This randomized controlled trial investigated the effects of a psychosocial nurse-led intervention on the depressive and cancer-related physical symptoms of patients with head and neck cancer. The intervention was found to be effective at reducing depressive symptoms 1 year after cancer treatment, especially in patients with raised levels of depressive symptoms.
Keywords: Head and neck, Nurse-led, Psychosocial, Intervention, Depressive symptoms
Abstract
Background.
Many patients with head and neck cancer (HNC) experience depressive symptoms after treatment. This randomized controlled trial investigated the effects of a psychosocial nurse counseling and after intervention (NUCAI) versus usual care on the depressive and HNC-related physical symptoms of patients with HNC at 1 year after diagnosis.
Methods.
A total of 205 patients with HNC were randomly assigned to either intervention (n = 103) or usual care (n = 102), with stratification for gender and tumor stage. The NUCAI, which consisted of six bimonthly 45-minute counseling sessions, was a problem-focused intervention aimed at helping patients to manage the physical, psychological, and social consequences of HNC and its treatment. It was nurse-led and offered in combination with regular medical follow-up visits at the University Medical Center Utrecht, the Netherlands. Depressive symptoms at 1 year after diagnosis were the primary outcome. Analyses were performed on an intention-to-treat basis for the total sample and for a predefined subgroup of patients with raised levels of depressive symptoms (Center for Epidemiologic Studies–Depression score ≥12; n = 91) at baseline using mixed-effect models.
Results.
One year after HNC treatment, levels of depressive symptoms were significantly lower in the intervention group than in the control group in the total sample and in the subgroup of patients with raised levels of depressive symptoms.
Conclusion.
The NUCAI was feasible and effective in reducing depressive symptoms in patients with HNC 1 year after HNC treatment, and especially in patients with raised levels of depressive symptoms. The results of this study need to be confirmed in future studies before the NUCAI can be used in daily clinical practice.
Implications for Practice:
Head and neck cancer patients are prone to physical problems, depressive symptoms, and decreased quality of life. The nurse counselling and after intervention (NUCAI) is a nurse-led psychosocial intervention. The NUCAI has been shown to be effective in decreasing depressive symptoms and tumor-and-treatment-related symptoms at one year after treatment in head and neck cancer patients, and especially in patients with raised levels of depressive symptoms. This nurse-led intervention is less intensive compared with other psychosocial interventions and is easy to combine with regular medical follow up. It is, therefore, promising to implement in daily clinical practice.
Introduction
Treatment of head and neck cancer (HNC) frequently results in long-term physical problems, such as changes in taste and smell, dry mouth, sticky saliva [1, 2], and problems with mastication [3]. In part because of these physical and functional impairments, patients with HNC are prone to depressive symptoms [4]. The prevalence of depressive symptoms in patients with HNC varies from 0.05% to 48% [5, 6], and the proportion of patients with possible depression ranges from 27%–28% at diagnosis to 9%–20% at 36 months after treatment [2, 7–9]. Even though the risk of depressive symptoms slowly decreases, it remains substantial 3 years after treatment. Depression is strongly associated with—and is a major predictor of—a decreased quality of life [10–13] and is accompanied by anxiety disorders [4] and fear of recurrence [14, 15]. Unfortunately, patients with HNC are not yet routinely screened for depressive symptoms.
Several meta-analyses and reviews have shown that psychosocial interventions are effective in diminishing depressive symptoms in the general cancer population [16–19]. Examples of such interventions include cognitive behavioral therapy [17, 19], counseling/psychotherapy [20], counseling/relaxation [21], computer-based assessment and individually tailored care plans [22, 23], supportive interventions [24–26], and multicomponent interventions [27, 28]. There is no evidence that one intervention is superior to another. Four studies reported psychosocial interventions, aimed at coping behavior, to be fairly successful in decreasing depressive symptoms in patients with HNC [29–32]. Literature suggests that it might be appropriate to offer such therapies only to those patients with a significant psychological burden [19, 33].
Psychosocial interventions are usually given by psychologists [20], mental health professionals [24], social workers [34], or nurses [22, 23, 26]. Although interventions and standard care are usually offered separately, one study showed the combination to be effective [21]. It might be advantageous, in terms of time and compliance, to incorporate the intervention in the medical follow-up of patients with HNC, with the intervention being administered by nurses who are familiar with the care and problems of patients with HNC. Working in close cooperation with attending physicians, these nurses can have an important role in the management of symptoms, assessment of depressive symptoms, and support and education about depression and its effects [35]. In the past, interventions for patients with cancer that were led by nurses have proven effective in reducing depressive symptoms [27, 28, 30] and physical symptoms, helping patients cope with physical impairments, and reducing emotional distress [22, 36].
The primary aim of this randomized controlled trial was to investigate the effectiveness of a comprehensive nurse-led intervention focused on decreasing depressive symptoms of patients with HNC after their cancer treatment. The secondary aim was to investigate the effect of the intervention on physical symptoms. We hypothesized that patients in the intervention group would show fewer depressive symptoms and fewer physical symptoms than patients in the control group 1 year after HNC treatment.
Patients and Methods
This randomized controlled trial (RCT) evaluated the 1-year effect of a comprehensive nurse-led intervention on depressive symptoms and HNC-related physical symptoms in patients treated for HNC and in a subgroup of patients with raised levels of depressive symptoms. Recruitment took place between January 2005 and September 2007. Patients were enrolled by the researcher before the start of cancer treatment; the nurse counseling and after intervention (NUCAI) was started after completion of treatment. Eligibility criteria included a primary diagnosis of squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx; treatment with curative intent; ability to complete questionnaires; and ability to participate in the intervention. Patients were excluded if they had a previous or concomitant malignancy and/or were being treated for depression, diagnosed according to Diagnostic and Statistical Manual of Mental Disorders criteria, as stated in their medical record [37].
The study was approved by the Medical Ethics Committee of the University Medical Center Utrecht (registered as ISRCTN06768231). Before randomization, each patient received written information about the study, in which they were told that two different types of aftercare were being investigated but that medical care would remain the same. Each participant signed the informed consent form and received general information about post-cancer treatment care, but no specific information about the NUCAI. After the completion of cancer treatment, the patients were randomized using an open block procedure to receive NUCAI or care as usual, stratified by gender and tumor stage. Each participant received a letter from the researchers on which type of care they would receive, but did not know whether this treatment was the control or intervention treatment.
Participants completed five questionnaires at home and returned them using a prepaid return envelope at baseline, before the start of cancer treatment, and at 3, 6, 9, and 12 months after the completion of cancer treatment. The primary endpoint was 12 months after completion of cancer treatment; the other measurements were taken to gain insight into the pattern of change in depressive and physical symptoms.
Care as Usual
Care as usual was provided by HNC specialists and was primarily aimed at the treatment of complications and the detection of recurrences or second primary tumors. Patients were seen at 2-month intervals for a 10-minute appointment, during which they were examined, their physical history was reviewed, and ancillary tests were ordered if necessary. If the patient had psychosocial problems, the HNC specialist could refer the patient to psychological aftercare.
Intervention
The NUCAI was designed to help patients manage the physical, psychological, and social consequences of their disease and its treatment, by means of restructuring cognitions and beliefs (part of the after intervention), educational and behavioral training and advice, and provision of emotional support. The NUCAI is problem focused and patient led.
A manual was developed to help nurses to structure the counseling sessions and to assist them in discussing problems and in choosing appropriate nursing interventions. The nurses kept a treatment file for each of their patients, in which they recorded the topics discussed—namely, the home situation, physical functioning, social functioning, mental functioning, and nursing interventions. Patients received six counseling sessions of 45–60 minutes during 1 year given by a trained nurse in the outpatient clinic. The counseling session was always combined with the patient's 2-month medical check-up, and each patient saw the same nurse for all six sessions. If needed, the patient could continue counseling after the first year.
The NUCAI consists of six components: evaluating current mental status with the Hospital Anxiety and Depression Scale (HADS); discussing current problems; systematically asking about physical problems and functioning in six relevant life domains; providing the Adjustment to Fear, Threat or Expectation of Recurrence (AFTER) intervention, if indicated; providing general medical assistance and advice, if indicated; and referring patients to psychological aftercare, if indicated. Before each counseling session, the patients completed HADS [38, 39] at home. Nurses used the HADS score to screen for anxiety and/or depressive symptoms (cutoff >10 points). The outcome was used to gain insight into the needs of the patient, and discussing the results of the HADS often made it easier for patients to talk about their problems (e.g., “I reviewed your answers on the questionnaire [HADS] and they show that you sometimes feel down, in despair, or worried. Is this correct? Do you feel down or worried?” Furthermore, the nurse checked the patient history to screen for the presence of psychosocial morbidity. This information was used to guide counseling.
The session started with a discussion of current problems and topics brought up by the patient. Patients were then systematically asked about physical problems related to HNC, such as mastication, swallowing, shoulder function, sense of taste or smell, breathing, restrictions in speech, pain, and fatigue (e.g., “Some patients feel that their shoulder is painful and stiff after surgery. Is your shoulder painful or stiff?”). Then patients were asked about their functioning in six relevant life domains: home situation, (resuming) work, household and leisure activities, mood and emotional distress, partner relationship and intimacy, and family and social life. (e.g., “Has your relationship with your partner/family changed since your treatment?”).
When indicated, the nurse gave information and advice, provided minor medical and/or behavioral treatment, and offered support in accordance with the Dutch oncological nursing guidelines [40], the cancer clinical practice guidelines of the Dutch Association of Comprehensive Cancer Centers [41], and the guidelines of the Nurse Intervention Classification [42]. For example, the nurses taught patients a relaxation exercise. If necessary, patients were referred to physicians, health care professionals specialized in psychosocial problems (e.g., psychologist or social worker), or a relevant patient program (e.g., oncological rehabilitation or patient support groups).
If indicated, the AFTER intervention [43] was carried out. This cognitive behavioral intervention, which is based on the self-regulation model by Leventhal et al. [44], was designed to reduce irrational thoughts and to help patients with orofacial cancer to handle excessive fear of recurrence and psychological distress. It consist of four components: expressing fear of recurrence, identifying beliefs about sensations and their interpretation as recurrence, evaluating the function of self-examination and reducing excessive checking behavior, and relaxation.
Training
Three experienced oncology nurses were selected from the oral maxillofacial and the otorhinolaryngology department of the University Medical Center Utrecht. Before the start of the study, the nurses were intensively trained by two psychologists (R.L. and W.R.) and one of the investigators (M.O.) to carry out the NUCAI by learning on the job. They also completed a comprehensive 1-day training, given by an expert, in how to administer the AFTER intervention [43], to ensure that the intervention was given in a standardized way. During the intervention period, the nurses, the psychologists, and the investigator reviewed selected tape-recorded intervention sessions every 2 months to monitor and, where necessary, improve the quality of the intervention sessions. No midcourse corrections and/or adaptions were needed.
Measures
Information about age, gender, educational level, and social status was collected by means of self-report questionnaires. Information about treatment, tumor type, and stage was obtained from the medical records. Depressive symptoms were measured with the Center for Epidemiologic Studies-Depression (CES-D) scale [45, 46], with a cutoff score of 16 or higher being considered indicative of clinical depression [47]. This 20-item self-report questionnaire has shown good psychometric properties in medically ill populations [48] and cancer populations [49, 50], including HNC [1, 51]. The mean (±SD) CES-D score in the Dutch population is 8.2 ± 7.2 [52]. Because a difference of half a standard deviation may be interpreted as a clinically relevant raised level of depressive symptoms [53], a CES-D score ≥12 was used in this study as indicative of a raised level of depressive symptoms.
As a secondary outcome, physical symptoms were assessed using the head and neck module of the Quality of Life Questionnaire (QLQ) of the European Organization for Research and Treatment of Cancer (EORTC).The EORTC QLQ head and neck module is a widely used and validated questionnaire [54]. For all functional items, high scores indicate more problems. During each counseling session, the nurse recorded if the following topics were discussed for each patient: home situation, physical functioning, social functioning, psychological functioning, and nursing interventions.
Analysis
The primary endpoint was depressive symptoms and the secondary endpoint was physical symptoms at 12 months after completion of cancer treatment. The sample size was based on the prevalence of patients with raised levels of depressive symptoms (CES-D ≥12). Power analysis for analysis of variance procedures showed that assuming an effect size of 0.32 with a two-sided test, a sample size of 45 patients with raised levels of depressive symptoms in each arm would suffice (power = 80%, α = 0.05). Data from our previous study [7, 55] showed that 56% of patients had a CES-D score ≥12 before cancer treatment. Therefore, a minimum of 160 patients with HNC would be needed; 205 were enrolled to allow for study dropout.
The effect of the intervention in the total group and in the predefined depressive subgroup was assessed on an intention-to-treat basis (all patients with complete data at baseline and at least one follow-up measurement), using a linear mixed model approach. Because some 1-year data were missing, we also included data for 3, 6, and 9 months after HNC treatment in the model to estimate the 1-year effect. In these analyses, the program accounts for missing data based on observed data by maximum likelihood estimations [56]. Pearson's correlation coefficients were used to explore the correlation between depressive and physical symptoms at baseline and 12 months after treatment and the relationship between changes from baseline to 12 months. To verify the comparability of prognostic factors in the depressive subgroup between the intervention and control conditions, Student's t test and Mann-Whitney test were used for continuous data and χ2 tests for categorical data. Results for the subgroup were controlled for any between-group differences in baseline characteristics. Two-sided significant tests were used (α < 0.05). Statistical analyses were performed using R software version 2.10.0. (www.r-project.org) and SPSS version 20 (SPSS, Chicago, IL). The content of 10 treatment files kept by the nurses was systematically analyzed in order to determine whether the intervention had been carried out as intended and to identify the topics discussed.
Results
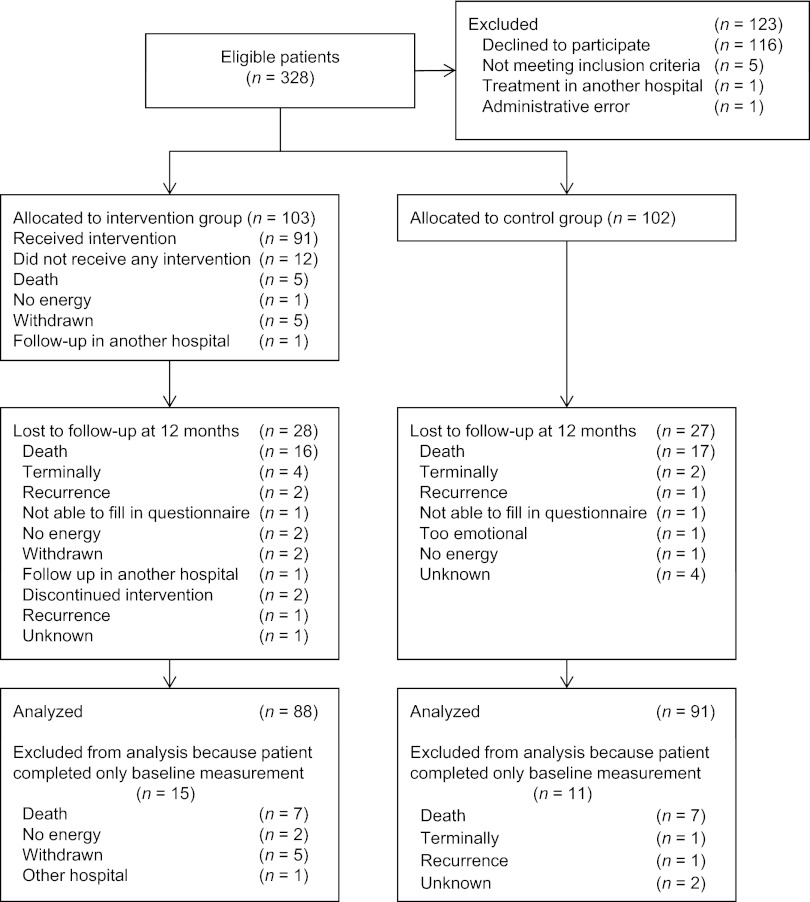
A total of 328 eligible patients were identified, of which 205 (62.5%) were eligible for participation. Reasons for nonparticipation are shown in Figure 1. In total, 103 patients were randomized to the intervention and 102 patients to the control group. At 12 months, 55 patients were lost to follow-up, 33 of whom had died. At baseline, the intervention and control groups were comparable in terms of demographic variables, clinical characteristics, and baseline CES-D scores (Table 1). Baseline demographic values for the subgroup of patients with raised levels of depressive symptoms were not statistically different, with the exception that patients in the intervention group had a higher educational level than the patients in the control group (p = .05; Table 2).
Figure 1.
Diagram of participants' progress through the study.
Abbreviation: CONSORT, Consolidated Standards of Reporting Trials.
Table 1.
Demographics and clinical characteristics at baseline by study arm
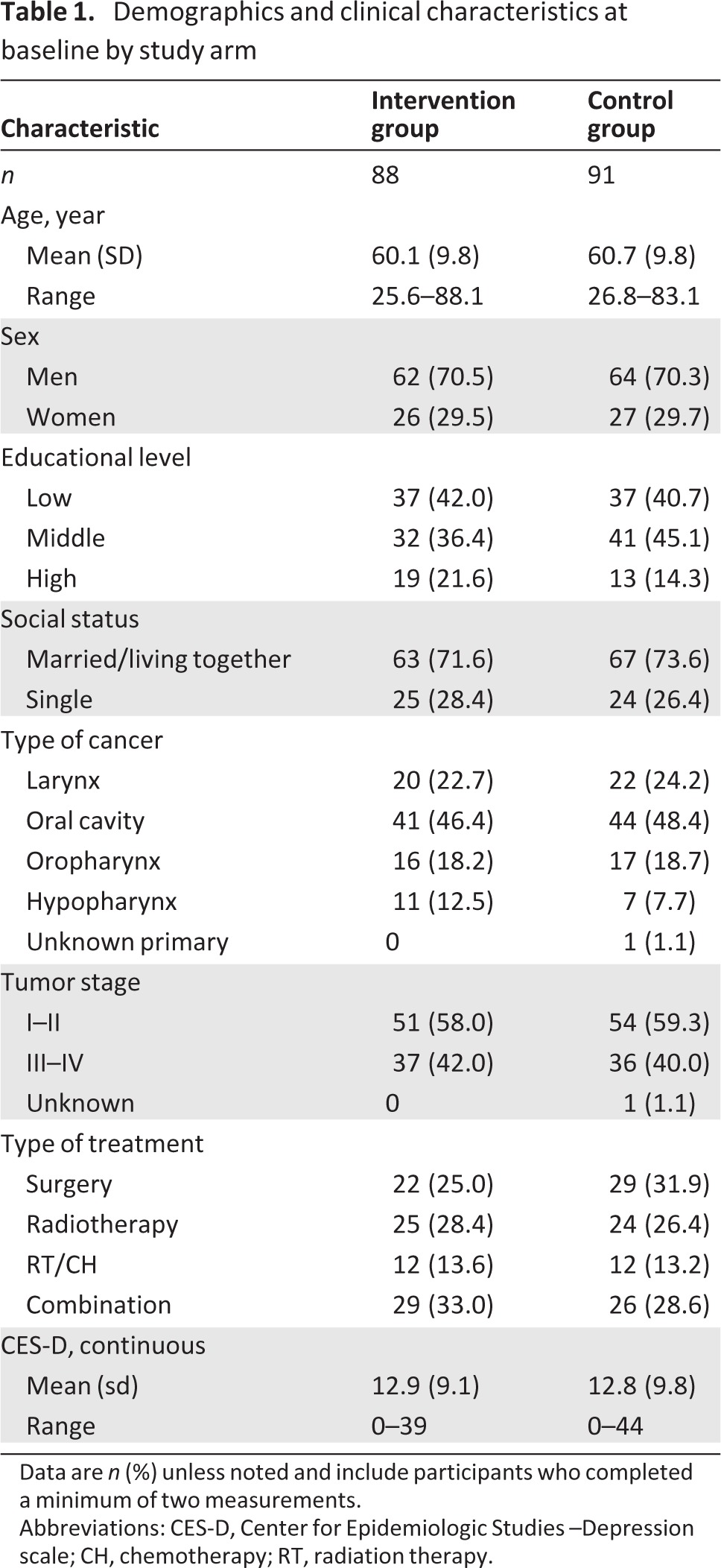
Data are n (%) unless noted and include participants who completed a minimum of two measurements.
Abbreviations: CES-D, Center for Epidemiologic Studies –Depression scale; CH, chemotherapy; RT, radiation therapy.
Table 2.
Subgroup of patients with a CES-D score ≥12 at baseline
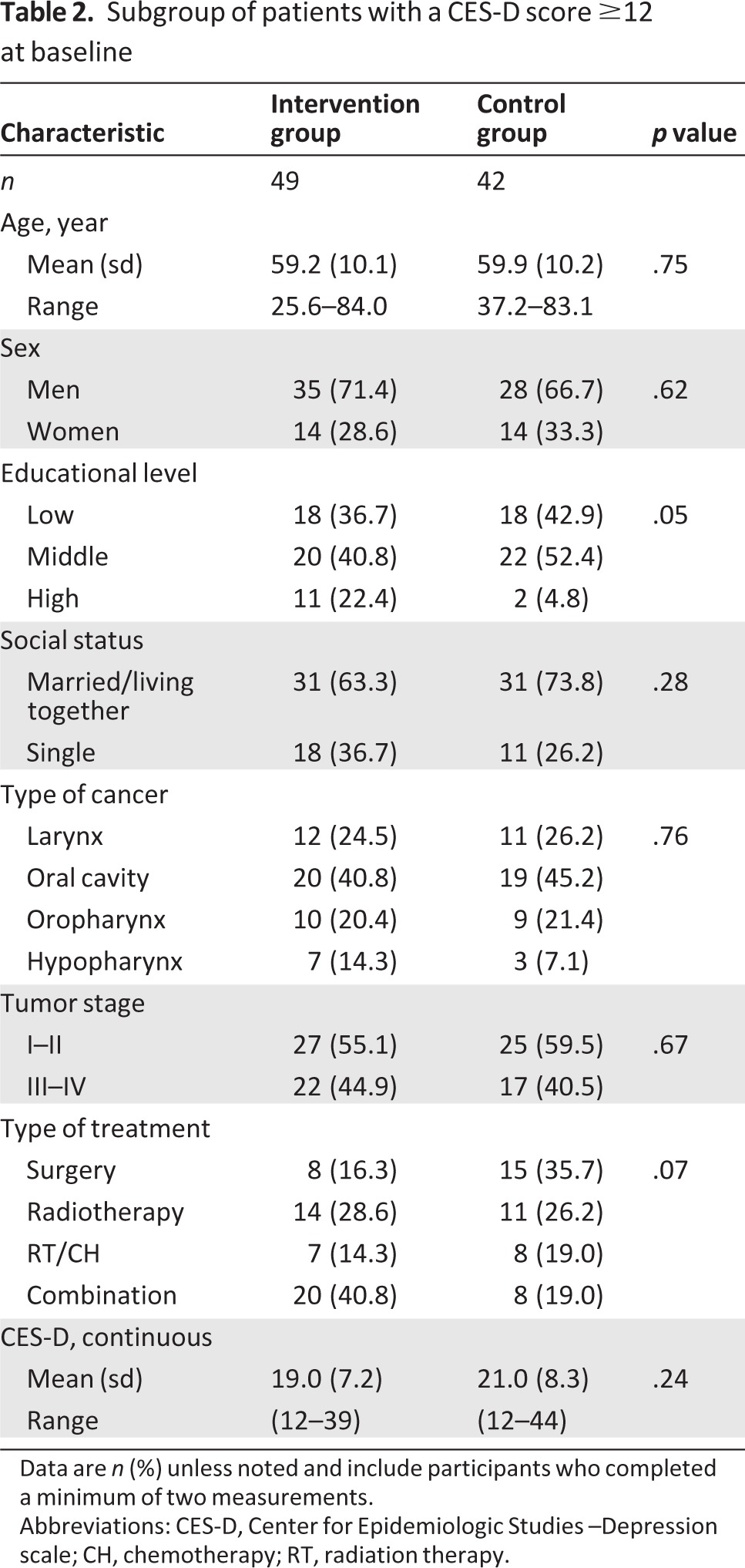
Data are n (%) unless noted and include participants who completed a minimum of two measurements.
Abbreviations: CES-D, Center for Epidemiologic Studies –Depression scale; CH, chemotherapy; RT, radiation therapy.
Significant differences were found between patients who were lost to follow-up (n = 55) and patients who completed the study (n = 150): patients who were lost to follow-up were older (p = .019), more educated (p = .011), and had an advanced tumor stage (p = .026). There were no differences in sociodemographic variables, clinical variables, and depressive symptoms between patients lost to follow-up in the intervention and control groups, indicating that loss was not selective. Antidepressant medication was used by one patient at baseline, by two patients at 6 months, and by three patients at 12 months in the intervention group. In the control group, antidepressant medication was used by one patient at baseline, by five patients at 6 months, and by six patients at 12 months.
Intervention Adherence
Of the 103 patients allocated to the intervention group, 12 (11.7%) did not attend any of the counseling sessions. Reasons for nonattendance are presented in Figure 1. Of the 91 (88.3%) patients who received counseling, 15 (16.5%) attended one or two sessions, 39 (42.9%) attended three or four sessions, and 37 (40.7%) attended five or six sessions. Three patients did not consider it necessary to complete the intervention and stopped after four (n = 1) or five sessions (n = 2). At 12 months, 65 patients (63.1%) still had one or more counseling sessions planned. The counseling sessions were sometimes delayed because it was not always possible for physicians to hold follow-up visits at 2-month intervals, and the NUCAI was always given in combination with these appointments. At 12 months, one participant in the intervention group and five participants in the control group had seen a psychologist. Two participants in the intervention group and two in the control group had received religious guidance.
Content of the Intervention
The analyses of a random selection of 10 patient treatment files showed that the nurses followed the manual, implementing the six components of the intervention. The treatment files also showed that patients were quite open and frank in discussing their history and emotions with the nurses. In some cases, the nurses provided minor medical assistance, such as prescribing an oral gel for dry mouth or giving information about stopping or cutting down smoking if smoking was a problem. The sessions were homogenous in content, although the first sessions focused more on physical symptoms, whereas the later sessions concentrated more on emotional, relational, and social problems. Patients' fear of reoccurrence was discussed in all sessions, if relevant. If indicated, patients were referred to a social worker or psychologist.
Depressive Symptoms
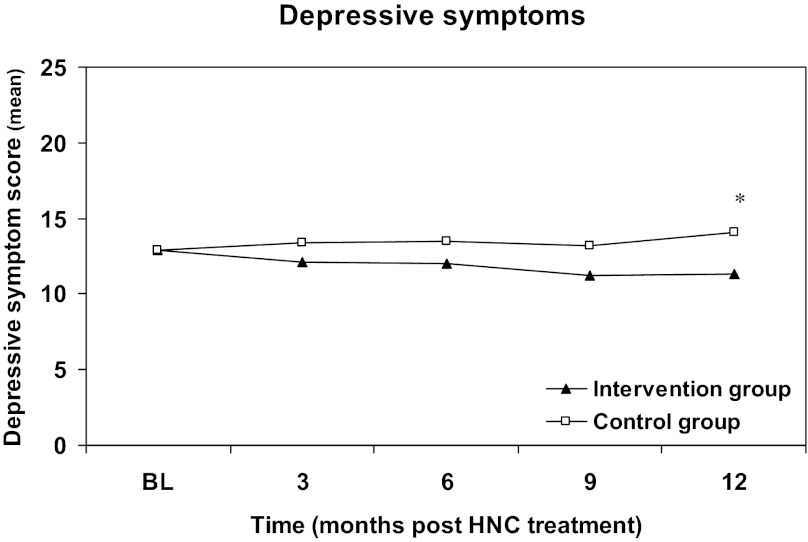
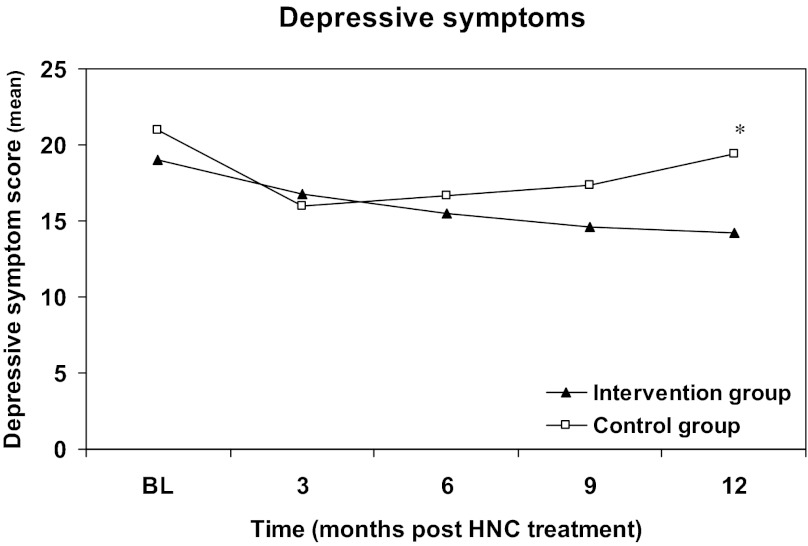
Intention-to-treat analysis revealed that the decrease in depressive symptoms was significant in the intervention group compared with the control group (−2.8, 95% confidence interval [CI]: −5.2 to −0.3) at 12 months after treatment (Table 3). On average, depressive symptoms in the intervention group decreased from 12.9 (SD 9.1) at baseline to 10.9 (SD 9.1) at 12 months, whereas symptoms increased in the control group from 12.8 (SD 9.8) to 13.8 (SD 12.3). Changes in depressive symptoms over 12 months for the total study sample are shown in Figure 2. For the depressive subgroup of patients (CES-D ≥12), analysis revealed a significant decrease in depressive symptoms in the intervention group compared with the control group at 12 months (−5.2, 95% CI: −9.1 to −1.2; Table 3). After adjustment for the between-group difference in baseline educational level, depressive symptoms decreased significantly from 19.0 (SD 7.3) at baseline to 13.8 (SD 10.1) in the intervention group but increased from 21.0 (SD 8.3) to 22.0 (SD 12.6) in the control group at 12 months. Changes in depressive symptoms over 12 months in the subgroup are shown in Figure 3.
Table 3.
Depressive symptoms and head and neck related physical symptoms during the 12-month study period
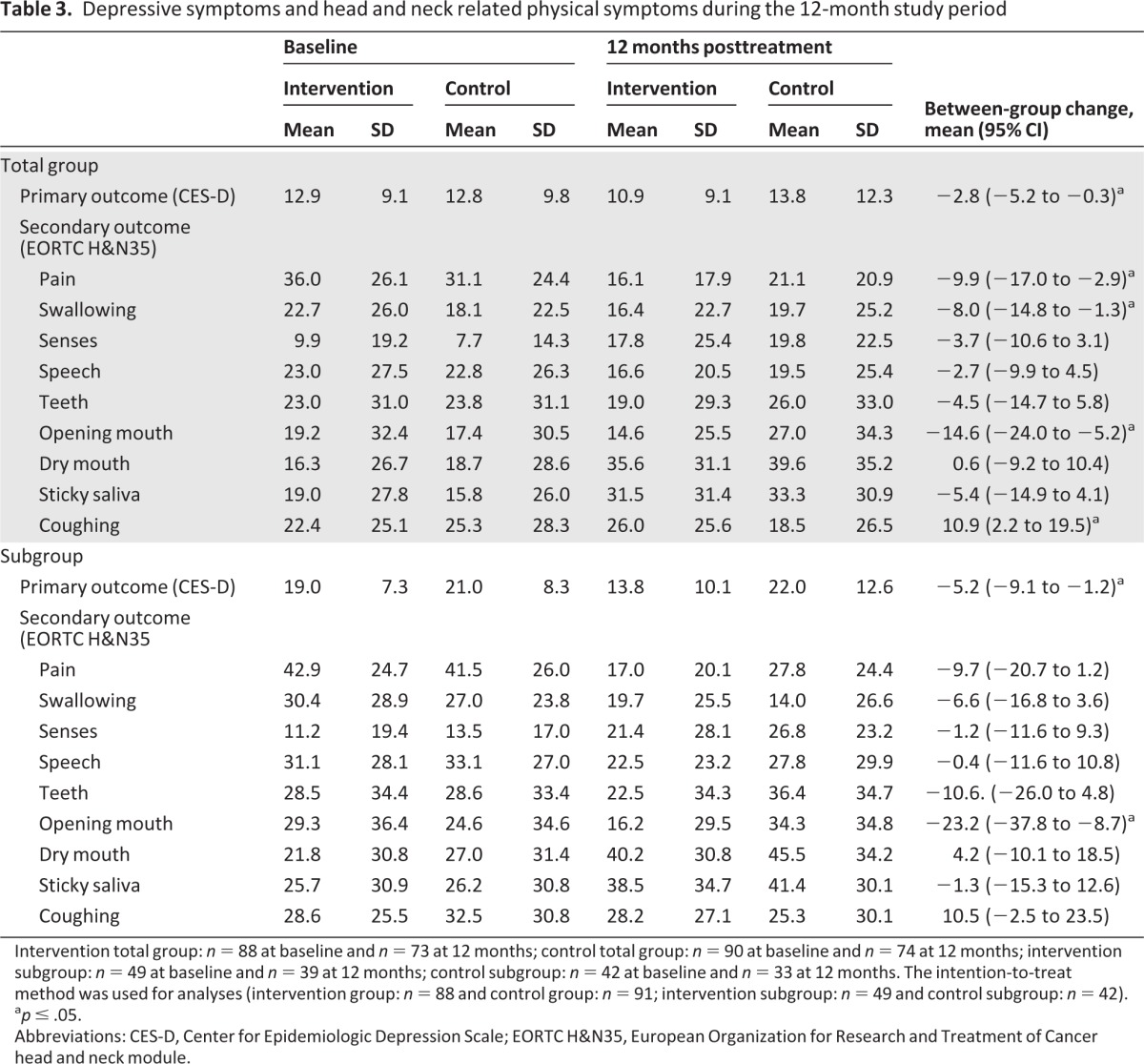
Intervention total group: n = 88 at baseline and n = 73 at 12 months; control total group: n = 90 at baseline and n = 74 at 12 months; intervention subgroup: n = 49 at baseline and n = 39 at 12 months; control subgroup: n = 42 at baseline and n = 33 at 12 months. The intention-to-treat method was used for analyses (intervention group: n = 88 and control group: n = 91; intervention subgroup: n = 49 and control subgroup: n = 42).
ap ≤ .05.
Abbreviations: CES-D, Center for Epidemiologic Depression Scale; EORTC H&N35, European Organization for Research and Treatment of Cancer head and neck module.
Figure 2.
Change in depressive symptoms in the total study sample (intention-to-treat) from baseline to 12 months after completion of cancer treatment. The intervention was started 2 months after completion of cancer treatment. Primary endpoint was the level of depression 12 months after end of HNC treatment. Intervention group: 88 patients; control group: 91 patients. *p < .05.
Abbreviations: BL, baseline; HNC, head and neck cancer.
Figure 3.
Change in depressive symptoms in patients with raised levels of depressive symptoms (intention-to-treat) from baseline to 12 months after completion of cancer treatment. The intervention was started at 2 months. Primary endpoint was the level of depressive symptoms 12 months after end of cancer treatment. Intervention subgroup: 49 patients; control subgroup: 42 patients. *p < .05.
Abbreviations: BL, baseline; HNC, head and neck cancer.
Physical Symptoms
Overall, physical symptoms decreased in the intervention group compared with the control group, with there being significant decreases in pain, swallowing, and opening mouth (p > .05). Symptoms of dry mouth and coughing increased in the intervention group compared with the control group, but the increase was significant for coughing only. Similar results were obtained for the depressive subgroup (CES-D ≥12), but only the decrease in opening mouth was significant. Detailed data for both groups are given in Table 3.
Overall, depressive symptoms were significantly associated with HNC-related symptoms at baseline and 12 months after treatment and with changes from baseline to 12 months after treatment. Correlation coefficients ranged from .18 to .58; only the correlation between changes from baseline to 12 months for dry mouth was not significant (r = 0.024, p = .8). Detailed data are given in supplemental online Table 1.
Discussion
In this RCT, we evaluated the effectiveness of the NUCAI in reducing depressive and HNC-related physical symptoms in patients with HNC. Twelve months after completion of cancer treatment, depressive symptoms were significantly lower in the intervention group than in the control group and physical symptoms generally decreased, showing the effectiveness of the NUCAI.
Our results are in agreement with the findings of a systematic review showing that psychotherapeutic interventions are effective in reducing depressive symptoms in general cancer patients [19]. Williams and Dale [19] suggested that insignificant findings in trials might be due to the inclusion of all patients with cancer instead of only patients with cancer and meaningful levels of depressive symptoms. We found that the nurse-led intervention significantly decreased depressive symptoms in the total group of patients with HNC, but the effect of the intervention was greater in the subgroup of patients with raised levels of depressive symptoms. Depressive symptoms appeared to be related to physical symptoms; that is, patients with more physical problems also had higher levels of depressive symptoms. The change in physical symptoms from baseline to 12 months after treatment was also related to changes in depressive symptoms. The intervention also had beneficial effects on physical symptoms, such as pain, swallowing, and opening mouth. Unexpectedly, symptoms coughing were higher in the intervention group. The intervention was carried out as intended and the patients were, if indicated, referred to other psychological healthcare professionals. However, few patients actually saw a psychologist or psychiatrist.
Four studies have reported beneficial effects in patients with HNC [29–32]. Allison et al. [29] used a small group intervention, which consisted of individual contact with a therapist and materials for use at home. Fifty patients with HNC were treated in two or three sessions of 2 hours over a period of 4 weeks. Semple et al. [30] used a combined intervention in a quasi-experimental study involving 54 patients with HNC. The intervention consisted of two to six sessions of 90 minutes given by a clinical nurse specialist at the patient's home. Both studies reported a decrease in depressive symptoms with the intervention but had methodological shortcomings, such as a nonrandomized and small sample and/or lack of control group.
The third study was a RCT showing that individualized psychological counseling significantly decreased depressive tendency in patients with HNC with raised scores on the Personality Traits Inventory (PTI; measures dominance, emotional instability, depressive tendency) [31]. In total, 47 patients received pre- and postoperative psychological counseling. If they still had a high PTI score 3 months postoperatively, they received another 12 sessions of individualized psychological counseling provided by a clinical psychologist over 6 months. However, the sample size was relatively small, depressive tendency was measured instead of symptoms, and the study took place in India, which makes it difficult to compare and generalize the findings.
The fourth study, a pilot RCT (n=35), reported that early implementation of a cognitive-behavioral therapy program had a beneficial effect, compared with a supportive counseling intervention, on depressive symptoms in newly diagnosed and distressed patients with HNC [32]. The intervention comprised six weekly 90-minute sessions given by a clinical psychologist during radiotherapy and one session 4 weeks after completion of radiotherapy. The improvement in the distress status was sustained for 12 months. However, the study only included patients aged 18 to 70 years and the dropout rate was relatively high (study results were based on intention-to-treat analyses). The latter two interventions [31, 32] were intensive with more than 12 and 7 sessions, respectively, which were led by a clinical psychologist. Our intervention was less intensive with five or six sessions of 45 minutes and led by experienced nurses. Our intervention would be expected to be less expensive than a more intensive 12-session intervention led by a clinical psychologist.
A review of psychosocial interventions for anxiety and depression in patients with cancer also emphasized the importance of cheap interventions requiring few professional resources [16]. In addition, nurses are already involved in patient care and do not have the stigma attached to them that a mental health provider might have. Hence, combining the intervention with the medical check-up is efficient in terms of patient time and might reduce noncompliance. Because the intervention is dependent on the time schedule of the physician, there were occasionally longer than intended intervals between the counseling sessions. Therefore, not all participants received the same number of counseling sessions; however, there were no significant differences in the decrease in depressive symptom levels between the participants who received different numbers of counseling sessions (data not shown). It could be argued that a nurse-initiated review, not in combination with the medical follow-up, might be beneficial for patients with more severe depressive symptoms, who could then be seen at shorter intervals, as determined by the nurse. This remains to be investigated.
Given that only two patients dropped out of the intervention group and that the overall dropout rate of 27% was mainly due to death, we can conclude the intervention is feasible for patients who need or want counseling after cancer treatment. The dropout rate in the control group was similar. The characteristics of the patients in our sample at baseline were comparable to those of other HNC samples (mean age: 60 years, 74%–78% men, 70%–77% living together [29, 30, 57], oral cavity as main tumor site [48%], and a baseline CES-D score of 12.4 [57]), which supports the generalizability of the results to other Dutch HNC samples. Further strengths of the study were its randomized controlled design with intention-to-treat analyses; the supervised, standardized, theory-based, and nurse-led intervention; and the regular screening for depressive symptoms using a validated instrument. In addition, the participants did not know the nature of the aftercare they would receive, which reduced the possibility of contamination.
Conclusion
This RCT showed that the NUCAI is feasible and effective for reducing depressive symptoms of patients with HNC, particularly for patients with raised levels of depressive symptoms. In addition, HNC-specific physical symptoms decreased. The results of this study need to be confirmed in future studies so that the NUCAI can be implemented in daily clinical practice.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This research was funded by a grant from the Dutch Cancer Society. We thank H. den Duyn, K. van Schothorst, and C. Winter for providing the intervention.
Author Contributions
Conception/Design: Wynand J.G. Ros, Miriam Oosterom, Gert-Jan Hordijk, Ron Koole, J. Rob. J. de Leeuw
Provision of study material or patients: Wynand J.G. Ros, Miriam Oosterom, Gert-Jan Hordijk, Ron Koole, J. Rob. J. de Leeuw
Collection and/or assembly of data: Wynand J.G. Ros, Miriam Oosterom
Data analysis and interpretation: Ingeborg C. van der Meulen, Anne M. May, Wynand J.G. Ros, Miriam Oosterom, Gert-Jan Hordijk, Ron Koole, J. Rob. J. de Leeuw
Manuscript writing: Ingeborg C. van der Meulen, Anne M. May, Wynand J.G. Ros, Miriam Oosterom, Gert-Jan Hordijk, Ron Koole, J. Rob. J. de Leeuw
Final approval of manuscript: Ingeborg C. van der Meulen, Anne M. May, Wynand J.G. Ros, Miriam Oosterom, Gert-Jan Hordijk, Ron Koole, J. Rob. J. de Leeuw
Disclosures
The authors reported no financial relationships.
References
- 1.de Graeff A, de Leeuw JRJ, Ros WJG, et al. Long-term quality of life of patients with head and neck cancer. Laryngoscope. 2000;110:98–106. doi: 10.1097/00005537-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Hammerlid E, Silander E, Hornestam L, et al. Health-related quality of life three years after diagnosis of head and neck cancer–A longitudinal study. Head Neck. 2001;23:113–125. doi: 10.1002/1097-0347(200102)23:2<113::aid-hed1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Speksnijder CM, van der Bilt A, Abbink JH, et al. Mastication in patients treated for malignancies in tongue and/or floor of mouth: A 1-year prospective study. Head Neck. 2011;33:1013–1020. doi: 10.1002/hed.21573. [DOI] [PubMed] [Google Scholar]
- 4.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 5.Archer J, Hutchison I, Korszun A. Mood and malignancy: Head and neck cancer and depression. J Oral Pathol Med. 2008;37:255–270. doi: 10.1111/j.1600-0714.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 6.Rogers SN, Miller RD, Ali K, et al. Patients' perceived health status following primary surgery for oral and oropharyngeal cancer. Int J Oral Maxillofac Surg. 2006;35:913–919. doi: 10.1016/j.ijom.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 7.de Leeuw JR, de Graeff A, Ros WJ, et al. Prediction of depressive symptomatology after treatment of head and neck cancer: the influence of pre-treatment physical and depressive symptoms, coping, and social support. Head Neck. 2000;22:799–807. doi: 10.1002/1097-0347(200012)22:8<799::aid-hed9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.de Leeuw JR, de Graeff A, Ros WJ, et al. Prediction of depression 6 months to 3 years after treatment of head and neck cancer. Head Neck. 2001;23:892–898. doi: 10.1002/hed.1129. [DOI] [PubMed] [Google Scholar]
- 9.Petruson KM, Silander EM, Hammerlid EB. Effects of psychosocial intervention on quality of life in patients with head and neck cancer. Head Neck. 2003;25:576–584. doi: 10.1002/hed.10243. [DOI] [PubMed] [Google Scholar]
- 10.D'Antonio LL, Long SA, Zimmerman GJ, et al. Relationship between quality of life and depression in patients with head and neck cancer. Laryngoscope. 1998;108:806–811. doi: 10.1097/00005537-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Duffy SA, Terrell JE, Valenstein M, et al. Effect of smoking, alcohol, and depression on the quality of life of head and neck cancer patients. Gen Hosp Psychiatry. 2002;24:140–147. doi: 10.1016/s0163-8343(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 12.de Graeff A, de Leeuw JR, Ros WJ, et al. Pretreatment factors predicting quality of life after treatment for head and neck cancer. Head Neck. 2000;22:398–407. doi: 10.1002/1097-0347(200007)22:4<398::aid-hed14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Ronis DL, Duffy SA, Fowler KE, et al. Changes in quality of life over 1 year in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134:241–248. doi: 10.1001/archoto.2007.43. [DOI] [PubMed] [Google Scholar]
- 14.Humphris GM, Rogers S, McNally D, et al. Fear of recurrence and possible cases of anxiety and depression in orofacial cancer patients. Int J Oral Maxillofac Surg. 2003;32:486–491. [PubMed] [Google Scholar]
- 15.Llewellyn CD, Weinman J, McGurk M, et al. Can we predict which head and neck cancer survivors develop fears of recurrence? J Psychosom Res. 2008;65:525–532. doi: 10.1016/j.jpsychores.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen PB, Jim HS. Psychosocial Interventions for anxiety and depression in adult cancer patients: Achievements and challenges. CA: A Cancer Journal for Clinicians. 2008;58:214–230. doi: 10.3322/CA.2008.0003. [DOI] [PubMed] [Google Scholar]
- 17.Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: Meta-analyses. Int J Psychiatry Med. 2006;36:13–34. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L. [DOI] [PubMed] [Google Scholar]
- 18.Rodin G, Lloyd N, Katz M, et al. The treatment of depression in cancer patients: A systematic review. Support Care Cancer. 2007;15:123–136. doi: 10.1007/s00520-006-0145-3. [DOI] [PubMed] [Google Scholar]
- 19.Williams S, Dale J. The effectiveness of treatment for depression/depressive symptoms in adults with cancer: A systematic review. Br J Cancer. 2006;94:372–390. doi: 10.1038/sj.bjc.6602949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lurati C, Riva M, Resega R, et al. A mono-institutional prospective study on the effectiveness of a specialist psychotherapeutic intervention (POI) started at the diagnosis of cancer. Support Care Cancer. 2012;20:475–481. doi: 10.1007/s00520-011-1096-x. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RW, Quinlivan JA. Preventing anxiety and depression in gynaecological cancer: A randomised controlled trial. BJOG. 2002;109:386–394. doi: 10.1111/j.1471-0528.2002.01271.x. [DOI] [PubMed] [Google Scholar]
- 22.Rawl SM, Given BA, Given CW, et al. Intervention to improve psychological functioning for newly diagnosed patients with cancer. Oncol Nurs Forum. 2002;29:967–975. doi: 10.1188/02.ONF.967-975. [DOI] [PubMed] [Google Scholar]
- 23.McLachlan S, Allenby A, Matthews J, et al. Randomized trial of coordinated psychosocial interventions based on patient self-assessments versus standard care to improve the psychosocial functioning of patients with cancer. J Clin Oncol. 2001;19:4117–4125. doi: 10.1200/JCO.2001.19.21.4117. [DOI] [PubMed] [Google Scholar]
- 24.Winzelberg AJ, Classen C, Alpers GW, et al. Evaluation of an internet support group for women with primary breast cancer. Cancer. 2003;97:1164–1173. doi: 10.1002/cncr.11174. [DOI] [PubMed] [Google Scholar]
- 25.Weber BA, Roberts BL, Resnick M, et al. The effect of dyadic intervention on self-efficacy, social support, and depression for men with prostate cancer. Psychooncology. 2004;13:47–60. doi: 10.1002/pon.718. [DOI] [PubMed] [Google Scholar]
- 26.Porter LS, Keefe FJ, Garst J, et al. Caregiver-assisted coping skills training for lung cancer: Results of a randomized clinical trial. J Pain Symptom Manage. 2011;41:1–13. doi: 10.1016/j.jpainsymman.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharpe M, Strong V, Allen K, et al. Management of major depression in outpatients attending a cancer centre: A preliminary evaluation of a multicomponent cancer nurse-delivered intervention. Br J Cancer. 2004;90:310–313. doi: 10.1038/sj.bjc.6601546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): A randomised trial. Lancet. 2008;372:40–48. doi: 10.1016/S0140-6736(08)60991-5. [DOI] [PubMed] [Google Scholar]
- 29.Allison PJ, Nicolau B, Edgar L, et al. Teaching head and neck cancer patients coping strategies: Results of a feasibility study. Oral Oncol. 2004;40:538–544. doi: 10.1016/j.oraloncology.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Semple CJ, Dunwoody L, Kernohan WG, et al. Development and evaluation of a problem-focused psychosocial intervention for patients with head and neck cancer. Support Care Cancer. 2009;17:379–388. doi: 10.1007/s00520-008-0480-7. [DOI] [PubMed] [Google Scholar]
- 31.Sharma D, Nagarkar AN, Jindal P, et al. Personality changes and the role of counseling in the rehabilitation of patients with laryngeal cancer. Ear Nose Throat J. 2008:3–3. [PubMed] [Google Scholar]
- 32.Kangas M, Milross C, Taylor A, et al. A pilot randomized controlled trial of a brief early intervention for reducing posttraumatic stress disorder, anxiety and depressive symptoms in newly diagnosed head and neck cancer patients. Psychooncology. 2013 doi: 10.1002/pon.3208. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Sheard T, Maguire P. The effect of psychological interventions on anxiety and depression in cancer patients: Results of two meta-analyses. Br J Cancer. 1999;80:1770–1780. doi: 10.1038/sj.bjc.6690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Söllner W, Maislinger S, König A, et al. Providing psychosocial support for breast cancer patients based on screening for distress within a consultation-liaison service. Psychooncology. 2004;13:893–897. doi: 10.1002/pon.867. [DOI] [PubMed] [Google Scholar]
- 35.Fulcher CD, Badger T, Gunter AK, et al. Putting evidence into practice: Interventions for depression. Clin J Oncol Nurs. 2008;12:131–140. doi: 10.1188/08.CJON.131-140. [DOI] [PubMed] [Google Scholar]
- 36.Badger T, Segrin C, Meek P, et al. Telephone interpersonal counseling with women with breast cancer: Symptom management and quality of life. Oncol Nurs Forum. 2005;32:273–279. doi: 10.1188/05.ONF.273-279. [DOI] [PubMed] [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 38.Spinhoven P, Ormel J, Sloekers PP, et al. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363–370. doi: 10.1017/s0033291796004382. [DOI] [PubMed] [Google Scholar]
- 39.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 40.Vereniging Van Integrale Kankercentra. Landelijke Oncologische Verpleegkundige Richtlijnen (National Oncological Nursing Guidelines) Leens, The Netherlands: Grafische Industrie De Marne; 2000. [Google Scholar]
- 41.Dutch Association of Comprehensive Cancer Centers. Oncoline, Cancer Clinical Practice Guidelines. [Accessed 2004]. Available at http://www.oncoline.nl/index.php.
- 42.McCloskey J, Bulechek GM. Nursing Interventions Classification (NIC) Philadelphia, PA: Elsevier; 2004. [PubMed] [Google Scholar]
- 43.Humphris G, Ozakinci G. The AFTER intervention: A structured psychological approach to reduce fears of recurrence in patients with head and neck cancer. Br J Health Psychol. 2008;13:223–230. doi: 10.1348/135910708X283751. [DOI] [PubMed] [Google Scholar]
- 44.Leventhal H, Halm E, Horowitz C, et al. Living with chronic illness: A contextualized, self-regulation approach. In: Sutton S, Johnston M, Baum A, editors. The Sage Handbook of Health Psychology. Thousand Oaks, CA: Sage Publications; 2005. pp. 197–240. [Google Scholar]
- 45.Hanewald GJFP. Een onderzoek naar de betrouwbaarheid en de validiteit. Amsterdam, The Netherlands: Vakgroep Klinische Psychologie, Universiteit Van Amsterdam; 1992. CES-D: De Nederlandse versie. [Google Scholar]
- 46.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 47.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 48.Devins GM, Orme CM, Costello CG, et al. Measuring depressive symptoms in illness populations: Psychometric properties of the Center for Epidemiologic Studies Depression (CES-D) scale. Psychol Health. 1988;2:139–156. [Google Scholar]
- 49.Beeber LS, Shea J, McCorkle R. The Center for Epidemiologic Studies Depression Scale as a measure of depressive symptoms in newly diagnosed patients. J Psychosoc Oncol. 1998;16:1–20. [Google Scholar]
- 50.Pasacreta JV. Depressive phenomena, physical symptom distress, and functional status among women with breast cancer. Nurs Res. 1997;46:214–221. doi: 10.1097/00006199-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 51.de Leeuw JR, de Graeff A, Ros WJ, et al. Negative and positive influences of social support on depression in patients with head and neck cancer: A prospective study. Psychooncology. 2000;9:20–28. doi: 10.1002/(sici)1099-1611(200001/02)9:1<20::aid-pon425>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 52.Bouma J, Ranchor AV, Sanderman R, et al. Het meten van symptomen van depressie met de CES-D: Een handleiding. Rijksuniverstiteit Groningen, Nederland: Noordelijk Centrum voor Gezondheidsvraagstukken; 1995. [Google Scholar]
- 53.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 54.Bjordal K, de Graeff A, Fayers PM, et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module in head and neck patients. Eur J Cancer. 2000;36:1796–1807. doi: 10.1016/s0959-8049(00)00186-6. [DOI] [PubMed] [Google Scholar]
- 55.de Graeff A, de Leeuw RJ, Ros WJ, et al. A prospective study on quality of life of laryngeal cancer patients treated with radiotherapy. Head Neck. 1999;21:291–296. doi: 10.1002/(sici)1097-0347(199907)21:4<291::aid-hed1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 56.Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. Vol. 202. Cambridge, UK: Cambridge University Press; 2003. Chapter 10: Missing data in longitudinal studies. [Google Scholar]
- 57.de Graeff A, de Leeuw JRJ, Ros WJG, et al. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37:332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.