Tolerability of pertuzumab plus trastuzumab plus docetaxel versus placebo plus trastuzumab plus docetaxel was observed in patients with HER2-positive metastatic breast cancer. Declines in LVEF were reported and the incidence of symptomatic LVSD was low. The majority of cardiac adverse events were reversible.
Keywords: Cardiac safety, HER2, LVSD, Metastatic breast cancer, Pertuzumab, Trastuzumab
Abstract
Introduction.
We report cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel versus placebo plus trastuzumab plus docetaxel observed in the phase III study CLEOPATRA in patients with HER2-positive first-line metastatic breast cancer (MBC).
Patients and Methods.
Left ventricular ejection fraction (LVEF) ≥50% and ECOG performance status of 0 or 1 were required for study entry. During the study, LVEF assessments took place every 9 weeks. Pertuzumab/placebo was given at 840 mg, then 420 mg q3w; trastuzumab was administered at 8 mg/kg, then 6 mg/kg q3w, and docetaxel was initiated at 75 mg/m2 q3w.
Results.
The incidence of cardiac adverse events (all grades) was 16.4% in the placebo arm and 14.5% in the pertuzumab arm, with left ventricular systolic dysfunction (LVSD, all grades) being the most frequently reported event (8.3% versus 4.4% in the placebo and pertuzumab arm). Declines in LVEF by ≥10% points from baseline and to <50% were reported in 6.6% and 3.8% of patients in the placebo and pertuzumab arm, respectively. Seventy-two percent (placebo arm) and 86.7% (pertuzumab arm) of those patients recovered to a value ≥50%. The incidence of symptomatic LVSD was low, occurring in 1.8% (n = 7) versus 1.0% (n = 4) of patients in the placebo and pertuzumab arm. In 8/11 patients, the symptomatic LVSD had resolved at data cutoff.
Conclusion.
The combination of pertuzumab plus trastuzumab plus docetaxel did not increase the incidence of cardiac adverse events, including LVSD, compared with the control arm in HER2-positive MBC. The majority of cardiac adverse events were reversible.
Implications for Practice:
CLEOPATRA was the first phase III trial in which the combination of pertuzumab with trastuzumab and docetaxel was studied in patients with HER2-positive metastatic breast cancer in the first line. As therapy with trastuzumab, especially in combination with anthracyclines, has been associated with cardiac dysfunction, it was important to investigate the cardiac tolerability of the study combination of two HER2-targeted antibodies, trastuzumab and pertuzumab, with docetaxel. Our analyses showed that the combination of pertuzumab, trastuzumab, and docetaxel was not associated with an increase in cardiac dysfunction, especially LVSD, compared with placebo, trastuzumab and docetaxel. Cardiac adverse events were largely reversible and clinically manageable. Despite our encouraging findings, we recommend the regular cardiac monitoring of patients while long-term safety data with pertuzumab-trastuzumab-based treatment are still being accrued in clinical practice.
Introduction
The addition of trastuzumab to chemotherapy significantly improved survival outcomes in patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer [1–4]. Increased rates of cardiac dysfunction with trastuzumab [1] were an unexpected observation and led to the formation of an independent cardiac review and evaluation committee that retrospectively reviewed seven phase II and III trials with trastuzumab [5]. Patients receiving trastuzumab, particularly when given in combination with anthracyclines, were found to be at increased risk of cardiac dysfunction [5]. These findings influenced the design of subsequent trials, in which left ventricular ejection fraction (LVEF) was monitored more frequently, concomitant use of trastuzumab and anthracyclines was avoided, and more rigorous cardiac eligibility criteria were applied [5, 6].
Cardiac dysfunction ascribed to trastuzumab seems to differ from that related to anthracyclines [7], which is dose-dependent [8, 9], induced by oxidative stress leading to myocyte cell death, and considered to be irreversible [10–13]. Trastuzumab-related cardiac dysfunction, on the other hand, is dose-independent and reversible in the majority of patients after discontinuation of trastuzumab and initiation of cardiac medication [5, 14–17]. Many patients are able to resume trastuzumab therapy after recovery from their transient decline in systolic function [18, 19]. Trastuzumab-associated cardiac dysfunction is believed to be related to HER2 expression on cardiomyocytes. HER2 is essential for cardiac development, as well as repair and survival of cardiomyocytes [20–24], pathways possibly impaired by trastuzumab.
As new anti-HER2 therapies enter the clinic, it is important to gauge their potential effect on cardiac function. Among them is pertuzumab, another humanized monoclonal antibody, targeting HER2 at a different epitope from trastuzumab, resulting in inhibition of HER2 heterodimerization [25–27]. In carefully selected patients, pertuzumab used alone or in combination with trastuzumab or nonanthracycline-based chemotherapy was associated with a low incidence of left ventricular systolic dysfunction (LVSD) [28].
CLEOPATRA (NCT00567190) was a randomized, double-blind, placebo-controlled phase III trial to compare the efficacy and safety of pertuzumab plus trastuzumab plus docetaxel with placebo plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer receiving first-line treatment. Pertuzumab, trastuzumab, and docetaxel significantly improved median progression-free survival by 6.1 months and resulted in a significant improvement in overall survival, with the safety profile being generally similar in both arms [29, 36]. Based on the study results, pertuzumab in combination with trastuzumab and docetaxel received FDA approval for treatment of HER2-positive first-line metastatic breast cancer in June 2012. This report details analyses of cardiac tolerability observed in CLEOPATRA.
Patients and Methods
The study was conducted in accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Protocol approval was obtained from an independent ethics committee for each site, and written informed consent was obtained from each participant.
Eligibility Criteria
Eligible patients had locally recurrent, unresectable, or metastatic HER2-positive breast cancer [29]. At baseline, an LVEF of ≥50%, determined by echocardiography (ECHO; preferred method) or multiple-gated acquisition scanning (MUGA), and an Eastern Cooperative Oncology Group (ECOG) performance status [30] of 0 or 1 were required. Patients may have received neoadjuvant and adjuvant chemotherapy with or without trastuzumab (Herceptin, F. Hoffmann-La Roche, Basel, Switzerland; Genentech Inc., South San Francisco, CA). Exclusion criteria included: prior exposure to a cumulative dose of doxorubicin of >360 mg/m2 or its equivalent; LVEF decline to <50% during or after prior trastuzumab therapy; history of congestive heart failure (CHF); history of serious cardiac arrhythmia requiring treatment; history of myocardial infarction within 6 months of randomization; current uncontrolled hypertension; and current dyspnea at rest, or other diseases that require continuous oxygen therapy.
Study Treatment
Study drugs were administered intravenously on a 3-week schedule. Patients received pertuzumab (Perjeta, F. Hoffmann-La Roche, Basel, Switzerland; Genentech Inc., South San Francisco, CA) or placebo at an initial dose of 840 mg, followed by 420 mg. Trastuzumab was given at an initial dose of 8 mg/kg, followed by 6 mg/kg. Pertuzumab/placebo and trastuzumab were given until disease progression as assessed by the investigator or unacceptable toxicity; dose modifications were not permitted. Docetaxel (Taxotere, Sanofi-Aventis, Paris, France) was administered at 75 mg/m2; dose escalation to 100 mg/m2 was allowed if the patient tolerated at least one cycle of 75 mg/m2 without experiencing febrile neutropenia, grade 4 neutropenia for >5 days, an absolute neutrophil count of <100/μL for >1 day, or other grade ≥3 nonhematologic toxicities. At least six cycles of docetaxel were recommended; fewer cycles were allowed for unacceptable toxicity or disease progression, and more cycles were allowed at the discretion of the investigator. Docetaxel dose reductions by 25% to 55 mg/m2 (or 75 mg/m2) were allowed to manage toxicity.
Cardiac Monitoring
LVEF was assessed at baseline, 9-weekly during study treatment, at treatment discontinuation, every 6 months in the first year after discontinuation, and annually thereafter for up to 3 years. The same method of LVEF assessment, ECHO or MUGA, was to be used throughout the study for an individual patient and, to the extent possible, to be obtained using the same equipment. Supplemental online Figure 1 presents the study treatment algorithm based on LVEF assessments. Patients for whom study treatment was permanently discontinued because of LVEF decline continued LVEF assessments at least every 3 months until the LVEF values improved to ≥50% or until 1 year after treatment discontinuation. Thereafter, LVEF assessments were performed annually for up to 3 years after treatment discontinuation.
Adverse events (AEs) were monitored continuously and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v3.0. Asymptomatic declines in LVEF were reported as AE only if the decline was ≥10% points from baseline and to <50%, or if it required treatment or led to discontinuation of study treatment. Asymptomatic LVEF declines of either <10% points from baseline or to a value ≥50% were neither reported as AE, nor did they result in treatment discontinuation, but were collected as LVEF data only. Grade 3 LVSD was defined as either symptomatic CHF responsive to intervention, or LVEF 20%–39%. Grade 4 LVSD was defined as refractory CHF or poorly controlled; or LVEF <20%; or intervention such as ventricular assist device, ventricular reduction surgery, or heart transplant indicated. An AE was reported as serious AE (SAE) if it was either fatal; life-threatening; required in-patient hospitalization or prolongation of existing hospitalization; resulted in persistent or significant disability/incapacity; was a congenital anomaly; or was medically significant or required intervention to prevent any of the aforementioned criteria. Symptomatic LVSD was reported as an SAE and led to discontinuation of study treatment.
Cardiac AEs ongoing at study discontinuation were followed until resolution or stabilization up to 1 year after the final dose. Patients with cardiac AEs that started up to 1 year after the final study dose were followed until resolution or stabilization up to 1 year after onset. Events of symptomatic LVSD that started up to 3 years after the final dose were followed until resolution, stabilization, confirmation by the investigator that no further improvement could be expected, or until completion of survival follow-up. Treatment-related SAEs were to be reported at any time regardless of the start date and were followed until resolution or stabilization up to 1 year after onset.
Statistical Methods
AEs were reported and summarized for descriptive purposes only. The time to first LVSD event was evaluated by the Kaplan-Meier approach. A Cox regression analysis of time to first LVSD event was performed to investigate predefined potential risk factors for cardiac dysfunction: prior anthracycline exposure; prior radiotherapy; prior trastuzumab therapy; age; smoking history; and underlying conditions of diabetes and hypertension. The baseline LVEF value and the maximum absolute decrease (or minimum absolute increase if all of the patient's post-baseline LVEF measurements were higher than at baseline) in LVEF measurement from baseline were summarized. The difference in the maximum absolute decrease in LVEF measurement between both arms was assessed by the Wilcoxon Rank Sum Test. The time to first decrease in LVEF to a value <50% and by ≥10% points from baseline was summarized by treatment arm using the Kaplan-Meier approach. A cumulative incidence analysis was performed on the time to first decrease in LVEF to <50% and by ≥10% points from baseline, with death as a competing risk event.
Results
Study Population
Between February 2008 and July 2010, 808 patients were enrolled at 204 centers in 25 countries and randomized to receive placebo plus trastuzumab plus docetaxel (n = 406) or pertuzumab plus trastuzumab plus docetaxel (n = 402). Two patients in each arm did not receive any treatment. In the placebo arm, eight patients received at least one dose of pertuzumab. In the pertuzumab arm, one patient received treatment allocated to the placebo arm only. The safety population therefore comprised 397 patients in the placebo arm and 407 patients in the pertuzumab arm (supplemental online Fig. 2). The data cutoff date for the primary analysis was in May 2011.
Baseline characteristics were similar in the safety population of both arms; slightly more patients in the pertuzumab arm presented with ECOG performance status of 0 (68.3% vs. 60.7% in the placebo arm).
Cardiac Adverse Events
The incidence of cardiac AEs (all grades) was 16.4% (n = 65) in the placebo arm and 14.5% (n = 59) in the pertuzumab arm (Table 1). The most frequently reported cardiac AE was LVSD (all grades), which was more common in the placebo arm (8.3%, n = 33) compared with the pertuzumab arm (4.4%, n = 18), and which led to a delay of study treatment in 10 patients in the placebo arm and four patients in the pertuzumab arm. The proportion of patients experiencing a cardiac AE of grade ≥3 was higher in the placebo arm (3.8%, n = 15) than the pertuzumab arm (1.5%, n = 6) (Table 1). LVSD was the most commonly reported grade ≥3 cardiac AE and was more frequent in the placebo arm (2.8%, n = 11) than the pertuzumab arm (1.2%, n = 5). The proportion of patients who experienced serious cardiac AEs, including symptomatic LVSD, atrial fibrillation, myocardial infarction, and ventricular fibrillation, was higher in the placebo arm (3.3%, n = 13) compared with the pertuzumab arm (1.2%, n = 5). Two patients in the placebo arm died as a result of myocardial infarction.
Table 1.
Cardiac disorders (NCI-CTCAE v3.0, grades 1–5)
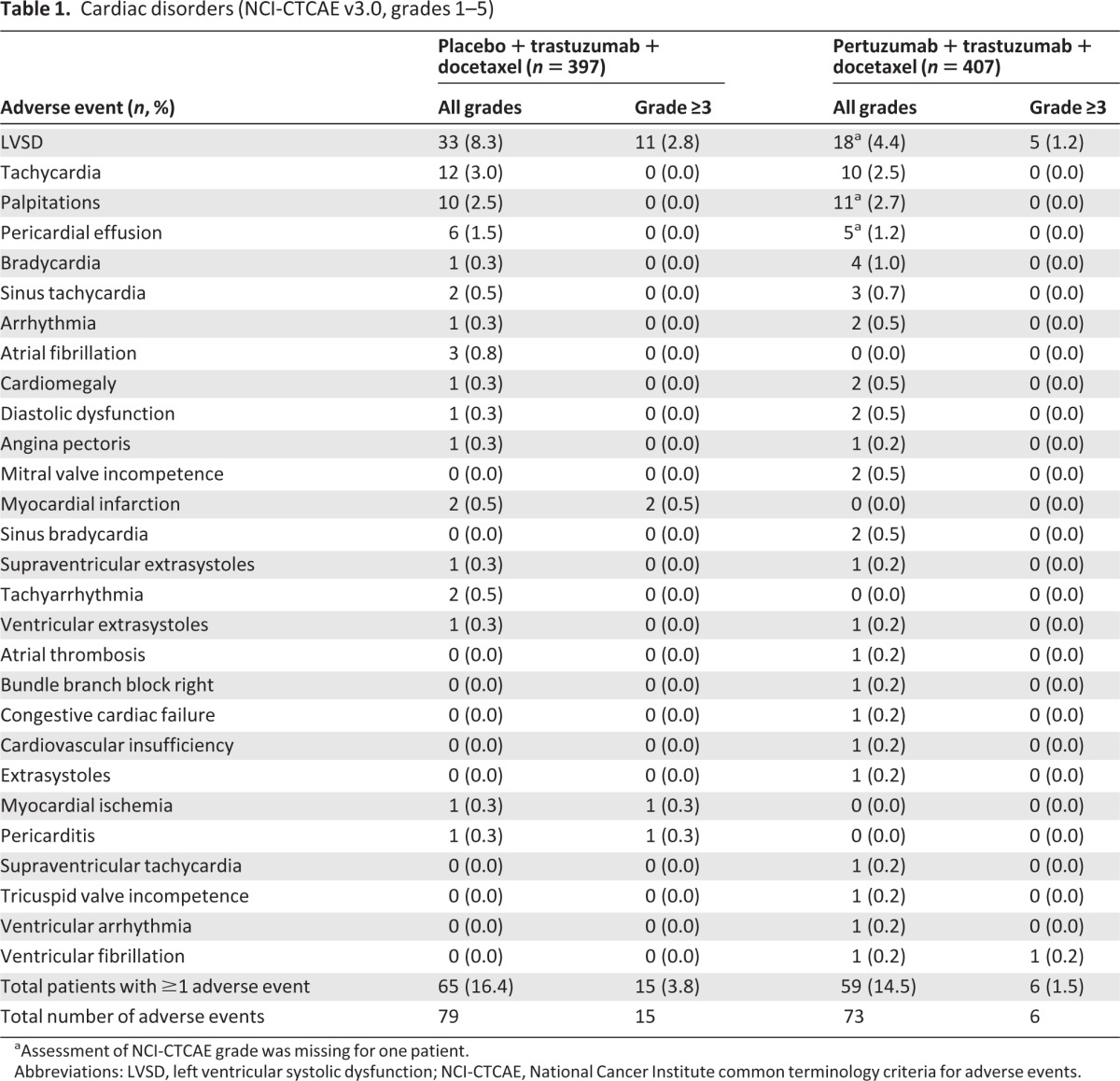
aAssessment of NCI-CTCAE grade was missing for one patient.
Abbreviations: LVSD, left ventricular systolic dysfunction; NCI-CTCAE, National Cancer Institute common terminology criteria for adverse events.
LVEF Measurements
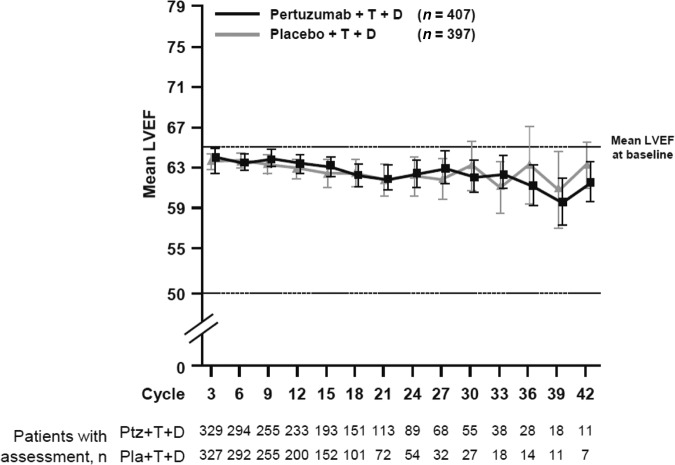
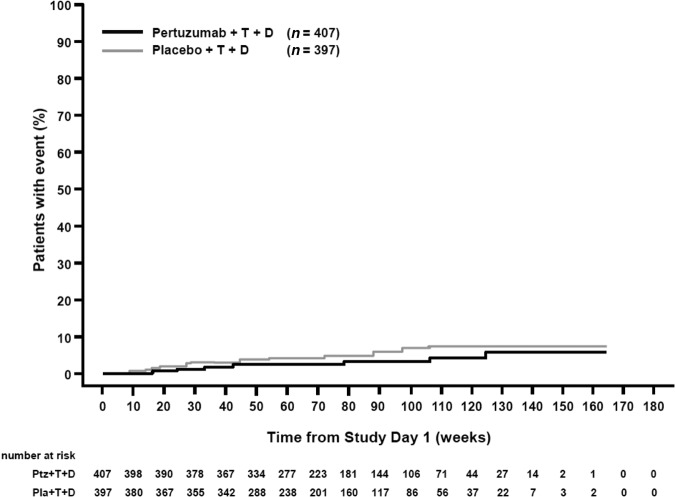
In both arms, LVEF was assessed by ECHO only in 77% of patients; by MUGA only in 18% of patients; and by both ECHO and MUGA in 5% of patients. The mean LVEF at baseline was 65.6% in the placebo arm and 64.8% in the pertuzumab arm (range 50%–88% in both arms). For 25.6% of patients in the placebo arm and for 29.4% of patients in the pertuzumab arm, at least one LVEF assessment was missing or not performed within the protocol-specified 9-week interval. Mean LVEF values remained generally stable over the treatment period in both arms (Fig. 1). It should be noted that, at later cycles, the number of patients with LVEF assessment was low, and that graphs have been truncated when patient numbers fall below 10 per arm. In 84.2% of patients in both arms, the final treatment value (the last available LVEF value up to the end of the study treatment period) was almost unchanged compared with baseline (no change, increase, or decrease from baseline by <10% points). The overall incidence of clinically significant declines in LVEF (≥10% points from baseline to an absolute value <50%) was low, although higher in the placebo arm (6.6%, n = 25) than the pertuzumab arm (3.8%, n = 15) (Table 2). The LVEF value recovered to ≥50% in 72.0% (18/25, placebo arm) and 86.7% (13/15, pertuzumab arm) of those patients. Six patients (three in each arm) experienced an LVEF decline to <40% at any time during the study. The analysis of the cumulative incidence rate of first decline in LVEF to an absolute value <50% and by ≥10% points from baseline, controlling for the competing risk of death, demonstrated no difference between arms (Fig. 2).
Figure 1.
Mean LVEF over time. The graph has been truncated when numbers fall below 10 per arm.
Abbreviations: D, docetaxel; LVEF, left ventricular ejection fraction; Pla, placebo; Ptz, pertuzumab; T, trastuzumab.
Table 2.
LVEF decline of ≥10% points from baseline to an absolute value <50% and recovery to ≥50%
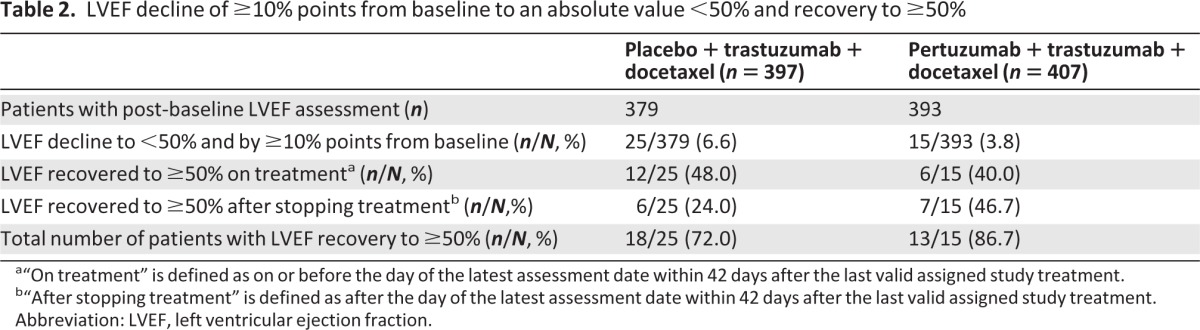
a“On treatment” is defined as on or before the day of the latest assessment date within 42 days after the last valid assigned study treatment.
b“After stopping treatment” is defined as after the day of the latest assessment date within 42 days after the last valid assigned study treatment.
Abbreviation: LVEF, left ventricular ejection fraction.
Figure 2.
Cumulative incidence of time to first decrease in LVEF by ≥10% points from baseline and to a value <50% with death as a competing risk event.
Abbreviations: D, docetaxel; LVEF, left ventricular ejection fraction; Pla, placebo; Ptz, pertuzumab; T, trastuzumab.
LVSD
Asymptomatic LVSD (grades 1–3) was reported for 26 patients (6.5%) in the placebo arm and 14 patients (3.4%) in the pertuzumab arm. Two patients in each arm discontinued study treatment as a result of asymptomatic LVSD. More patients experienced symptomatic LVSD in the placebo arm (1.8%, n = 7) compared with the pertuzumab arm (1.0%, n = 4) (Table 3). All of these events were considered possibly related to study treatment and were reported as SAEs. Of the 11 symptomatic LVSD events, 10 led to discontinuation of study medication (6/7 patients, placebo arm; 4/4 patients, pertuzumab arm). One patient in the placebo arm discontinued study treatment because of disease progression, which was diagnosed at the same time. The most common symptoms of LVSD were dyspnea on exertion (five patients in the placebo arm, four patients in the pertuzumab arm), cough (three patients in the placebo arm), and fatigue (two patients in the placebo arm, one patient in the pertuzumab arm). Ten of 11 patients who developed symptomatic LVSD received cardiac medication; one patient in the placebo arm was not treated for the event (supplemental online Table 1). At the time of data cutoff, symptomatic LVSD had resolved in 8/11 patients (5/7 in the placebo arm, 3/4 in the pertuzumab arm). None of the LVSD events was fatal.
Table 3.
LVSD (grading according to NCI-CTCAE v3.0)
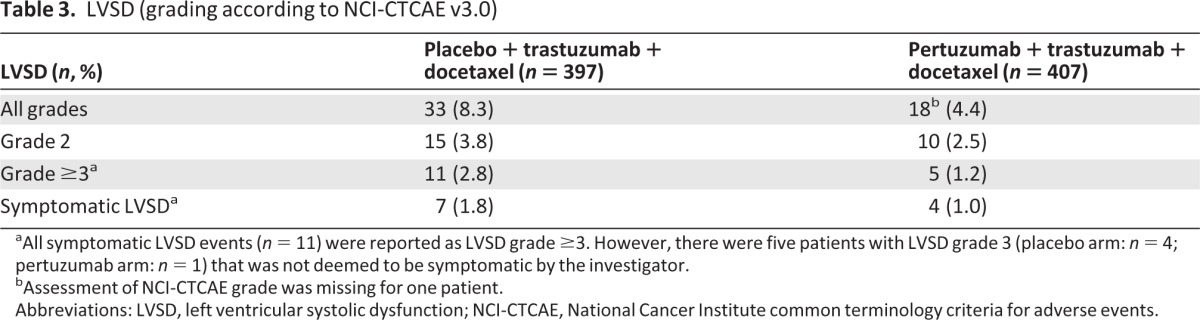
aAll symptomatic LVSD events (n = 11) were reported as LVSD grade ≥3. However, there were five patients with LVSD grade 3 (placebo arm: n = 4; pertuzumab arm: n = 1) that was not deemed to be symptomatic by the investigator.
bAssessment of NCI-CTCAE grade was missing for one patient.
Abbreviations: LVSD, left ventricular systolic dysfunction; NCI-CTCAE, National Cancer Institute common terminology criteria for adverse events.
Analysis of time to first LVSD event (all grades, n = 51) indicated a lower risk of LVSD event for patients in the pertuzumab arm [hazard ratio (HR), 0.42; 95% confidence interval (CI), 0.24 to 0.76; p = .0036] (supplemental online Table 2).
Risk Factors for Development of LVSD
The incidence of potential risk factors for LVSD at baseline was compared among patients who developed symptomatic LVSD (n = 11) and the overall patient population (Table 4). Patients who developed symptomatic LVSD had a higher incidence of previous anthracycline therapy and radiotherapy. Although prior radiotherapy was not a stratification factor, there was no imbalance between treatment arms, with 43% of patients in each arm (170/397 vs. 175/407) having received radiotherapy before study entry. For patients who developed symptomatic LVSD (seven in the placebo arm, four in the pertuzumab arm), the side of the chest exposed to radiation was investigated. Prior radiotherapy to the right breast/chest wall was received by two patients in each arm. Prior radiotherapy to the left breast/chest wall was received by one patient in the placebo arm and two patients in the pertuzumab arm. For one patient with symptomatic LVSD in the placebo arm, the side of radiation was not specified; three patients had not received prior radiotherapy. Prior therapy with anthracyclines in the neoadjuvant and adjuvant setting was received by 40.1% (159/397, placebo arm) and 37.8% (154/407, pertuzumab arm) of patients. In these patients, the median cumulative dose of doxorubicin (or its equivalent) was 240 mg/m2 (range 25–600 mg/m2) in the placebo arm and 240 mg/m2 (range 38–480 mg/m2) in the pertuzumab arm. In the placebo arm, 5/7 patients who developed symptomatic LVSD had been previously treated with anthracyclines; the median cumulative doxorubicin (or equivalent) dose was 240 mg/m2 (range 37.5–360 mg/m2). In the pertuzumab arm, 3/4 patients who developed symptomatic LVSD had been exposed to anthracyclines, with a median cumulative doxorubicin (or equivalent) dose of 300 mg/m2 (range 270–360 mg/m2). It should be noted, however, that the low number of patients with symptomatic LVSD (n = 11) limits the interpretation of potential risk factors at baseline for development of symptomatic LVSD. A univariate Cox regression analysis was performed to assess the influence of the predefined potential risk factors, prior anthracycline exposure, prior radiotherapy, prior trastuzumab therapy, age, smoking history, and underlying conditions of diabetes and hypertension, on the time to LVSD (all grades, n = 51). Prior anthracycline exposure (HR, 2.21; 95% CI, 1.27 to 3.86; p = .0053) and prior radiotherapy (HR, 2.43; 95% CI, 1.37 to 4.31; p = .0025) were identified as potentially important risk factors for the development of LVSD irrespective of treatment. These two significant risk factors had no influence on the overall analysis of the time to first LVSD event, with an HR of 0.44 when controlling for prior anthracycline therapy and 0.43 when controlling for prior radiotherapy (supplementary online Table 2). None of the other predefined potential risk factors, including prior therapy with trastuzumab, was significantly associated with the development of LVSD.
Table 4.
Comparison of risk factors between patients who developed symptomatic LVSD and the overall patient population
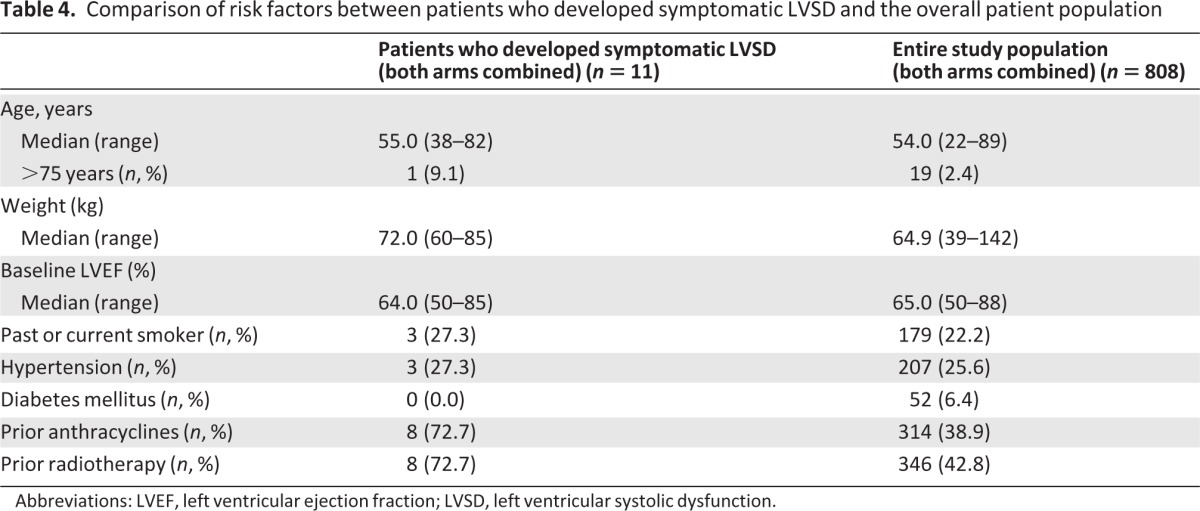
Abbreviations: LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction.
Discussion
CLEOPATRA was the first phase III study of the combination of two anti-HER2-targeted antibodies, pertuzumab and trastuzumab, with docetaxel in HER2-positive first-line metastatic breast cancer. The overall incidence of cardiac AEs was low; importantly, the combination of pertuzumab with trastuzumab and docetaxel did not increase the incidence of cardiac AEs compared with the placebo arm. Prior neoadjuvant and adjuvant therapy with trastuzumab was not found to be significantly associated with the development of LVSD. Even with these encouraging findings in CLEOPATRA, routine cardiac monitoring of patients should be performed in clinical practice. Measurement of LVEF is recommended before starting the regimen of pertuzumab, trastuzumab, and docetaxel, followed by repeat LVEF assessments at regular 3-month intervals during treatment. However, the optimal schedule of such monitoring in clinical practice remains to be established, as does the potential benefit to patients of long-term scrutiny of cardiac function, especially in view of the imperfect predictive value of commonly used parameters of systolic contractility (i.e., cardiac ultrasound [ECHO] and multigated [MUGA] blood-pool imaging). As additional data regarding the long-term cardiac safety of trastuzumab and pertuzumab become available, the need for cardiac monitoring using parameters of cardiac dysfunction as well as newer modalities such as biomarkers should be reassessed; this is an important consideration at a time when health care expenditures are of concern.
Compared with historic data, the incidence of cardiac dysfunction reported in either arm in CLEOPATRA was not increased [5]. In the pivotal phase III study in patients with HER2-positive metastatic breast cancer receiving trastuzumab-based therapy in the first-line setting, asymptomatic and symptomatic cardiac dysfunction were reported in 13% of patients who received trastuzumab in combination with paclitaxel; in 2% of patients, those events were NYHA class III or IV [1]. In a small phase II study (N = 11) of the combination of pertuzumab and trastuzumab in patients with HER2-positive metastatic breast cancer whose disease had progressed on prior trastuzumab-based therapy for advanced disease, six patients developed LVSD, which was symptomatic in one of these patients [31]. All six patients had received prior anthracycline therapy. This high incidence of cardiac dysfunction was not confirmed in subsequent studies with the pertuzumab–trastuzumab combination in advanced [32, 33] or early breast cancer [34]. Another study in the neoadjuvant setting showed that pertuzumab and trastuzumab, given concurrently or sequentially with an anthracycline-based chemotherapy regimen, or concurrently with a carboplatin-based chemotherapy regimen, result in a low incidence of LVSD [35]. Taken together, CLEOPATRA and other studies using the pertuzumab–trastuzumab combination do not give evidence that pertuzumab has an additive effect on cardiac dysfunction when combined with trastuzumab-based therapy.
In conclusion, the safety profile of the pertuzumab plus trastuzumab plus docetaxel regimen reported in CLEOPATRA, in addition to that seen in previous studies [28], together with the superior efficacy of the antibody combination, are encouraging, particularly with regard to an ongoing phase III study of pertuzumab and trastuzumab with standard chemotherapy in the adjuvant setting of HER2-positive early breast cancer (APHINITY; NCT01358877).
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This study was funded by F. Hoffmann-La Roche Ltd., Basel, Switzerland and Genentech, a member of the Roche Group, South San Francisco, CA. Targos Molecular Pathology, Kassel, Germany conducted central HER2 testing. Support for third-party writing assistance for this manuscript, furnished by Vilma Graupner, Ph.D. (Health Interactions, London), was provided by F. Hoffmann-La Roche Ltd.
Author Contributions
Conception/Design: Sandra M. Swain, Javier Cortés, Graham Ross, José Baselga
Provision of study material or patients: Sandra M. Swain, Javier Cortés, Dino Amadori, David Miles, José Baselga
Collection and/or assembly of data: Michael S. Ewer, Dino Amadori, Mark C. Benyunes
Data analysis and interpretation: Sandra M. Swain, Javier Cortés, David Miles, Adam Knott, Emma Clark, Mark C. Benyunes, Graham Ross, José Baselga
Manuscript writing: Sandra M. Swain, Michael S. Ewer, Javier Cortés, Dino Amadori, David Miles, Adam Knott, Emma Clark, Mark C. Benyunes, Graham Ross, José Baselga
Final approval of manuscript: Sandra M. Swain, Michael S. Ewer, Javier Cortés, Dino Amadori, David Miles, Adam Knott, Emma Clark, Mark C. Benyunes, Graham Ross, José Baselga
Disclosures
Sandra M. Swain: Roche/Genentech (C/A); Roche/Genentech, Pfizer/Puma Biotechnology (RF); Michael S. Ewer: GlaxoSmithKline, Boehringer Ingelheim, Roche (C/A, H); Javier Cortés: Celgene, Novartis, Roche (C/A, H); Eisai (H); David Miles: Roche (C/A, H); Adam Knott: Roche (E); Emma Clark: Roche (E); AstraZeneca (OI); José Baselga: Roche/Genentech (C/A); Graham Ross: Roche Products (E, OI, IP). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB)Scientific advisory board
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 5.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 6.Perez EA, Rodeheffer R. Clinical cardiac tolerability of trastuzumab. J Clin Oncol. 2004;22:322–329. doi: 10.1200/JCO.2004.01.120. [DOI] [PubMed] [Google Scholar]
- 7.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 8.Lefrak EA, Pitha J, Rosenheim S, et al. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 10.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 11.Kotamraju S, Konorev EA, Joseph J, et al. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- 12.Billingham ME, Mason JW, Bristow MR, et al. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep. 1978;62:865–872. [PubMed] [Google Scholar]
- 13.Mackay B, Ewer MS, Carrasco CH, et al. Assessment of anthracycline cardiomyopathy by endomyocardial biopsy. Ultrastruct Pathol. 1994;18:203–211. doi: 10.3109/01913129409016291. [DOI] [PubMed] [Google Scholar]
- 14.Suter TM, Cook-Bruns N, Barton C. Cardiotoxicity associated with trastuzumab (Herceptin) therapy in the treatment of metastatic breast cancer. Breast. 2004;13:173–183. doi: 10.1016/j.breast.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J Clin Oncol. 2005;23:2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 16.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 17.Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the Herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–3865. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 18.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 19.Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: The M.D. Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107–4115. doi: 10.1200/JCO.2005.04.9551. [DOI] [PubMed] [Google Scholar]
- 20.Lee KF, Simon H, Chen H, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 21.Erickson SL, O'Shea KS, Ghaboosi N, et al. ErbB3 is required for normal cerebellar and cardiac development: A comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 22.Zhao YY, Sawyer DR, Baliga RR, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 23.Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 24.Negro A, Brar BK, Lee KF. Essential roles of Her2/erbB2 in cardiac development and function. Rec Prog Horm Res. 2004;59:1–12. doi: 10.1210/rp.59.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Cho HS, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 26.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2–pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 27.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 28.Lenihan D, Suter T, Brammer M, et al. Pooled analysis of cardiac safety in patients with cancer treated with pertuzumab. Ann Oncol. 2012;23:791–800. doi: 10.1093/annonc/mdr294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baselga J, Cortes J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 31.Portera CC, Walshe JM, Rosing DR, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–2716. doi: 10.1158/1078-0432.CCR-07-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortes J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: Activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:1594–1600. doi: 10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
- 34.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 35.Schneeweiss A, Chia S, Hickish T, et al. Neoadjuvant pertuzumab and trastuzumab concurrent or sequential with an anthracycline-containing or concurrent with an anthracycline-free standard regimen: A randomized phase II study (TRYPHAENA) Cancer Res. 2011;71(24 suppl):S5–6. [Google Scholar]
- 36.Swain SM, Kim S-B, Cortes J, et al. Confirmatory overall survival analysis of CLEOPATRA: A randomized, double-blind, placebo-controlled phase III study with pertuzumab, trastuzumab, and docetaxel in patients with HER2-positive first-line metastatic breast cancer. Cancer Res. 2012;72(24 suppl):P5–18-26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.