Vemurafenib has been approved for the treatment of patients with advanced BRAFV600E-mutant melanoma. The most commonly reported adverse events were dermatologic conditions, occurring in 92%–95% of patients. Dose interruptions and/or reductions were required in <10% of patients.
Keywords: Vemurafenib, Dermatologic, cuSCC, Keratoacanthoma
Abstract
Background.
Vemurafenib has been approved for the treatment of patients with advanced BRAFV600E-mutant melanoma. This report by the Vemurafenib Dermatology Working Group presents the characteristics of dermatologic adverse events (AEs) that occur in vemurafenib-treated patients, including cutaneous squamous cell carcinoma (cuSCC).
Methods.
Dermatologic AEs were assessed from three ongoing trials of BRAFV600E mutation-positive advanced melanoma. Histologic central review and genetic characterization were completed for a subset of cuSCC lesions.
Results.
A total of 520 patients received vemurafenib. The most commonly reported AEs were dermatologic AEs, occurring in 92%–95% of patients. Rash was the most common AE (64%–75% of patients), and the most common types were rash not otherwise specified, erythema, maculopapular rash, and folliculitis. Rash development did not appear to correlate with tumor response. Photosensitivity occurred in 35%–63% of patients, and palmar-plantar erythrodysesthesia (PPE) occurred in 8%–10% of patients. The severity of rash, photosensitivity, and PPE were mainly grade 1 or 2. In all, 19%–26% of patients developed cuSCC, mostly keratoacanthomas (KAs). The majority of patients with cuSCC continued therapy without dose reduction after resection. Genetic analysis of 29 cuSCC/KA samples demonstrated HRAS mutations in 41%.
Conclusions.
Dermatologic AEs associated with vemurafenib treatment in patients with melanoma were generally manageable with supportive care measures. Dose interruptions and/or reductions were required in <10% of patients.
Implications for Practice:
Vemurafenib has been approved for the treatment of patients with advanced BRAF-mutant melanoma. Skin toxicity is common with vemurafenib therapy, and the majority of patients are able to tolerate and continue therapy following symptomatic and/or local management (e.g., surgical resection). Clinicians should be aware of these toxicities to better educate and manage their patients.
Introduction
Activating mutations of BRAF are pivotal to the malignant phenotype of approximately 60% of melanomas [1–4]. The BRAFV600E mutation, in which valine is substituted by glutamic acid, is the most prevalent [1] and results in constitutive ERK signaling via the MAPK pathway, leading to the various hallmarks of malignancy [5]. A multinational phase III trial of 675 treatment-naïve patients with advanced melanoma harboring the BRAFV600E mutation demonstrated that the BRAF inhibitor vemurafenib led to a 63% reduction in the risk of death and a 74% decrease in the risk of disease progression or death compared with dacarbazine [6]. In addition, phase I and II trials reported response rates greater than 50% and median overall survival (OS) times of 14–16 months [7, 8]. Based on these results, vemurafenib was approved by regulatory agencies for the treatment of metastatic or unresectable melanoma harboring the BRAFV600E mutation.
The development of cutaneous toxicities (i.e., dermatologic adverse events [AEs]) with vemurafenib is common [6–8]. Although these do not generally lead to treatment discontinuation, they can affect consistent dosing and dictate the need for antitoxicity interventions. Common AEs include rash, photosensitivity, palmar-plantar erythrodysesthesia (PPE; also known as hand–foot syndrome), and cutaneous squamous cell carcinoma (cuSCC), including a well-differentiated type with low potential for invasive or metastatic disease—keratoacanthoma (KA). In this report, we describe the clinical and histologic characteristics of common dermatologic AEs reported in vemurafenib-treated patients. In addition, we present the results of molecular studies of cuSCC lesions and discuss proposed management strategies for dermatologic AEs. An understanding of these AEs with thorough evaluations and appropriate management are important because these events may affect the dosing regimen and patient quality of life.
Materials and Methods
Study Design
Data were analyzed from three ongoing trials: phase III randomized NO25026 (BRAF Inhibitor in Melanoma [BRIM]-3; NCT01006980), phase II NP22657 (BRIM-2; NCT00949702), and phase I NP25163 (BRIM-4; NCT01107418; Table 1). Protocols were approved by the institutional review board at each participating institution and were conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, as defined by the International Conference on Harmonisation. All patients provided written informed consent before enrollment.
Table 1.
Characteristics of study populations
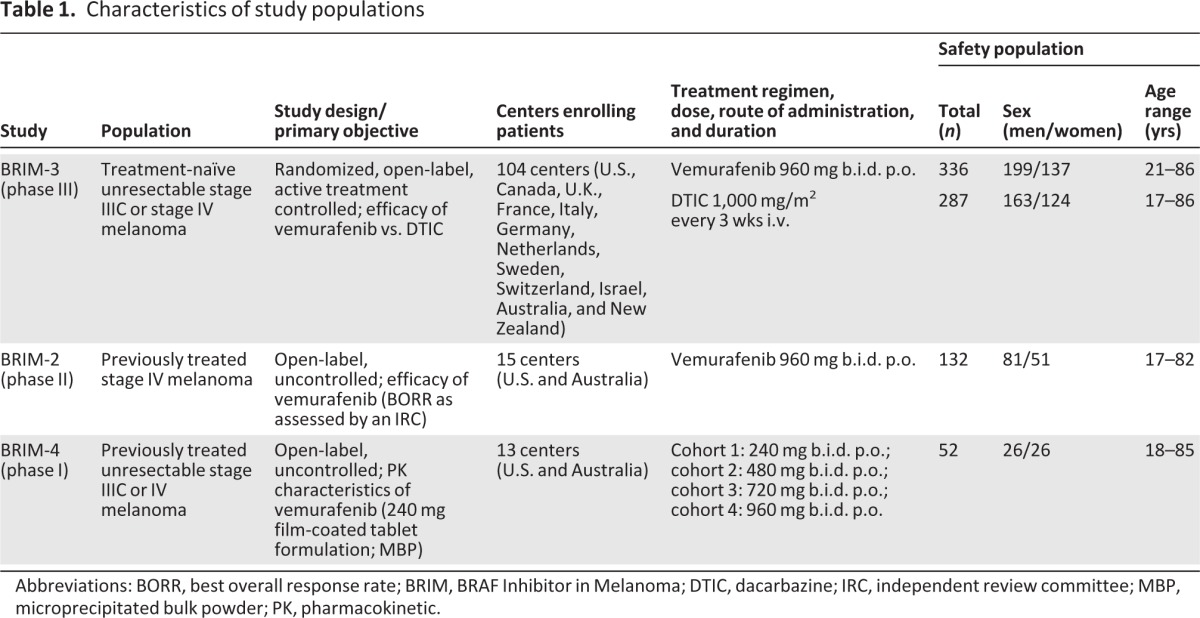
Abbreviations: BORR, best overall response rate; BRIM, BRAF Inhibitor in Melanoma; DTIC, dacarbazine; IRC, independent review committee; MBP, microprecipitated bulk powder; PK, pharmacokinetic.
Eligible patients had metastatic or advanced melanoma harboring the BRAFV600E mutation and disease that was previously untreated (BRIM-3) or refractory to standard therapy (BRIM-2, BRIM-4). In BRIM-3, patients were randomized to oral vemurafenib 960 mg twice daily or intravenous dacarbazine 1,000 mg/m2 of body surface area every 3 weeks. In BRIM-2, patients received oral vemurafenib 960 mg twice daily. In BRIM-4, patients received oral vemurafenib 960 mg twice daily after an initial 21-day dose escalation (240, 480, 720, or 960 mg twice daily). The 240-mg tablet was used in all trials.
All patients completed an initial dermatologic history, which included a history of chronic sun exposure, tanning bed use, previous skin lesions (including cuSCC, KA, actinic keratosis), and other factors that may increase the risk of cuSCC (e.g., sorafenib use, photochemotherapy for psoriasis, and immunosuppression). All patients underwent evaluations by a dermatologist or equivalent physician prior to enrollment, after 22–30 days of therapy, and then every 3 months. Suspicious skin lesions were biopsied or excised, and cuSCC lesions were submitted for both central dermatopathology review and molecular characterization.
Treatment interruptions were prespecified for intolerable grade 2 or higher AEs (except cuSCC; see below), during which vemurafenib was held until improvement of toxicity to grade ≤1, and then resumed at a reduced dose of 720 mg twice daily (or 480 mg twice daily for grade 4 or recurrent AEs). Therapy was discontinued with disease progression unless continued administration was in the best interest of the patient.
Clinical Reviews of Adverse Events
The cutoff dates used for data analysis were March 1, 2011 (BRIM-3, BRIM-4) and January 31, 2011 (BRIM-2). The safety population was comprised of all treated patients who had at least one on-study safety assessment.
In November 2009, the Vemurafenib Dermatology Working Group (DWG) was assembled and included experts in dermatology, oncology, and dermatopathology. The objectives were to evaluate and provide management recommendations for dermatologic AEs in vemurafenib clinical trials. The Medical Dictionary of Regulatory Activities was used to identify searchable AE terms related to the system organ class of skin and subcutaneous disorders. Severity of AEs was graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.0 [35]. AE summaries were sorted by descending frequency (number of patients with the event/number of patients at risk). For patients who experienced the same AE of varying severity, the highest grade was selected for the summary. Investigators were instructed to report all cuSCC and KA cases as treatment-related grade 3 events.
Statistical Analyses
For patients in BRIM-3, the following descriptive summaries were performed using the primary efficacy dataset (i.e., that led to study unblinding), which had an earlier cutoff date (December 30, 2010): occurrence of AE before or after day 120; relationship between best confirmed tumor response (complete response [CR] or partial response [PR]) and both occurrence of rash AE and worst grade of rash AE; outcome (resolved, unresolved, outcome unknown) of each rash event according to dosage (modified, discontinued, no action taken) and grade (1, 2, ≥3, missing); and relationship between onset of AEs that may result from sun exposure (phototoxicity and cuSCC). The median time to first onset was estimated for each AE by the Kaplan-Meier method. When median times were not reached, summary statistics were calculated for time to first onset among patients with the event.
Central Dermatopathologic Review
Clinically suspicious skin lesions were reported as AEs, biopsied, and sent for local pathologic analysis. For a subset of cuSCC specimens, central dermatopathology review was conducted by an independent group (Dermpath Diagnostics, Brookfield, WI). Two reviews were conducted for each specimen in a manner blinded to the reported diagnosis. Each reviewer examined the same sections and graded them on a three-point scale:
Squamous cell carcinoma (SCC), KA type, with features sufficiently expressed to be diagnostic (SCC, KA type)
SCC, with at least some features suggesting KA type, but with partial expression of usual features or with some features uncommon in KA type (SCC, mixture KA type)
SCC, with no features suggesting KA type (SCC, no KA type)
Features used to distinguish KA included a central keratin-filled crater, abrupt transition to normal epidermis at lesion edge, and neoplastic cells with abundant pale-pink glassy cytoplasm. In the event of a discrepancy in diagnosis or scaling, adjudication by a third dermatopathologist would occur.
Molecular Studies
Mutation analysis was performed on centrally confirmed cases of cuSCC (including KA). HRAS (exons 2 and 3), NRAS (exons 2 and 3), KRAS (exons 2 and 3), and BRAF (exon 15) gene sequences were investigated using direct DNA [9]. Remaining DNA was analyzed for known hotspot mutations using the Mass Array System (Sequenom, San Diego, CA).
Role of the Funding Source
The corresponding author had full access to the data in this manuscript and had final responsibility for the decision for publication. The study sponsors have contributed to the study designs; collection, analysis, and interpretation of data; writing of the report; and decision to submit the paper for publication.
Results
Clinical Characteristics
A total of 520 patients with BRAFV600E mutation-positive unresectable stage IIIC or stage IV melanoma received at least one dose of vemurafenib (Table 1). Dermatologic AEs were the most commonly reported body system class and occurred in 93% of patients in BRIM-3 (312/336), 95% in BRIM-2 (125/132), and 92% in BRIM-4 (48/52).
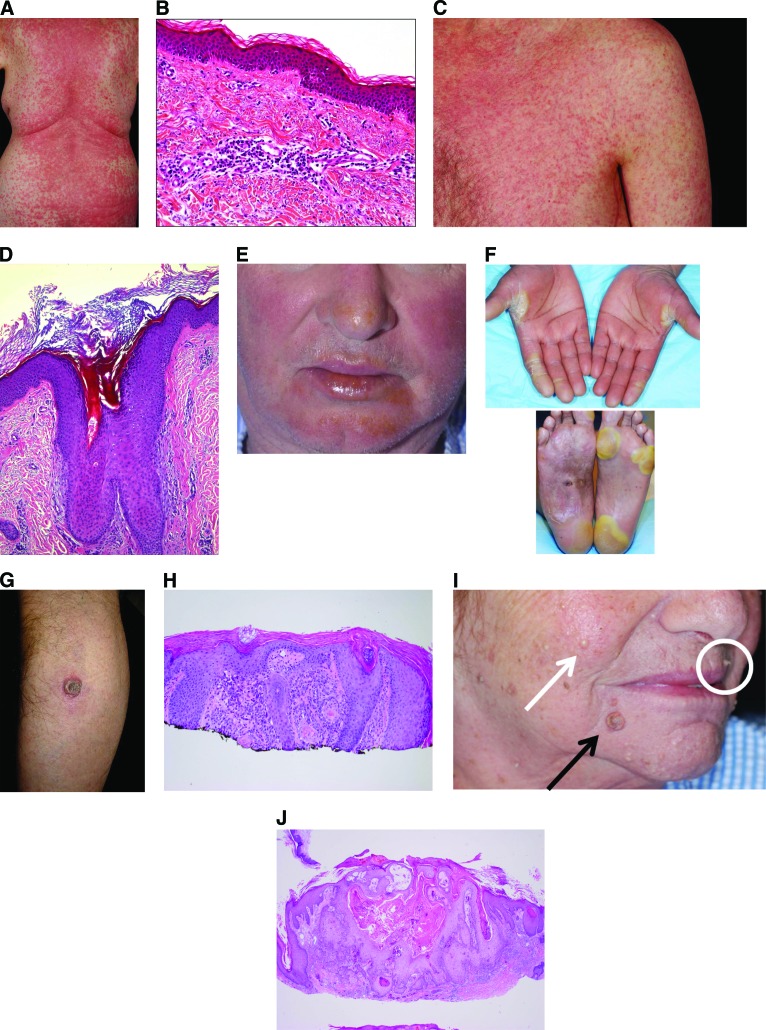
The most commonly reported AEs were rash and photosensitivity (Table 2). Rash incidence ranged from 64%–75%, most commonly rash not otherwise specified (37%–54%), erythema (10%–14%), maculopapular rash (4%–21%), folliculitis (6%–9%), and keratosis pilaris-like eruption (6%–10%; Fig. 1A–D). Photosensitivity ranged from 35%–63%, and PPE was reported in 8%–10% of patients (Fig. 1E, 1F). CuSCC lesions (including KA) developed in 79 patients (23.5%) in BRIM-3, 34 patients (25.8%) in BRIM-2, and 10 patients (19.2%) in BRIM-4 (Table 2; Fig. 1G–J). Other common AEs occurring in ≥10% of patients included alopecia (29%–45%), pruritus (10%–32%), skin papilloma (17%–31%), hyperkeratosis (23%–30%), and dry skin (15%–19%). In some cases of papilloma, eruption of multiple lesions occurred. Vasculitis, erythema nodosum, and panniculitis were uncommon (all ≤2%).
Table 2.
Common (>5%) dermatologic adverse events with vemurafenib treatment
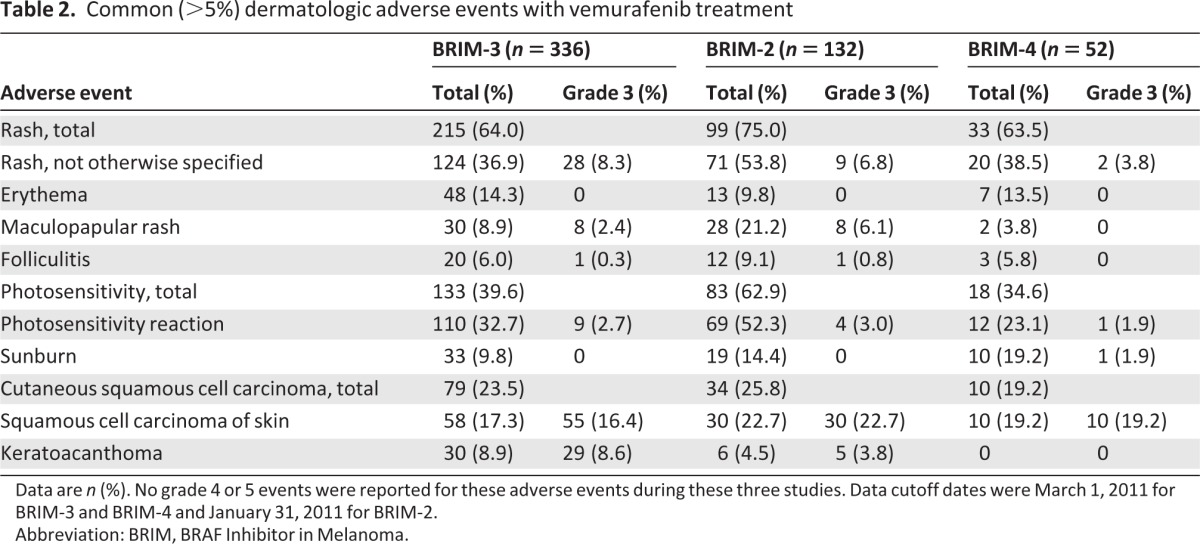
Data are n (%). No grade 4 or 5 events were reported for these adverse events during these three studies. Data cutoff dates were March 1, 2011 for BRIM-3 and BRIM-4 and January 31, 2011 for BRIM-2.
Abbreviation: BRIM, BRAF Inhibitor in Melanoma.
Figure 1.
Clinical and histologic presentation. (A): Maculopapular rash. (B): Maculopapular rash (histology). (C): Keratosis pilaris. (D): Keratosis pilaris rash (histology). (E): Photosensitivity. (F): Palmar-plantar erythrodysesthesia. (G): Cutaneous squamous cell carcinoma. (H): Cutaneous squamous cell carcinoma (histology). (I): Multiple cutaneous lesions: keratoacanthoma (black arrow), verruca (white circle), milia (white arrow). (J): Keratoacanthoma (histology).
The majority of AEs (aside from cuSCC) were of grade 1 or 2 severity. Only one patient discontinued vemurafenib for rash (grade 3; BRIM-3). Four additional patients discontinued vemurafenib due to a dermatologic AE (all one case each): Stevens-Johnson syndrome (SJS), toxic skin eruption (toxic epidermal necrolysis, TEN), grade 3 cellulitis, and grade 3 flushing. No patients discontinued vemurafenib for either photosensitivity or PPE. CuSCC lesions were managed with surgical excision, and no patient discontinued vemurafenib therapy due to the development of cuSCC or KA. The majority of patients continued therapy without dose reduction after resection.
Most AEs occurred early during the initial 4 months of therapy. Of the 126 patients who were treated for more than 120 days in BRIM-3, the first onset of most AEs was reported prior to day 120 (vs. after day 120): alopecia (86%), photosensitivity (98%), rash (100%), hyperkeratosis (87%), pruritus (88%), and skin papilloma (84%). The median times to first onset of cuSCC, rash, and photosensitivity were 7.1 weeks, 1.6 weeks, and 1.7 weeks, respectively.
The occurrence or severity of rash did not appear to be associated with treatment response. Of the 146 patients who were evaluable for response and reported an AE of rash in BRIM-3, 72 patients (49%) were responders (i.e., achieved a PR or CR). Similarly, among the 73 patients who did not develop a rash, 34 patients (47%) responded. Incidence of the most severe grade of rash was similarly distributed between responders and nonresponders: severity of rash was mostly grade 1 or 2 (78% responders, 82% nonresponders). Development of grade 3 rash was slightly higher in responding versus nonresponding patients (22% vs. 15%, respectively); there were no reported grade 4 or 5 rash AEs. These data suggest no association between the incidence or severity of rash and response to treatment.
In BRIM-3, cases of rash were analyzed to determine whether dose interruption or reduction was required for resolution. Approximately 82% (291/357) of cases resulted in no action taken. Of these, 152 (52%) resolved and 139 (48%) were unresolved or the outcome was unknown at the time of the analysis; the majority were grade 1 (n = 202) or 2 (n = 69). Of the grade 1 cases, 119 resolved and 83 were either unresolved or unknown. There were 66 cases of rash for which dose modification or interruption or discontinuation occurred. The majority of these were grade 3 (n = 36) or grade 2 (n = 22). Of note, the protocol required dose modification and/or interruption for grade 3 and intolerable grade 2 AEs. In cases when the dose was modified or discontinued, 41 (62%) resolved and 25 (38%) were unresolved or the outcome was unknown. These data indicate that at least 59% (119 of 202) and 43% (30 of 69) of grade 1 and grade 2 cases of rash, respectively, resolved without dose modification or interruption.
The relationship between phototoxicity and cuSCC events, both related to sun exposure, was evaluated in BRIM-3. In all, 29 of the 336 patients developed both cuSCC and photosensitivity; 179 patients did not develop either toxicity. Of patients who developed cuSCC, the proportion with photosensitivity (29 of 124; 23%) was similar to those without photosensitivity AE (33 of 212; 16%), suggesting no association between these toxicities. For those patients who developed both AEs, the majority (90%) had a first onset of photosensitivity on or before their first onset of cuSCC.
Central Review and Genetic Analysis of cuSCC
Of the 268 suspicious lesions that were collected from 76 patients and submitted for central review, 136 (51%) were cuSCC or KA (Table 3) and 132 (49%) were other lesions, including actinic keratosis, basal cell carcinoma, and verruca vulgaris. The majority of cuSCC or KA cases were diagnosed locally as cuSCC (rather than KA): 58 cuSCC and 30 KA in BRIM-3, 30 cuSCC and 6 KA in BRIM-2, and 10 cuSCC and 0 KA in BRIM-4. Conversely, the majority of lesions centrally diagnosed as SCC were classified as KA type (103 of 136 lesions, 76%; Table 3) or with some features of KA (27 of 136 lesions, 20%), with a minority reviewed as SCC with no KA features (6 of 136 lesions, 4%). Molecular characterization was performed on 29 samples (Table 4). Eleven samples carried HRAS mutations as determined by sequencing; Sequenom testing detected an HRAS mutation (HRAS G12V/D) in an additional case (patient 100,003). In total, 41% (12 of 29) carried HRAS mutations in either exon 2 or 3. No specimens had a BRAF mutation in exon 15.
Table 3.
Central dermatopathology classification of selected biopsies
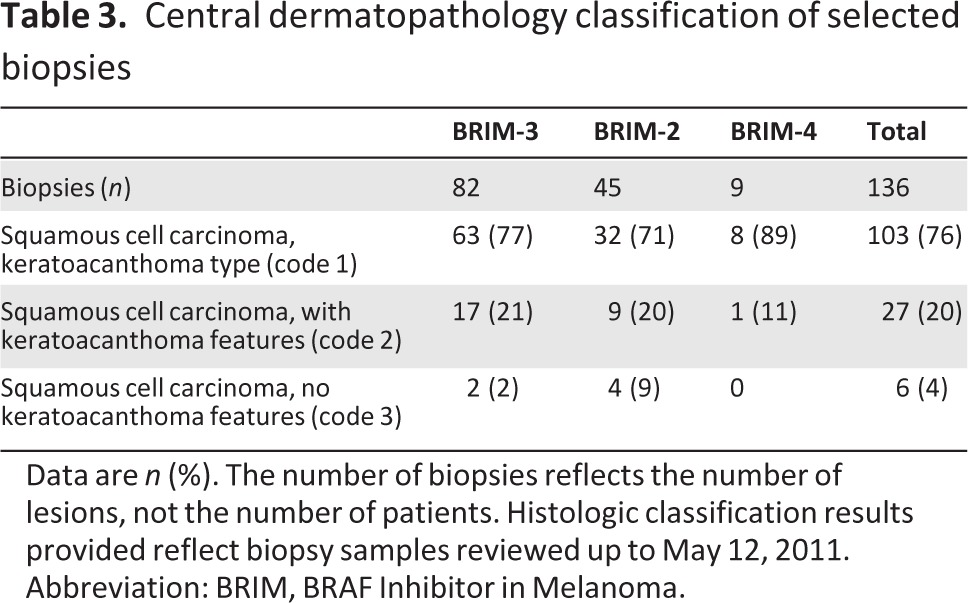
Data are n (%). The number of biopsies reflects the number of lesions, not the number of patients. Histologic classification results provided reflect biopsy samples reviewed up to May 12, 2011.
Abbreviation: BRIM, BRAF Inhibitor in Melanoma.
Table 4.
Summary of genetic analysis of cutaneous squamous cell carcinoma lesions

Discussion
This comprehensive review of dermatologic AEs that occurred during three separate vemurafenib trials demonstrates that these events are common but generally manageable with supportive care measures, in addition to dose modifications in a subset of patients. The frequency of these events and their potential impact on patient quality of life dictates comprehensive and thorough dermatologic evaluations by health care providers, preferably as conducted in clinical trials that include skin assessments prior to therapy initiation, followed by periodic evaluations at week 4, week 12, and every 12 weeks thereafter.
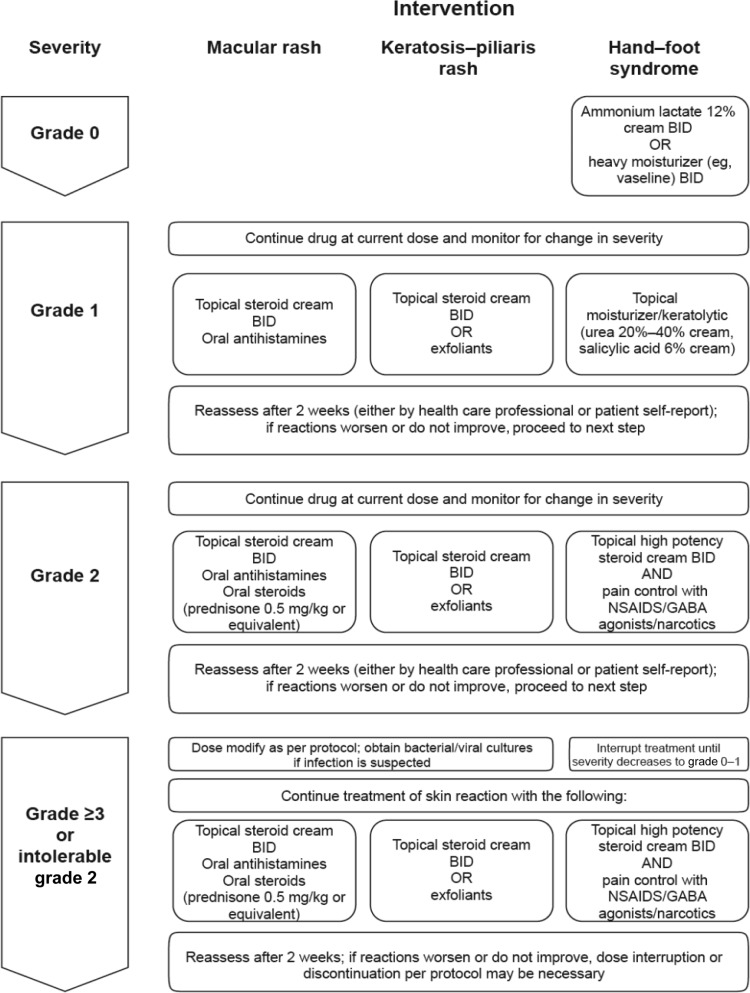
The spectrum of AEs, including the clinical presentation of rash, is variable and therefore does not suggest a particular mechanism of development, unlike the acneiform rash that develops with epidermal growth factor receptor inhibitors [10]. With vemurafenib, a frequently pruritic maculopapular rash is likely a hypersensitivity reaction that, however, does not preclude rechallenge in most cases. Signs suggestive of a type 1 hypersensitivity reaction, such as angioedema, blistering, and anaphylaxis, have not been observed; serious AEs, such as SJS and TEN, were rare (one case each in BRIM-3). Another cutaneous feature seen with vemurafenib is the appearance of asymptomatic, disseminated pink-to-red hair follicle openings with tiny spicules, which resembles or may be identical to the more limited autosomal-dominant condition of keratosis pilaris, which has not been previously reported as a drug-induced rash [11]. Most patients with rash were able to maintain full dose intensity or continue therapy after one dose-level reduction. A spontaneous recovery rate of approximately 50% for grade 1 and 2 rash without dose reduction was noted.
Treatment recommendations for rash, as well as for PPE, are based on the experience of the authors in managing patients in the aforementioned trials; recommendations are similar to those used in general dermatology practice (Fig. 2). For photosensitivity protection, patients should avoid prolonged sun exposure and use protective hats, clothing, sunglasses, and sunscreens (SPF ≥15), especially those providing broad ultraviolet (UV), including UV-A, protection [12]. For less frequent events (<2%), such as second primary melanomas [13, 14], vasculitis, erythema nodosum, and panniculitis, a phenotype-specific management approach is recommended [15].
Figure 2.
Management strategies for vemurafenib-associated macular rash, keratosis-pilaris rash, and hand–foot syndrome.
Abbreviations: BID, twice daily; GABA, γ-aminobutyric acid; NSAID, nonsteroidal anti-inflammatory drug.
CuSCC and/or KA were found in 19%–26% of patients. KAs are common, low-grade, skin neoplasms that are usually found on sun-exposed areas [16]. Surgical or destructive treatment is usually indicated; however, they can regress spontaneously. They are considered a type of SCC by some pathologists, although others consider them to be distinct tumors [17]. Diagnosis requires complete or near-complete sampling, and routine or partial histologic sections may make a diagnosis inaccurate [16, 18]. Thus, inadequate sampling and nosological diversity may contribute to the large discordance between rates of SCC versus KA reported by the local pathologists and central dermatopathologists in the BRIM trials.
The development of cuSCC and/or KA has been reported with other agents that inhibit RAF signaling, such as sorafenib (11% of patients) [11, 19–22] and dabrafenib (6% of patients) [23–25]; however, these reports have not always commented on KAs or similar lesions. These lower rates of SCC are consistent with the low incidence of SCC without KA features reported centrally in the BRIM trials (4% of total lesions, Table 3). Differences in cuSCC prevalence may also result from differences in dose, treatment duration, pathological classification, RAF inhibitor potency, AE reporting, as well as differences in the enrolled study populations (e.g., UV exposure and inherent SCC risk).
Risk factors for vemurafenib-associated cuSCC are similar to those for sporadic cases, including chronic sun exposure, and lesions develop more frequently on sun-exposed areas [26]. Body distribution of lesions from the phase I trial included head/neck (41%), upper extremities (16%), trunk/back/abdomen (19%), and lower extremities (25%) in sun-exposed skin [26]. In our analysis, 41% of lesions were found to have RAS mutations in codons commonly mutated in sporadic, sun exposure-related cuSCC/KA [27], which is similar to mutation rates reported with other RAF inhibitors [28, 29]. The short latency of onset (median: 8 weeks) [7, 8] suggests that skin cells with pre-existing RAS mutations may develop into cuSCC/KA lesions with exposure to vemurafenib. RAF inhibitors can paradoxically induce ERK signaling by transactivating CRAF in cells with mutant RAS [30]. Moreover, hyperkeratotic lesions (23%–30%), including verruca vulgaris and milia, and multiple keratotic warty papules were commonly reported, suggesting a spectrum of proliferative lesions of keratinocytes from hyperkeratosis and keratosis pilaris, to KA and cuSCC. It is not known whether these lesions are associated with HPV infection, which may be pathogenic in other squamous malignancies [31]. Of note, a recent clinical and molecular analysis of lesions collected from dabrafenib-treated patients suggested no association between HPV and the development of keratotic proliferative lesions, including SCC [32]. Second or new primary melanomas and atypical melanocytic lesions have also been reported in patients treated with vemurafenib and other RAF inhibitors [10, 14]. Genetic analysis of melanocytic lesions that have developed on vemurafenib is ongoing, and results of similar analyses have been described elsewhere [10].
Although there have been no reported cases of locally advanced or metastatic SCC associated with vemurafenib (or other RAF inhibitors) [28], strict adherence to management guidelines must be followed, including regular dermatologic surveillance and surgical excision. For patients with multiple SCCs/KAs, multiple excisions may not be feasible, and clinicians should be sensitive to resulting morbidity and possible functional consequences. Alternative surgical procedures (e.g., saucerization), nonsurgical modalities (e.g., curettage, electrodessication, cryosurgery, photodynamic therapy), and other medical therapies (e.g., topical fluorouracil, systemic acitretin) have been reported [33, 34] and can be considered; however, there are no conclusive data on the safety of these treatments. Of the 34 patients in BRIM-2 in whom cuSCC/KA lesions were reported, the majority of patients (76%) developed only one (20 patients) or two (6 patients) lesions [8]. Four patients developed three lesions, and one patient each developed four, five, six, and seven lesions.
In summary, although cutaneous toxicities are common with vemurafenib therapy, the majority of patients are able to tolerate and continue therapy following symptomatic (e.g., for rash, photosensitivity, PPE) and/or local (e.g., cuSCC, KA) management. Clinicians should be aware of these AEs to better educate and manage their patients, as well as minimize impact on patient quality of life.
Acknowledgments
This work was supported by F. Hoffmann-La Roche. Trials were sponsored by F. Hoffmann-La Roche. Editorial assistance in the preparation of this manuscript was provided by Dominic Sloane, Ph.D., of Adelphi Communications, including editing of a draft prepared by the authors and administrative assistance in the formatting and submission of the paper; authorship criteria were not met as the content of the manuscript was driven solely by the listed authors. Support for third-party editing assistance for this manuscript was provided by F. Hoffmann-La Roche. The study sponsors have contributed to the study designs; collection, analysis, and interpretation of data; writing of the report; and decision to submit the paper for publication.
We thank the BRAF Inhibitor in Melanoma (BRIM)-3 investigators led by P. Chapman, A. Hauschild, and G. McArthur; BRIM-2 investigators led by J. Sosman and A. Ribas; and BRIM-4 investigators led by A. Daud; and their study teams (including research nurses, study coordinators, and data coordinators). We also thank other members of F. Hoffmann-La Roche (including Victor Alejandro Iglesias) and the Dermatology Working Group, including J. Hou, B. Lestini, G. Bray, L. Burdette, K. Winson, P. Bridge, J. Zeffren, C. Shiomos, S. Nanni, S. Kaesshaefer, and E. Roberts-Thomson. We appreciate the commitment of all the patients who were screened for and enrolled in these trials.
Author Contributions
Conception/Design: Mario Lacouture, Madeleine Duvic, Axel Hauschild, Caroline Robert, Dirk Schadendorf, Patricia Myskowski, Keith Nolop, Joseph Grippo, Richard Lee, Andrew Joe
Provision of study material or patients: Mario Lacouture, Madeleine Duvic, Axel Hauschild, Victor Prieto, Caroline Robert, Dirk Schadendorf
Collection and/or assembly of data: Mario Lacouture, Axel Hauschild, Victor Prieto, Caroline Robert, Dirk Schadendorf, Patricia Myskowski, Olivia Spleiss, Kerstin Trunzer, Keith Nolop, James Troy, Andrew Joe
Data analysis and interpretation: Mario Lacouture, Madeleine Duvic, Axel Hauschild, Victor Prieto, Caroline Robert, Olivia Spleiss, Kerstin Trunzer, Fei Su, Betty Nelson, Keith Nolop, James Troy, Andrew Joe
Manuscript writing: Mario Lacouture, Madeleine Duvic, Axel Hauschild, Victor Prieto, Caroline Robert, Dirk Schadendorf, Caroline Kim, Christopher McCormack, Patricia Myskowski, Olivia Spleiss, Kerstin Trunzer, Fei Su, Betty Nelson, Keith Nolop, Joseph Grippo, Richard Lee, Matthew Klimek, James Troy, Andrew Joe
Final approval of manuscript: Mario Lacouture, Madeleine Duvic, Axel Hauschild, Victor Prieto, Caroline Robert, Dirk Schadendorf, Caroline Kim, Christopher McCormack, Patricia Myskowski, Olivia Spleiss, Kerstin Trunzer, Fei Su, Betty Nelson, Keith Nolop, Joseph Grippo, Richard Lee, Matthew Klimek, James Troy, Andrew Joe
Disclosures
Mario Lacouture: Genentech/Roche (C/A); Madeleine Duvic: Roche (C/A); Axel Hauschild: Amgen, AstraZeneca, Biovex, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eisai, GlaxoSmithKline, IGEA, Lilly, Medac (C/A, H, RF); Caroline Robert: Roche, GlaxoSmithKline, Bristol-Myers Squibb (C/A); Dirk Schadendorf: GlaxoSmithKline, Roche, Novartis, Amgen, Merck/MSD, Bristol-Myers Squibb (C/A); GlaxoSmithKline, Roche, Novartis, Amgen, MSD, Bristol-Myers Squibb (H); Merck (RF); Caroline C. Kim: Hoffman La Roche (C/A); Christopher J. McCormack: Vemurafenib Dermatology Working Group (H); Olivia Spleiss: Hoffmann La Roche (E); Kerstin Trunzer: F. Hoffmann-La Roche (E, OI); Fei Su: Roche Pharmaceuticals (E, OI); Betty Nelson: Genentech (E); Roche Pharmaceuticals (OI); Keith B. Nolop: Plexxikon (E, OI); Joseph F. Grippo: Hoffmann La Roche (E); Matthew J. Klimek: Roche (E); James L. Troy: Roche (C/A, RF); Andrew K. Joe: Hoffmann-La Roche (E)
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
References
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 3.Yazdi AS, Palmedo G, Flaig MJ, et al. Mutations of the BRAF gene in benign and malignant melanocytic lesions. J Invest Dermatol. 2003;121:1160–1162. doi: 10.1046/j.1523-1747.2003.12559.x. [DOI] [PubMed] [Google Scholar]
- 4.Ribas A, Flaherty KT. BRAF targeted therapy changes the treatment paradigm in melanoma. Nat Rev Clin Oncol. 2011;8:426–433. doi: 10.1038/nrclinonc.2011.69. [DOI] [PubMed] [Google Scholar]
- 5.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 6.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 11.Hwang S, Schwartz RA. Keratosis pilaris: A common follicular hyperkeratosis. Cutis. 2008;82:177–180. [PubMed] [Google Scholar]
- 12.Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemurafenib therapy. N Engl J Med. 2012;366:480–481. doi: 10.1056/NEJMc1113752. [DOI] [PubMed] [Google Scholar]
- 13.Dalle S, Poulalhon N, Thomas L. Vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;365:1448–1449. doi: 10.1056/NEJMc1108651. [DOI] [PubMed] [Google Scholar]
- 14.Zimmer L, Livingstone E, Hillen U, et al. Panniculitis with arthralgia in patients with melanoma treated with selective BRAF inhibitors and its management. Arch Dermatol. 2012;148:357–361. doi: 10.1001/archdermatol.2011.2842. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in advanced melanoma patients undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375–2383. doi: 10.1200/JCO.2011.41.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1–19. doi: 10.1016/s0190-9622(94)70001-x. [DOI] [PubMed] [Google Scholar]
- 17.Kossard S, Tan KB, Choy C. Keratoacanthoma and infundibulocystic squamous cell carcinoma. Am J Dermatopathol. 2008;30:127–134. doi: 10.1097/DAD.0b013e318161310c. [DOI] [PubMed] [Google Scholar]
- 18.Ko CJ. Keratoacanthoma: Facts and controversies. Clin Dermatol. 2010;28:254–261. doi: 10.1016/j.clindermatol.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Kong HH, Cowen EW, Azad NS, et al. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol. 2007;56:171–172. doi: 10.1016/j.jaad.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnault JP, Wechsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59–e61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 21.Dubauskas Z, Kunishige J, Prieto VG, et al. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20–23. doi: 10.3816/CGC.2009.n.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnault JP, Mateus C, Escudier B, et al. Skin tumors induced by sorafenib; paradoxic RAS-RAF pathway activation and oncogenic mutations of HRAS, TP53, and TGFBR1. Clin Cancer Res. 2012;18:263–272. doi: 10.1158/1078-0432.CCR-11-1344. [DOI] [PubMed] [Google Scholar]
- 23.Kefford R, Arkenau H, Brown MP, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28(suppl 15):8503. [Google Scholar]
- 24.Trefzer U. BREAK-2: A phase IIA trial of the selective BRAF kinase inhibitor GSK2118436 in patients with BRAF (V600E/K) positive metastatic melanoma. Abstract presented at: 8th International Melanoma Congress of the Society for Melanoma Research; November 9–13, 2011; Tampa, FL. [Google Scholar]
- 25.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicenter, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 26.Lacouture ME, McArthur GA, Chapman PB, et al. PLX4032 (RG7204), a selective mutant RAF inhibitor: Clinical and histologic characteristics of therapy-associated cutaneous neoplasms in a phase I trial. J Clin Oncol. 2010;28(suppl 15):8592. [Google Scholar]
- 27.Corominas M, Kamino H, Leon J, et al. Oncogene activation in human benign tumors of the skin (keratoacanthomas): Is HRAS involved in differentiation as well as proliferation? Proc Natl Acad Sci U S A. 1989;86:6372–6376. doi: 10.1073/pnas.86.16.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberholzer PA, Kee D, Dziunycz P, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30:316–321. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubina M, Goldenberg G. Viral-associated nonmelanoma skin cancers: A review. Am J Dermatopathol. 2009;31:561–573. doi: 10.1097/DAD.0b013e3181a58234. [DOI] [PubMed] [Google Scholar]
- 32.Anforth RM, Blumetti TC, Kefford RF, et al. Cutaneous manifestations of dabrafenib (GSK2118436): A selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br J Dermatol. 2012;167:1153–1160. doi: 10.1111/j.1365-2133.2012.11155.x. [DOI] [PubMed] [Google Scholar]
- 33.Alloo A, Garibyan L, LeBoeuf N, et al. Photodynamic therapy for multiple eruptive keratoacanthomas associated with vemurafenib treatment for metastatic melanoma. Arch Dermatol. 2012;148:363–366. doi: 10.1001/archdermatol.2011.3080. [DOI] [PubMed] [Google Scholar]
- 34.Anforth R, Bluemetti TC, Mohd Affandi A, et al. Systemic retanoid therapy for chemoprevention of nonmelanoma skin cancer in a patient treated with vemurafenib. J Clin Oncol. 2012;30:e165–e167. doi: 10.1200/JCO.2011.39.8594. [DOI] [PubMed] [Google Scholar]
- 35.Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol. 2012;67:1025–1039. doi: 10.1016/j.jaad.2012.02.010. [DOI] [PubMed] [Google Scholar]