Salivary duct carcinoma (SDC) is a rare and aggressive malignancy with high mortality and poor response to treatment. This retrospective study found that HER2/neu positivity and treatment with trastuzumab correlated well with long-term survival and response in adjuvant and palliative settings.
Keywords: Salivary duct cancer, Chemotherapy, Targeted therapy
Abstract
Objectives.
Salivary duct carcinoma (SDC) is a rare and aggressive malignancy with high mortality and poor response to treatment. A significant fraction of SDCs are HER2 positive. This retrospective review examines HER2 testing in SDC and the outcome of trastuzumab-based therapy in adjuvant and palliative settings.
Methods.
A total of 13 patients with SDC and HER2/neu expression by immunohistochemistry of 1–3+ were treated with trastuzumab in adjuvant (n = 8) or palliative (n = 5) setting. Adjuvant therapy consisted of concurrent radiation and chemotherapy with weekly paclitaxel, carboplatin, and trastuzumab (TCH) for 6 weeks followed by TCH for 12 weeks and trastuzumab alone for 1 year. Palliative treatment for metastatic disease consisted of TCH every 3 weeks for 6 cycles followed by trastuzumab for variable time periods with or without second-line chemotherapy for progression. All patients had fluorescence in situ hybridization testing for HER2/neu gene amplification.
Results.
The median duration of follow-up was 27 months (range: 8–48 months). In all, 62% of adjuvant patients (5/8) had no evidence of disease more than 2 years from completion of therapy. All patients with metastatic disease (5/5 patients) responded to treatment with TCH. One patient achieved a complete response and remains with no evidence of disease 52 months after initiation of TCH. The median duration of response was 18 months (range: 8–52 months).
Conclusion.
HER2/neu positivity and treatment with trastuzumab correlated well with long-term survival and response in our patients. Based on this data, we propose that HER2/neu status be examined routinely in all patients with SDCs and the treatment be directed accordingly.
Implications for Practice:
Salivary duct carcinoma (SDC) is an extremely rare and aggressive type of salivary gland cancer with high morbidity and mortality. Current treatment options of surgery with or without radiation therapy provide limited benefit. Conventional chemotherapeutic agents have been utilized in the palliative setting with modest results. Randomized prospective clinical trials are difficult due to the rarity of the disease. Newer treatment strategies are needed to improve outcomes. At Dana-Farber Cancer Institute, we treated HER2 positive SDCs with trastuzumab-based therapy with excellent results. Patients with HER2 positive SDC received a combination of paclitaxel, carboplatin and trastuzumab (TCH) in the palliative or adjuvant setting. Although the results below are from a small cohort of patients, treatment with trastuzumab correlated well with long-term survival and response to therapy. The unique application of this treatment in the adjuvant and palliative setting is practice changing and could have direct clinical implications.
Introduction
Malignant salivary gland tumors form 11% of all head and neck cancers, accounting for nearly 10,000 cases per year in United States [1]. Salivary duct carcinoma (SDC) is a rare type of salivary gland cancer that was first defined as a separate pathologic entity in 1991 [2]. Nearly 100 cases of SDC are diagnosed each year, with a male-to-female ratio of 3:1. SDC is now well recognized as an aggressive malignancy, frequently presenting as stage IV disease with large primaries, early facial nerve involvement, extra parotid growth, frequent regional lymph node involvement, and distant spread [2, 3].
The standard treatment for SDC is surgical resection with or without adjuvant radiation. However, the results of standard therapy have been disappointing. The survival rate is poor, with more than 60% of patients dying of recurrent disease within 3 years despite aggressive surgical resection and radiotherapy [3, 4]. In one of the largest reviews of 104 patients, 33% of curatively treated cases relapsed with local recurrence, 59% developed regional nodal recurrences, and 46% developed systemic disease. Ultimately, 65% of patients died from distant disease [4].
SDC histologically resembles ductal carcinoma of the breast, with both intraductal (or in situ) and invasive components [2]. The resemblance of SDC to ductal carcinoma of the breast led to the study of hormone receptor status and HER2/neu expression [5]. HER2/neu overexpression or amplification is seen in 15%–20% of patients with invasive breast cancers and is considered an adverse prognostic factor [6]. Strong immunohistochemical (IHC) staining for HER2/neu protein has been identified in 25%–90% of SDCs and is associated with a poor prognosis [3, 5, 7, 8]. SDCs can be IHC 1–3+ for HER2 in the absence of amplification. The discordance between HER2/neu expression by IHC and fluorescence in situ hybridization (FISH) also has been of concern in SDC [7]. HER2/neu 3+ positive/FISH nonamplified tumors are considered to be false positive in breast cancer. Such false-positive cases have been reported at 3% in breast cancer versus 27%–43% in SDCs [7].
Single-agent trastuzumab (Herceptin; F. Hoffmann-La Roche, Basel, Switzerland) was previously studied in a phase II trial of multiple histologies of advanced salivary gland carcinomas with minimal benefit. One patient with advanced salivary gland cancer had stabilization of disease for 40 weeks [9]. Based on the palliative and adjuvant data for combination activity of trastuzumab in breast cancer, we treated patients with SDC with trastuzumab-based therapy and present the results in this retrospective analysis. Because of the data regarding discordance in IHC expression and FISH positivity, all patients who were IHC 1–3+ were included in the study.
Patients and Methods
Thirteen patients with SDC who were treated with trastuzumab-based therapy as the first treatment for adjuvant or recurrent metastatic disease between 2005 and 2010 were identified using the pathology and chemotherapy pharmacy database. All patients were initially evaluated at our institution. A detailed physical examination was done and staging scans were reviewed. Histologic confirmation of disease was made before initiating treatment. Data were reviewed under a retrospective protocol approved by the institutional review board.
Pathologic Analysis
Immunohistochemistry
IHC was performed on 4-μm tissue sections using the EnVision+ System (Dako, Carpinteria, CA). Briefly, slides were deparaffinized and rehydrated, with endogenous peroxidase activity blocked using 3% hydrogen peroxide in methanol. Antigen retrieval was performed using 10 mM of citrate buffer pH 6.0 (Target Retrieval Solution S1699, Dako) and pressure cooking (Biocare Medical, Concord, CA) at 122°C (14 and 17 psi) for 45 minutes. The primary antibody for HER2/neu (SP3 1:100; Labvision, Fremont, CA) was incubated for 40 minutes at room temperature. A Dako polymer secondary antibody system was used and incubated at room temperature for 30 minutes in a humid chamber. 3,3′-diaminobenzidine (Sigma Chemical, St. Louis, MO) was used for detection with counterstaining using Mayer hematoxylin. External positive controls were included with each run.
Slides were scored by a pathologist at Brigham and Women's Hospital (J.K.) as positive 3+ (strong complete membrane immunoreactivity in >30% of tumor cells), equivocal 2+ (weak to moderate complete membrane immunoreactivity in at least 10% of tumor cells), negative 1+ (faint, weak partial membrane immunoreactivity in at least 10% of tumor cells), or negative 0 (no immunoreactivity or immunoreactivity in <10% of tumor cells) according to guidelines from the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) [10].
Fluorescence In Situ Hybridization
Bacterial artificial chromosome clones RP11–62N23, RP11–1065L22, and RP11–1044P23 were obtained from Children's Hospital (Oakland, CA) and used in construction of FISH probes for a 340-kb region including ERBB2. Probes were labeled with biotin using the Random Prime DNA Labeling System (Invitrogen, Grand Island, NY) according to the manufacturer's protocol and detected with rhodamine (red). A probe to the chromosome 17 centromeric region labeled with fluorescein isothiocyanate was purchased from Abbott Laboratories (Des Plaines, IL)/Vysis Corp (Downers Grove, IL). Specificity of probe binding was verified using normal lymphocyte metaphase spreads.
Dual-color FISH was performed on 4-μm tissue sections. Slides were counterstained with 4',6-diamidino-2-phenylindole (DAPI)/Antifade (Vector Labs, Burlingame, CA) and evaluated using an Olympus BX51 fluorescence microscope. Hybridization signals were scored in at least 20 tumor cells according to ASCO/CAP guidelines [10]. A ratio of HER2 probe signals to centromere probe signals was calculated, with a ratio of >2.2 reported as amplified and a ratio of <1.8 as not amplified.
Primary and Adjuvant Treatment
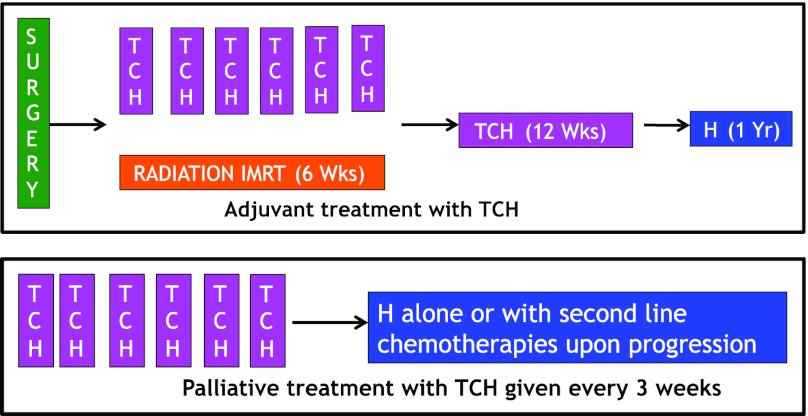
Patients were first considered for primary surgical management if resectable. Postoperative concurrent chemoradiotherapy (CRT) and adjuvant chemotherapy was given in all cases. Radiotherapy was delivered using intensity-modulated radiation therapy for all cases (Fig. 1A; Table 1). All patients received a median of 66 Gy of radiation mostly to the tumor bed and ipsilateral neck. The CRT was given over 6 weeks.
Figure 1.
Chemotherapy design for adjuvant therapy (top) and palliative therapy (bottom).
Abbreviations: H, Herceptin/trastuzumab; IMRT, intensity modulated radiation therapy; TCH, paclitaxel, carboplatin, Herceptin/trastuzumab.
Table 1.
Adjuvant therapy with trastuzumab for salivary duct carcinoma
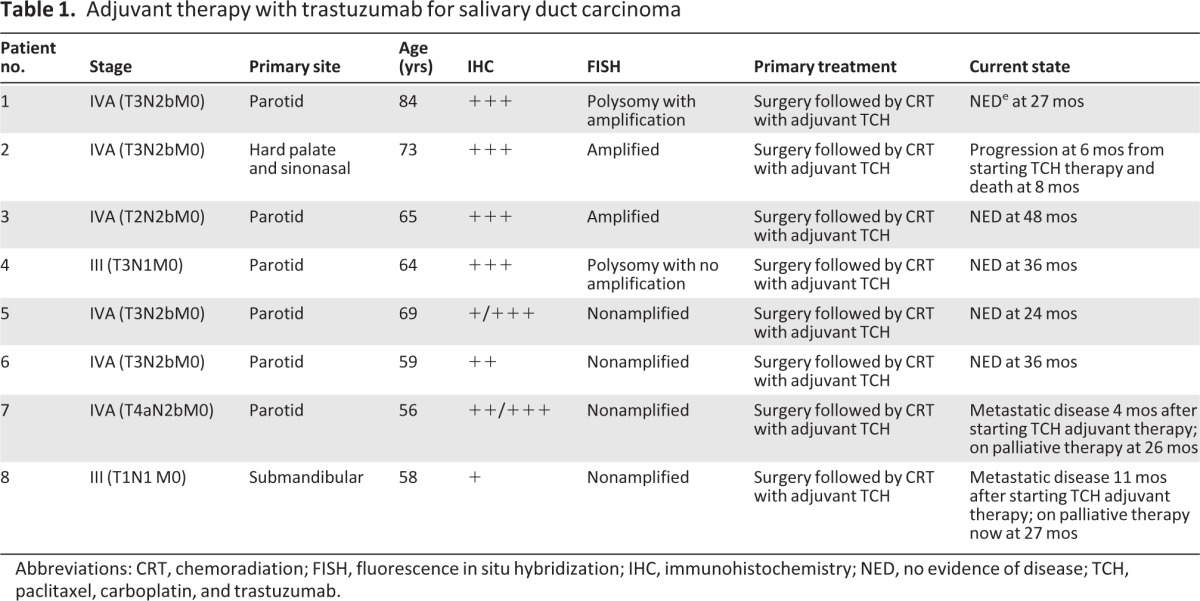
Abbreviations: CRT, chemoradiation; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NED, no evidence of disease; TCH, paclitaxel, carboplatin, and trastuzumab.
Chemotherapy consisted of weekly carboplatin (area under the curve [AUC] 1.5), paclitaxel (45 mg/m2), and trastuzumab (2 mg/kg; TCH) in all patients who were HER2/neu positive by IHC 1–3+. Adjuvant chemotherapy comprising the same doses of TCH was continued on a weekly basis for 12 additional weeks after completion of CRT. Adjuvant trastuzumab was then continued for a total of 1 year in accordance with breast cancer treatment paradigms.
Palliative Treatment
Patients who were treated with palliative therapy had received their primary treatment prior to assessment by our multidisciplinary team. Patients generally (a) had initial surgical resection of the primary with adjuvant radiation with or without chemotherapy and then developed recurrence or (b) presented with systemic disease (Fig. 1B; Table 2). Three of five patients had received adjuvant radiation at outside centers.
Table 2.
Palliative therapy with trastuzumab for salivary duct carcinoma
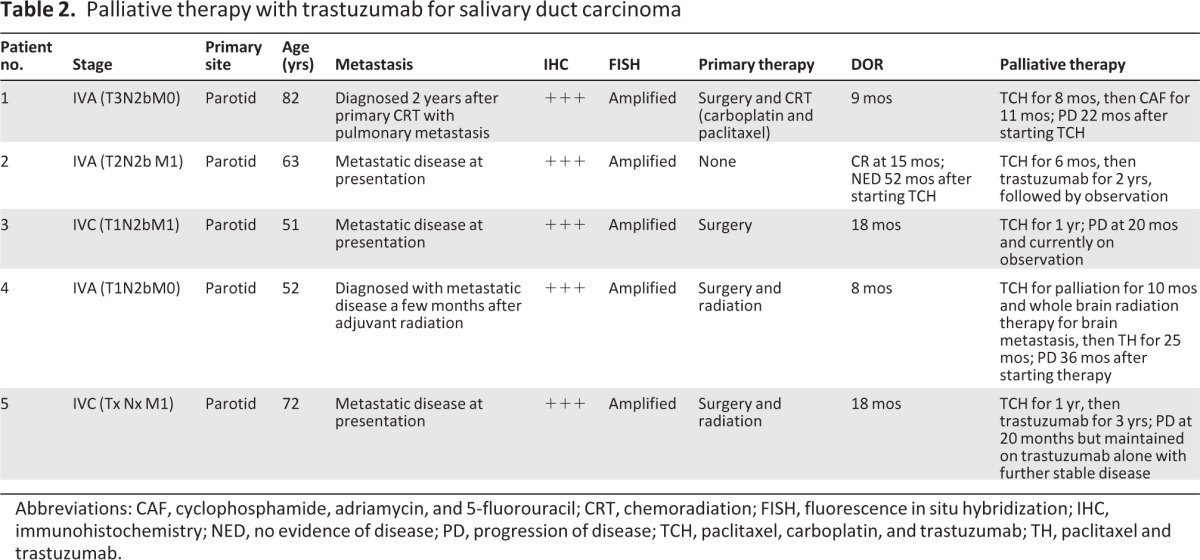
Abbreviations: CAF, cyclophosphamide, adriamycin, and 5-fluorouracil; CRT, chemoradiation; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NED, no evidence of disease; PD, progression of disease; TCH, paclitaxel, carboplatin, and trastuzumab; TH, paclitaxel and trastuzumab.
After evaluation by our team, palliative treatment was initiated with carboplatin (AUC 5–6), paclitaxel (175 mg/m2), and weekly trastuzumab (2 mg/kg) every 3 weeks for six cycles. Trastuzumab was then given every 2 weeks at 4 mg/kg for variable time periods or until progression. Second-line therapies were determined at the time of progression.
Follow-up and Evaluation of Response
Adjuvant patients were followed every 6 weeks in the first year after treatment and every 3 months in the second year. They were evaluated by imaging (computed tomography [CT]/magnetic resonance imaging and/or positron-emission tomography [PET]) after 3 months of completion of radiation therapy, then every 6 months for the first 2 years, and yearly thereafter.
Palliative patients were evaluated by imaging (CT and/or PET) every 3–6 months to monitor for response to therapy. In the patient who had complete response from treatment, yearly PET scans were done to monitor for sustained response from the third year. Radiologists at our institution or at local imaging centers reviewed all follow-up/restaging imaging. Cardiac function was monitored every 3 months by echocardiogram.
Toxicity
Acute (≤3 months from treatment) and late (>3 months from treatment) toxicities were analyzed by retrospective chart review and defined according to the Common Terminology Criteria for Adverse Events (version 4.0, http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40).
Results
The median age of presentation for all 13 patients was 64 years (range: 51–84 years). The majority of patients (9/13) were men and the most common primary site was the parotid gland (11/13; Tables 1, 2). Eight patients presented for initial treatment of localized disease and five presented for recurrent or metastatic disease. The 13 patients with HER2/neu overexpression represented 20% of all cases of SDC seen between 2005 and 2010.
Patients Undergoing Adjuvant Treatment
All eight patients presented with locally advanced stage IVA disease. Six of eight patients had primary SDC of the parotid gland, one patient had a submandibular gland primary, and one patient had a hard palate and paranasal sinus primary. Six patients had T3 disease with extraparotid extension; another patient had T4a disease with facial nerve involvement. Six of eight patients had multiple ipsilateral lymph node involvement (N2b).
HER2/neu status was analyzed by IHC prior to initiating therapy in all eight patients. Four patients had IHC 3+. Two patients had variable HER2/neu expression of IHC 1–3+ and 2–3+, respectively. One patient had IHC 2+ and another had IHC 1+. FISH testing was performed retrospectively for all eight patients. Three patients were found to have HER2/neu gene amplification by FISH. One of these patients had polysomy of chromosome 17. Another patient had polysomy of chromosome 17 without any HER2/neu gene amplification on FISH. Four patients had no HER2/neu amplification by FISH. There was 75% (3/4) concordance between IHC 3+ and FISH amplification.
Patients With Metastatic Disease
Five patients had been treated for metastatic disease with TCH. All five patients had a primary malignancy of the parotid gland. Two patients had initially presented with localized stage IVA disease, and three patients presented with metastatic stage IVC disease. All five patients presented to our institution with distant metastatic disease. Three out of five patients had multiple pulmonary and bony metastases, one had skeletal metastasis alone, and one had metastasis to the lung and bones and later to the brain.
For the primary treatment elsewhere, one patient was treated with surgical resection followed by concurrent CRT with weekly carboplatin and paclitaxel; this patient developed distant disease 2 years later. Two other patients had primary surgical resection followed by adjuvant radiation. One patient had metastatic disease at diagnosis and the other developed metastatic disease after finishing adjuvant radiotherapy. One patient had primary surgical resection alone and had metastasis at diagnosis. Another patient was found to have metastatic disease at the time of diagnosis and no primary surgical resection was performed. No patient had received prior chemotherapy for recurrent/metastatic disease.
HER2/neu status was analyzed by IHC prior to initiating therapy. All five patients had IHC 3+ for HER2/neu protein. All five patients were to have amplification of the HER2/neu gene retrospectively on FISH. There was 100% concordance between IHC 3+ and FISH.
Outcomes of Treatment and Correlation With HER2/neu Expression and Amplification
Adjuvant Therapy
Three patients were HER2/neu amplified by FISH (one with polysomy 17). Of these, one patient developed progressive disease (base of skull) at 6 months after beginning TCH therapy and died after 8 months. One patient had no evidence of disease at 48 months. One patient with HER2/neu gene amplification on FISH and polysomy of chromosome 17 had no evidence of disease at 27 months. One patient with polysomy of chromosome 17 and no amplification remained with no evidence of disease at 36 months.
Four patients had no HER2/neu gene amplification on FISH. One patient with IHC 2–3+ at presentation developed metastatic disease at 4 months. Another patient who had HER2/neu IHC 1+ at presentation developed metastatic disease 11 months after beginning TCH therapy. Two other patients who had no HER2/neu gene amplification retrospectively on FISH were IHC 1–3+ and IHC 2+, respectively; these patients continued to have no evidence of disease 24 months and 36 months after beginning TCH therapy.
The median duration of follow-up was 27 months (range: 8–48 months). Five out of eight patients (62%) remained without any evidence of disease after more than 2 years of therapy. All three recurrences were seen within the first 12 months of starting CRT and adjuvant therapy.
Palliative Therapy
All five patients presenting with metastatic disease were IHC 3+ and had HER2/neu gene amplification on FISH. All five patients had TCH as initial treatment for metastatic disease. Two patients showed partial response (PR), one patient had a complete response (CR), and two patients had progressive disease (PD) at the time of this analysis. One patient who had biopsy-proven widespread skeletal metastasis at the time of initial presentation was treated with TCH followed by trastuzumab alone for 2 years; this patient had a CR and remained without any evidence of disease 52 months after beginning therapy.
One patient was treated with TCH followed by trastuzumab for 3 years for a PR; this patient has since been followed clinically for minimal disease without any treatment. One patient was treated with TCH followed by trastuzumab for 1 year for multiple pulmonary and skeletal metastases; this patient developed minimal progression in the pulmonary metastasis at 20 months. The lesions subsequently remained stable for over 16 months without any further treatment.
Two patients died from progressive disease. One patient developed brain metastasis while on treatment with TCH. This patient required whole brain radiation followed by weekly trastuzumab and paclitaxel; the patient developed progressive disease and died 42 months after starting therapy with TCH. The other patient received TCH followed by trastuzumab for 8 months. The patient developed progressive disease and died 25 months after beginning TCH.
Toxicity From Adjuvant and Palliative Therapy
The acute toxicities (any grade) reported during adjuvant therapy were mucositis, dermatitis, esophagitis, and otitis externa. The late toxicities (any grade) reported were xerostomia, increased sensitivity of the skin and ear (serous otitis media), and neuropathy (Table 3). One patient developed grade 3 mucositis and dermatitis acutely. The most common toxicities (any grade) of palliative treatment were fatigue, anorexia, myelopathy, neuropathy, and arthralgia. Grade 3 fatigue and thrombocytopenia were seen in one patient each. There were no grade 4 or 5 toxicities reported. No cardiac dysfunction was seen as a result of trastuzumab-based therapy.
Table 3.
Toxicity from treatment
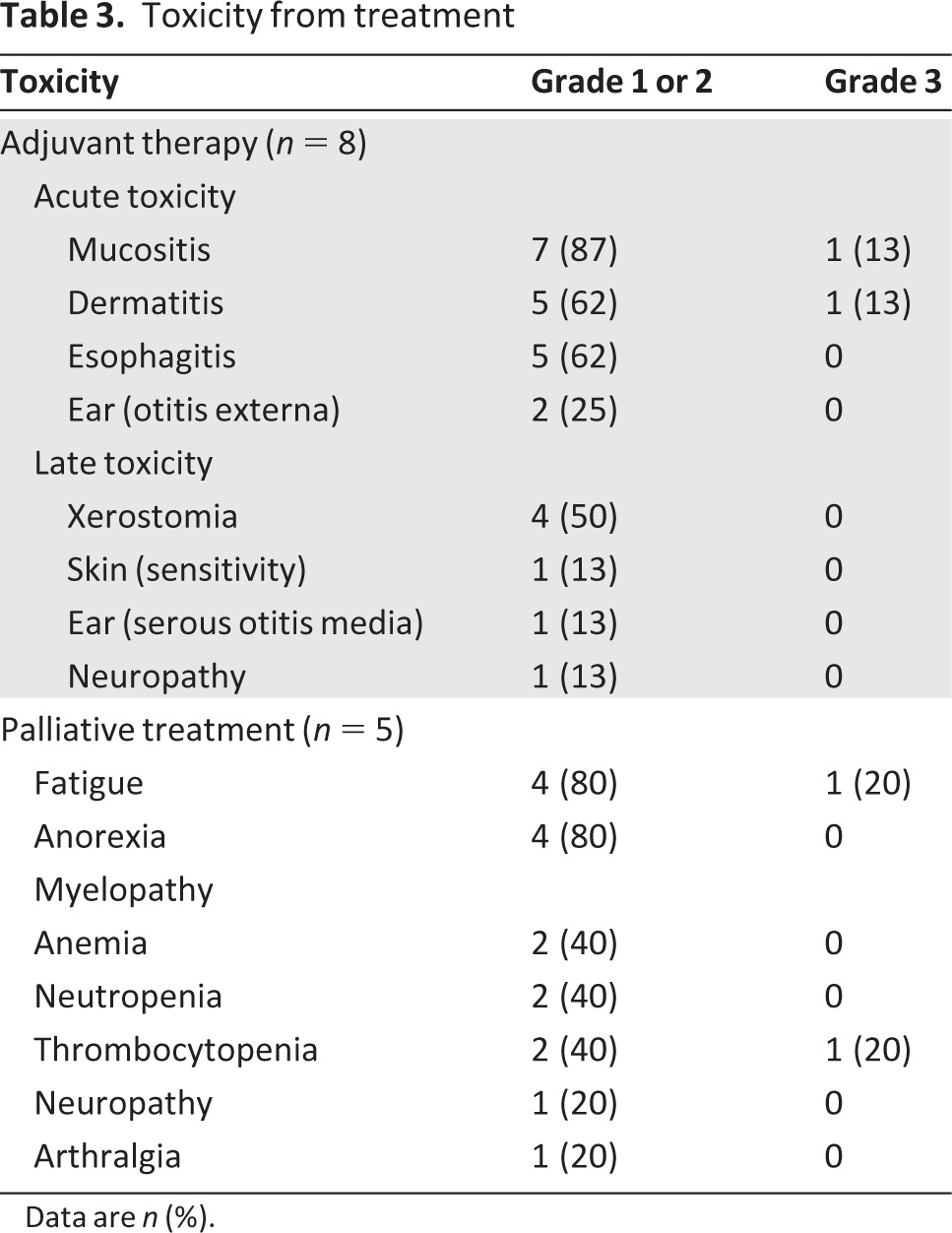
Data are n (%).
Discussion
Systemic therapies are generally ineffective in the management of salivary gland cancers. Chemotherapy is reserved for palliative management, although adjuvant CRT has been advocated in selected settings [11]. Conventional agents have limited response rates that vary with the histologic types and few long-term responses are observed [12]. SDC is a rare, distinct, and aggressive subtype of salivary gland cancer with early metastasis and a median overall survival of <3 years from the time of diagnosis [4]. The risk of recurrence is >60% at 2 years. Primary treatment is surgery with or without adjuvant radiation. Because of the rarity of disease and lack of prospective studies, there are no standard or evidence-based chemotherapeutic recommendations for the treatment of SDC. The poor survival seen in locally advanced and metastatic settings warrants a change in strategy of treatment, even though the pursuit of a prospective trial is not realistic.
Because of the efficacy of trastuzumab in HER2+ breast cancer and the observed similarities between SDC and breast cancer, we treated HER2+ SDC with TCH in the adjuvant and palliative setting and achieved excellent results. We saw benefit in all five of our patients with metastatic SDC who showed HER2 amplification on FISH. The median survival for metastatic patients is 40 months, and three of our five patients were alive at the time of this analysis. Notably, one patient with widely metastatic disease had complete response and remained free of disease 52 months after beginning therapy.
Five of eight adjuvant patients also remained disease free >2 years after treatment. Of the five patients who did not show HER2/neu gene amplification on FISH, two have remained free of disease. Because of concerns regarding the discordance reported between IHC and FISH for HER2/neu positivity in SDC, we treated all patients with IHC 1–3+ expression. It is unclear with these small numbers if the adjuvant patients derived any benefit from adjuvant trastuzumab therapy or maintained disease control due to the aggressive chemotherapy and radiation strategy. However, the treatment appears reasonable in light of their advanced disease and poor prognosis. The median survival for the IHC3+ adjuvant patients is 32 months.
The major limitations of our study were the retrospective nature of our study, the small sample size, and the lack of a complete analysis of androgen receptor status in our patients. These limitations make interpretation of the impact of trastuzumab-based therapy on response and survival difficult.
Trastuzumab has proven benefit for HER2/neu+ breast cancer in the adjuvant and metastatic setting and for advanced gastric cancer [13–15]. Trastuzumab has a favorable safety profile with reversible cardiac dysfunction in <5%, which is significantly reduced with nonanthracycline-based chemotherapy [14]. Trastuzumab use was studied prospectively in a phase II trial of advanced salivary gland carcinomas with minimal benefit. However, one patient with advanced salivary gland cancer had stabilization of disease for 40 weeks [9]. There are now multiple case reports of benefit from trastuzumab in treatment of advanced SDC [16–18]. In our report, all five patients with HER2/neu+ metastatic SDC showed benefit from treatment with trastuzumab-based therapy.
The combination of carboplatin and paclitaxel has shown benefit in several malignancies, including lung cancer, ovarian cancer, and squamous cell carcinoma of head and neck. The combination of TCH has synergistic properties and is effective in HER2+ breast cancer in the adjuvant setting [14]. Weekly carboplatin and paclitaxel has radiation-sensitizing effects [14, 19]. Based on data of the benefit from adjuvant use of trastuzumab for HER2+ breast cancer [14], we treated our patients with trastuzumab-based adjuvant therapy and used a similar combination in the metastatic setting.
Because of similarities to breast cancer, hormone receptor expression has been studied widely for SDC. Estrogen receptor (ER) and progesterone receptor (PR) expression was shown in 2% and 12% of patients with SDC, respectively [2]. Androgen receptor (AR) expression has been ubiquitously reported in SDC (range: 66%–91%), with reports of response to androgen deprivation therapy [20, 21]. AR positivity has been shown to segregate with HER2 positivity and is well described in ER/PR negative, HER2+ breast cancer. These data in breast cancer have initiated new discussions regarding the role of AR in the management of HER2+ disease [22].
The assessment of AR in HER2+ SDC also will indicate an alternative treatment for this aggressive disease. Locati et al. analyzed HER+ SDCs for AR positivity and found equal percentages of AR and HER2/neu expression in their SDC samples [23]. Although HER2+ SDCs are known to have a high coexpression of AR, this assessment was not complete in our SDC cases. Because taxane-based treatment is known to be effective in AR-positive cancers, it is difficult to negate the response as a result of this mechanism, especially due to lack of details regarding AR status in our patients. However, HER2 positivity is known to contribute to taxane/chemotherapy resistance [24]. In our view, the response to treatment seen in our patients mostly resulted from overcoming this resistance with trastuzumab and the synergistic effects of the trastuzumab, taxanes, and platinum combination. Trastuzumab has been widely shown to overcome chemotherapy resistance in HER2+ breast cancer and enhance the effects of chemotherapy, especially taxanes [25].
EGFR expression was reported in nearly 70% of SDCs, and 10% of patients had an EGFR mutation, although no EGFR amplification was seen [8]. There was no significant benefit noted with gefitinib in a study on salivary gland cancers [26]. Wirth and Juric detected mutually exclusive genetic aberrations (BRAF, PI3K, HER2/neu) in 13 of 24 (54%) patients with SDCs and facilitated treatment in a phase I study where appropriate [27]. A case report also described benefit from mammalian target of rapamycin in two patients with SDC [28]. New molecular targets are being identified in SDC and might be used for treatment.
SDC is an extremely rare disease with variable HER2/neu overexpression. Prospective, randomized trial-based verification of the benefits of trastuzumab use is, therefore, nearly impossible. Based on the results in our case series and evidence from other case reports, we propose that HER2/neu expression and amplification be ascertained in every patient with SDC. Palliative and adjuvant trastuzumab-based therapy also should be considered in all patients with SDC and HER2+ disease.
Acknowledgments
This study was supported by the Head and Neck Cancer Research Fund at the Dana-Farber Cancer Institute. We thank Mei Zheng and Krishan Taneja for expert technical assistance with immunohistochemistry and fluorescence in situ hybridization, respectively.
Author Contributions
Conception/Design: Sewanti Limaye, Marshall Posner, Jeffrey Krane, Jochen Lorch, Robert Haddad
Provision of study materials or patients: Sewanti Limaye, Marshall Posner, Jeffrey Krane, Deborah Dillon, Robert Haddad
Collection and/or assembly of data: Sewanti Limaye, Jeffrey Krane, Deborah Dillon
Data analysis and interpretation: Sewanti Limaye, Jeffrey Krane, Maria Fonfria, Jochen Lorch, Deborah Dillon, Aditya Shreenivas, Roy Tishler, Robert Haddad
Manuscript writing: Sewanti Limaye, Marshall Posner, Jeffrey Krane, Jochen Lorch, Deborah Dillon, Roy Tishler, Robert Haddad
Final approval of manuscript: Sewanti Limaye, Marshall Posner, Jeffrey Krane, Maria Fonfria, Jochen Lorch, Deborah Dillon, Aditya Shreenivas, Roy Tishler, Robert Haddad
Disclosures
Jochen H. Lorch: Novartis (RF); Deborah A. Dillon: Axela (RF). The other authors indicated no financial relationships.
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Boukheris H, Curtis RE, Land CE, et al. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: A population-based study in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:2899–2906. doi: 10.1158/1055-9965.EPI-09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McHugh JB, Visscher DW, Barnes EL. Update on selected salivary gland neoplasms. Arch Pathol Lab Med. 2009;133:1763–1774. doi: 10.5858/133.11.1763. [DOI] [PubMed] [Google Scholar]
- 3.Jaehne M, Roeser K, Jaekel T, et al. Clinical and immunohistologic typing of salivary duct carcinoma: A report of 50 cases. Cancer. 2005;103:2526–2533. doi: 10.1002/cncr.21116. [DOI] [PubMed] [Google Scholar]
- 4.Barnes L, Rao U, Krause J, et al. Salivary duct carcinoma. Part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol. 1994;78:64–73. doi: 10.1016/0030-4220(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 5.Glisson B, Colevas AD, Haddad R, et al. HER2 expression in salivary gland carcinomas: Dependence on histological subtype. Clin Cancer Res. 2004;10:944–946. doi: 10.1158/1078-0432.ccr-03-0253. [DOI] [PubMed] [Google Scholar]
- 6.Vargas-Roig LM, Gago FE, Tello O, et al. c-erbB-2 (HER-2/neu) protein and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1999;84:129–134. doi: 10.1002/(sici)1097-0215(19990420)84:2<129::aid-ijc6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Cornolti G, Ungari M, Morassi ML, et al. Amplification and overexpression of HER2/neu gene and HER2/neu protein in salivary duct carcinoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 2007;133:1031–1036. doi: 10.1001/archotol.133.10.1031. [DOI] [PubMed] [Google Scholar]
- 8.Williams MD, Roberts DB, Kies MS, et al. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: Empirical and therapeutic significance. Clin Cancer Res. 2010;16:2266–2274. doi: 10.1158/1078-0432.CCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haddad R, Colevas AD, Krane JF, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39:724–727. doi: 10.1016/s1368-8375(03)00097-6. [DOI] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 11.Haddad RI, Posner MR, Busse PM, et al. Chemoradiotherapy for adenoid cystic carcinoma: Preliminary results of an organ sparing approach. Am J Clin Oncol. 2006;29:153–157. doi: 10.1097/01.coc.0000203756.36866.17. [DOI] [PubMed] [Google Scholar]
- 12.Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006;24:2673–2678. doi: 10.1200/JCO.2005.05.3025. [DOI] [PubMed] [Google Scholar]
- 13.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 14.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 16.Nashed M, Casasola RJ. Biological therapy of salivary duct carcinoma. J Laryngol Otol. 2009;123:250–252. doi: 10.1017/S0022215108002314. [DOI] [PubMed] [Google Scholar]
- 17.Prat A, Parera M, Reyes V, et al. Successful treatment of pulmonary metastatic salivary ductal carcinoma with trastuzumab-based therapy. Head Neck. 2008;30:680–683. doi: 10.1002/hed.20714. [DOI] [PubMed] [Google Scholar]
- 18.Nabili V, Tan JW, Bhuta S, et al. Salivary duct carcinoma: A clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29:907–912. doi: 10.1002/hed.20614. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld JD, Sher DJ, Norris CM, Jr., et al. Salivary gland tumors treated with adjuvant intensity-modulated radiotherapy with or without concurrent chemotherapy. Int J Radiat Oncol Biol Phys. 2012;82:308–314. doi: 10.1016/j.ijrobp.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 20.Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: A disease entity with promising new treatment options. J Clin Oncol. 2011;29:e473–e476. doi: 10.1200/JCO.2010.32.8351. [DOI] [PubMed] [Google Scholar]
- 21.Williams MD, Roberts D, Blumenschein GR, Jr., et al. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: Biologic significance and potential role in therapeutic stratification of patients. Am J Surg Pathol. 2007;31:1645–1652. doi: 10.1097/PAS.0b013e3180caa099. [DOI] [PubMed] [Google Scholar]
- 22.Micello D, Marando A, Sahnane N, et al. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch. 2010;457:467–476. doi: 10.1007/s00428-010-0964-y. [DOI] [PubMed] [Google Scholar]
- 23.Locati LD, Perrone F, Losa M, et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs) Oral Oncol. 2009;45:986–990. doi: 10.1016/j.oraloncology.2009.05.635. [DOI] [PubMed] [Google Scholar]
- 24.Yu D, Jing T, Liu B, et al. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell. 1998;2:581–591. doi: 10.1016/s1097-2765(00)80157-4. [DOI] [PubMed] [Google Scholar]
- 25.Azambuja E, Durbecq V, Rosa DD, et al. HER-2 overexpression/amplification and its interaction with taxane-based therapy in breast cancer. Ann Oncol. 2008;19:223–232. doi: 10.1093/annonc/mdm352. [DOI] [PubMed] [Google Scholar]
- 26.Glisson B, Blumenschein G, Francisco M, et al. Phase II trial of gefitinib in patients with incurable salivary gland cancer. J Clin Oncol. 2005;23:580S. [Google Scholar]
- 27.Wirth LJ, Juric D. Detection of novel genetic aberrations in salivary duct carcinoma (SDC) by SNaPshot analysis. J Clin Oncol. 2011;29(suppl):5579. [Google Scholar]
- 28.Piha-Paul SA, Cohen PR, Kurzrock R. Salivary duct carcinoma: Targeting the phosphatidylinositol 3-kinase pathway by blocking mammalian target of rapamycin with temsirolimus. J Clin Oncol. 2011;29:e727–e730. doi: 10.1200/JCO.2011.36.2095. [DOI] [PubMed] [Google Scholar]