 dB SPL | 5.7 ± 1.24 | 2.1 ± 1.71 | 0.12 |
| 55 dB SPL | 15.6 ± 1.17 | 11.2 ± 2.26 | 0.12 | |
| 65 dB SPL | 23.5 ± 0.80 | 19.1 ± 2.77 | 0.18 | |
| 75 dB SPL | 37.0 ± 1.53 | 32.8 ± 3.25 | 0.30 | |
| Group 3 (female guinea pigs fed a high-fat diet) | 45 dB SPL | 2.6 ± 1.25 | −0.7 ± 2.61 | 0.26 |
| 55 dB SPL | 13.0 ± 1.02 | 6.5 ± 3.13 | 0.10 | |
| 65 dB SPL | 20.5 ± 0.99 | 13.1 ± 3.79 | 0.11 | |
| 75 dB SPL | 32.3 ± 1.60 | 24.1 ± 4.22 | 0.12 | |
DPOAE indicates distortion product otoacoustic emission; SEM, standard error of the mean; SPL, sound pressure level.
Significant values were taken to be p < 0.05.
Further analysis was undertaken by comparing the DPOAE magnitudes at Weeks 0 and 14 for each individual frequency and intensity level. Again, a paired Student’s t test was used for statistical analysis. For the control group, there were statistically significant decreases in the magnitude of the DPOAE at 12 and 14 kHz when presented at a stimulus level of 45 dB SPL (p < 0.05). In contrast, there was a significant increase in the magnitude of the DPOAE at 14 kHz when delivered at a stimulus level of 55 dB SPL (p < 0.05). For the experimental group of male guinea pigs (Group 2), there was a significant decrease in the DPOAE magnitude at 6 kHz when tested at a stimulus level of 45 dB SPL (p < 0.05). In addition, there was also a significant decrease in the DPOAE magnitude at 12 kHz when tested at stimulus frequencies of 45, 55, and 65 dB SPL (p < 0.05). All other frequencies and stimulus levels tested showed no significant change in the DPOAE magnitude. For the experimental group of female guinea pigs (Group 3), there was a significant decrease in the DPOAE magnitude at 12 kHz when tested at a stimulus of 65 dB SPL. Other than these presumably spurious results, there were no significant changes in the DPOAE magnitudes at any other frequency or stimulus level tested.
The data were also analyzed by averaging the DPOAE magnitudes at all frequencies together. The male and female experimental groups were also combined into one large experimental group. When the average DPOAEs were compared between Weeks 0 and 14 at each of the presented intensity levels for the combined groups, there was found to be a statistically significant change in the DPOAE magnitude for each of the intensity levels (4.8, 5.2, 5.6, and 4.7 dB for 45, 55, 65, and 75 dB SPL, respectively). However, similar reductions in DPOAE magnitudes were found in our control group (3.5, 5.3, 5.8, and 6.1 dB for 45, 55, 65, and 75 dB SPL, respectively). The nonpaired Student’s t test demonstrated that there was no statistically significant difference between the change in DPOAE magnitude in the controls and the combined experimental group (p > 0.6). Thus, the high-fat diet was not associated with any change in DPOAE magnitude.
DISCUSSION
In the cross-sectional human study arm, elevated triglyceride levels were found to be predictors of decreased auditory function, whereas serum LDL and HDL were not. These data were consistent for both pure tone thresholds and DPOAE responses. Older age and male sex were also found to be associated with poorer auditory function, as expected (8). In the prospective animal study, there were no changes in auditory function associated with a high-fat diet, although there were statistically significant increases in the weights and cholesterol levels of the same groups over the study period. Our data indicate that dyslipidemia with elevated triglyceride levels is associated with sensorineural hearing loss, but that short-term diet modifications are not. Excess triglycerides are primarily derived from foods containing high levels of carbohydrates, especially fructose containing simple sugars and concentrated sweets.
There are several important caveats concerning the conclusions from this study. All subjects in this study were hypercholesterolemic by standards of the American Heart Association; therefore, generalizations of these findings are limited to groups with significantly elevated lipid levels, that is, serum total cholesterol >220 mg/dl. As well, one should not conclude from these data that modest elevations of triglycerides are associated with hearing loss, as there were no patients in this study with normal triglyceride levels for comparison. Noise exposure history was not controlled for in this study and may be a confounding variable. For example, patients with lower socioeconomic status are more likely to hunt and have occupational noise exposure, and have been found to have higher triglycerides (9). Additionally, our guinea pigs were not subject to acoustic stress and this may affect our findings. Finally, it is unclear whether our sample is representative of all people with dyslipidemia because the subjects who volunteered for this study may have participated because of their interest or concern in their hearing status.
Although several studies have sought to define the relationship between dyslipidemia and cochlear function, controversy still exists. In 1964, Rosen et al. (10) were the first to publish a report raising the possibility that dyslipidemia was associated with reduced hearing. They conducted several epidemiologic studies in different areas of the world and suggested that diet is an important factor for prevention of coronary artery disease and hearing loss (10). There have also been several other studies conducted that suggest a relationship between hyperlipidemia and hearing loss, especially presbycusis and noise-induced hearing loss (11,12). Others describe a relationship between specific lipid fractions and hearing disturbances (13,14). In addition, a few reports have been published that not only describe a relationship between dyslipidemia and hearing loss, but suggest that the hearing loss is potentially reversible if dietary measures to reduce cholesterol are instituted (12,15).
Conversely, there have been several reports that call into question the relationship between dyslipidemia and auditory function. In a retrospective review of 100 patients with hearing loss, Lowry and Isaacson (16) found a lower prevalence of hyperlipidemia in their subjects than would be expected in the general population. In addition, other studies have concluded that there may not be a significant relationship between increased blood lipids and hearing loss (17,18).
The largest human study to date that has examined the relationship between dyslipidemia and hearing loss is by Gates et al. (14). In their study, 1,662 patients from the Framingham Heart Study cohort were examined to determine the relationship between cardiovascular risk factors and hearing during 6 years. The only significant finding was an inverse relationship of HDL level and low-frequency hearing thresholds in women. There was no relationship between hearing and serum total cholesterol, LDL, or triglyceride levels.
In 2000, Jones and Davis (19) reported a retrospective study of 1,490 patients who had presented to their neurotology clinic. All patients had had fasting lipid profiles performed regardless of their presenting problem. Groups were then separated and compared on the basis of whether they had normal hearing or hearing loss. Interestingly, they found that an elevated fasting cholesterol level was associated with significantly better hearing threshold levels (19). In a similar study, Pulec et al. (12) reported on all new patients presenting to their clinic (4,251) during an 8-year period. Of this cohort, 2,332 had complaints of hearing loss. All patients had lipid testing performed, and it was found that only 120 were dyslipidemic (5.1%). Preyer et al. (20) compared pure tone audiometry and DPOAEs in 20 healthy subjects and 20 patients with familial hypercholesterolemia. Their results indicated that although hearing thresholds were not statistically different between the two groups, speech scores were significantly reduced in the hypercholesterolemic subjects.
Sex effects on pure tone thresholds and otoacoustic emissions are also well established (8). Although the mechanism of the sex effect on auditory function is unclear, it has been suggested that different serum levels of circulating androgens may account for these observed effects. Thus, it is possible that dyslipidemia affects serum androgen levels, which then act to modulate auditory function.
In summary, we have found that elevated triglycerides along with the known factors of age and sex are associated with elevated pure tone thresholds and reduced DPOAE responses in a population of dyslipidemic human subjects. However, no differences in DPOAE magnitudes were found in response to a high-fat diet in our prospective guinea pig study arm. These results suggest that whereas chronic dyslipidemia associated with elevated triglycerides may reduce auditory function, short-term dietary changes may not.
Acknowledgments
This study was funded by NIH grants DC006671 (to J. S. O.) and DC 02775 (to W. E. B.). Statistical consultation was provided through the Baylor College of Medicine Department of Medicine, statistics section.
References
- 1.Oghalai JS. Chlorpromazine inhibits cochlear function in guinea pigs. Hear Res. 2004;198:59–68. doi: 10.1016/j.heares.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Oghalai JS. The cochlear amplifier: augmentation of the traveling wave within the inner ear. Curr Opin Otolaryngol Head Neck Surg. 2004;12:431–8. doi: 10.1097/01.moo.0000134449.05454.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oghalai JS, Patel AA, Nakagawa T, et al. Fluorescence-imaged microdeformation of the outer hair cell lateral wall. J Neurosci. 1998;18:48–58. doi: 10.1523/JNEUROSCI.18-01-00048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oghalai JS, Tran TD, Raphael RM, et al. Transverse and lateral mobility in outer hair cell lateral wall membranes. Hear Res. 1999;135:19–28. doi: 10.1016/s0378-5955(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 5.Oghalai JS, Zhao HB, Kutz JW, et al. Voltage- and tension-dependent lipid mobility in the outer hair cell plasma membrane. Science. 2000;287:658–61. doi: 10.1126/science.287.5453.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen TV, Brownell WE. Contribution of membrane cholesterol to outer hair cell lateral wall stiffness. Otolaryngol Head Neck Surg. 1998;119:14–20. doi: 10.1016/S0194-5998(98)70167-6. [DOI] [PubMed] [Google Scholar]
- 7.Gratton MA, Wright CG. Alterations of inner ear morphology in experimental hypercholesterolemia. Hear Res. 1992;61:97–105. doi: 10.1016/0378-5955(92)90040-t. [DOI] [PubMed] [Google Scholar]
- 8.Jerger J, Chmiel R, Stach B, et al. Gender affects audiometric shape in presbyacusis. J Am Acad Audiol. 1993;4:42–9. [PubMed] [Google Scholar]
- 9.Whitty CJ, Brunner EJ, Shipley MJ, et al. Differences in biological risk factors for cardiovascular disease between three ethnic groups in the Whitehall II study. Atherosclerosis. 1999;142:279–86. doi: 10.1016/s0021-9150(98)00239-1. [DOI] [PubMed] [Google Scholar]
- 10.Rosen S, Plester D, El-Mofty A, et al. Relation of hearing loss to cardiovascular disease. Trans Am Acad Ophthalmol Otolaryngol. 1964;68:433–44. [PubMed] [Google Scholar]
- 11.Axelsson A, Lindgren F. Is there a relationship between hypercholesterolaemia and noise-induced hearing loss? Acta Otolaryngol. 1985;100:379–86. doi: 10.3109/00016488509126561. [DOI] [PubMed] [Google Scholar]
- 12.Pulec JL, Pulec MB, Mendoza I. Progressive sensorineural hearing loss, subjective tinnitus and vertigo caused by elevated blood lipids. Ear Nose Throat J. 1997;76:716–20. 725–6, 728. passim. [PubMed] [Google Scholar]
- 13.Suzuki K, Kaneko M, Murai K. Influence of serum lipids on auditory function. Laryngoscope. 2000;110:1736–8. doi: 10.1097/00005537-200010000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Gates GA, Cobb JL, D’Agostino RB, et al. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. 1993;119:156–61. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- 15.Strome M, Topf P, Vernick DM. Hyperlipidemia in association with childhood sensorineural hearing loss. Laryngoscope. 1988;98:165–9. doi: 10.1288/00005537-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Lowry LD, Isaacson SR. Study of 100 patients with bilateral sensorineural hearing loss for lipid abnormalities. Ann Otol Rhinol Laryngol. 1978;87:404–8. doi: 10.1177/000348947808700321. [DOI] [PubMed] [Google Scholar]
- 17.Ullrich D, Aurbach G, Drobik C. A prospective study of hyperlipidemia as a pathogenic factor in sudden hearing loss. Eur Arch Otorhinolaryngol. 1992;249:273–6. doi: 10.1007/BF00714491. [DOI] [PubMed] [Google Scholar]
- 18.Lee FS, Matthews LJ, Mills JH, et al. Analysis of blood chemistry and hearing levels in a sample of older persons. Ear Hear. 1998;19:180–90. doi: 10.1097/00003446-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Jones NS, Davis A. A retrospective case-controlled study of 1490 consecutive patients presenting to a neuro-otology clinic to examine the relationship between blood lipid levels and sensorineural hearing loss. Clin Otolaryngol. 2000;25:511–7. doi: 10.1046/j.1365-2273.2000.00408.x. [DOI] [PubMed] [Google Scholar]
- 20.Preyer S, Baisch A, Bless D, et al. Distortion product otoacoustic emissions in human hypercholesterolemia. Hear Res. 2001;152:139–51. doi: 10.1016/s0378-5955(00)00245-8. [DOI] [PubMed] [Google Scholar]