Abstract
Background
Although controversial, reducing low-density lipoprotein cholesterol (LDL-C) to target levels remains a common therapeutic goal after acute myocardial infarction (AMI). We sought to illuminate patient and provider characteristics associated with LDL-C goal nonattainment after AMI.
Methods
In an observational registry of 24 United States hospitals, we included 366 AMI patients who had baseline LDL-C levels ≥100 mg/dl and underwent 6-month fasting LDL-C reassessment. Our primary outcome was failure to reach the guidelines recommended LDL-C goal of <100 mg/dl at 6-months post-AMI.
Results
One in 3 AMI patients with initially elevated LDL-C failed to attain LDL-C goal at 6-months. Compared with those who attained LDL-C goal, those who did not were more often discharged without a statin (21% vs. 9%, p<0.001), despite only 4% having documented contraindications. Patients not achieving LDL-C goal also more frequently discontinued statin use by 6-months (24% vs. 6%, p<0.001). Multivariable modeling (c index 0.78) revealed the absence of a statin prescription at discharge and lack of persistence on statin therapy as the strongest independent factors associated with failure to reach LDL-C goal. Additional independent risk factors were patient report of not consistently adhering to prescribed medications, not participating in cardiac rehabilitation, nonwhite race, and lack of insurance.
Conclusions
One-third of AMI patients with baseline hyperlipidemia do not attain the LDL-C goal of <100 mg/dl at 6-months. Our findings support targeted interventions in the transition of AMI care to promote affordable statin prescription at discharge, medication persistence and adherence, and cardiac rehabilitation participation.
Keywords: myocardial infarction, hyperlipidemia, LDL cholesterol, goal attainment, barriers
Low-density lipoprotein cholesterol (LDL-C) of <100 mg/dl is a cornerstone of secondary prevention and an integral part of a multifaceted, evidence-based approach to risk reduction after acute myocardial infarction (AMI). In particular, LDL-C lowering by HMG-CoA reductase inhibitors (statins) has been shown in multiple randomized clinical trials to reduce coronary heart disease (CHD) events. Therefore, statins are a Class IA American College of Cardiology/American Heart Association (ACC/AHA) recommendation for secondary prevention of atherosclerotic CHD.
Yet, in translating this evidence into practice, there may be substantial room for improvement. Recent studies suggest that we are reaching a plateau with 1 in 3 patients failing to attain LDL-C levels <100 mg/dl. There is thus a need to better understand the relative importance of various patient and provider factors and whether such factors are potentially actionable. Therefore, we investigated patient and provider factors associated with LDL-C goal nonattainment after AMI using detailed clinical and longitudinal data from the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patient’s Health status (TRIUMPH) observational study.
METHODS
TRIUMPH Study
TRIUMPH is a prospective cohort study of 4,340 AMI patients enrolled between April 11, 2005 and December 31, 2008 at 24 academic and nonacademic centers across the United States. Participants were 18 years of age or older and presented or transferred to an enrolling center within 24 hours of an AMI diagnosis. AMI was defined as clinical features of ischemia (e.g. prolonged ischemic signs/symptoms, ST-segment changes in ≥2 contiguous leads on ECG) combined with elevated troponin (per local laboratory cutoff) outside the setting of elective coronary revascularization. Research staff conducted detailed baseline, 1-month, 6-month, and 12-month patient interviews in person or over the phone using standardized questions to capture clinical and sociodemographic data, which was complemented by chart abstraction. All data were entered into a Web-based program then underwent front-end logic and range checks and directed queries to ensure data accuracy. If a statin was not prescribed at discharge, then data collectors sought documentation of an allergy, intolerance, patient refusal, or other reason documented by a physician, nurse practitioner, or physician assistant. All patients provided informed consent and each enrolling center obtained institutional review board approval.
Study Population
Serial laboratory testing was a voluntary sub-study in TRIUMPH. Patients were asked if they would be willing to provide fasting blood solely for study purposes at baseline, 1-month, and 6-months. For patients who agreed, baseline blood was drawn near discharge during the AMI hospitalization. Follow-up blood was collected by trained medical personnel at an in-home visit, or if this was not possible, kits were mailed to the patients for collection at their physician’s office. All blood samples were processed, refrigerated and sent by overnight mail in freezer packs to the core laboratory (Clinical Reference Laboratory, Lenexa, KS). Lipid analyses employed the vertical auto profile method developed by Atherotech (Birmingham, AL), which directly measures cholesterol values including LDL-C.
From the TRIUMPH inception cohort, 886 subjects had 6-month lipid measurement. In this group, baseline LDL-C level was not at goal (≥100 mg/dl) in 366 subjects, who comprised our primary analysis study population. Our study population had lower rates of diabetes and prior MI and higher baseline LDL-C levels compared with the overall TRIUMPH population but had similar rates of statin use (Appendix 1). No subjects were excluded from this group.
Definitions
In our primary analysis, failure to achieve goal was defined as an LDL-C level ≥100 mg/dl at 6-months. We chose the NCEP ATP III conservative rather than aggressive goal because it is more commonly attained. We selected the 6-month time-point, as opposed to 1-month, because this provided a greater opportunity for patients to respond to new therapy and for risk factors for failure to reach goal to emerge.
A statin was considered not to have been prescribed if it was absent from the discharge medication list. Subjects were considered not to have persisted with statin treatment if a statin was on the discharge medication list but not on the 6-month medication list. Medication nonadherence was considered present if a patient reported taking medications <90% of the time at 6-months. Alternative lipid lowering therapy included the prescription of niacin, ezetimibe, a fibrate, or a bile acid sequestrant (combination lipid lowering therapy was defined as any one or more of these plus a statin). Lack of diet and activity counseling were defined as the absence of documentation of counseling on the patient’s discharge paperwork.
Physical function was quantified by the Short Form-12 Physical Component Summary score (SF-12 PCS). Social support was evaluated by the 7-item ENRICHD Social Support Inventory. Patients’ desires for shared medical decision making were examined with the Deber instrument. Standardized questions also assessed patients’ attitudes on self-efficacy and locus of control.
Statistical Analyses
Based on 6-month LDL-C levels, our primary analysis classified subjects as those who attained an LDL-C <100 mg/dl (n = 245) and those who did not (n = 121). Descriptive statistics were generated for both groups. Continuous variables with a Gaussian distribution were reported as mean (standard deviation) and their differences were assessed by independent t tests. Continuous variables without a Gaussian distribution were reported as median (25th percentile, 75th percentile) with differences compared by the Wilcoxon rank-sum test. Categorical variables were reported as proportions and differences were compared using the Chi-square method and Fisher exact test.
Given that not achieving LDL-C goal was common, we used a modified Poisson regression with a robust error variance to examine independent correlates of poor LDL-C control. Variables were selected for multivariable analysis based on clinical judgment of a priori importance and statistically significant differences between the groups in bivariate comparisons. A majority of patients (89%) were not missing any covariate information; 97% of patients were missing data for fewer than 2 covariates. The covariate missing rate was 0–4% with baseline SF-12 PCS, medication adherence, and insurance status having the highest rates. Data were assumed to be missing at random and were imputed using a single imputation model that contained all of the variables from the multivariable model.
In sensitivity analyses, we tested whether risk factors were similar for failure to attain the more aggressive LDL-C goal of <70 mg/dl and non-HDL-C goal of <130 mg/dl. The sample size increased (n = 695) by lowering the LDL-C goal as more patients were above goal at baseline. However, the sample size was reduced (n = 174) in the non-HDL-C analysis as some patients in TRIUMPH only reported LDL-C levels.
All analyses were conducted with SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.7.2 (R Foundation for Statistical Computing, Vienna, Austria).
The TRIUMPH registry was funded by the National Heart, Lung and Blood Institute (P50 HL 077113). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
RESULTS
One-third of subjects did not attain LDL-C levels <100 mg/dl by 6 months post-AMI (Figure 1). This group started with a median LDL-C level of 124 mg/dl that remained unchanged at 125 mg/dl at 6-months. In contrast, the median LDL-C level among those who attained LDL-C levels <100 mg/dl fell from 118 mg/dl to 74 mg/dl by 6-months.
Figure 1. Low-density lipoprotein cholesterol (LDL-C) levels at baseline and 6-months post-myocardial infarction in those who attained (-) and those who did not attain (- - -) LDL-C goal at 6 months.
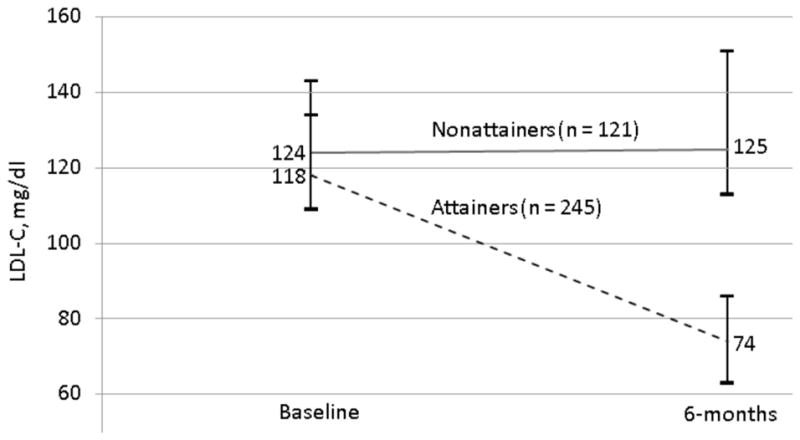
Numeric values are medians and whiskers indicate interquartile ranges.
Baseline Clinical and Sociodemographic Patient Factors
Compared with those who attained LDL-C goal, those who did not were younger and more likely to be nonwhite (Table 1). Type of AMI (ST-segment elevation MI vs. non-ST-segment elevation MI) did not differ between the groups, nor did baseline lipid parameters. Those who did not attain LDC-C goal more often had diabetes or a prior MI. The groups were not different by stroke, peripheral arterial disease, hypertension, body mass index, or statin use on arrival.
Table 1.
Baseline Clinical Characteristics.
| All Subjects n = 366 | Did Not Attain Goal n = 121 | Attained Goal n = 245 | p value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 58.9 (11.0) | 57.0 (12.0) | 59.8 (10.4) | 0.02 |
| Female | 30% | 36% | 27% | 0.08 |
| Nonwhite | 28% | 37% | 23% | 0.01 |
| STEMI (vs. Non-STEMI) | 51% | 50% | 51% | 0.91 |
| Prior MI | 16% | 23% | 12% | 0.01 |
| Diabetes | 24% | 32% | 19% | 0.01 |
| Prior stroke | 6% | 7% | 5% | 0.24 |
| Peripheral arterial disease | 4% | 5% | 4% | 0.58 |
| Hypertension | 59% | 59% | 59% | 0.99 |
| Body mass index | 29.7 (5.9) | 29.6 (6.8) | 29.8 (5.4) | 0.82 |
| Lipid parameters | ||||
| Statin on arrival | 19% | 22% | 18% | 0.32 |
| LDL-C, mg/dl | 120 (109, 137) | 124 (109, 143) | 118 (109, 134) | 0.06 |
| Triglycerides, mg/dl | 152 (114, 209) | 152 (115, 199) | 154 (114, 218) | 0.57 |
| HDL-C, mg/dl | 40 (35, 47) | 40 (33, 50) | 40 (35, 46) | 0.85 |
Variables are expressed as percentages, mean (standard deviation), or median (25th percentile, 75th percentile). STEMI = ST-segment elevation myocardial infarction; MI = myocardial infarction; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol.
Considering factors that may influence medication-taking behavior, the groups were similar by discharge disposition to home/self-care and the number of medications prescribed at discharge (Table 2). Compared with those who achieved goal, those who did not were twice as likely to be uninsured (30% vs. 15%, p<0.001) and were more likely to avoid care and medications due to cost. Although those who did not achieve goal were less often married, they had similar social support and frequency of depressive symptoms relative to those who attained LDL-C goal. The groups were also similar by high school education, employment, and attitudes on locus of control and shared decision making. Overall rates of alcohol and illicit drug use were low and numerically higher (not statistically significant) among those who did not attain goal. Physical function was modestly lower compared with those who attained goal (Table 1).
Table 2.
Patient Factors That May Influence Medication Taking Behavior.
| All Subjects n = 366 | Did Not Attain Goal n = 121 | Attained Goal n = 245 | p value | |
|---|---|---|---|---|
|
| ||||
| Discharged to home/self-care | 94% | 93% | 95% | 0.33 |
|
| ||||
| Number of medications prescribed at discharge | 8.0 (1.7) | 8.1 (1.9) | 8.0 (1.6) | 0.31 |
|
| ||||
| Uninsured | 20% | 30% | 15% | <0.001 |
|
| ||||
| Avoid care due to cost | 25% | 32% | 21% | 0.03 |
|
| ||||
| Avoid medications due to cost | 0.002 | |||
| Always | 4% | 7% | 2% | |
| Frequently | 7% | 7% | 6% | |
| Occasionally | 9% | 12% | 7% | |
| Rarely | 7% | 11% | 5% | |
| Never | 75% | 63% | 80% | |
|
| ||||
| Married | 58% | 49% | 63% | 0.01 |
|
| ||||
| ENRICHD Social Support Score | 21.5 (4.7) | 21.4 (5.2) | 21.6 (4.5) | 0.68 |
|
| ||||
| Depression requiring treatment | 7% | 6% | 8% | 0.41 |
|
| ||||
| High school education | 82% | 80% | 83% | 0.45 |
|
| ||||
| Employed (work full or part time) | 51% | 48% | 53% | 0.35 |
|
| ||||
| Locus of control: I can reduce my risk of MI. | 0.86 | |||
| Strongly agree | 81% | 81% | 80% | |
| Somewhat agree | 12% | 11% | 13% | |
| Agree a little | 0.8% | 0.8% | 0.8% | |
| Don’t know | 5% | 6% | 5% | |
| Disagree a little | 0.8% | 0.8% | 0.8% | |
| Somewhat disagree | 0.3% | 0.0% | 0.4% | |
| Strongly disagree | 0.3% | 0.8% | 0.0% | |
|
| ||||
| Shared decision making: who decides treatment? | 0.38 | |||
| Doctor alone | 17% | 22% | 14% | |
| Mostly the doctor | 12% | 13% | 11% | |
| Doctor and you equally | 39% | 36% | 41% | |
| Mostly you | 12% | 12% | 13% | |
| You alone | 20% | 18% | 22% | |
|
| ||||
| Alcohol abuse | 8% | 12% | 7% | 0.10 |
|
| ||||
| Cocaine use | 3% | 6% | 2% | 0.07 |
|
| ||||
| Other illicit drug use | 2% | 3% | 1% | 0.40 |
|
| ||||
| Physical function (SF-12 PCS) | 44.2 (11.9) | 41.6 (12.0) | 45.4 (11.6) | 0.01 |
Variables are expressed as percentages and mean (standard deviation).
Provider Factors in Lipid Management at Discharge and Shared Patient-Provider Factors in Follow-up
Providers did not prescribe a statin at discharge in 21% of those who did not achieve goal LDL-C compared with 9% of those who did (Table 3). Only 4% of those who did not attain goal LDL-C had documented contraindications to statin therapy. There was considerable variability between hospitals in discharge statin prescription rates after accounting for documented contraindications or any reason for withholding (range 76–100%; 11 hospitals >90%, 10 hospitals 80–90%, and 3 hospitals <80%). When a statin was prescribed, generic, lower-cost statins at that time (pravastatin, lovastatin, fluvastatin) were used uncommonly in both groups. Diet and activity counseling were also provided less frequently among those who did not reach goal compared with those who did. In contrast, ≥95% of patients in both groups were scheduled for follow-up care by hospital discharge without significant differences between groups.
Table 3.
Provider Factors in Lipid Management at Discharge and Shared Patient-Provider Factors in Follow-up.
| All Subjects n = 366 | Did Not Attain Goal n = 121 | Attained Goal n = 245 | p value | |
|---|---|---|---|---|
|
| ||||
| Discharge | ||||
|
| ||||
| Statin | ||||
|
| ||||
| Not prescribed | 13% | 21% | 9% | 0.001 |
|
| ||||
| Atorvastatin | 42% | 31% | 47% | 0.01 |
|
| ||||
| Simvastatin | 34% | 33% | 35% | 0.82 |
|
| ||||
| Rosuvastatin | 5% | 3% | 5% | 0.39 |
|
| ||||
| Pravastatin | 4% | 6% | 3% | 0.27 |
|
| ||||
| Lovastatin | 3% | 4% | 2% | 0.31 |
|
| ||||
| Fluvastatin | 0.3% | 1% | 0% | 0.33 |
|
| ||||
| Statin (type unknown) | 0.5% | 2% | 0% | 0.11 |
|
| ||||
| Statin contraindication | 1% | 4% | 0% | 0.04 |
|
| ||||
| Alternative lipid lowering therapy prescribed | 11% | 15% | 9% | 0.09 |
|
| ||||
| Combination lipid lowering therapy prescribed | 9% | 11% | 8% | 0.34 |
|
| ||||
| Omega-3 supplement prescribed | 20% | 18% | 21% | 0.43 |
|
| ||||
| Diet/activity instructions | 93% | 89% | 96% | 0.02 |
|
| ||||
| Scheduled follow-up | 97% | 98% | 96% | 0.76 |
|
| ||||
| Follow-up | ||||
|
| ||||
| Statin discontinued by 6-months | 12% | 24% | 6% | <0.001 |
|
| ||||
| How often do you take your meds? | 0.01 | |||
| All of the time (100%) | 84% | 78% | 86% | |
| Nearly all of the time (90%) | 13% | 14% | 12% | |
| Most of the time (75%) | 2% | 3% | 0.8% | |
| About half the time (50%) | 1% | 2% | 0.8% | |
| Less than half the time (<50%) | 1% | 3% | 0% | |
|
| ||||
| Cardiac rehabilitation participation | 39% | 23% | 46% | <0.001 |
|
| ||||
| How active are you during leisure time? | 0.003 | |||
| Mainly sedentary | 41% | 52% | 36% | |
| Mild exercise | 28% | 29% | 28% | |
| Moderate exercise | 25% | 18% | 29% | |
| Strenuous exercise | 6% | 2% | 8% | |
|
| ||||
| How often do you eat fast food? | 0.27 | |||
| Less than once a month | 53% | 49% | 55% | |
| 1–3 times a month | 27% | 31% | 25% | |
| 1–2 times a week | 14% | 16% | 13% | |
| 3–4 times a week | 5% | 4% | 5% | |
| 5 or more times a week | 2% | 0% | 3% | |
|
| ||||
| How well does your doctor know you? | 0.61 | |||
| Extremely well | 60% | 56% | 62% | |
| Fairly well | 30% | 31% | 30% | |
| Somewhat | 6% | 9% | 5% | |
| Not very well | 4% | 4% | 3% | |
| Not at all | 0% | 0% | 0% | |
Variables are expressed as percentages and median (25th percentile, 75th percentile).
In follow-up, statin therapy was discontinued by 6-months in 24% of those who did not reach goal compared with 6% among those who did. Only one patient not discharged on a statin reported initiation after discharge and persistence through 6 months. By 1 year, 43% of those who did not attain LDL-C goal were no longer taking statin therapy compared with only 12% of those who attained goal (p <0.001). Patients who reached goal were more adherent to medications though both groups reported high adherence. Patients who did not attain LDL-C goal participated in cardiac rehabilitation at half the rate of those who did and were also more sedentary. The groups had similarly low frequencies of fast food intake. The majority of patients in both groups reported that their doctor knew them well.
Multivariable Analyses
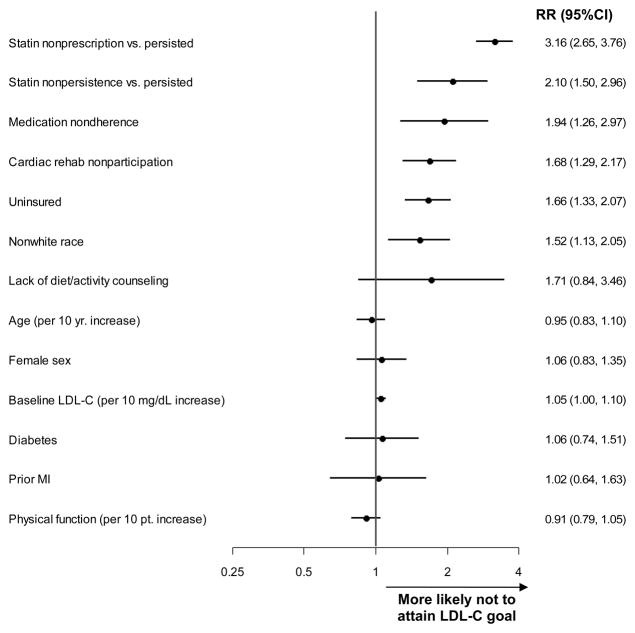
In multivariable analysis (c statistic 0.78 for final model), the factors most strongly associated with not reaching LDL-C goal were no statin prescription at discharge and failure to continue statin therapy for the duration of follow up (Figure 2). Other independent risk factors included medication nonadherence, not participating in cardiac rehab, lack of insurance, and nonwhite race. Factors that lost significance after multivariable adjustment were lack of diet/activity counseling, age, sex, baseline LDL-C, diabetes, prior MI, and physical function.
Figure 2. Multivariable analysis of factors associated with failure to attain the low-density lipoprotein cholesterol (LDL-C) goal of <100 mg/dL at 6-months.
All factors in the forest plot were included in the multivariable analysis. Relative risks (RR) and 95% confidence intervals (CI) are presented. MI = myocardial infarction.
In sensitivity analyses examining failure to attain the LDL-C goal of <70 mg/dl (Appendix Figure 1) and the non-HDL-C goal of <130 mg/dl (Appendix Figure 2), lack of statin and cardiac rehabilitation use were consistently independent risk factors.
DISCUSSION
The TRIUMPH study offered a unique opportunity to examine several patient and provider factors that may be modified to maximize LDL-C treatment goal attainment among AMI patients. We found that, in contemporary practice, one in three AMI patients with baseline LDL-C levels ≥100 mg/dl still do not attain the LDL-C goal of <100 mg/dl by 6-months. Failure to prescribe statin therapy and lack of statin persistence emerged as the strongest independent risk factors associated with not reaching LDL-C goal. Medication adherence and cardiac rehabilitation participation were also independent risk factors.
Key Barriers: Suboptimal statin prescription, persistence, and adherence
AMI is an opportune time to institute lipid lowering therapy, but rates of discharge statin use among patients with diagnosed hyperlipidemia were lower than anticipated in this prospective observational study. Documented contraindications explained 4% of statin nonprescription among those who did not achieve LDL-C goal at 6 months. Yet, an additional 17% of those who did not reach goal were not prescribed a statin at discharge and another 24% discontinued use before 6-months. Underlying these population-level observations are individual patients with unique clinical contexts and challenges to the practical application of lipid therapy. Undocumented contraindications to treatment are possible; however, these rates are well beyond what would be expected based on the statin safety literature.16–18 Therefore, it seems that a considerable number of untreated AMI patients are eligible for statin therapy.
While patients in our study reported high overall rates of medication adherence, nonadherence remained an independent risk factor for lipid goal nonattainment in our study. Staying on (persistence) and consistently taking (adherence) statin therapy drives therapeutic success and clinical outcomes.19 Importantly, the timing of the statin prescription may modify long-term persistence. For example, in the Cardiac Hospitalization Atherosclerosis Management Program (CHAMP),20 prescribing a statin at discharge, rather than deferring to follow-up, improved the proportion of patients on a statin at 1 year from 10% to 91%. Statin adherence cannot be robustly explained in large databases,21 attesting to complex mechanisms underlying it. Despite this, development of a positive therapeutic relationship3,22 and use of medication reminder tools (e.g. pillbox) are possible methods through which providers can promote adherence.22
Low-cost statins and cardiac rehabilitation: additional actionable barriers?
Our study suggests the importance of two other tangible ways to foster adherence, persistence, and therapeutic goal attainment: use of lower cost therapies and cardiac rehabilitation. Higher medication cost is strongly associated with nonadherence.21,23–25 In TRIUMPH, lower cost generic statins were used in only 1 in 10 patients who did not achieve LDL-C goal while 4 in 10 reported avoiding medications due to cost, suggesting a disconnect between prescribing patterns and the socioeconomic context of patients. Lack of insurance was also an independent risk factor for reaching an LCL-C goal of <100 mg/dl, and may serve as a flag for patients who cannot fill a prescription for a higher priced statin. Since our study, generic forms of simvastatin, and atorvastatin, have become available. On average, LDL-C is reduced by ~35% with generic simvastatin 40 mg daily and ~50% with generic atorvastatin 80 mg daily. Both regimens safely reduce cardiovascular events in high risk patients.26,27 Therefore, greater use of these regimens could improve cost-effectiveness.
Beyond cost, providers may further enhance adherence and persistence by encouraging patient participation in cardiac rehabilitation.2,3,28 In another analysis of the TRIUMPH population, cardiac rehabilitation participation was associated with greater persistence of secondary prevention medications including statins.28 In the present analysis, it is notable that patients who did not attain LDL-C goal participated in cardiac rehabilitation at half the rate of those who did. The association of cardiac rehabilitation and LDL-C goal attainment may reflect improved medication adherence and persistence as well as enhanced physical activity and health behaviors as a result of cardiac rehabilitation.
Can we continue to raise the ceiling in LDL-C goal attainment?
Finally, if we consider LDL-C goal attainment rates in our study in the context of prior studies, it seems that we could be hitting a ceiling. In studies between 1996 and 2003, <20% of chronic coronary artery disease patients attained LDL-C levels <100 mg/dl.29,30 In a study conducted in 2003, 67% of coronary artery disease patients attained LDL-C levels <100 mg/dl, a rate that remained static in more contemporary studies. In contrast to these, our study focused on AMI patients with baseline hyperlipidemia and revealed a sizable remaining treatment gap. In striving for greater quality of care, our data strongly support the introduction of discharge statin prescription as the 10th Joint Commission Quality Performance Measurement in AMI.31 Our data also indicate that this single metric, while key, is not enough. For example, it does not address statin affordability and promotion of long-term statin persistence. Refining quality performance measurement will gain increased importance as we develop the ability to leverage outpatient data for quality of care assessment and improvement.
Limitations
First, our analyses depended upon patients who participated in serial labs and had baseline LDL-C levels ≥100 mg/dl, which could make them less representative of the general AMI population. In comparing our primary analysis sample to all other TRIUMPH participants, we found differences in diabetes, prior MI, and baseline LDL-C (Appendix Table 1), but no differences in statin use, cardiac rehabilitation, or the other independent, modifiable factors identified in our analysis. Second, TRIUMPH hospitals were predominantly academic, which may limit the generalizability of our results and detailed probing of provider differences in LDL-C goal achievement. Third, while we performed extensive bivariate comparisons of relevant factors in our well characterized sample and used adjusted multivariable modeling, unmeasured confounders are possible in this observational analysis. Fourth, statin doses were missing in 60 subjects and therefore not included in analyses. However, dose was unlikely to be an important factor in our primary analysis given even low to moderate doses would generally yield sufficient LDL-C reduction for goal attainment in our population with only modest baseline LDL-C elevation. Fifth, patient adherence to therapy was self-reported, not validated with pharmacy level data or direct monitoring.
Conclusion
One-third of hyperlipidemic AMI patients in current practice do not attain the guideline-recommended conservative LDL-C goal by 6-months. Among modifiable patient and provider factors, discharge statin prescription, persistence of and adherence to statin therapy, and participation in cardiac rehabilitation were associated with lipid goal attainment. Our results serve as a call to action for greater patient-physician alliance to ensure effective translation of evidence into practice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction. J Am Coll Cardiol. 2008;51:210–47. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. J Am Coll Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 6.O’Keefe JH, Jr, Cordain L, Harris WH, et al. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43:2142–6. doi: 10.1016/j.jacc.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Davidson MH, Maki KC, Pearson TA, et al. Results of the National Cholesterol Education (NCEP) Program Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendations. Am J Cardiol. 2005;96:556–63. doi: 10.1016/j.amjcard.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Santos RD, Waters DD, Tarasenko L, et al. Low- and high-density lipoprotein cholesterol goal attainment in dyslipidemic women: The Lipid Treatment Assessment Project (L-TAP) 2. Am Heart J. 2009;158:860–6. doi: 10.1016/j.ahj.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Melloni C, Shah BR, Ou FS, et al. Lipid-lowering intensification and low-density lipoprotein cholesterol achievement from hospital admission to 1-year follow-up after an acute coronary syndrome event: results from the Medications ApplIed aNd SusTAINed Over Time (MAINTAIN) registry. Am Heart J. 2010;160:1121–9. doi: 10.1016/j.ahj.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–76. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006;26:787–802. doi: 10.1016/j.cll.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Vaglio J, Jr, Conard M, Poston WS, et al. Testing the performance of the ENRICHD Social Support Instrument in cardiac patients. Health Qual Life Outcomes. 2004;2:24. doi: 10.1186/1477-7525-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Arch Intern Med. 1996;156:1414–20. [PubMed] [Google Scholar]
- 15.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 16.Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther. 2007;29:1761–70. doi: 10.1016/j.clinthera.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–22. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–86. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 20.Fonarow GC, Gawlinski A, Moughrabi S, et al. Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP) Am J Cardiol. 2001;87:819–22. doi: 10.1016/s0002-9149(00)01519-8. [DOI] [PubMed] [Google Scholar]
- 21.Chan DC, Shrank WH, Cutler D, et al. Patient, physician, and payment predictors of statin adherence. Med Care. 2010;48:196–202. doi: 10.1097/MLR.0b013e3181c132ad. [DOI] [PubMed] [Google Scholar]
- 22.Melloni C, Alexander KP, Ou FS, et al. Predictors of early discontinuation of evidence-based medicine after acute coronary syndrome. Am J Cardiol. 2009;104:175–81. doi: 10.1016/j.amjcard.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Shrank WH, Choudhry NK, Fischer MA, et al. The epidemiology of prescriptions abandoned at the pharmacy. Ann Intern Med. 2010;153:633–40. doi: 10.7326/0003-4819-153-10-201011160-00005. [DOI] [PubMed] [Google Scholar]
- 24.Shrank WH, Hoang T, Ettner SL, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006;166:332–7. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- 25.Ye X, Gross CR, Schommer J, et al. Association between copayment and adherence to statin treatment initiated after coronary heart disease hospitalization: a longitudinal, retrospective, cohort study. Clin Ther. 2007;29:2748–57. doi: 10.1016/j.clinthera.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 27.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 28.Boyden TF, Reid KJ, Buchanan DM, et al. Is cardiac rehabilitation associated with improved medication persistence following acute myocardial infarction? J Am Coll Cardiol. 2011;57:E485. [Google Scholar]
- 29.Pearson TA, Laurora I, Chu H, et al. The lipid treatment assessment project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med. 2000;160:459–67. doi: 10.1001/archinte.160.4.459. [DOI] [PubMed] [Google Scholar]
- 30.Malek F, Dvorak IJ, Strieborna H, et al. Reaching target lipid levels and the natural history of diabetes mellitus in patients surviving acute coronary syndrome: A retrospective cohort study from a tertiary care outpatient clinic. Exp Clin Cardiol. 2008;13:25–8. [PMC free article] [PubMed] [Google Scholar]
- 31. [Accessed on 4/28/12]; http://www.jointcommission.org/assets/1/6/AMI_Statin_factsheet_10_1_10_revised.pdf.