Abstract
The goal of the present study was to examine how social subordination stress and 5HTT polymorphisms affect the development of brain serotonin (5HT) systems during the pubertal transition in female rhesus monkeys. We also examined associations with developmental changes in emotional reactivity in response to a standardized behavioral test, the Human Intruder (HI). Our findings provide the first longitudinal evidence of developmental increases in 5HT1A receptor and 5HTT binding in the brain of female primates from pre- to peripuberty. The increase in 5HT1A BPND in these socially housed female rhesus monkeys is a robust finding, occurring across all groups, regardless of social status or 5HTT genotype, and occurring in left and right hemispheres of all prefrontal regions studied, as well as amygdala, hippocampus, hypothalamus, and raphe nuclei. 5HTT BPND also showed an increase with age in raphe, anterior cingulate cortex, and dorsolateral prefrontal cortex. These changes in brain 5HT systems take place as females establish more adult-like patterns of social behavior, as well as during the HI paradigm. Indeed, the main developmental changes in behavior during the HI (increase in freezing and decrease in submission/appeasement) were related to neurodevelopmental increases in 5HT1A receptors and 5HTT, because the associations between these behaviors and 5HT endpoints emerge at peripuberty. We detected an effect of social status on 5HT1A BPND in the hypothalamus and on 5HTT BPND in the orbitofrontal cortex, with subordinates showing higher BPND than dominants in both cases during the pubertal transition. No main effects of 5HTT genotype were observed for 5HT1A or 5HTT BPND. Our findings indicate that adolescence in female rhesus monkeys is a period of central 5HT reorganization, partly influenced by exposure to the social stress of subordination, that likely functions to integrate adrenal and gonadal systems and shape the behavioral response to emotionally challenging social situations.
Keywords: social stress, serotonin transporter polymorphisms, nonhuman primates, [18F]FEmZIENT, p-[18F]MPPF
1. Introduction
Exposure to chronic stress, whether physical or psychosocial, adversely affects many aspects of health (McEwen, 2008; Juster et al., 2010) by repeated or sustained activation of cortico-limbic circuits that trigger sympathetic and limbic-hypothalamic-pituitary-adrenal (LHPA) responses (Herman et al., 2003; Choi et al., 2008; Jankord and Herman, 2008; Ulrich-Lai and Herman, 2009). Chronic-stress related adverse health outcomes include cardiovascular, immune, reproductive, and psychiatric illness (McEwen, 1998), such as anxiety disorders and depression. The brain serotonin (5HT) neurotransmitter system is an important modulator of mood, emotional reactivity and social behaviors via actions at corticolimbic circuits (Owens and Nemeroff, 1994; RL Sanchez et al., 2010), and the serotonin transporter (5HTT) is a critical regulator of serotonergic transmission, as it removes 5HT from the synapse (Persico, 2010). 5HTT is encoded by the gene SLC6A4, and the short “s” allele in the polymorphic region of the promoter (5HTTLPR) is associated with lower transcriptional activity in vitro than the long “l” allele in humans (Lesch et al., 1996). Individuals carrying one or two salleles show increased vulnerability to stress-induced psychopathology, including anxiety and major depression (Caspi et al., 2003; Nordquist and Oreland, 2010; Lesch, 2011). Further support for a role of brain 5HT systems in psychopathology is provided by evidence of alterations in presynaptic binding to 5HTT (Ichise et al., 2006; Cannon et al., 2007; Murrough et al., 2011) and postsynaptic binding to the 5HT1A receptor (Drevets et al., 2007; Law et al., 2008; Spinelli et al., 2010) in adult humans with depressive disorders and in juvenile nonhuman primates exposed to early adversity.
The risk of stress-related disorders, such as anxiety or major depression, is nearly twice as high for women as for men (Kessler et al., 1993), and the increased incidence of mood disorders emerges during adolescence in girls (Nolen-Hoeksema and Girgus, 1994; Forbes et al., 2004). Pubertal status rather than age may be the critical variable (Zahn-Waxler et al., 2000), as stress-induced mood disorders increase in girls coincident with the appearance of secondary sexual characteristics (Angold et al., 1999; Ge et al., 2001). Importantly, the adverse consequences of stress during puberty can be attenuated by a supportive social environment (Born et al., 2002; Masten, 2004) such that for most girls adjustment problems are transient, being resolved by early adulthood (Walker et al., 2004). However, continued exposure to psychosocial stressors may place adolescent females on a high-risk trajectory for further emotional problems and emergence of psychopathology in adulthood (Pine et al., 1998).
A key question is why puberty is a vulnerable period for the emergence of stress-related mood disorders (Steiner et al., 2003). Estradiol (E2) is known to facilitate central 5HT activity (Bethea et al., 2002) and improves mood in some women (McEwen, 2001), so it seems counterintuitive that puberty onset is associated with an increase in vulnerability to mood disorders for girls. However, data from a number of models suggest that a changing E2 milieu may indeed affect measures of emotionality, such as acoustic startle in adult humans (Swerdlow et al., 1997) and animals (Vaillancourt et al., 2002) and negative affect in girls (Buchanan et al., 1992). Epidemiological evidence of a temporal relation between puberty and emergence of mood disorders in at-risk girls has not identified a clear role for E2 (Zahn-Waxler et al., 2000), and it is not understood how E2 affects LHPA regulation in girls exposed to social stressors (Young and Altemus, 2004), suggesting that pubertal changes are complex and potentially modulated by additional biological signals including brain neurochemical signals and social factors. Thus, emotional dysregulation that occurs coincident with puberty in girls may not be due to developmental increases in of E2 per se but rather the result of an increased exposure to socially stressful situations that occur as a consequence of the activational effects of E2 on prosocial behaviors. Given this, a main question that remains unanswered, and the main focus of this paper, is whether pubertal changes in female emotional behavior are predicted by developmental changes in central 5HT activity, and modulated by 5HTT polymorphisms and social status.
The socially-housed rhesus monkey (Macaca mulatta) is an excellent animal model to examine the effects of social stress and 5HTT genetic variation on maturation of the brain 5HT system and behavior in primates. The brain distribution of 5HTT and 5HT-1 and 5HT-2 receptors (Lidow et al., 1989) and 5HT axon terminals (MA Wilson and Molliver, 1991), as well as the neurodevelopmental patterns of monoamine neurotransmitters and their receptors (Goldman-Rakic and Brown, 1982; Lidow et al., 1991) have been characterized, and many aspects of neuroanatomy and neurochemistry are comparable between rhesus monkeys and humans (Christian et al., 2009). The rhesus monkey also has polymorphisms in the 5HTTLPR (rh5HTTLPR) that are structurally and functionally orthologous to those in humans (Lesch et al., 1997), and confer similar risk for stress-mediated pathophysiology and psychopathology (Bethea et al., 2004; Michopoulos et al., 2009; Lesch, 2011). In addition, the macaque social structure is organized by a linear dominance hierarchy that functions to maintain group stability although it is enforced by more dominant animals aggressing subordinates (Bernstein and Gordon, 1974; Bernstein et al., 1974). As a result of this continual harassment and low social status, lower-ranking animals are exposed to unpredictable, repeated social stress and have less control over their social and physical environment (Silk, 2002). Thus, social subordination in adult female macaques is a well-established model to study the adverse health effects of psychosocial stress exposure, including cardiovascular disease, neuroendocrine and reproductive dysfunction and immune compromise (Adams et al., 1985; Gust et al., 1991; Kaplan et al., 1996; Morgan et al., 2002; ME Wilson et al., 2008; Michopoulos et al., 2009; Paiardini et al., 2009).
The goal of the present study was to determine how social subordination and 5HTT genotype affect the development of brain 5HTT and 5HT1A receptors during the pubertal transition in female rhesus monkeys and their associations with developmental changes in emotional reactivity in response to the standardized Human Intruder (HI) test (Kalin and Shelton, 1989). We predicted that developmental exposure to social subordination stress would result in differences in central 5HT activity prior to puberty, which would be magnified during the pubertal transition, particularly in cortico-limbic regions involved in emotional regulation (such as the prefrontal cortex, amygdala and hippocampus) and in 5HT-synthesizing neurons in the raphe nuclei. Based on findings of reduced brain 5HTT binding density in these regions associated with stress-related disorders (Owens and Nemeroff, 1994; Malison et al., 1998), and in other nonhuman primate models of early life stress (Ichise et al., 2006), we hypothesized that subordinate female macaques, particularly those with the s allele, would exhibit reduced 5HTT binding potential. We also predicted that 5HT1A receptor binding would be reduced in subordinates, as stress-induced elevations in glucocorticoids reduce 5HT1A receptor binding in limbic regions such as hippocampus (Fernandes et al., 1997; Sargent et al., 2000).
2. Experimental Procedures
2.1. Subjects
Subjects were twenty-five female juvenile rhesus monkeys living since birth with their mothers and siblings in four complex social groups at the Yerkes National Primate Research Center (YNPRC) Field Station (Lawrenceville, GA) in outdoor, half-acre compounds with an attached indoor area. Each social group consisted of 60-80 animals organized in 7 to 10 matrilines with multiple related adult females and their juvenile offspring as well as two to four adult males. Subjects were reared by their mothers in these social groups where they remained for the duration of this study, which did not impose any experimental intervention to the social structure/environment. At the beginning of the study, subjects ranged from 10-16 months of age, about 14 months prior to puberty onset (defined as menarche, which for this cohort occurred at 29.92±0.62 mo (range: 25.9 to 36.7 mo), consistent with previous reports in rhesus monkeys housed outdoors (ME Wilson et al., 1988)). Animals were fed standard low-fat, high-fiber monkey chow (Purina Mills International, Lab Diets, #5038, St. Louis MO) twice daily, ad libitum, and had continuous access to water and a daily supplement of fresh fruit and vegetables. The Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Guide for Use of Laboratory Animals and the Animal Welfare Act.
Social organization in rhesus monkey groups is defined by linear dominance hierarchy, with matrilines ranking over one another (Bernstein et al., 1974), and offspring assuming the relative rank of their mothers. In this study, each subject’s relative dominance rank within the natal group was determined based on outcomes of dyadic agonistic interactions, with the subordinate animal defined as the one who exhibits unequivocal submissive behavior towards another animal. These interactions were recorded as a part of formal behavioral observations collected every two months from the average age of 14.24±0.24 until 36.04±0.21 mo as well as during group checks conducted multiple times each week by research staff. At each formal sampling interval, two 30-minute focal observations (Altmann and Altmann, 1977) were collected on each subject in the format of actor-behavior-recipient, using an established ethogram (Altmann, 1962) to obtain rates of aggressive and submissive behavior directed to and received from group members. For the present analysis, agonistic behavior involving non-family members was used, as this reflects more consequential interactions than with kin (Bernstein and Ehardt, 1985). Each female’s relative rank, determined from these formal observations and periodic group checks, was calculated as the ratio of her rank to the total number of animals in her group, excluding animals <12 mo, such that a female with a rank of 25 out of a group of 100 would have a relative rank of 0.25. For the present analysis, those ranked in the upper 30% (.01-.30) were considered dominant (n=11), and those in the lower 70% were considered subordinate (n=14). Of these 14 subordinate females, five ranked between 0.50 and 0.59 while nine ranked lower than 0.66.
Prior to the start of the study, all subjects were genotyped for polymorphisms in SLC6A4, as described previously (Hoffman et al., 2007). Of the 25 females, 12 (6 dominant & 6 subordinate) were homozygous for the long promoter variant (l/l) and 13 (5 dominant & 8 subordinate) had at least one allele of the short promoter variant (s-variant). 5HTT l/s and s/s genotypes (s-variants) were combined based on the low occurrence of the s/s genotype and previous reports that the l/s genotype produces a phenotype similar to that of the s/s genotype (Champoux et al., 2002).
2.2. Positron Emission Tomography (PET) Radiochemistry
The radiotracers 4-(2′-Methoxyphenyl)-1-[2′-(N-2″-pyridinyl)-p[18F]fluorobenzamido]ethylpiperazine (p-[18F]MPPF), a fluoro analog of WAY-100635 (Le Bars et al., 1998; Kumar and Mann, 2007), and [18F]-[2 -carbo-(2-fluoroethoxy)-3 -(3′-((Z)-2-iodoethenyl)phenyl)nortropane], ([18F]FEmZIENT) (Stehouwer et al., 2008) were synthesized in the YNPRC Radiochemistry Laboratory, with a radiochemical purity over 99%. p-[18F]MPPF and [18F]FEmZIENT were used to assess the binding potentials of brain 5HT1A receptor and 5HTT, respectively. Binding potential is defined here as BPND, the ratio at equilibrium of specifically bound radioligand to that of nondisplaceable radioligand in tissue, a measurement often used to correlate receptor or transporter density with experimental conditions (Innis et al., 2007).
2.3. PET Image Acquisition, Post-processing and Analysis
Each subject received two PET scans, separated by at least 1 month, to assess 5HT1A receptor BPND and 5HTT BPND at prepuberty (average ages: 19.37±0.59 and 21.32±0.60 mo, respectively), and peripuberty (average ages: 34.73±0.65 mo and 32.88±0.53 mo, respectively). This timeframe encompassed the transition from a prepubertal period through menarche (29.92±0.62 mo) and occurrence of first ovulation (33.67±0.92 mo) for these females (ME Wilson et al., in press). Beginning at menarche, each female’s blood was sampled twice weekly to assay progesterone levels in order to document her first ovulation (defined as a luteal phase increase in serum progesterone), and subsequent ovulations as well as pregnancy. This information was included in the analyses to rule out effects of “reproductive stage” on BPND at peripuberty (age 2). At the time of the peripubertal PET scans, all but two females (both subordinate, one l/l and one s-variant), had reached menarche, and all but six (one dominant l/l, three subordinate l/l, and two subordinate s-variant females) had attained first ovulation.
PET scans were collected on a Focus 220 microPET scanner (Siemens, Concorde Microsystems, Knoxville, TN; 26 cm transaxial field of view (FOV), 8 cm axial FOV; 2.1 mm isotropic reconstructed resolution) located at the YNPRC Imaging Center (see exceptions below). Subjects were transported from their groups to the Imaging Center the day before the scans. Because this temporary social separation and transport constitutes a potential stressor, all procedures were standardized across subjects and in a manner to minimize stress, to control for potential group effects. Animals were prepped with an i.v. catheter for radioligand infusion and administration of hydration fluids, and scanned supine, with head positioned at standardized coordinates and secured using a soft elastic wrap. Scans were performed under isoflurane anesthesia (1.2%), and subjects received a five-minute pump infusion of ~4 mCi (1 Bq = 2.703 × 10−11 Ci) of p-[18F]MPPF or [18F]FEmZIENT. Specific activity at infusion time was greater than 0.15 Ci/μmol for p-[18F]MPPF and greater than 0.5 Ci/μmol for [18F]FEmZIENT in all cases. Anesthesia, heart rate, blood oxygenation levels and respiration were monitored by veterinarian staff throughout the scanning period.
After an initial transmission scan was performed using a Co-57 point source, listmode emission data were acquired for 120 min. The data were rebinned into 21 dynamic frames ranging in duration from 30 sec to 10 min. The attenuation image was reconstructed, segmented into air, tissue (water), and bone and then the segments were replaced with the appropriate 511 keV attenuation coefficients. Images were reconstructed with the manufacturer-supplied FastMAP algorithm and included corrections for attenuation, deadtime, and detector normalization. Each subject’s PET images were summed across frames and then manually (rigid-body) registered to her age-appropriate (pre- or peripuberty) T1-weighted MRI (Figs. 1A and 3A) using in-house routines written in the IDL language, as described below. Regions of interest (ROIs) were manually drawn on the MRI image and then propagated to the PET images. Time-activity curves (TACs) were generated and BPND calculated by Logan analysis using the cerebellum as reference region (Logan et al., 1990; Saigal et al., 2006; Yasuno et al., 2006).
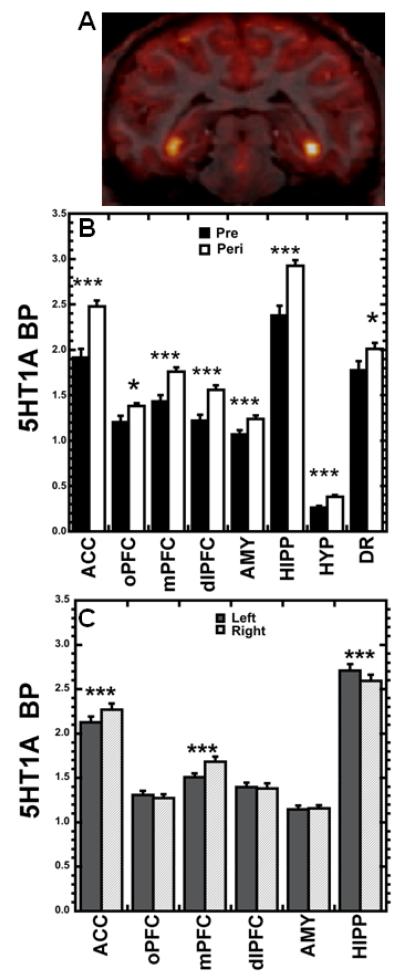
Figure 1.
(A) Representative example of 5HT1A receptor binding: co-registration of PET and T1-MRI images. (B) 5HT1A BPND age effects: increases occurred from pre- to peripuberty in prefrontal regions (ACC, oPFC, mPFC, dlPFC), temporal regions (AMY, HIPP), and HYP and DR. (C) 5HT1A BPND hemisphere effects: left differed significantly from right in ACC, mPFC, and HIPP. *p<0.05, ***p<0.001, RM-ANOVA.
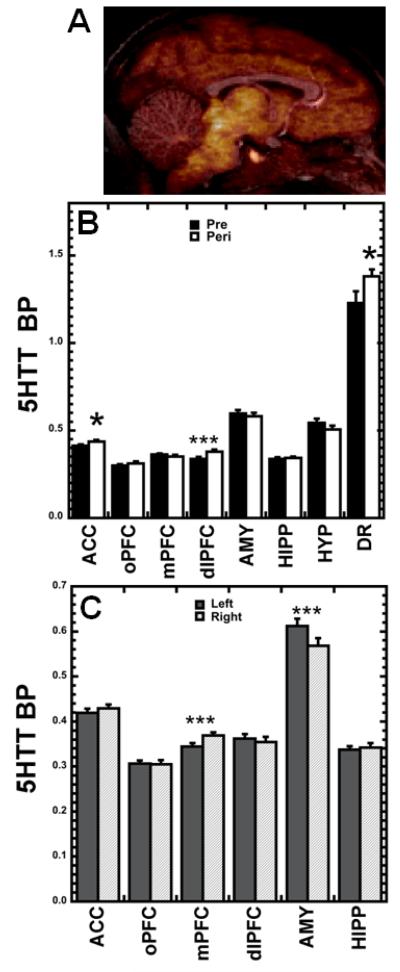
Figure 3.
(A) Representative example of 5HTT binding: co-registration of PET and T1-MRI images. (B) 5HTT BPND age effects: increases occurred with age in ACC, dlPFC, and DR, but not in other ROIs. (C) 5HTT BPND hemispheric effects: left differed significantly from right in mPFC and AMY. *p<0.05, ***p<0.001, RM-ANOVA.
During this longitudinal study, a Siemens P4 microPET scanner (Siemens, Concorde Microsystems, Knoxville, TN; Cherry et al., 1997) was set up at the YNPRC Field Station. The last 25% of the 5HT1A BPND data (the peripubertal scans from 12 out of 25 subjects) were collected on this system using identical scanning procedures by the same personnel, and balancing experimental groups for status and genotype. A MANOVA performed to evaluate scanner effect on 5HT1A BPND data revealed a significant effect of age (F8,15=10.898, p=.001), and age × scanner interaction (F8,15=4.186, p=.008), indicating higher BPND estimation on the P4 microPET scanner than on the Focus 220. Test-retest studies in 6 additional adult animals confirmed instrumentation bias. In order to control for this bias, the peripubertal 5HT1A data were normalized by 1) calculating a z-distribution separately for the data from each scanner, and 2) converting the mean and variation of the P4 dataset to the Focus 220 distribution. Thus, scanner effect was controlled for by normalizing the smaller subset of data collected on the P4 to the distribution of the larger dataset collected on the Focus 220.
2.4. MRI Image Acquisition & Template
Magnetic resonance imaging (MRI) scans were acquired from all subjects at prepuberty (average age: 21.38±0.95 mo) and peripuberty (31.19±0.83) for co-registration to PET scans and tracing of the Regions of Interest (ROI) in the structural MRI images. Scans were acquired at the YNPRC Imaging Center using a 3T Siemens scanner, an 8-channel phase array knee coil and a T1-weighted MPRAGE sequence (TI/TR/TE=950/3000/3.49ms, FOV=96 mm, 8 averages) with a 0.5×0.5×0.5mm3 voxel size, under anesthesia (1-1.5% isoflurane, inhalation to effect). Each subject’s MRI images were reconstructed into a 3D volume and registered (rigid-body) to a custom-made rhesus monkey MRI template created from 16 animals in our colony using non-linear registration algorithms (FSL software, FMRIB Oxford, UK), as previously described (Parr et al., 2012). The template was aligned to the Wisconsin 112RM-SL rhesus T1 atlas (McLaren et al., 2009; McLaren et al., 2010), to allow drawing the ROIs in Saleem and Logothetis macaque brain atlas space (Saleem and Logothetis, 2007).
2.5. ROI Tracing Procedures and Anatomical Definitions
An ROI analysis was used to examine group differences in brain regions known to be affected by early stress (Damsa et al., 2008), following landmarks and procedures previously published in rhesus by our group (Parr et al., 2012). Briefly, ROI-drawing was guided by macaque brain atlases (Paxinos et al., 2000; Schmahmann and Pandya, 2006; Saleem and Logothetis, 2007) and traced in coronal and sagittal views in the brain structural MRI images, after alignment into atlas space (Saleem and Logothetis, 2007) by a rater blind to experimental groups. ROIs were traced following established anatomical landmarks in macaques and humans (Rosene and Van Hoesen, 1987; Bertolino et al., 1997; MM Sanchez et al., 1998; Giedd et al., 1999; Mathew et al., 2003; McCormick et al., 2005; Machado and Bachevalier, 2007; Spinelli et al., 2009; Payne et al., 2010; Parr et al., 2012), and included amygdala (AMY), medial and dorsolateral prefrontal cortex (mPFC and dlPFC), anterior cingulate cortex (ACC), orbitofrontal cortex (oPFC), hippocampus (HIPP), and hypothalamus (HYP) and the raphe nuclei. All of these regions are involved in emotional regulation and stress response and are strongly regulated by the serotonin system (Lundberg et al., 2007; Ulrich-Lai and Herman, 2009). They have also been the focus of previous studies examining the effects of stress on the nonhuman primate brain 5HT system using PET neuroimaging (Ichise et al., 2006; Shively et al., 2006; Spinelli et al., 2010). Cerebellum (CER) was included as reference region (Yasuno et al., 2006). When applicable, right and left ROIs were traced separately. The midbrain raphe nuclei (DR) were also included in the analyses due to the high density of somatodendritic 5HT1A receptors working as autoreceptors and 5HTT that regulate 5HT neurotransmission (Stockmeier, 2003). The raphe nuclei are not easily identified on the MR images so ROIs of this structure were drawn with the aid of the PET data. The ROI was manually drawn on a summed image of the PET data to include all pixels that had values greater than 50% of the peak value.
2.6. Behavioral Data Assessment
Each subject’s behavioral reactivity was also tested in response to the Human Intruder (HI) paradigm (Kalin and Shelton, 1989), a standardized test of emotional reactivity, during prepuberty (18.45±0.09 mo), and again during peripuberty (27.32±0.09 mo), for analysis of associations with neuroimaging data. The HI paradigm evokes strong and distinct behavioral responses to novel and threatening stimuli in rhesus monkeys and has been used to examine the role of limbic regions such as AMY and oPFC on emotional regulation (Kalin et al., 2007; Machado et al., 2009) as well as the effects of 5HTT genotype (Bethea et al., 2004). The HI paradigm consists of three consecutive ten-minute conditions: (1) an alone (AL) condition that elicits distress vocalizations and locomotion; (2) a profile (PR) condition where an unfamiliar experimenter enters the testing room and presents his/her facial profile towards the monkey, who typically stops locomoting/vocalizing and freezes while scanning the intruder; and a stare (ST) condition, during which the experimenter faces the animal and makes continuous eye contact with it, which is a threatening behavior for rhesus macaques that induces aggressive and submissive behaviors towards the intruder. Sessions were videotaped and scored for frequencies and durations of behaviors following previously published ethogram and procedures (Machado and Bachevalier, 2006) , with an inter-rater reliability >92%. For analyses of associations with PET data, we selected a set of most salient behaviors (freezing, coo vocalizations, threat, anxiety-like, and lipsmack) reflective of emotional reactivity to the threatening conditions (PR and ST), based on the well-established patterns of defensive behaviors elicited by the different HI conditions (Kalin and Shelton, 1989; Kalin et al., 1991; Kalin and Shelton, 1998), and excluding behaviors with low occurrence across subjects.
2.7. Statistical Analysis
5HT1A receptor and 5HTT PET binding potential data were analyzed with PASW Statistics v18.0 RM-ANOVA, using Status (Dominant vs. Subordinate) and 5HTT genotype (l/l vs. s-variant) as fixed factors, and Age (prepuberty vs. peripuberty) and brain hemisphere (Left vs. Right) as repeated factors. Fisher’s LSD tests were conducted for post-hoc comparisons. For analyses yielding significant effects of status and 5HTT genotype, multiple regression analyses were run adding age at first ovulation to the model to test its potential contribution to the effects on the dependent variable BPND at peripuberty. To evaluate the potential effect of ovarian hormones (i.e. menstrual cycle phase) on the peripubertal scans, we conducted RM-ANOVAs of 5HT1A BP and 5HTT BP with subject’s “reproductive stage” as fixed factor (based on progesterone levels, there were 5 potential categories: prepubertal, follicular phase, luteal phase, pregnant, or post-pubertal but seasonally suppressed) and hemisphere as within-subject factor. RM-ANOVA was also used to test whether rates of group agonistic behavior varied by age, status and genotype. Stepwise multiple regression models were used with a female’s relative rank, 5HTT genotype status (0=s-variant, 1=l/l) and BPND in each ROI (hemispheres averaged) as predictors of each behavior from the HI test (freeze during PR, and freeze, coo, threat, anxiety, and lipsmack during ST) for prepuberty and peripuberty. These analyses were done for each year and each ligand separately. In addition, for those HI behaviors that changed significantly from pre- to peripuberty, models were used to predict the change using relative rank, genotype, and the change in ROI BPND. Level of statistical significance was set at p<0.05 for all analyses.
3. Results
3.1. 5HT1A receptor BPND
5HT1A receptor BPND increased significantly with age in every ROI we examined by univariate RM-ANOVA (Fig. 1B). Main hemisphere effects were also detected (Fig. 1C), such that 5HT1A receptor BPND was greater in the right hemisphere for ACC (F1,21=30.501, p<.001) and mPFC (F1,21=37.042, p<.001) and greater in the left hemisphere for HIPP (F1,21=22.518, p<.001). HIPP also showed a significant age × hemisphere interaction (F1,21=9.855, p=.005), with greater 5HT1A BPND in left hemisphere than right at peripuberty (p<.001, Fisher’s LSD).
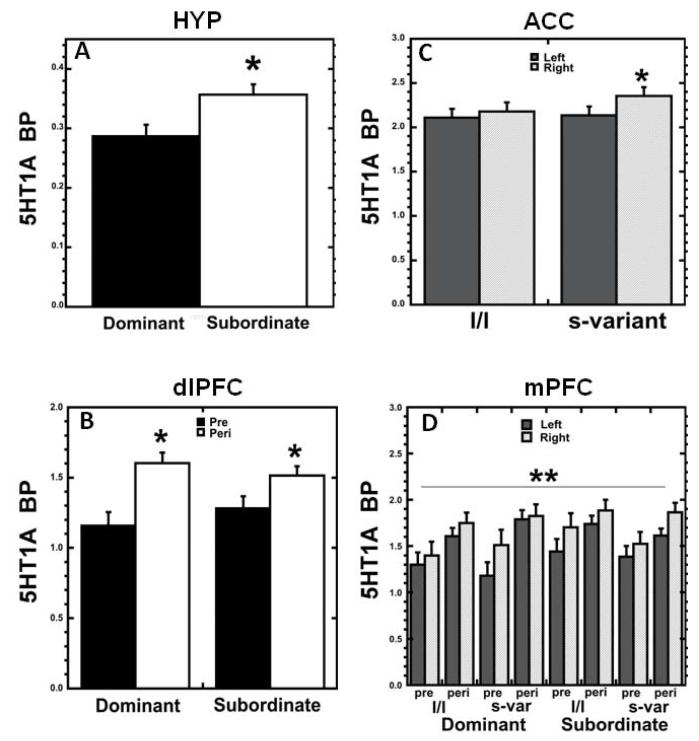
A main effect of social status was observed in HYP, with subordinate females exhibiting higher 5HT1A BPND than dominants independent of age (F1,21=7.544, p=.012; Fig. 2A). Follow-up multiple regression analysis indicated that age at first ovulation did not significantly predict peripubertal HYP 5HT1A BPND (p>.05), or modify the significant effect of status on 5HT1A BPND (p=.022). A status × age interaction was detected in the dlPFC (F1,21=4.647, p=.043; Fig. 2B) and although post-hoc comparison of means did not yield statistically significant differences, visual inspection of the data suggests that dominant females show a greater increase in binding with age than subordinates.
Figure 2.
(A) 5HT1A BPND status effect in HYP: subordinates showed higher BPND than dominant animals. (B) 5HT1A BPND showed a significant status × age interaction in dlPFC. (C) 5HT1A BPND in ACC showed a 5HTT genotype × hemisphere interaction: s-variants had greater right hemisphere 5HT1A BPND than l/l individuals. (D) 5HT1A BPND in mPFC showed a complex status × 5HTT genotype × age × hemisphere interaction. *p<0.05,**p<0.01, RM-ANOVA.
A 5HTT genotype × hemisphere interaction was detected in the ACC (F1,21=8.044, p=.010; Fig. 2C), with l/l subjects showing similar 5HT1A BPND in both hemispheres (p=.073, Fisher’s LSD), while s-variant subjects showed greater BPND in the right hemisphere (p<.001, Fisher’s LSD). The mPFC showed a complex interaction of age × hemisphere × status × genotype (F1,21=10.684, p=.004; Fig. 2D). No other status or 5HTT genotype effects were detected in the ROIs. Follow-up RM-ANOVA to evaluate the potential effects of reproductive stage at peripuberty on 5HT1A BP showed no main effect of this factor (reproductive stage categories based on progesterone levels at age 2: prepubertal (n=5 females), pregnant (n=7), postpubertal but seasonally suppressed (n=13)) or interaction with hemisphere (p>.05).
3.2. 5HTT BPND
Unlike the consistent increase with age in BPND identified for the 5HT1A receptor, transporter BPND increased with age in only three ROIs (ACC: F1,21=8.113, p=.010; dlPFC: F1,21=18.147, p<.001; DR: F1,21=5.013, p=.036; Fig. 3A) and remained unchanged in others, as shown by RM-ANOVA. Main effects of hemisphere were also detected (Fig. 3B), such that 5HTT BPND was lower in left mPFC than in right (F1,21=36.011, p<.001), showing the same pattern of asymmetry as the receptor in this prefrontal region. In contrast, left AMY 5HTT BPND was greater than right (F1,21=26.596, p<.001).
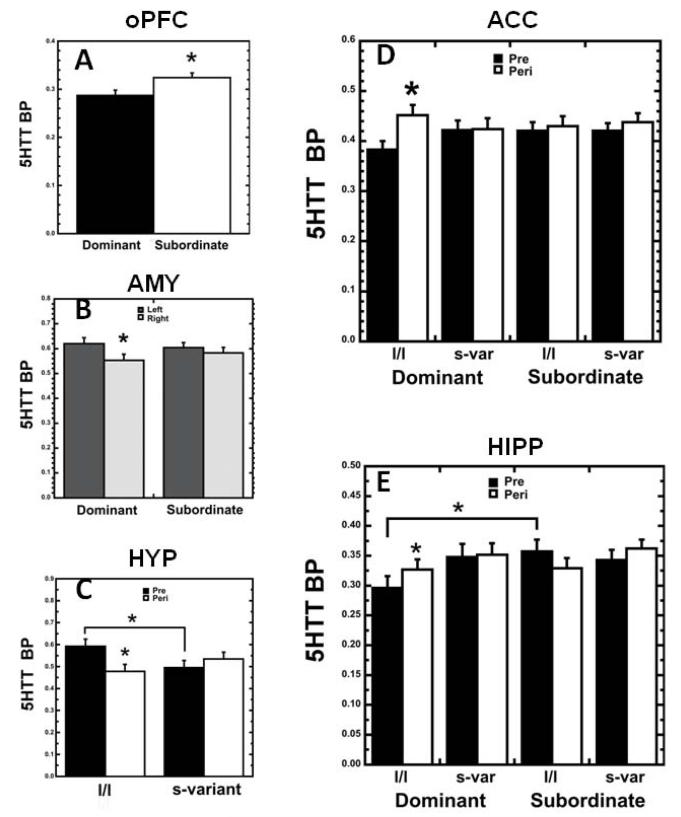
Subordinate females had higher 5HTT BPND in oPFC than dominant females (F1,21=6.049, p=.023; Fig. 4A), the only main effect of status detected for 5HTT BPND. Follow-up multiple regression analysis indicated that age at first ovulation did not significantly predict peripubertal oPFC 5HTT BPND (p>.05). A status × hemisphere interaction was detected in the AMY (F1,21=7.078, p=.015; Fig. 4B), with dominant, but not subordinate females, showing significant asymmetry (left hemisphere greater than right) in 5HTT BPND (p<.001, Fisher’s LSD).
Figure 4.
(A) 5HTT BPND status effect: subordinates showed higher levels of 5HTT BPND in oPFC than dominants. (B) 5HTT BPND in AMY showed a status × hemisphere interaction: dominants, but not subordinates, showed left greater than right hemisphere BPND. (C) 5HTT BPND in HYP showed a 5HTT genotype × age interaction: BPND was higher for l/l than s-variant prepubertal animals, and decreased with age for l/l monkeys. (D) 5HTT BPND in ACC showed a status × 5HTT genotype × age interaction effect: dominant l/l monkeys showed increase with age in BPND while other subgroups did not. (E) 5HTT BPND in HIPP showed a status × 5HTT genotype × age interaction. *p<0.05, RM-ANOVA.
A significant status × 5HTT genotype × age interaction was found in ACC (F1,21=4.758, p=.041; Fig. 4D), where dominant l/l monkeys showed an increase with age in 5HTT BPND (p<.001, Fisher’s LSD) while other groups did not. A similar three-way interaction was seen in the HIPP (F1,21=6.458, p=.019; Fig 4E), where dominant l/l monkeys showed lower 5HTT BPND than subordinate l/l monkeys during prepuberty (p=0.045, Fisher’s LSD), and dominant l/l’s BPND increased from prepuberty to peripuberty (p=0.046, Fisher’s LSD) while other groups’ BPND did not. Finally, a genotype × age interaction was detected in HYP (F1,21=8.932, p=.007; Fig. 4C), such that 5HTT BPND was higher in l/l than s-variant prepubertal animals (p=0.049, Fisher’s LSD), and decreased with age in l/l’s (p=.005, Fisher’s LSD), but not in s-variants. No other status or 5HTT genotype effects were detected in the ROIs. A follow-up RM-ANOVA to evaluate the potential effect of reproductive stage at peripuberty on 5HTT BP (categories based on progesterone levels at age 2: prepubertal (n=5 females), follicular (n=4), luteal (n=7), pregnant (n=5), or postpubertal but seasonally suppressed (n=4)) showed no main effect of reproductive stage for any ROI, and only a reproductive stage × hemisphere interaction for AMY (p=.03). The interaction appears due to lower BP in the left compared to the right hemisphere in pregnant females in contrast to the opposite direction in the four other groups and higher BP in the right amygdala in post-pubertal seasonally suppressed females compared to others.
3.3. Behavioral Context for Social Subordination Model of Chronic Stress
Over the 20-mo study period, agonistic behavior of the juvenile females involving non-family members remained stable (p>0.05) except the frequency of submissive behavior received from others in the group was higher at peripuberty than in prepuberty (F1,21=4.75, p=0.041). Significant main effects of status on rates of agonistic behavior were observed independent of age (Table 1), as dominant females were more aggressive (F1,21=4.12, p=0.05) and received significantly more submissions from others (F1,21=5.53, p=0.028) whereas subordinates directed more submissive behaviors towards others (F1,21=6.57, p=0.018). Rates of aggression received during these group tests did not vary by status (F1,21=0.82, p=0.385). Agonistic behavior was not influenced by genotype or status by genotype interaction (data not shown, p>0.05).
Table 1.
Mean ± sem rates or durations of agonistic behavior per half-hour from the social group observations for dominant and subordinate females.
| Behavior | Dom | Sub | P |
|---|---|---|---|
|
Aggression
actor |
2.75 ± .88 | .36 ±.78 | 0.05 |
|
Aggression
recipient |
1.63 ± .48 | 1.04 ± .43 | 0.385 |
|
Submission
actor |
1.85 ± .45 | 3.41 ± .40 | 0.018 |
|
Submission
recipient |
1.32 ± .25 | .52 ±.23 | 0.028 |
3.4. Multiple regression models of behavioral changes with age in HI test context
Table 2 shows the results of the multiple regression analyses using relative rank, 5HTT genotype, and 5HT1A and 5HTT BPND as predictors of change in HI test behaviors at both ages. Only models that yielded significant results are shown. Two behaviors changed significantly from pre- to peripuberty, both during ST (the most threatening condition): freezing duration increased, and lip-smacking (appeasement) decreased. Table 3 provides descriptive statistics, by dominance status, for the behaviors significantly predicted by rank, genotype or BP in Table 2: lipsmacking, freezing and threat behavior during ST.
Table 2.
Multiple stepwise regression models using relative dominance rank (0.01 – 0.99, with higher number indicating lower status), 5HTT genotype (0 = s-variant, 1 = l/l), and binding potential for 5HT1A receptor and 5HTT in each ROI (hemispheres combined) as predictors for behaviors from the Human Intruder profile (PR) and stare (ST) conditions in prepuberty (Age 1) and peripuberty (Age 2). Also shown are models predicting the change in behavior from pre- to peripuberty, using relative rank, genotype, and the change with age in binding potential in each ROI (anterior cingulate cortex, ACC; medial prefrontal cortex, mPFC; orbital prefrontal cortex, oPFC; dorsolateral prefrontal cortex, dlPFC; amygdala, AMY; hippocampus, HIPP; hypothalamus, HYP; dorsal raphe nuclei, DR). B is the unstandardized beta coefficient and SE is the standard error of this coefficient; ß is the standardized coefficient; R2 is the measure of variability in each outcome accounted for by the predictors; and the p-value is the probability that the model would be obtained by chance. Models not yielding significant results are not shown.
| Behavior | Factor | B | SEB | ß | R2 | p-value |
|---|---|---|---|---|---|---|
| Freeze PR Age 2 | HYP 5HTT mPFC 5HTT DR 5HT1A |
17.809 −30.245 −2.924 |
5.042 11.579 1.4 |
0.624 −0.475 −0.334 |
0.48 | 0.003 |
| Freeze ST Age 1 | Relative Rank | −2.74 | 1.267 | −0.422 | 0.18 | 0.045 |
| Freeze ST Age 2 | Relative Ranks oPFC 5HTT |
−5.889 −28.074 |
2.015 10.641 |
−0.465 −0.419 |
0.47 | 0.001 |
| Increase in Freeze ST Age 1 to 2 | ACC 5HTT Relative Rank |
−41.799 5.649 |
12.185 2.058 |
−0.617 0.493 |
0.42 | 0.004 |
| Threat ST Age 1 | Relative Rank | 130.401 | 60.904 | 0.423 | 0.18 | 0.044 |
| Lipsmack ST Age 1 | Relative Rank dlPFC 5HTT |
11.363 32.425 |
2.875 14.962 |
0.618 0.339 |
0.51 | 0.001 |
| LipsmackST Age 2 | oPFC 5HT1A | −15.192 | 5.426 | −0.504 | 0.25 | 0.01 |
| Decrease in Lipsmack ST Age 1 to 2 | 5HTT Genotype | −5.129 | 2.311 | −0.436 | 0.19 | 0.038 |
Table 3.
Mean ± sem durations and frequencies of freezing, lipsmack and threat behaviors during the stare condition of the Human Intruder test at prepuberty (Age 1) and peripuberty (Age 2) for dominant and subordinate females.
| Dom | Sub | |
|---|---|---|
| Freeze ST | ||
| Age 1 | 1.70 ± .52 | .64 ± .45 |
| Age 2 | 5.04 ± 1.04 | 1.74 ± .89 |
| Lipsmack ST | ||
| Age 1 | 3.58 ± 1.27 | 10.18 ± 1.10 |
| Age 2 | 1.88 ± 1.65 | 4.20 + 1.43 |
| Threat ST | ||
| Age 1 | 77.92 ± 26.71 | 150.73 ± 23.02 |
| Age 2 | 53.83 ± 24.89 | 90.96 ± 21.45 |
The duration of freezing during PR at peripuberty was significantly predicted by higher transporter BPND in HYP, lower transporter BPND in mPFC, and lower 5HT1A BPND in the DR. No predictors emerged for freezing during PR in prepuberty. In contrast, relative rank emerged as a significant predictor of the duration of freezing during ST. At prepuberty, longer durations of freezing were predicted by higher dominance rank. This relation with rank held up at peripuberty but greater 5HTT BPND in oPFC also significantly contributed to the duration of freezing during ST at that later age. As noted above, the duration of freezing during ST increased from pre- to peripuberty. A greater increase was predicted by higher dominance status and a smaller increase in 5HTT BPND in ACC.
Threat during ST was also predicted by rank only at prepuberty, with more subordinate females showing more threat behavior (Table 3). No predictors emerged for threat frequency during PR in peripuberty or for the change from pre- to peripuberty. Predictors for appeasement behavior (lipsmack) during ST showed a developmental difference. At prepuberty, more subordination and higher 5HTT BPND in dlPFC predicted more lipsmack. However, at peripuberty, greater appeasement was predicted by lower 5HT1A BPND in oPFC. When the decrease in appeasement with age was evaluated, a bigger decrease was predicted by the 5HTT s-variant genotype.
4. Discussion
This study provides the first longitudinal evidence of a developmental increase in 5HT1A receptor binding across several brain regions during the female rhesus monkey pubertal transition. The increase in 5HT1A BPND in these socially housed females is a robust finding, occurring across all groups, regardless of social status or 5HTT genotype, and occurring in left and right hemispheres of all prefrontal regions studied, as well as amygdala, hippocampus, hypothalamus, and raphe nuclei. 5HTT BPND also showed an increase with age in raphe, ACC, and dlPFC. These changes in brain 5HT systems take place as rhesus females establish more adult-like patterns of behavior within their natal social group, as well as during the Human Intruder paradigm. Indeed, the main developmental changes in behavior during the HI (increase in freezing and decrease in submission/appeasement) could potentially be related to the neurodevelopmental increases in 5HT1A receptors and 5HTT, because the associations between these behaviors and receptors/transporters emerged at peripuberty. We detected an effect of social status on 5HT1A BPND in the HYP and on 5HTT BPND in the oPFC, with subordinates showing higher BPND than dominants in both cases, during the pubertal transition. No main effects of 5HTT genotype were observed for 5HT1A or 5HTT BPND, though.
The widespread increase of 5HT1A BPND in prefrontal, limbic, and brainstem regions by up to 50% from pre- to peripuberty in female rhesus monkeys was unexpected, because of previous autoradiographic evidence that 5HT receptor systems (among other neurotransmitters) decline gradually in cortical regions from the juvenile to adolescent period (12 versus 36 months) (Lidow et al., 1991). Bar-Peled and colleagues (1991) showed that 5HT1A receptors peak prenatally in humans, with greater density in frontal regions in fetuses than in adults, while Lidow and colleagues (1991) found a postnatal peak in 5HT1 & 5HT2 receptors in rhesus monkeys at 2-4 mo, followed by gradual decline until young adulthood, tapering from 36-60 mo (the last age studied), with adult receptor densities higher than those at birth. However, neither study focused on developmental changes during the pubertal transition, which is captured in our data, nor provided information about the sex of the animals included in the study. Theoretically, the striking age-related increase in 5HT1A BPND detected in our study could reflect a change in receptor density, affinity, or a combination of both (Innis et al., 2007). Using in vitro autoradiography, Lidow and colleagues (1991) observed no change in apparent affinity for 5HT1 & 5HT2 receptors in rhesus from 0-60 mo of age, inferring that the kinetic properties of these receptors are likely established at birth. Evidence supporting that argument includes the adult-like affinity detected for the 5HT1A receptor during the 2nd-trimester of pregnancy in human fetuses (Bar-Peled et al., 1991). Based on this and evidence that the maximum specific binding observable in vivo is more appropriately characterized as the subset of available receptors or transporters (Innis et al., 2007), and can be modulated by factors such as extracellular 5HT levels or receptor internalization, the age-related increases in 5HT1A BPND in the present study would seem to more likely reflect an increase in the number of available receptors (and transporters) throughout the pubertal transition, than changes in their respective affinities. However, a recent report of general brain reduction in 5HT1A receptor density and affinity in male and female monkeys with early adverse experience, imposed by peer-rearing, compared to mother-reared controls at 24 mo of age (Spinelli et al., 2010), suggests potential developmental effects in affinity from birth to the juvenile period. Therefore, because we were not able to measure whether the developmental changes here were due to changes in receptor density or affinity, this remains an important question for further studies.
These age-related increases in 5HT1A receptor and 5HTT BPND occur as rhesus females establish more adult-like patterns of behavior within the social group and during the Human Intruder paradigm. The main developmental changes in behavior during the HI (increase in freezing and decrease in submission/appeasement during the ST condition) are correlated with the age-related increases in 5HT1A receptor and 5HTT BPND, at least based on the emergence of associations between these behaviors and the increased binding potential of these receptors/transporters in specific brain regions at peripuberty, particularly in prefrontal cortex. Similar age-related changes in behavior (reduction in appeasement/lipsmacking) during the HI have been previously reported in male rhesus monkeys (Kalin et al, 1998).
As expected, dominant females exhibited more aggression and subordinate monkeys exhibited more submission, consistent with the behavioral phenotypes reported for this social subordination stress animal model (Bernstein and Ehardt, 1985). Although the rate of aggression received by subordinate females did not reach statistical significance in this small cohort of animals in comparison to the bigger dataset (Bernstein and Gordon, 1974; ME Wilson et al., in press), the unpredictable, continual harassment experienced by subordinate females in these groups is a known chronic stressor that results in elevated cortisol and dysregulation of the LHPA axis (Shively et al., 1997a; Shively et al., 1997b; Jarrell et al., 2007; Kaplan et al., 2010; Howell et al., under review). We observed two main effects of social status on central 5HT measures, both in the opposite direction of our original hypotheses: subordinate females showed higher 5HT1A BPND in HYP, and higher 5HTT BPND in oPFC, than dominants. In the human literature, most studies have found reductions in brain 5HT1A BPND associated with stress-related disorders, in particular, anxiety and depression (Drevets et al., 2007; Hirvonen et al., 2008; Moses-Kolko et al., 2008; Nash et al., 2008; Sullivan et al., 2009), though some studies have also reported no differences or increased BP in comparison to healthy controls (Bailer et al., 2007; Sullivan et al., 2009). Drevets and colleagues (2007) replicated previous findings of reduced 5HT1A BPND in mesiotemporal cortex and raphe in depressed male and female subjects, although, in a review of the field, noted that inconsistencies in the literature may arise from brain region-specific effects or pathophysiological heterogeneity within depressive disorders. Age, sex, and reproductive hormones are also significant factors found to affect 5HT1A BPND (Moller et al., 2007; Raje et al., 2008; Moses-Kolko et al., 2011).
Most non-human primate models of stress, early life adversity, or depression have also reported reduced brain 5HT1A BPND associated with behavioral alterations. Adult female cynomolgus monkeys with depressive-like behavior showed overall lower 5HT1A BPND across AMY, HIPP, cingulate cortex and raphe than controls (Shively et al., 2006). Male and female marmosets exposed as infants to daily parental deprivation showed lower hippocampal 5HT1A mRNA expression and BPND compared to their undeprived siblings (Law et al., 2008). Similarly, using peer-rearing as a model of early adversity, Spinelli and colleagues (2010) found a trend for lower 5HT1A receptor density and significantly lower radioligand affinity overall across all regions studied (AMY, HIPP, ACC, dorsomedial prefrontal cortex and medial cingulate cortex) in peer-reared 24 month-old (male and female) rhesus monkeys compared to their mother-reared counterparts. In contrast to that overall finding, their ROI analysis showed higher 5HT1A BPND in dorsomedial prefrontal cortex of peer- than mother-reared females, but not males, supporting the notion that inconsistencies in the field may relate to factors such as sex and brain regional-specific effects. In comparison to these studies, we found no effect of social status on 5HT1A BPND in prefrontal or limbic regions, and the other studies did not assess binding potential in HYP. Thus, our finding of higher 5HT1A BPND in HYP of subordinate juvenile female rhesus monkeys is a novel observation.
We chose the HYP as an ROI due to the central role of 5HT neurotransmission in this area in regulating both neuroendocrine stress responses and reproductive function. Although the PET spatial resolution does not allow differentiation of the specific HYP nuclei contributing to the differences in binding, both the paraventricular (PVN) and ventromedial hypothalamic (VMH) nuclei are enriched with postsynaptic 5HT1A receptors. Several studies have reported a stimulatory effect of PVN 5HT1A receptor activation on HPA axis, evidenced by elevated cortisol secretion, which can be prevented with Selective Serotonin Reuptake Inhibitors - SSRIs (Nikisch et al., 2005; Navinés et al., 2007; Gómez-Gil et al., 2010). Thus, the higher levels of 5HT1A receptor binding detected in the HYP of subordinate females could potentially underlie the elevated levels of cortisol found in parallel studies with the same cohort of animals (ME Wilson et al., in press). In female macaques, 5HT1A receptor is also very densely expressed in the VMH (Gundlah et al., 1999; Centeno et al., 2007) and although 5HT1A mRNA expression did not differ between stress-sensitive and stress-resilient females in those studies, the VMH’s role in feeding and sexual behavior (Aiello-Zaldivar et al., 1992; Spiteri et al., 2010), suggests that the difference in 5HT1A BPND between dominant and subordinate monkeys in our study could be related to emerging differences in their expression of sexual behavior at peripuberty. Given that 5HT1A receptors in HYP are regulated by ovarian hormones, as one month of estradiol replacement reduces binding sites in HYP of adult ovariectomized macaques (Lu and Bethea, 2002), it is possible that the increased receptor sites in subordinate females are related to their greater incidence of delayed puberty onset (ME Wilson et al., 1986; Zehr et al., 2005) and reproductive compromise in adults (Michopoulos et al., 2009; Kaplan et al., 2010).
Our finding of increased oPFC 5HTT BPND in subordinate females is also interesting, given the bidirectional connections between the oPFC and AMY (Amaral et al., 1992) and its reported role in mediating trait-like unconditioned behavioral responses to fearful stimuli in rhesus monkeys (Kalin et al., 2001). For example, lesions to oPFC in adolescent male monkeys reduced freezing behavior in the HI test without affecting other behaviors (Kalin et al., 2007). Despite the evidence of an important involvement of the oPFC-limbic circuits in the control of emotional reactivity and threat-response, the specific role of 5HT’s action through its receptors and transporters in the primate AMY (Oler et al., 2009), HIPP, and the complex PFC neurocircuitry (Puig and Gulledge, 2011) is not clearly understood, which complicates interpretation of our findings. Mice 5HTT knockout studies have shown increases in extracellular levels of 5HT in the forebrain (including the PFC), associated with a phenotype of increased anxiety (Li, 2010), whereas 5HTT transgenic overexpressing mice result in the opposite phenotype (Holmes, 2008). These effects on emotional regulation are proposed to be modulated by a ventromedial PFC-AMY pathway, and fMRI studies in humans contribute further evidence that PFC-AMY circuitry is critical in emotional regulation (for review, see Hariri and Holmes, 2006). However, findings from 5HTT knockout studies have to be interpreted with caution in the context of current 5HTT binding potential in primates and humans, given the profound structural effects that alterations in extracellular 5HT levels can have during early development (Hariri and Holmes, 2006), as well as important cross-species neuroanatomical differences (Uylings et al., 2003; Smith and Porrino, 2008).
Studies in humans have shown lower 5HTT BPND associated with mood disorders in men and women (Frankle et al., 2005; Murrough et al., 2011), but also, the opposite or more complex associations by brain region and disorder heterogeneity (Cannon et al., 2006; Cannon et al., 2007). Few studies have examined 5HTT BPND levels in rhesus monkeys, and results are similarly mixed. A recent study (Ichise et al., 2006) found reduced 5HTT BPND across DR, thalamus, HYP, basal ganglia, ACC, AMY, and HIPP in peer-reared compared to mother-reared male 3 year-old rhesus monkeys. In the opposite direction, although not directly addressing the functional role of 5HTT in primate PFC, a behavioral and neuroendocrine composite measure of anxious temperament correlated positively with 5HTT BPND in the bed nucleus of the stria terminalis (BNST) and amygdalo-hippocampal region (bilaterally) among adult mother-reared male and female rhesus monkeys (Oler et al., 2009). Similarly, 5HTT availability in the raphe nuclei correlated positively with amount of alcohol intake in adult male rhesus monkeys who had undergone social isolation at 6 mo of age followed by peer-rearing (Heinz et al., 2003). Thus, stress-related psychopathology is not consistently associated with reduced levels of 5HTT BPND in humans or non-human primates, suggesting more complex, region-specific effects.
To summarize the social status effects on 5HT1A receptor and 5HTT binding potential, a few points are noteworthy. Our findings highlight specific associations of social subordination with 5HT function in localized brain regions of juvenile female macaques, in particular, increases in HYP 5HT1A receptors and oPFC 5HTT. These specific effects found during pubertal development, which could be associational and not necessarily due to a causal relationship, contrast with other data reporting long-term brain-wide changes in other nonhuman primate models of stress and adversity (Ichise et al., 2006; Shively et al., 2006; Spinelli et al., 2010). However, this social subordination model, which relies on the natural hierarchy and normal variation of behavior of young female rhesus monkeys in their complex, natal social groups is an ethologically valid model of the psychosocial stressors humans face in daily life, and therefore, not necessarily comparable with the early adversity experienced in other primate models such as peer-rearing. Shively and colleagues (2006) showed a stress-induced, dose-effect of depressive-like behavior in stress-sensitive macaque females in association to reduced 5HT1A BPND. Thus, it is possible that the “dose” of chronic stress to mother-reared, socially-housed subordinate females up to 34 mo of age has not been sufficient to affect brain-wide 5HT neurochemistry and that we would see the development of more striking differences as the animals get older, when a combination of chronic stress, its consequent inhibition of 5HT neural function (Tokuyama et al., 2008; RL Sanchez et al., 2010; Bethea and Reddy, 2012) and potentially reduced exposure to E2 in subordinates could induce a phenotype of maladaptation observable in PET imaging of 5HT function.
The 5HTTLPR short allelic variant has been associated with vulnerabilities to psychopathology and pathophysiology in humans and nonhuman primates (for review, see Nordquist and Oreland, 2010; Lesch, 2011), including HPA dysregulation (McCormack et al., 2009), metabolic and reproductive consequences (Hoffman et al., 2007; Michopoulos et al., 2009; Michopoulos et al., 2012), as well as differences in cognition and brain morphology (Borg et al., 2009; Jedema et al., 2010), and brain metabolism (Kalin et al., 2008). Extracellular levels of 5HT (regulated by functional 5HTT protein) have been the hypothesized mechanism underlying the behavioral and clinical effects of 5HTTLPR allelic variation, based on consistent in vitro results (Jedema et al., 2010). Therefore, 5HT neurotransmission and, consequently, 5HT1A and 5HTT BPND levels might be expected to differ by 5HTT genotype. However, a consensus is now emerging that current brain 5HT1A or 5HTT BPND levels, at least in adults, do not relate to 5HTT genotype (Borg et al., 2009; Christian et al., 2009; Murthy et al., 2010; Nordquist and Oreland, 2010; Bose et al., 2011). In agreement with those reports, the present study found no major effects of rh5HTTLPR genotype on brain 5HT1A or 5HTT BPND. Thus, the present study extends the finding of lack of genotype effects on markers of 5HT neurotransmission from adults to pre- and peripubertal female primates. Studies showing no effects of rh5HTTLPR genotype on peripheral blood mononuclear cells 5HTT expression, though not a brain measure, in rhesus infants (Kinnally et al., 2010) suggests that the direct effects of genotype on 5HT function may occur earlier, probably prenatally. Thus, the evidence of increased vulnerability of s-variant carriers to psychopathology and pathophysiology, without a current correlation with differential brain 5HTT or 5HT1A BPND point toward structural and functional brain changes occurring early in development, due to neurotrophic effects of different brain 5HT levels (Daubert and Condron, 2010; Jedema et al., 2010). This hypothesis is bolstered by evidence from rodent knockout models (5HTT, monoamine oxidase A enzyme that degrades serotonin, 5HT1A receptor) demonstrating clear effects of serotonin homeostasis disruption during early development on lifelong brain structure, function and biobehavioral traits (Nordquist and Oreland, 2010).
As mentioned above, the developmental increases detected in brain 5HT1A receptors and 5HTTs from pre- to peripuberty take place in parallel to changes in behavior of these females. In fact, our regression analyses suggest that the main developmental changes in behavioral reactivity during the HI test (increase in freezing and decrease in submission/appeasement) may be related to increases in the receptors and transporters, because associations between these behaviors and markers of 5HT function emerge mostly at peripuberty. As noted above, 5HTT BPND in the amygdalo-hippocampal region and BNST has been positively associated with a composite measure of anxious temperament (freezing + cortisol + coo) in rhesus monkeys (Oler et al., 2009). Further, lesions to oPFC in adolescent monkeys reduced freezing behavior in the HI test without affecting other behaviors (Kalin et al., 2007). In our study, freezing during PR showed no 5HT associations at prepuberty, but was associated with higher 5HTT BPND in HYP and lower 5HTT mPFC BPND and 5HT1A BPND in DR at peripuberty. Similarly, no relationship between 5HT measures and freezing during the stare condition was found at prepuberty, but at peripuberty lower 5HTT BPND in oPFC significantly predicted longer freezing durations. In addition, developmental changes in levels of 5HTT in another prefrontal region, the ACC, were associated with freezing behavior in ST: blunted developmental increases in ACC 5HTT BPND were associated with higher increases in freezing behavior with age. These relationships with markers of prefrontal 5HT function at peripuberty are interesting as oPFC and mPFC have been implicated in the regulation of emotional behavior (Kalin et al., 2001; Oler et al., 2009), and the ACC implicated in performance monitoring (Jedema et al., 2010) and regulation of impulsive aggression (Frankle et al., 2005). Finally, lipsmacking (appeasement) behavior during ST decreased with age, and while higher dlPFC 5HTT BPND was related to more lipsmacking in prepuberty, at peripuberty appeasement was negatively correlated with oPFC 5HT1A BPND.
Higher social status predicted longer freezing durations during ST and greater increases in this behavior at peripuberty. While such an observation may appear counterintuitive, the longer durations of freezing by more dominant females could reflect more adaptive strategies in response to threat in this group of animals than in the subordinate females, supported by the general increase in this behavior with age. Freezing during the HI, at least during the profile condition, is considered an adaptive behavior to escape detection by a predator (Kalin et al., 2005). In contrast, subordinate status predicted higher submissive behaviors, maybe a generalization of their increased submission rates in the social context, but only during prepuberty; as the females decreased lipsmack with age, social status no longer differentiated females at peripuberty. Similarly, both dominants and subordinates decreased the frequency of threat behavior during ST with age in the HI test (Table 3), and subordinate social status predicted more threat behavior only in prepuberty. The age-related changes in these behaviors are interesting, as the changes suggest the emergence of adaptive behavioral responses to environmental stimuli with maturity.
Limitations are unavoidable, particularly in large-scale, longitudinal, developmental studies of multiple outcome measures. As 5HT1A and 5HTT binding are modified by exposure to estrogen and progesterone (Bethea et al., 2002; Lu et al., 2003), the question of differential hormone exposure during the pubertal transition and reproductive life remains important. Although we did not control for developmental changes in ovarian hormones or menstrual cycle phase at the time of the peripubertal PET scans, we did determine that (1) the timing of first ovulation did not predict differences in 5HT1A receptor or 5HTT binding potential at peripuberty; and (2) based on progesterone levels collected the week before the scan, we were able to establish the “reproductive stage” of the females at the age 2 scans and we detected no main effects of reproductive stage on either 5HT1A BP or 5HTT BP in this study. Despite this important information provided, it has to be acknowledged that the lack of a experimental design at peripuberty where the scans were done at a specific phase of the menstrual cycle, in parallel to the lack of specific estrogen and progesterone levels on the date of the scan represent limitations of this study. The brief social separation and transport prior to scanning constitute likely acute stressors, which could potentially influence rapidly changing components of the serotonin system in the brain (e.g., local 5HT release: Keeney et al., 2006) and our binding potential measures as suggested by evidence that acute stress can lead to 5HT1A and 5HTT expression changes (López et al., 1999; Vollmayr et al., 2000). In order to control for the potential effects of these unavoidable manipulations, all subjects underwent identical procedures (e.g., handling, transport) according to a standardized protocol and efforts were made to minimize stress. Unfortunately, no blood samples were taken at the time of scan, so cortisol data were not available as a covariate in our analyses. However, the potential acute effects of stress should wash out statistically across groups, allowing our measures of 5HT1A and 5HTT BP to reflect group differences in the chronic effects of psychosocial stress experienced by subordinate females. Similarly, isoflurane anesthesia can also cause acute changes (reductions) in extracellular 5HT levels (Whittington and Virág, 2006), and receptor binding (Seeman and Kapur, 2003), at least in rodents. However, anesthesia is an intrinsic limitation when scanning rhesus monkeys, as commonly done in this field (Ichise et al., 2006; Shively et al., 2006; Christian et al., 2009; Oler et al., 2009; Jedema et al., 2010; Spinelli et al., 2010), and was applied in a similar way across all subjects according to a standardized protocol to control for its potential effects.
4.1 Conclusions
This study provides the first longitudinal evidence of developmental increases in 5HT1A receptor and 5HTT binding in the brain of female primates during the pubertal transition. These changes in brain 5HT systems took place as rhesus females established more adult-like patterns of behavior within the social group, as well as during the Human Intruder paradigm. Indeed, the main developmental changes in behavior during the HI (increase in freezing and decrease in submission/appeasement) could be related to the neurodevelopmental increases in 5HT1A receptors and 5HTT, since the associations between these behaviors and receptors/transporters emerge at peripuberty. In addition, the higher 5HT1A BPND detected in the HYP of subordinate females could underlie the neuroendocrine and reproductive alterations previously reported in this model. Though the underlying mechanisms remain to be clarified, increasing evidence points towards reciprocal and interacting effects of the 5HT, hypothalamic-pituitary-adrenal, hypothalamic-pituitary-gonadal, and other systems. In the adolescent period of increased social stress and simultaneous synaptic pruning, these interactions strongly suggest that, in addition to possible prenatal 5HT effects on brain development, adult behavior and brain function may be critically shaped during the pubertal transition, that is in part modified by exposure to social stressors.
Highlights for review.
5HT1A & 5HTT BP increased in female rhesus brain regions through puberty
Hypothalamic 5HT1A receptor binding was increased in subordinate juvenile females
Higher orbitofrontal cortex 5HTT BP was detected in subordinate females
No main effects of 5HTT genotype were observed for 5HT1A or 5HTT BP
Developmental changes in 5HT systems related to different behavioral reactivity
Acknowledgements
We would like to thank Jennifer Whitley, Jodi Godfrey, Marta Checchi, Shannon Bounar, Erin Olmoguez and the entire staff of the Yerkes National Primate Research Center Field Station for their invaluable help in collecting the behavioral data presented here. The project described was supported by Grant Numbers MH079100, F31 MH086203 (BRH) and F31 MH085445 (VM) from the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, NICHD or the National Institutes of Health. The project was also funded by the National Center for Research Resources P51RR165 (YNPRC Base grant) and is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132. The YNPRC is fully accredited by the American for the Assessment and Accreditation of Laboratory Care, International.
Abbreviations
- 5HT
5-hydroxytryptamine, serotonin
- 5HT1A
serotonin 1A (receptor)
- 5HTT
serotonin transporter
- 5HTTLPR
serotonin transporter-linked polymorphic region
- ACC
anterior cingulate cortex
- AL
alone condition of HI test
- AMY
amygdala
- BNST
bed nucleus of the stria terminalis
- BPND
binding potential (ratio at equilibrium of specifically bound radioligand to that of nondisplaceable radioligand in tissue)
- CER
cerebellum
- dlPFC
dorsolateral prefrontal cortex
- DR
dorsal raphe (nuclei)
- E2
estradiol
- [18F]FEmZIENT
[18F]-[2 -carbo-(2-fluoroethoxy)-3 -(3′-((Z)-2-iodoethenyl)phenyl)nortropane]
- HI
Human Intruder (paradigm)
- HIPP
hippocampus
- HYP
hypothalamus
- i.v.
intravenous
- LHPA
limbic-hypothalamo-pituitary-adrenal
- l/l
long promoter variant
- mPFC
medial prefrontal cortex
- MRI
magnetic resonance image
- oPFC
orbital prefrontal cortex
- p-[18F]MPPF
4-(2′-Methoxyphenyl)-1-[2′-(N-2″-pyridinyl)-p[18F]fluorobenzamido]ethylpiperazine
- Peri
peripuberty
- PET
positron emission tomography
- PR
profile condition of HI test
- Pre
prepuberty
- PVN
paraventricular nucleus (of the hypothalamus)
- rh5HTTLPR
rhesus serotonin transporter-linked polymorphic region
- ROI
region of interest
- ST
stare condition of the HI test
- s-variant
short promoter variant
- VMH
ventromedial hypothalamic (nucleus)
- YNPRC
Yerkes National Primate Research Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Drs. Embree, Michopoulos, Votaw, Voll, Mun, Stehouwer, Goodman, Wilson, and Sanchez declare no actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three (3) years of beginning the work submitted that could inappropriately influence (bias) their work.
References
- Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol Behav. 1985;35:935–940. doi: 10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Aiello-Zaldivar M, Luine V, Frankfurt M. 5,7-DHT facilitated lordosis: effects of 5-HT agonists. Neuroreport. 1992;3:542–544. doi: 10.1097/00001756-199206000-00024. [DOI] [PubMed] [Google Scholar]
- Altmann SA. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Annals of the New York Academy of Sciences. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Altmann SA, Altmann J. On the analysis of rates of behaviour. Animal Behaviour. 1977;25(Part 2):364–372. doi: 10.1016/0003-3472(77)90011-2. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The amygdala. Wiley; New York: 1992. pp. 1–67. [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Mathis CA, Wagner A, Thornton L, Hoge J, Ziolko SK, Becker CR, McConaha CW, Kaye WH. Exaggerated 5-HT1A but Normal 5-HT2A Receptor Activity in Individuals Ill with Anorexia Nervosa. Biological Psychiatry. 2007;61:1090–1099. doi: 10.1016/j.biopsych.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Bar-Peled O, Gross-Isseroff R, Ben-Hur H, Hoskins I, Groner Y, Biegon A. Fetal human brain exhibits a prenatal peak in the density of serotonin 5-HT1A receptors. Neuroscience Letters. 1991;127:173–176. doi: 10.1016/0304-3940(91)90787-t. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Ehardt CL. Age-sex differences in the expression of agonistic behavior in rhesus monkey (Macaca mulatta) groups. Journal of Comparative Psychology. 1985;99:115–132. [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci. 1974;62:304–311. [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatologica. 1974;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Saunders RC, Mattay VS, Bachevalier J, Frank JA, Weinberger DR. Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporo-limbic lesions: a proton magnetic resonance spectroscopic imaging study. Cereb Cortex. 1997;7:740–748. doi: 10.1093/cercor/7.8.740. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse Actions of Ovarian Steroids in the Serotonin Neural System. Frontiers in Neuroendocrinology. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. Effect of ovarian steroids on gene expression related to synapse assembly in serotonin neurons of macaques. Journal of Neuroscience Research n/a-n/a. 2012 doi: 10.1002/jnr.23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious Behavior and Fenfluramine-Induced Prolactin Secretion in Young Rhesus Macaques with Different Alleles of the Serotonin Reuptake Transporter Polymorphism (5HTTLPR) Behav Genet. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Borg J, Henningsson S, Saijo T, Inoue M, Bah J, Westberg L, Lundberg J, Jovanovic H, Andree B, Nordstrom A-L, Halldin C, Eriksson E, Farde L. Serotonin transporter genotype is associated with cognitive performance but not regional 5-HT1A receptor binding in humans. The International Journal of Neuropsychopharmacology. 2009;12:783–792. doi: 10.1017/S1461145708009759. [DOI] [PubMed] [Google Scholar]
- Born L, Shea A, Steiner M. The roots of depression in adolescent girls: is menarche the key? Curr Psychiatry Rep. 2002;4:449–460. doi: 10.1007/s11920-002-0073-y. [DOI] [PubMed] [Google Scholar]
- Bose SK, Mehta MA, Selvaraj S, Howes OD, Hinz R, Rabiner EA, Grasby PM, Turkheimer FE, Murthy V. Presynaptic 5-HT1A is related to 5-HTT receptor density in the human brain. Neuropsychopharmacology. 2011;36:2258–2265. doi: 10.1038/npp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones? Evidence for activational effects of hormones on moods and behavior at adolescence. Psychological Bulletin. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Fromm SJ, Nugent AC, Rollis D, Gandhi SK, Klaver JM, Charney DS, Manji HK, Drevets WC. Serotonin Transporter Binding in Bipolar Disorder Assessed using [11C]DASB and Positron Emission Tomography. Biological Psychiatry. 2006;60:207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated Serotonin Transporter Binding in Major Depressive Disorder Assessed Using Positron Emission Tomography and [11C]DASB; Comparison with Bipolar Disorder. Biological Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science. 2003;301:386. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Centeno M-L, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic expression of serotonin 1A, 2A and 2C receptor and GAD67 mRNA in female cynomolgus monkeys with different sensitivity to stress. Brain Research. 2007;1142:1–12. doi: 10.1016/j.brainres.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley J, Lesch K, Suomi S. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Cherry SR, Shao Y, Silverman RW, Meadors K, Siegel S, Chatziioannou A, Young JW, Jones W, Moyers JC, Newport D, Boutefnouchet A, Farquhar TH, Andreaco M, Paulus MJ, Binkley DM, Nutt R, Phelps ME. MicroPET: a high resolution PET scanner for imaging small animals. Nuclear Science, IEEE Transactions on. 1997;44:1161–1166. [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MMN, Ostrander MM, Herman JP. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008;33:659–669. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian BT, Fox AS, Oler JA, Vandehey NT, Murali D, Rogers J, Oakes TR, Shelton SE, Davidson RJ, Kalin NH. Serotonin transporter binding and genotype in the nonhuman primate brain using [C-11]DASB PET. NeuroImage. 2009;47:1230–1236. doi: 10.1016/j.neuroimage.2009.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Current Opinion in Psychiatry. 2008;22:96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- Daubert EA, Condron BG. Serotonin: a regulator of neuronal morphology and circuitry. Trends in Neurosciences. 2010;33:424–434. doi: 10.1016/j.tins.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nuclear Medicine and Biology. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, McKittrick CR, File SE, McEwen BS. Decreased 5-HT1A and increased 5-HT2A receptor binding after chronic corticosterone associated with a behavioural indication of depression but not anxiety. Psychoneuroendocrinology. 1997;22:477–491. doi: 10.1016/s0306-4530(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Dahl RE. Positive and Negative Affect in Depression: Influence of Sex and Puberty. Annals of the New York Academy of Sciences. 2004;1021:341–347. doi: 10.1196/annals.1308.042. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Lombardo I, New AS, Goodman M, Talbot PS, Huang Y, Hwang D, Slifstein M, Curry S, Abi-Dargham A, Laruelle MA, Siever LJ. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. Am J Psychiatry. 2005;162:915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Developmental Brain Research. 1982;4:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Gómez-Gil E, Navinés R, Martínez De Osaba MJ, Díaz-Ricart M, Escolar G, Salamero M, Martín-Santos R, Galán A, Gastó C. Hormonal responses to the 5-HT1A agonist buspirone in remitted endogenous depressive patients after long-term imipramine treatment. Psychoneuroendocrinology. 2010;35:481–489. doi: 10.1016/j.psyneuen.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Molecular Brain Research. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain, Behavior, and Immunity. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Gorey JG, Bennet A, Suomi SJ, Weinberger DR, Higley JD. Serotonin transporter availability correlates with alcohol intake in non-human primates. Mol Psychiatry. 2003;8:231–234. doi: 10.1038/sj.mp.4001214. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo HF, Mueller NK, Ulrich-Lai YM, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, Nagren K, Salminen JK, Hietala J. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. The International Journal of Neuropsychopharmacology. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- Hoffman JB, Kaplan JR, Kinkead B, Berga SL, Wilson ME. Metabolic and reproductive consequences of the serotonin transporter promoter polymorphism (5-HTTLPR) in adult female rhesus monkeys (Macaca mulatta) Endocrine. 2007;31:202–211. doi: 10.1007/s12020-007-0017-8. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neuroscience & Biobehavioral Reviews. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, Godfrey J, Michopoulos V, Zhang X, Nair G, Hu X, Wilson ME, Sanchez MM. (under review) Social subordination stress and serotonin transporter genotype affect brain tracts in juvenile female macaques. Biol Psychiatry. doi: 10.1093/cercor/bht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. Effects of Early Life Stress on [11C]DASB Positron Emission Tomography Imaging of Serotonin Transporters in Adolescent Peer- and Mother-Reared Rhesus Monkeys. The Journal of Neuroscience. 2006;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang S-C, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle MA, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic Regulation of Hypothalamo-Pituitary-Adrenocortical Function during Acute and Chronic Stress. Annals of the New York Academy of Sciences. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga SL, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in females rhesus monkeys. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.11.042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Suomi SJ, Olsen AS, Porter JN, Lopresti BJ, Hariri AR, Bradberry CW. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry. 2010;15:512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive Behaviors in Infant Rhesus Monkeys: Environmental Cues and Neurochemical Regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Ontogeny and stability of separation and threat-induced defensive behaviors in rhesus monkeys during the first year of life. Am J Primatol. 1998;44:125–135. doi: 10.1002/(SICI)1098-2345(1998)44:2<125::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the Primate Orbitofrontal Cortex in Mediating Anxious Temperament. Biological Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The Primate Amygdala Mediates Acute Fear But Not the Behavioral and Physiological Components of Anxious Temperament. The Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain Regions Associated with the Expression and Contextual Regulation of Anxiety in Primates. Biological Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Molecular Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive Behaviors in Infant Rhesus Monkeys: Ontogeny and Context-dependent Selective Expression. Child Development. 1991;62:1175. [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosom Med. 1996;58:598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, Wilson ME, Manuck SB, Clarkson TB. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Human Reproduction. 2010;25:3083–3094. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential Effects of Acute and Chronic Social Defeat Stress on Hypothalamic-Pituitary-Adrenal Axis Function and Hippocampal Serotonin Release in Mice. Journal of Neuroendocrinology. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Tarara ER, Mason WA, Mendoza SP, Abel K, Lyons LA, Capitanio JP. Serotonin transporter expression is predicted by early life stress and is associated with disinhibited behavior in infant rhesus macaques. Genes, Brain and Behavior. 2010;9:45–52. doi: 10.1111/j.1601-183X.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JSD, Mann JJ. PET tracers for 5-HT1A receptors and uses thereof. Drug Discovery Today. 2007;12:748–756. doi: 10.1016/j.drudis.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Walker M, Gordon-Andrews H, Weickert CS, Feldon J, Pryce CR, Harrison PJ. Early Parental Deprivation in the Marmoset Monkey Produces Long-Term Changes in Hippocampal Expression of Genes Involved in Synaptic Plasticity and Implicated in Mood Disorder. Neuropsychopharmacology. 2008;34:1381–1394. doi: 10.1038/npp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D, Lemaire C, Ginovart N, Plenevaux A, Aerts J, Brihaye C, Hassoun W, Leviel V, Mekhsian P, Weissmann D, Pujol JF, Luxen A, Comar D. High-Yield Radiosynthesis and Preliminary In Vivo Evaluation of p-[18F]MPPF, a Fluoro Analog of WAY-100635. Nuclear Medicine and Biology. 1998;25:343–350. doi: 10.1016/s0969-8051(97)00229-1. [DOI] [PubMed] [Google Scholar]
- Lesch KP. In: When the Serotonin Transporter Gene Meets Adversity: The Contribution of Animal Models to Understanding Epigenetic Mechanisms in Affective Disorders and Resilience Molecular and Functional Models in Neuropsychiatry. Hagan JJ, editor. Springer Berlin Heidelberg; 2011. pp. 251–280. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Li Q. Cellular and molecular alterations in animal models of serotonin transporter disruption: a comparison between developmental and adult ages. In: Kalueff AV, LaPorte JL, editors. Experimental Models in Serotonin Transporter Research. Cambridge University Press; Cambridge, UK: 2010. pp. 43–77. [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallagher DW, Rakic P. Quantitative autoradiographic mapping of serotonin 5-HT1 and 5-HT2 receptors and uptake sites in the neocortex of the rhesus monkey. J Comp Neurol. 1989;280:27–42. doi: 10.1002/cne.902800104. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proc Natl Acad Sci U S A. 1991;88:10218–10221. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, Christman DR. Graphical Analysis of Reversible Radioligand Binding from Time-Activity Measurements Applied to [N-11C-methyl]-(-)-Cocaine PET Studies in Human Subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- López JF, Liberzon I, Vázquez DM, Young EA, Watson SJ. Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biological Psychiatry. 1999;45:934–937. doi: 10.1016/s0006-3223(98)00224-8. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Mol Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Lundberg J, Borg J, Halldin C, Farde L. A PET study on regional coexpression of 5-HT1A receptors and 5-HTT in the human brain. Psychopharmacology (Berl) 2007;195:425–433. doi: 10.1007/s00213-007-0928-3. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. European Journal of Neuroscience. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–1098. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Masten AS. Regulatory Processes, Risk, and Resilience in Adolescent Development. Annals of the New York Academy of Sciences. 2004;1021:310–319. doi: 10.1196/annals.1308.036. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Shungu DC, Mao X, Smith EL, Perera GM, Kegeles LS, Perera T, Lisanby SH, Rosenblum LA, Gorman JM, Coplan JD. A magnetic resonance spectroscopic imaging study of adult nonhuman primates exposed to early-life stressors. Biol Psychiatry. 2003;54:727–735. doi: 10.1016/s0006-3223(03)00004-0. [DOI] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and Behavior. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick L, Decker L, Nopoulos P, Ho BC, Andreasen N. Effects of atypical and typical neuroleptics on anterior cingulate volume in schizophrenia. Schizophr Res. 2005;80:73–84. doi: 10.1016/j.schres.2005.06.022. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited Review: Estrogens effects on the brain: multiple sites and molecular mechanisms. Journal of Applied Physiology. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Kastman EK, Bendlin BB, Johnson SC. Rhesus macaque brain morphometry: a methodological comparison of voxel-wise approaches. Methods. 2010;50:157–165. doi: 10.1016/j.ymeth.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Oakes TR, Kroenke CD, Kohama SG, Matochik JA, Ingram DK, Johnson SC. A population-average MRI-based atlas collection of the rhesus macaque. Neuroimage. 2009;45:52–59. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social Subordination and Polymorphisms in the Gene Encoding the Serotonin Transporter Enhance Estradiol Inhibition of Luteinizing Hormone Secretion in Female Rhesus Monkeys. Biology of Reproduction. 2009;81:1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller M, Jakobsen S, Gjedde A. Parametric and Regional Maps of Free Serotonin 5HT1A Receptor Sites in Human Brain as Function of Age in Healthy Humans. Neuropsychopharmacology. 2007;32:1707–1714. doi: 10.1038/sj.npp.1301310. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Shah N, Berga S, Sereika SM, Fisher PM, Coleman R, Becker C, Mason NS, Loucks T, Meltzer CC. Age, Sex, and Reproductive Hormone Effects on Brain Serotonin-1A and Serotonin-2A Receptor Binding in a Healthy Population. Neuropsychopharmacology. 2011;36:2729–2740. doi: 10.1038/npp.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Wisner KL, Price JC, Berga SL, Drevets WC, Hanusa BH, Loucks TL, Meltzer CC. Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertility and Sterility. 2008;89:685–692. doi: 10.1016/j.fertnstert.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Huang Y, Hu J, Henry S, Williams W, Gallezot J-D, Bailey CR, Krystal JH, Carson RE, Neumeister A. Reduced Amygdala Serotonin Transporter Binding in Posttraumatic Stress Disorder. Biological Psychiatry. 2011;70:1033–1038. doi: 10.1016/j.biopsych.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy NV, Selvaraj S, Cowen PJ, Bhagwagar Z, Riedel WJ, Peers P, Kennedy JL, Sahakian BJ, Laruelle MA, Rabiner EA, Grasby PM. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5-HTT to [11C] DASB binding in the living human brain. NeuroImage. 2010;52:50–54. doi: 10.1016/j.neuroimage.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. The British Journal of Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Navinés R, Martín-Santos R, Gómez-Gil E, Martínez de Osaba MJ, Luisa Imaz M, Gastó C. Effects of citalopram treatment on hypothermic and hormonal responses to the 5-HT1A receptor agonist buspirone in patients with major depression and therapeutic response. Psychoneuroendocrinology. 2007;32:411–416. doi: 10.1016/j.psyneuen.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Mathé A, Czernik A, Thiele J, Bohner J, Eap C, Ågren H, Baumann P. Long-term citalopram administration reduces responsiveness of HPA axis in patients with major depression: relationship with S-citalopram concentrations in plasma and cerebrospinal fluid (CSF) and clinical response. Psychopharmacology. 2005;181:751–760. doi: 10.1007/s00213-005-0034-3. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Nordquist N, Oreland L. Serotonin, genetic variability, behaviour, and psychiatric disorders - a review. Upsala Journal of Medical Sciences. 2010;115:2–10. doi: 10.3109/03009730903573246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Christian BT, Murali D, Oakes TR, Davidson RJ, Kalin NH. Serotonin Transporter Availability in the Amygdala and Bed Nucleus of the Stria Terminalis Predicts Anxious Temperament and Brain Glucose Metabolic Activity. The Journal of Neuroscience. 2009;29:9961–9966. doi: 10.1523/JNEUROSCI.0795-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clinical Chemistry. 1994;40:288–295. [PubMed] [Google Scholar]
- Paiardini M, Hoffman JB, Cervasi B, Ortiz AM, Stroud F, Silvestri G, Wilson ME. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain, Behavior, and Immunity. 2009;23:286–293. doi: 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Boudreau M, Hecht E, Winslow JT, Noble PL, Nemeroff CB, Sanchez MM. Early life stress affects cerebral glucose metabolism in adult rhesus monkeys (Macaca mulatta) Developmental Cognitive Neuroscience. 2012;2:181–193. doi: 10.1016/j.dcn.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; San Diego: 2000. [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: A volumetric magnetic resonance imaging study. Hippocampus. 2010;20:922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM. Developmental roles for the serotonin transporter. In: Kalueff AV, LaPorte JL, editors. Experimental Models in Serotonin Transporter Research. Cambridge University Press; Cambridge, UK: 2010. pp. 78–104. [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The Risk for Early-Adulthood Anxiety and Depressive Disorders in Adolescents With Anxiety and Depressive Disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Puig M, Gulledge A. Serotonin and Prefrontal Cortex Function: Neurons, Networks, and Circuits. Molecular Neurobiology. 2011;44:449–464. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raje S, Patat AA, Parks V, Schechter L, Plotka A, Paul J, Langstrom B. A Positron Emission Tomography Study to Assess Binding of Lecozotan, a Novel 5-Hydroxytryptamine-1A Silent Antagonist, to Brain 5-HT1A Receptors in Healthy Young and Elderly Subjects, and in Patients With Alzheimer’s Disease. Clin Pharmacol Ther. 2008;83:86–96. doi: 10.1038/sj.clpt.6100232. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. The Hippocampal Formation of the Primate Brain: a Review of Some Comparative Aspects of Cytoarchitecture and Connections. Plenum Press; Oxford, UK: 1987. [Google Scholar]
- Saigal N, Pichika R, Easwaramoorthy B, Collins D, Christian BT, Shi B, Narayanan TK, Potkin SG, Mukherjee J. Synthesis and Biologic Evaluation of a Novel Serotonin 5-HT1A Receptor Radioligand, 18F-Labeled Mefway, in Rodents and Imaging by PET in a Nonhuman Primate. Journal of Nuclear Medicine. 2006;47:1697–1706. [PubMed] [Google Scholar]
- Saleem KS, Logothetis N. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Academic Press; London; Burlington, MA: 2007. [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Bethea CL. Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques. Neuroscience. 2010;171:893–909. doi: 10.1016/j.neuroscience.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Pandya DN. Fiber pathways of the brain. Oxford university Press; New York: 2006. [Google Scholar]
- Seeman P, Kapur S. Anesthetics inhibit high-affinity states of dopamine D2 and other G-linked receptors. Synapse. 2003;50:35–40. doi: 10.1002/syn.10221. [DOI] [PubMed] [Google Scholar]
- Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Henderson JA, Smith MA, Buchheimer N. Behavioral Depression and Positron Emission Tomography-Determined Serotonin 1A Receptor Binding Potential in Cynomolgus Monkeys. Arch Gen Psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA. Social stress, depression, and brain dopamine in female cynomolgus monkeys. Annals of the New York Academy of Sciences. 1997a;807:574–577. doi: 10.1111/j.1749-6632.1997.tb51972.x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological Psychiatry. 1997b;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Silk JB. Females, food, family, and friendship. Evolutionary Anthropology: Issues, News, and Reviews. 2002;11:85–87. [Google Scholar]
- Smith H, Porrino L. The comparative distributions of the monoamine transporters in the rodent, monkey, and human amygdala. Brain Structure and Function. 2008;213:73–91. doi: 10.1007/s00429-008-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, Barr CS, Suomi SJ, Higley JD, Stein EA. Effects of Early-Life Stress on Serotonin1A Receptors in Juvenile Rhesus Monkeys Measured by Positron Emission Tomography. Biological Psychiatry. 2010;67:1146–1153. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-Life Stress Induces Long-term Morphologic Changes in Primate Brain. Arch Gen Psychiatry. 2009;66:658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Ågmo A. Estrogen-Induced Sexual Incentive Motivation, Proceptivity and Receptivity Depend on a Functional Estrogen Receptor α in the Ventromedial Nucleus of the Hypothalamus but Not in the Amygdala. Neuroendocrinology. 2010;91:142–154. doi: 10.1159/000255766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehouwer JS, Jarkas N, Zeng F, Voll RJ, Williams L, Camp VM, Malveaux EJ, Votaw JR, Howell L, Owens MJ, Goodman MM. Synthesis, Radiosynthesis, and Biological Evaluation of Fluorine-18-Labeled 2β-Carbo(fluoroalkoxy)-3β-(3′-((Z)-2-haloethenyl)phenyl)nortropanes: Candidate Radioligands for In Vivo Imaging of the Serotonin Transporter with Positron Emission Tomography. Journal of Medicinal Chemistry. 2008;51:7788–7799. doi: 10.1021/jm800781a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. Journal of Affective Disorders. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. Journal of Psychiatric Research. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Ogden RT, Oquendo MA, Kumar JSD, Simpson N, Huang Y-y, Mann JJ, Parsey RV. Positron Emission Tomography Quantification of Serotonin-1A Receptor Binding in Medication-Free Bipolar Depression. Biological Psychiatry. 2009;66:223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: Implications for neuropsychiatric disorders. Biological Psychiatry. 1997;41:452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Reddy AP, Bethea CL. 2008;154:720–731. doi: 10.1016/j.neuroscience.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vaillancourt C, Cyr M, Rochford J, Boksa P, Di Paolo T. Effects of ovariectomy and estradiol on acoustic startle responses in rats. Pharmacol Biochem Behav. 2002;74:103–109. doi: 10.1016/s0091-3057(02)00967-x. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Keck S, Henn FA, Schloss P. Acute stress decreases serotonin transporter mRNA in the raphe pontis but not in other raphe nuclei of the rat. Neuroscience Letters. 2000;290:109–112. doi: 10.1016/s0304-3940(00)01346-x. [DOI] [PubMed] [Google Scholar]
- Walker EF, Sabuwalla Z, Huot R. Pubertal neuromaturation, stress sensitivity, and psychopathology. Dev Psychopathol. 2004;16:807–824. doi: 10.1017/s0954579404040027. [DOI] [PubMed] [Google Scholar]
- Whittington RA, Virág L. Isoflurane Decreases Extracellular Serotonin in the Mouse Hippocampus. Anesthesia & Analgesia. 2006;103:92–98. doi: 10.1213/01.ane.0000221488.48352.61. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: Regional distribution of axon terminals. Neuroscience. 1991;44:537–553. doi: 10.1016/0306-4522(91)90076-z. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Bounar S, Godfrey J, Michopoulos V, Higgins M, Sanchez MM. Social and emotional predictors of the tempo of puberty in female rhesus monkeys. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2012.04.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: Social status effects on caloric consumption. Physiology & Behavior. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Gordon TP, Collins DC. Ontogeny of luteinizing hormone secretion and first ovulation in seasonal breeding rhesus monkeys. Endocrinology. 1986;118:293–301. doi: 10.1210/endo-118-1-293. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Gordon TP, Rudman CG, Tanner JM. Effects of a natural versus artificial environment on the tempo of maturation in female rhesus monkeys. Endocrinology. 1988;123:2653–2661. doi: 10.1210/endo-123-6-2653. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Zoghbi SS, McCarron JA, Hong J, Ichise M, Brown AK, Gladding RL, Bacher JD, Pike VW, Innis RB. Quantification of serotonin 5-HT1A receptors in monkey brain with [11C](R)-(-)-RWAY. Synapse. 2006;60:510–520. doi: 10.1002/syn.20327. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Klimes-Dougan B, Slattery MJ. Internalizing problems of childhood and adolescence: prospects, pitfalls, and progress in understanding the development of anxiety and depression. Dev Psychopathol. 2000;12:443–466. [PubMed] [Google Scholar]
- Zehr JL, Van Meter PE, Wallen K. Factors Regulating the Timing of Puberty Onset in Female Rhesus Monkeys (Macaca mulatta): Role of Prenatal Androgens, Social Rank, and Adolescent Body Weight. Biology of Reproduction. 2005;72:1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]