Abstract
Objective
Previous studies have demonstrated consistent benefit in older adults undergoing cochlear implantation as compared with younger control groups, with age category thresholds between 60 and 70 years. The objective of this study is to report auditory performance in implant recipients older than 75 years, a cohort for which few data have been reported.
Study Design
Retrospective chart review.
Setting
Academic cochlear implant program in a tertiary-care hospital.
Patients
Twenty-eight cochlear implant recipients were subdivided into implant users older than 80 years (Group 1) and recipients currently older than 75 years (Group 2).
Intervention
Cochlear implantation.
Main Outcome Measures
Open-set speech perception scores.
Methods
Postoperative open-set speech perception scores were compared with preoperative scores in the best-aided condition. Criteria were developed to define situations where the implant was considered to be nonbeneficial or less beneficial than amplification, and those data were then subjected to Kaplan-Meier analysis.
Results
Group 1 included 13 patients with mean age of 80.7 years at the time of implantation. Group 2 included 15 patients with a mean age of 71.6 years. Scores were significantly better postoperatively at 6 months (p < 0.01) for Group 2 and at 12 months (p < 0.01) for both Groups 1 and 2. Kaplan-Meier curves were constructed for both groups.
Conclusion
Cochlear implantation in patients older than 75 years is beneficial, and Kaplan-Meier analysis demonstrates that the clinical benefit is durable over time. Patients older than 80 years obtain similar benefit, although auditory performance was less robust.
Keywords: Cochlear implantation, Elderly, Kaplan-Meier benefit analysis, Speech perception
Current US Census data indicate that persons older than 65 years comprise just more than 12% of the population (1). More importantly, US Census Bureau demographic projections predict that the age group older than 65 years will double by the year 2035, and the subsets of persons older than 85 and 100 years will triple in the next 3 decades (2). Highly prevalent conditions in this segment of the population will have the potential to consume significant healthcare-related resources, and options for treatment of those conditions clearly must be developed and expanded in the years ahead. Effective rehabilitation of hearing loss as the third most common condition in an aging population demographic will represent a significant challenge to clinicians (3).
Although cochlear implantation has proven to be successful in overcoming peripheral auditory dysfunction, the relationships between central auditory functional decline, or aging-related neurophysiologic changes and cochlear implantation are not well understood. Jerger et al. (4) has postulated that hearing loss with aging represents a type of disorder of central auditory processing not explained by a decline in peripheral auditory sensitivity alone. Pichora-Fuller and Souza (5) and Welsh et al. (6) have also characterized the concept of central presbycusis and its relationship to hearing loss with advancing age. Whether these factors and others represent a physiologic “ceiling” to the benefits of cochlear implantation remains a subject of debate.
Several studies have demonstrated the benefits of cochlear implantation in individuals older than 65 years, including improvements in hearing and communication abilities, emotional and psychological quality of life, and related benefits in relationships with spouses and peers in social settings (7–11). Surgical risks, cost-benefit ratios, and the availability and consumption of healthcare resources are also important factors to consider. However, there are few data reported in the literature on the benefits of cochlear implantation in individuals older than 75 years; earlier studies include data from small numbers of these individuals in reported series, but sample sizes have generally been small and have not routinely reported data in individuals older than 75 years and beyond (12). The purpose of this study was to investigate and report audiometric outcomes in patients older than 75 years undergoing cochlear implantation and to also apply Kaplan-Meier methods of analysis to the data to further understand long-term benefits of cochlear implantation in these individuals.
MATERIALS AND METHODS
This study was reviewed and approved by the Baylor College of Medicine Institutional Review Board (IRB no. H-24008).
Clinical databases in the Department of Otolaryngology–Head and Neck Surgery and Audiology (The Methodist Hospital) were reviewed to identify all adult cochlear implant recipients from 1995 to the present. Clinical and audiologic records were reviewed for all patients currently older than 75 years, which served as the initial study cohort. All patients underwent standard cochlear implant candidacy evaluation under the care of both the implanting surgeon and the implant audiologist. Initial inclusion criteria required that each patient had previous experience (at least 6 wk) with conventional amplification before implant surgery, had undergone cochlear implantation in at least 1 ear, and had audiologic follow-up data for at least 12 months postoperatively. Patients that developed significant cognitive deficits, neurologic decline, had cerebrovascular accidents with residual deficit, or died within the first 12 months after implant surgery were excluded from analysis. The remaining patients were then subdivided into 2 groups. Group 1 included patients older than 80 years at implantation or older than 75 years at implantation with at least 5 years of implant use. Group 2 included patients older than 75 years at implantation with less than 5 years of experience, patients older than 70 at implantation with between 5 and 10 years of implant experience, or patients older than 65 years at implantation with 10 or more years of implant experience.
Data sets were compiled, when available, for each patient and included their preoperative speech-perception scores on hearing in noise test (HINT), City University of New York, and Consonant-Nucleus-Consonant (CNC) open-set tests (either live, tape- or CD-recorded, or both), and sound field pure-tone thresholds in their best-aided condition. Postoperative scores for those open-set batteries were recorded at 1 (or at the time of device activation), 6, and 12 months, and then HINT scores were recorded annually thereafter. Sound field pure-tone data were also recorded in postoperative visits over 1 year. Pre-operative and postoperative data were then compiled and analyzed using Stata, version 7.0 (Stata Corporation, College Station, TX, USA).
Open-set speech perception data were then analyzed using Kaplan-Meier techniques to help evaluate the clinical benefit(s) of implantation over time in this population. For purposes of analysis, the binary condition was defined as “significant benefit” from the implant versus “nonbenefit” (indicating benefit less than that provided by amplification alone before implantation). Nonbenefit situations included device nonuse or open-set speech-perception scores that were lower than preoperative best-aided scores on HINT or City University of New York sentences or both. If speech perception scores were better than pre-operative scores but were less than 40% (representing the current Medicare criterion for cochlear implantation), that data point was considered nonbenefit and was censored from Kaplan-Meier analysis at that point in time.
A questionnaire was developed to obtain additional subjective information from the implant recipient and/or from spouses or close family members (see Appendix 1). Patients received the questionnaire over the course of the study provided that they had at least 1 year of experience with the implant. Questionnaire results were compiled for information purposes only to attempt to obtain additional long-term subjective, patient-perceived benefits but were not included in the statistical analysis.
RESULTS
The total number of implant recipients meeting inclusion criteria was 31; 2 patients had less than 1 year of implant experience and were excluded from analysis, and 1 patient had postoperative open-set data available only for the first 6 months after implantation and was excluded. Of all remaining patients, none experienced significant neurologic or cognitive decline or death within the first 12 months after implantation. The final cohort compiled for data analysis included 28 patients.
Of 28 patients, 27 (96%) received Nucleus devices (Cochlear Americas, Centennial, CO, USA); speech processors in use over the course of the study and during data acquisition included Sprint, Esprit 3G, and Freedom platforms, although most patients had upgraded to the Freedom processor when possible. One patient received a Clarion (Spiral) device (Advanced Bionics, Sylmar, CA, USA). No attempt was made to stratify patients or results based on processor, speech-processing, or mapping strategies. It was assumed that those patient-related factors were optimized by the implant audiologist on an individual basis to achieve maximal benefit in each case.
Table 1 summarizes demographic data for the study population. Group 1 included 13 patients with a mean age of 80.7 years at the time of implantation. There were 6 women and 7 men, with a mean current age of 85.7 years. Two patients died during the course of the study after implantation. All patients were implanted with Nucleus devices; 6 patients received implants in the left ear and 7 on the right. Mean number of years of implant use for the group was 5.3 years. The oldest current implant user was implanted at 89 years and is currently 91 years old, and 1 other recipient is currently 90 years old. Mean follow-up time (indicating open-set speech perception data were available) was 41.1 months. Group 2 included 15 patients, with 7 women and 8 men. Seven patients received implants in the left ear and 6 on the right; there were 2 bilateral recipients, both sequential. The mean age at implantation was 71.6 years. Mean follow-up time was 46.6 months. Two patients, both in Group 2, underwent bilateral, sequential implantation over the course of the study. Two patients were explanted over the course of the study for flap-related complications; only 1 underwent reimplantation.
TABLE 1.
Demographic data summarizing study population
| Group (no. subjects) | Age range at implantation, yr | Mean age at implantation, yr | Mean current age, yr | Mean years of implant use | Mean follow-up, mo |
|---|---|---|---|---|---|
| 1 (13) | 75–89 | 80.7 | 85.7 | 5.3 | 41.1 (SD, 30.3) |
| 2 (15) | 65–78 | 71.6 | 77.7 | 6.9 | 46.6 (SD, 47.3) |
| Total = 28 | 75.8 | 81.2a | 6.1 | 44.1 |
Two patients died during the course of the study period.
Preoperative and postoperative speech-perception scores for Group 1 are displayed graphically in Figure 1. Similarly, Group 2 preoperative and postoperative speech-perception scores are displayed in Figure 2. Six patients did not have open-set preoperative HINT or CNC scores available because they were implanted in the late 1990s before standardized application of those speech batteries in cochlear implant evaluations. Their postoperative data were not included in the statistical analysis comparing preoperative versus postoperative differences but were included in the Kaplan-Meier analysis (reported in succeeding sentences) as an indicator of patient benefit over time. Thus, there were 22 patients with evaluable data included in the preoperative versus postoperative data analysis. Paired t-test indicated a statistically significant difference in both groups between preoperative and postoperative HINT sentence scores (p < 0.001) after 1 year. Two-sample t-test indicated no statistically significant difference between the 2 groups in postoperative HINT scores (p = 0.13), although there was a trend for Group 2 to outperform Group 1. Group 1 mean HINT scores were 57.3% (standard deviation [SD], 29.48; 95% confidence interval, 39.49–75.12), and Group 2 mean HINT scores were 73.1% (SD, 22.89; 95% confidence interval, 59.92–86.36) after 1 year.
FIG. 1.
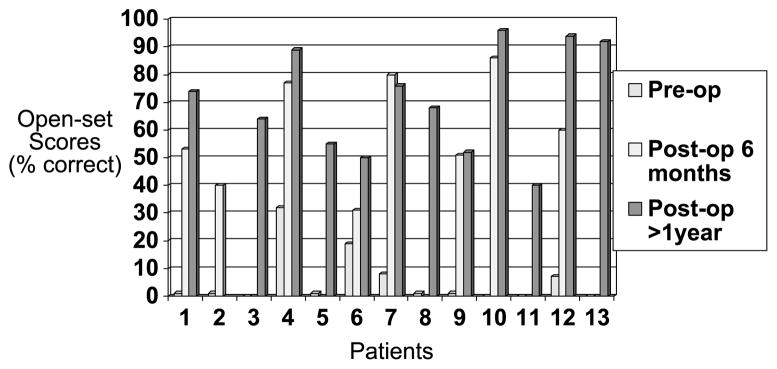
Scores indicate percent correct on HINT sentences for individuals in Group 1.
FIG. 2.
Scores indicate percent correct on HINT sentences for individuals in Group 2.
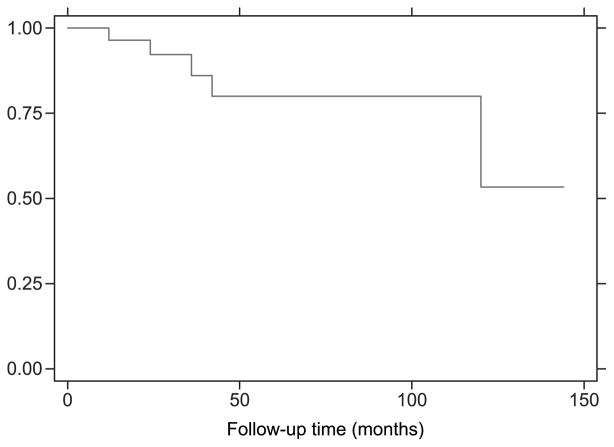
Kaplan-Meier curves were constructed for Groups 1 and 2 individually and are displayed in Figure 3. Outcomes depicted represent the proportion of patients who benefitted from implantation according to criteria previously outlined and the durability of those outcomes over time. Overall Kaplan-Meier outcome analysis representing all patients in the study is included in Figure 4.
FIG. 3.
Kaplan-Meier analysis indicating proportion of patients in each group receiving benefit from cochlear implantation plotted over follow-up period.
FIG. 4.
Proportion of all study participants receiving benefit from cochlear implantation plotted over follow-up period.
Questionnaire responses were obtained from 13 individuals for a response rate of 46%. Nine responses were interpreted as overall positive (69.2%) and reported a mean daily implant use of 14.5 hours. Two questionnaires were returned regarding patients who died, and 2 questionnaires were interpreted as overall negative (15.4%), indicating that the patient (and/or spouse) felt that the implant overall was not significantly better that amplification alone before surgery, or that the patient overall would not undergo implantation again or recommend implantation to others.
DISCUSSION
These data represent the first analysis and reporting of audiometric performance data in patients older than 75 and 80 years undergoing cochlear implantation as measured by open-set speech perception. The benefits of implantation are realized within the first 12 months and are durable over time (mean follow-up period of 44.1 mo for all participants) as represented by Kaplan-Meier methods of analysis. The younger cohort achieved statistically significant auditory benefit within the first 6 months of implantation (p = 0.004); adequate data for the older group were not available to evaluate at 6 months postoperatively, but a similar trend was observed. Subjective questionnaire data, although not statistically analyzed, indicate ongoing, patient-perceived benefits from cochlear implantation for even longer periods of follow-up, in some cases, up to 8 to 10 years later. Overall, the data support the concept that cochlear implantation is a beneficial and effective means of rehabilitating severe or severe-profound sensorineural hearing loss in older individuals, and that age alone, including age well into the ninth decade of life, is not a contraindication to cochlear implantation.
Chatelin et al. (12) and Vermeire et al. (10) have previously reported similar outcomes and benefit after cochlear implantation in patients older than 70 years, although mean follow-up was shorter in those studies (<24 mo). Both studies reported that auditory performance was statistically significantly less than younger control groups, but Vermeire et al. did demonstrate similar quality-of-life outcomes in the elderly. The data in the present study demonstrate a similar trend toward a decline in performance with advancing age, but results did not reach statistical significance. A number of other studies have reported similar, favorable results in implant recipients older than 65 years and included small numbers of patients in their ninth decade (13–16).
Leung et al. (16) reported auditory performance data in a large pooled group of individuals older than 65 years (mean age, 73.4 yr) and compared them against a younger cohort, with no statistically significant difference noted between groups based on postoperative CNC scores. They then developed mathematical predictive formulae (based on previously reported models) from the data that indicated that duration of deafness and preoperative Central Institute for the Deaf sentence scores carried greater predictive value than age at implantation. They proposed that a “foundation of central auditory processing” or internalized auditory memory might help to overcome any disadvantages in performance due to age alone. This is an important concept and an area that deserves further study, both in developing useful factors that predict postoperative implant outcomes and also in further elucidating the physiologic and neurocognitive changes that occur in central auditory pathways with aging.
The concept of aging is not universally understood, but it is known to be a multifactorial, complex, and highly variable process that begins early in life, typically measurable in the third decade, and continues thereafter. Neuronal functional changes and loss in the central nervous system become evident in the cortex and cerebellum first, followed by subcortical nuclei, and lastly in the brainstem. There are also known changes in processing abilities, including declines in working memory, recall of verbal information, episodic memory, and temporal processing (17). Histologic studies have documented postmortem changes in the central auditory pathway, including reduced cell numbers and degenerative changes in the cochlear and olivary nuclei, inferior colliculus, medial geniculate body, and auditory cortex (18–21). The relationships between these changes and the concept of central presbycusis are not well known, and additional research should be directed at further defining those relationships and the complex changes in hearing loss with aging.
Limitations to the current study include its retrospective nature and lack of control group for comparison and the attendant challenges in extrapolating to other patient populations in a prospective manner. Study participants were generally highly motivated and had adequate resources to seek evaluation and treatment at a tertiary-care center and optimize postoperative mapping strategies. There was limited variability in device implantation, and no attempts were made to stratify or analyze results based on external processor or speech-processing strategies, mapping data, or individual factors such as duration of hearing loss, or the importance of amplification in the preoperative or postoperative periods. As previously noted, there is no standardized, preoperative assessment of neurocognitive function in the cochlear implant evaluation process or in current guidelines for implant candidacy. Implanting surgeons and audiologists should consider all of these factors when evaluating and counseling their older patients and in deciding on appropriate options for rehabilitating hearing loss.
An additional important factor to consider is the spiral ganglion cell population in this cohort of patients. Progressive neuronal loss over the course of the study likely accounts for at least some of the changes in speech perception but was not controlled for in the present study. Although it would be difficult to objectively quantify neuronal populations in this study group, additional research might seek to measure neurocognitive function postoperatively and study its relationship to any changes in auditory performance. If a significant correlation could be shown, this would argue more strongly for the role of central decline over time as opposed to a loss of spiral ganglion cells.
With current projections in demographic data for individuals older than 65 years, cochlear implantation will become an increasingly important option for the rehabilitation of sensorineural hearing loss. The data in this study and those of Leung et al. and others support the concept that patient age does not represent a limitation to the benefits achieved with implantation, but rather a gradual decline that perhaps can be mitigated by further scientific inquiry and technological advances.
CONCLUSION
Cochlear implantation in individuals older than 75 and 80 years provides statistically significant benefits in auditory performance as measured by open-set speech perception scores, and those benefits are durable over time according to Kaplan-Meier methods of analysis.
APPENDIX 1
Name: _____________________________________
Date of Birth: ___________________
Contact Phone Number(s): _______________________
| 1. Are you still using your cochlear implant? | Yes | No | Sometimes |
| 2. If so, how many hours per day on average? | |||
| 3. Are you happy overall with the implant? | Yes | No | Somewhat |
| 4. Is it better for you than hearing aid(s) were? | Yes | No | Somewhat |
| 5. Does your spouse or family think it helps you? | Yes | No | Somewhat |
| 6. Do you have any difficulties using the implant? | Yes | No | Sometimes |
| 7. If so, what are they, or when do they occur (noisy situations, outside, etc.)? | |||
| 8. Please list any complaints or concerns about the implant that you would like to have changed: | |||
| 9. Would you recommend cochlear implants to others with hearing loss similar to yours? | Yes | No | Maybe |
| 10. Is there anything else you would like your doctor or the audiologist to know? |
References
- 1.U.S. Census Bureau. [Accessed May 2009];Census 2000 Summary File 1; Age Groups and Sex. Available at: www.census.gov.
- 2.U.S. Census Bureau. Projections of the Population by Selected Age Groups and Sex for the United States: 2010 to 2050, released 2008. Available at: www.census.gov.
- 3.Centers for Disease Control. Health Interview Survey (1999) [Accessed May 2009];MMWR CDC Surveill Summ. 1999 48:131–56. Available at: www.cdc.gov. [Google Scholar]
- 4.Jerger J, Jerger S, Oliver T, Pirozzolo F. Speech understanding in the elderly. Ear Hear. 1989;10:79–89. doi: 10.1097/00003446-198904000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Pichora-Fuller MK, Souza PE. Effects of aging on auditory processing of speech. Int J Audiol. 2003;42:S11–6. [PubMed] [Google Scholar]
- 6.Welsh LW, Welsh JJ, Healy MP. Central presbycusis. Laryngoscope. 1985;95:128–36. doi: 10.1288/00005537-198502000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Francis HW, Chee N, Yeagle J, Cheng A, Niparko JK. Impact of cochlear implants on the functional health status of older adults. Laryngoscope. 2002;112:1482–8. doi: 10.1097/00005537-200208000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Knutson JF, Murray KT, Husarek S, et al. Psychological change over 54 months of cochlear implant use. Ear Hear. 1998;19:191–201. doi: 10.1097/00003446-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy V, Stephens D, Fitzmaurice P. The impact of cochlear implants from the perspective of significant others of adult cochlear implant users. Otol Neurotol. 2008;29:607–14. doi: 10.1097/MAO.0b013e318165652c. [DOI] [PubMed] [Google Scholar]
- 10.Vermeire K, Brokx JPL, Wuyts FL, et al. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol. 2005;26:188–95. doi: 10.1097/00129492-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Orabi AA, Mawman D, Al-Zoubi F, Saeed SR, Remsden RT. Cochlear implant outcomes and quality of life in the elderly: Manchester experience over 13 years. Clin Otolaryngol. 2006;13:116–22. doi: 10.1111/j.1749-4486.2006.01156.x. [DOI] [PubMed] [Google Scholar]
- 12.Chatelin V, Kim EJ, Driscoll C, et al. Cochlear implant outcomes in the elderly. Otol Neurotol. 2004;25:298–301. doi: 10.1097/00129492-200405000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Facer GW, Peterson AM, Brey RH. Cochlear implantation in the senior citizen age group using the Nucleus 22-channel device. Ann Otol Rhinol Laryngol Suppl. 1995;166:187–90. [PubMed] [Google Scholar]
- 14.Labadie RF, Carrasco VN, Gilmer CH, Pillsbury HC. Cochlear implant performance in senior citizens. Otolaryngol Head Neck Surg. 2000;123:419–24. doi: 10.1067/mhn.2000.109759. [DOI] [PubMed] [Google Scholar]
- 15.Haensel J, Ilgner J, Chen YS, Thuermer C, Westhofen M. Speech perception in elderly patients following cochlear implantation. Acta Otolaryngol. 2005;125:1272–6. doi: 10.1080/00016480510044214. [DOI] [PubMed] [Google Scholar]
- 16.Leung J, Wang NY, Yeagle JD, et al. Predictive models for cochlear implantation in elderly candidates. Arch Otolaryngol Head Neck Surg. 2005;131:1049–54. doi: 10.1001/archotol.131.12.1049. [DOI] [PubMed] [Google Scholar]
- 17.Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol Mech Dis. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 18.Kirikae I, Sato T, Shitara T. A study of hearing in advanced age. Laryngoscope. 1963;74:205–20. doi: 10.1002/lary.5540740204. [DOI] [PubMed] [Google Scholar]
- 19.Ronning-Arnesen A. Presbyacusis-loss of neurons in the human cochlear nuclei. J Laryngol Otol. 1992;96:503–11. doi: 10.1017/s002221510009277x. [DOI] [PubMed] [Google Scholar]
- 20.Makishima K. Histopathological studies in presbycusis. J Otorhinolar Soc Jap. 1967;70:63. [Google Scholar]
- 21.Brody H. Organization of the cerebral cortex. J Comp Neurol. 1955;102:511–56. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]