Abstract
Background
Notch signaling has previously been shown to play an essential role in regulating cell fate decisions and differentiation during cardiogenesis in many systems including Drosophila, Xenopus and mammals. We hypothesized that Notch may also be involved in directing the progressive lineage restriction of cardiomyocytes into specialized conduction cells.
Methods and Results
In hearts where Notch signaling is activated within the myocardium from early development onwards, Notch promotes a conduction-like phenotype based on ectopic expression of conduction system-specific genes and cell autonomous changes in electrophysiology. Using an in vitro assay to activate Notch in newborn cardiomyocytes, we observed global changes in the transcriptome as well as in action potential characteristics consistent with reprogramming to a conduction-like phenotype.
Conclusions
Notch can instruct the differentiation of chamber cardiac progenitors into specialized conduction-like cells. Plasticity remains in late-stage cardiomyocytes, which has potential implications for engineering of specialized cardiovascular tissues.
Keywords: action potentials, conduction, pacemakers, Purkinje, reprogramming
Introduction
Diseases of the cardiac conduction system (CCS) are a significant cause of morbidity and mortality, where few therapeutic options exist for treatment outside of implantation of electronic pacemakers. Limitations of current pacemaker technology include minimal autonomic responsiveness, infection, the potential for contractile dyssynchrony that may contribute to heart failure, and the requirement for life-long maintenance and replacement, an especially troubling aspect in the pediatric population. Biologic pacemakers may eventually represent a viable alternative to electronic devices, even if only under limited circumstances, and proof of principle studies suggest initial promise (reviewed in 1). The ability to instruct cardiomyocytes to become “conduction-like”, either through directed differentiation of human embryonic stem cells or iPS cells, or through direct reprogramming of cardiomyocytes in vivo into conduction-like cells, may be an evolving paradigm for reversing or treating degenerative conduction disease. Deciphering signals that can instruct cardiomyocytes to adopt a conduction phenotype is a prerequisite for progress with this therapeutic approach.
The CCS consists of the sinoatrial (SA) node, which generates impulses that travel through atrial tissue to arrive at the atrioventricular (AV) node. At the AV node, there is a delay in impulse propagation to allow the atria to contract. Impulses then travel rapidly through the ventricular conduction system comprised of the His bundle, right and left bundle branches, and peripheral Purkinje fiber network which coordinates activation of the heart from apex to base. Cells of the CCS can be identified as distinct from atrial and ventricular “working” or “chamber” myocardium based on unique action potential morphologies and gene expression profiles. Lineage studies in both chick and mice have demonstrated that cells of the conduction system share a common origin with cardiomyocytes, with the exception of the sinus node, which is recruited from mesenchymal cells just outside the heart field.2–4 In the murine heart, Cx40-positive embryonic trabeculae give rise to both conduction and working myocytes at early embryonic stages.4 Whether the potential for cardiomyocyte plasticity between conduction and chamber myocardium exists at later stages of development remains to be elucidated.
Endothelin-1 and neuregulin-1 are two factors secreted by endothelial cells that play important roles in the development of ventricular trabeculae and can direct the differentiation of embryonic cardiomyocytes into Purkinje-like cells during discrete developmental windows.5, 6 However, the effects of these inductive signals are likely to be context dependent, as treatment of human embryonic stem cells with neuregulin-1s leads to increased working-type cells, while a neuregulin-1s inhibitor or ErbB inhibitor promotes an AV nodal phenotype.7 Several transcription factors operate both at the level of conduction system morphogenesis and differentiation to control the elecrophysiologic properties of cells. T-box containing transcriptional repressors including Tbx18, Tbx3 and Tbx5 play a role in the specification of the SA node, AV bundle and bundle branches. Tbx18 controls the formation of the SAN head from mesenchymal precursors, onto which Tbx3 subsequently imposes the pacemaker gene program by repressing expression of atrial “working” myocardial genes.8 Overexpression of Tbx3 in atrial tissue results in conversion of atrial cardiomyocytes to a nodal-like phenotype.9, 10 Nkx2.5 is a critically important transcription factor in conduction system formation and maintenance, as evidenced by a hypoplastic AV node and postnatal defects in Purkinje fiber differentiation in global haploinsufficient mice.11, 12 Tbx5 and Nkx2.5 cooperate to mediate expression of Id2, a helix-loop-helix transcription factor that represses myogenic genes and promotes ventricular conduction system differentiation.13
In the zebrafish heart notch1b is important for central conduction system formation.14 Our recent work has demonstrated that loss of myocardial Notch signaling in a murine model results in abnormal AV nodal formation and function, while activation of myocardial Notch signaling leads to ventricular preexcitation.14, 15 Based on this previous work, we hypothesized that Notch may influence progressive cardiomyocyte cell lineage decisions involving components of the conduction system, and we investigated this possibility further using both in vivo and in vitro approaches.
Materials and Methods
Mice
All mice were maintained on a mixed genetic background. Mlc2vCre/+ mice were genotyped using Cre-specific primers, CCS-LacZ16 with LacZ-specific primers, and Cntn2-EGFP17 with GFP-specific primers. We used mice in which a dominant-negative Mastermind-like gene and the Notch intracellular domain (DNMAML and NICD, respectively, knocked into the constitutively active Rosa26 locus) are expressed in a tissue-specific manner after activation by Cre recombinase.18 DNMAML and NICD mice were genotyped with ROSA26 locus primers. Littermate animals were compared in all experiments unless otherwise noted. All animal protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Histology and immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded sections with antibodies recognizing Contactin-2 (R&D Systems, AF4439), Tbx3 (sc-17871, Santa Cruz) and Ki67 (sc-15402, Santa Cruz). Secondary antibody-fluorescent conjugates included anti-rabbit Alexa 568 (Invitrogen) and anti-goat Alexa 488 (Invitrogen). Histology, immunohistochemistry and whole mount Xgal images were analyzed using Adobe Photoshop. Control and mutant images were treated identically in all cases where brightness and contrast were altered.
Proliferation Index of AV Node
Sections from 3 regions within newborn Mlc2vCre/+; NICD and NICD hearts were costained for Tbx3 and Ki67 as described above. The total number of Tbx3 positive and Tbx3/Ki67 double positive cells were counted in n=3 hearts of each genotype. The proliferation ratio was calculated by dividing the Tbx3/Ki67 double positive cells by the total number of Tbx3 positive cells.
Cardiomyocyte Culture and Viral Infection
Perinatal hearts were isolated in chilled PBS followed by digestion in 0.10% trypsin diluted in HBSS with 1mg/mL type IV collagenase (Sigma) for 20 minutes rotating at 37°C with gentle trituration every 5 minutes. FBS was then added at a 1:1 ratio, following by plating of the cells on gelatin-coated wells at a density of 1.5–2.0 × 10^6 cells per well. Cardiomyocytes were cultured in myocyte media (65% DMEM, 20% M-199, 1.7 mM L-glutamine, 85 mM HEPES, 10% horse serum, 5% FBS). Hearts from 14.5 dpc embryos were treated similarly except they were digested in 0.15% trypsin diluted in DMEM with 1ug/mL type IV collagenase (Sigma). After 24 hours in culture, cardiomyocytes were treated with either the constitutively active Notch1 adenovirus (ICNX19) or control adenovirus (EGFP or lacZ) at an MOI of 100, followed by analysis of the cells 48 hours after infection.
Reverse Transcription-Quantitative Real Time PCR (RT-qPCR)
Total mRNA was isolated from hearts or cultured cells using Trizol (Invitrogen) and DNase treated using TURBO DNA-free DNase Treatment Kit (Ambion). cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen). qPCR was performed with Power SYBR Green 2X Master Mix (Applied Biosystems) using the Applied Biosystems 7900 HT Fast Real-Time PCR System using SDS2.4 software. Relative gene expression was normalized to β actin. Primer sequences are provided in Supplemental Methods.
Isolation of Adult Ventricular Cardiomyocytes
Adult murine ventricular myocytes were isolated using the method described by Mitra and Morad.20 Briefly, mice were injected with 100 Units of heparin intraperitoneally, anesthetized with pentobarbital 50 mg/kg and hearts excised through a sternotomy. Hearts were mounted on a Langendorf apparatus, perfused with Ca2+-free Tyrode’s solution for 6 min at 3.0–3.5 ml/min and a temperature of 36–37 °C, followed by 12–15 min of perfusion with Ca2+-free Tyrode’s solution containing: collagenase B and collagenase D (Roche Chemical Co.) plus protease (Fraction IV, Sigma Chemical Co.). When the hearts appeared pale and flaccid they were removed from the Langendorf apparatus and the ventricles were dissected away and kept in Ca2+-free Tyrode’s solution with 1 mg/ml of bovine serum albumin (Fraction XIV, Sigma Chemical Co.). The ventricles were teased into small pieces and then triturated gently with a Pasteur pipette to dissociate individual myocytes.
Cellular electrophysiology
Current clamp recordings were performed using the patch clamp technique in the whole-cell configuration described by Hamill et al.21 Briefly, gigaohm seals were achieved using pipettes fashioned from borosilicate glass (Harvard Apparatus) with resistances of 2–2.5 MΩ after fire polishing. Action potentials were recorded using an Axopatch 200B amplifier running the pClamp program (v9.2) on a PC-based computer by injecting 0.2–0.5 nA current pulses at 1 Hz to 6 Hz with a 2-ms duration. Voltage recordings were filtered at 1–2 kHz and digitized at 25 kHz using the Digidata 1332A A/D converter (Molecular Devices). Only cells with resting potentials lower than −50 mV, seal resistances 1 GΩ, and access resistance less than 10 MΩ were accepted for analysis. For current clamp recordings, pipette solution contained 110 mM/l KCl, 5 mM/l Na2-ATP, 11 mM/l EGTA, 10 mM/l HEPES, 1 mM/l CaCl2, 1 mM/l MgCl2, pH 7.3, with KOH, and bath solution contained 132 mM/l NaCl, 4.8 mM/l KCl, 10 mM/l HEPES, 1.2 mM/l CaCl2, 2 mM/l MgCl2, pH 7.4, with NaOH.
Statistical Analysis
Student’s unpaired 2-tailed t-test was used to evaluate differences in gene expression and electrophysiologic parameters when two groups were compared. One-way ANOVA and Tukey-Kramer test for post-hoc analysis was used to evaluate differences when gene expression changes or electrophysiological parameters in more than two groups were compared. Chi square analysis was performed to evaluate for rescue of preexcitation. Sample sizes and statistical methods are provided in each figure legend. Data are represented as mean ± SEM. P values of less than 0.05 were considered statistically significant.
Results
Activation of Notch Signaling in Vivo Results in Up-regulation of Conduction System-Enriched Genes
The Notch signaling pathway regulates many developmental processes, including segregating cell lineages from fields of equivalent cells and defining boundaries between distinct cell populations. We have previously reported that activation of Notch signaling in the murine myocardium in Mlc2vCre/+; NICD mice, in which Mlc2vCre/+ activates expression of the Notch intracellular domain (NICD), results in altered boundary formation between AV canal myocardium and ventricular myocardium and leads to ventricular preexcitation.15 Additionally, constitutive activation of Notch signaling within the myocardium from early development onwards resulted in an enlarged AV node based on an expanded region of Tbx3 expression, while inhibition of canonical Notch signaling in Mlc2vCre/+; DNMAML mice results in smaller AV nodes with defective function.15 We hypothesized that AV nodal defects in Notch mutants may reflect an ability of Notch to bias cardiomyocytes towards a conduction phenotype during the progressive lineage restriction of cardiomyocytes and their precursors.
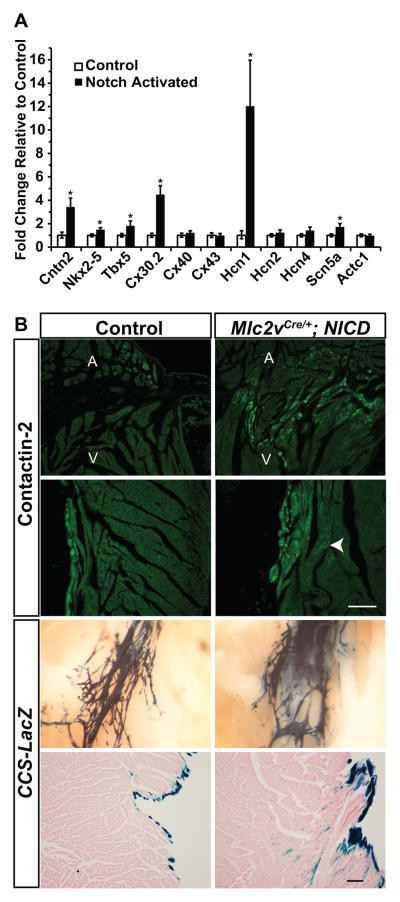
To test whether Notch causes gene expression changes consistent with recruitment to a conduction-like phenotype, we performed RT-qPCR on adult Notch activated hearts. Atria were removed, and the ventricles with the base of the interatrial septum (including the AV nodal region) were analyzed. Increased expression of the canonical Notch target Hes1 reflects activation of the Notch pathway (Supplemental Figure 1). Although few markers that are specific for the conduction system have been described, a recent transcriptome analysis of Purkinje fibers purified from CCS-LacZ mice identified Contactin2 (Cntn2), a cell adhesion molecule with unknown function within the heart, as a specific marker of conduction cells.16, 17 Therefore, we assayed for the expression of Contactin2, as well as other conduction system-enriched ion channels, gap junction isoforms, and transcription factors in adult Notch activated mice.
Cntn2 was significantly up-regulated 3.3-fold in ventricles from Notch activated mice when compared with controls (Figure 1A). Tbx5 and Nkx2-5, encoding transcription factors that play important roles in AV nodal and ventricular conduction system development with exquisite dosage-sensitivity, are up-regulated 1.8 fold and 1.5 fold, respectively (Figure 1A). Connexin isoforms multimerize to form gap junctions and are expressed preferentially within different regions of the heart. Cx30.2, encoding a low conductance gap junction isoform expressed exclusively within nodal tissues, is up-regulated 3.4-fold in Notch activated hearts. Hcn1, encoding a hyperpolarization-activated channel that contributes to the If pacemaker current, is up-regulated 12-fold. The gene encoding the major sodium channel expressed in the heart, Scn5a, which is also enriched in Purkinje fibers, is up-regulated 1.7 fold. Taken together, Notch activation in ventricular myocardium results in up-regulation of both nodal and Purkinje-enriched genes.
Figure 1.
Activation of Notch Signaling Upregulates Conduction System-Enriched Genes. (A) RT-qPCR of conduction-enriched genes in ventricular samples from Mlc2vCre/+; NICD mice with transcript levels normalized to control littermates. Whereas there was no change in expression of the pan-cardiac marker Actc1 (alpha-cardiac actin), Notch activation up-regulates conduction-enriched transcription factor, gap junction, and ion channel genes. (B) Immunohistochemistry for the conduction-specific protein Cntn2 (green) demonstrates ectopic expression in Notch activated hearts when compared with control, including within the right ventricle near the atrioventricular junction and in the interventricular septum near the right bundle. The arrow demonstrates Cntn2-expressing cells within the interventricular septum in a region distant from the endocardium. Whole mount analysis of CCS-LacZ expression similarly demarcates ectopic conduction tissue along the right bundle with a blurring of the boundary between conduction and chamber myocardium in Notch activated hearts. Eosin stained sections from Notch activated hearts along the left side of the interventricular septum demonstrate ectopic CCS-LacZ expression broadly in the subendocardial region. Scale bars = 100μM, whole mount CCS-LacZ images are at 6.3× magnification. A=atrium, V=ventricle. Control mice are NICD. N=3 each genotype. Group comparison was performed using a Student’s unpaired 2-tailed t-test. *p<0.05
To further probe the mechanism for enlarged AV nodes in Notch activated hearts, Tbx3-expressing cells in Notch activated versus control hearts were assessed for proliferation by Ki67 staining. Since AV nodal and AV junction defects are progressive during the early postnatal stages of development in Notch activated hearts,15 we assessed proliferation in newborn hearts. There was no significant difference in the percentage of Tbx3+ proliferating cells (Mlc2vCre/+; NICD 0.091% ± 0.014 versus NICD 0.094%± 0.010, p=0.88), consistent with a recruitment effect and not a proliferative effect. Expression of conduction-specific Cntn2 was similar at birth in control and Notch activated hearts (Supplemental Figure 2A,B), but by 3 weeks of age there was modest ectopic expansion of Cntn2 in Notch activated hearts in regions where conduction tissue is not characteristically found, most prominently in the right ventricle near the AV junction and in an expanded region in the interventricular septum near the right bundle branch (Figure 1B). CCS-LacZ, which labels the conduction system, is expressed normally in Notch activated hearts at birth (Supplemental Figure 2C), but by 3 weeks of age CCS-LacZ expression is ectopic in the mutants, resulting in blurring of the boundary between the conduction system and chamber myocardium (Figure 1B). Similar results were seen using Cntn2-EGFP mice to label the distal Purkinje fiber network (Supplemental Figure 2D, E). Many of the ectopic Cntn2-expressing cells also express Cx40, a marker of Purkinje cells (Supplemental Figure 3). These results support the conclusion that Notch is capable of influencing cardiomyocyte lineage decisions.
Activation of Notch Signaling In Vivo Results in Cell Autonomous Electrophysiologic Changes Consistent with a Conduction-like Phenotype
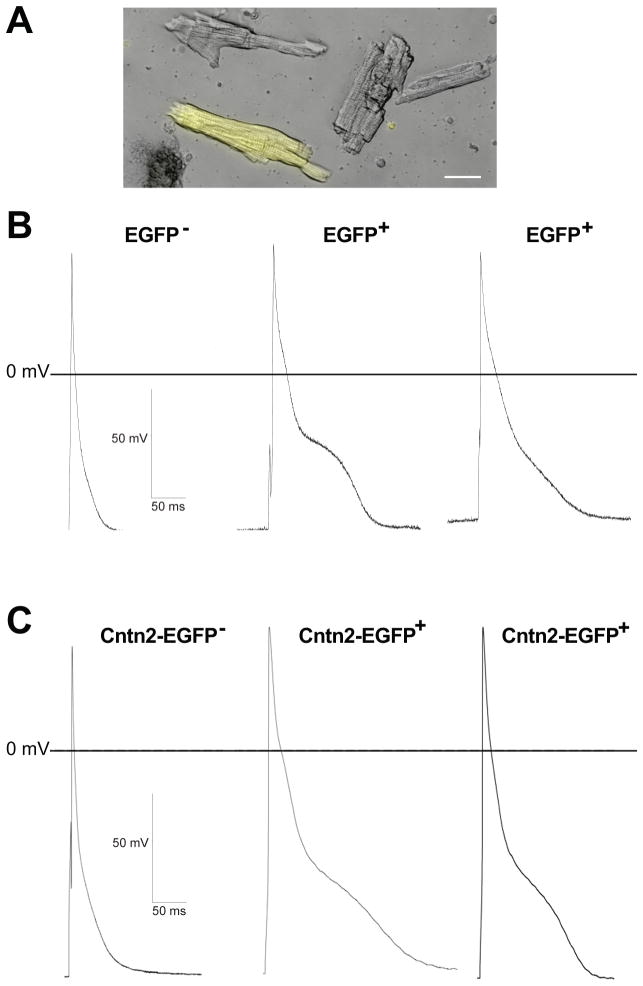
Individual Notch activated ventricular cells were identified by EGFP, which is activated by Cre recombinase in Mlc2vCre/+; Z/EG; NICD mice, and were compared with EGFP+ cells from control Mlc2vCre/+; Z/EG mice. Notch activated cardiomyocytes have a similar morphology to neighboring cardiomyocytes (Figure 2A), consistent with reports of some adult murine Cntn2-EGFP+ Purkinje fibers having an elongated, rod-shaped phenotype typical of the Purkinje fibers observed in many species, as well as other Purkinje fibers with morphologies similar to those of chamber cardiomyocytes.17 Adult mouse Purkinje fiber action potentials are distinguishable from ventricular cardiomyocyte action potentials by a prolonged duration and a distinct plateau in phase 2.17 The action potential characteristics of many Notch activated myocytes look remarkably similar to the action potentials of adult mouse Purkinje fibers (Figure 2B, C and Supplemental Figure 4). Some Notch activated myocytes displayed spontaneous depolarizations under current clamp conditions with phase 4 depolarization that further supports transition to a Purkinje-like phenotype (Supplemental Figure 5), whereas this was not seen in control cells. We noted a relatively wide range of action potential durations among Notch activated cells, with some cells having APD90 very similar to previously described adult murine Purkinje fibers (approximately 140 ms). When averaged, the APD90 was significantly prolonged in Notch activated cells compared to controls (87.5 ms vs. 41.0 ms, p=0.006) while there were no significant differences in the resting membrane potential or action potential amplitude (Table 1). There was no significant effect on electrophysiologic parameters in EGFP− cells between Notch activated Mlc2vCre/+; Z/EG; NICD and control Mlc2vCre/+; Z/EG hearts, suggesting a cell autonomous effect (Table 1). Taken together, in ventricular myocardium there are cells that shift their phenotype to become indistinguishable from Purkinje cells as evidenced by upregulation of both Cntn2 and Cx40 and a characteristic action potential morphology, though there are also cells that appear to be only partially reprogrammed, as well as non-responders (Supplemental Figure 6).
Figure 2.
Notch Activation in Ventricular Myocardium Converts Cellular Electrophysiology to Resemble Purkinje Cells. (A) Phase contrast image of ventricular cardiomyocytes isolated from Notch activated hearts with superimposed fluorescence microscopy to identify EGFP+ Notch activated cells. The cellular morphology of Notch activated adult ventricular cardiomyocytes is similar to that of surrounding EGFP− cells. (B) Action potential morphologies from EGFP+ Notch activated cells reveal a prolonged action potential duration and a plateau in phase 2 of the action potential, similar to adult mouse Purkinje cell action potentials recorded from Cntn2-EGFP+ mice (C). Scale bar A = 20μM.
Table 1.
Action Potential Characteristics
| RMP, mV | APA, mV | APD50, ms | APD90, ms | |
|---|---|---|---|---|
| EGFP+ Mlc2vCre/+ | −66.6 ± 0.7 | 123.8 ± 4.5 | 12.9 ± 0.7 | 41.0 ± 2.8 |
| EGFP+ Mlc2vCre/+; NICD | −62.6 ± 1.8 | 115.0 ± 2.1 | 27.2 ± 4.1* | 87.5 ± 12.3* |
| EGFP− Mlc2vCre/+ | 61.9 ± 3.3 | 110.5 ± 4.4 | 14.6 ± 0.8 | 40.8 ± 0.6 |
| EGFP− Mlc2vCre/+; NICD | 67.6 ± 1.6 | 116.2 ± 6.5 | 16.8 ± 2.1 | 50.4 ± 9.9 |
Data are expressed as mean ± SEM.
p<0.05.
n =9 EGFP+ Mlc2vCre/+, n=11 EGFP+ Mlc2vCre/+; NICD, n=4 EGFP− Mlc2vCre/+, n=5 EGFP− Mlc2vCre/+; NICD. RMP = resting membrane potential; APA = action potential
amplitude; APD50 = action potential at 50% repolarization; APD90 = action potential at 90% repolarization.
Transient Notch Activation Converts the Transcriptional Profile of Newborn Cardiomyocytes to a Conduction-like Phenotype
As mentioned above, inductive cues such as neuregulin-1 and endothelin-1, which can bias cardiomyocytes toward a conduction phenotype, are often context-dependent.6, 7, 17 Since our in vivo system activates Notch early and continuously throughout development into adulthood, we sought an alternative approach to determine whether transient Notch activation at specific times of cardiac maturity would be sufficient to reprogram cells towards a conduction-like phenotype. Evidence from transgenic mouse reporter lines suggests that conduction cells may be specified as early as embryonic day 8.5, and lineage tracing studies show that cardiomyocytes retain plasticity to become either conduction or working cardiomyocytes until mid-gestation.4, 16 Whether any cell fate plasticity remains at later stages of development, and whether fully-differentiated chamber cardiomyocytes retain the potential to become specialized conduction cells, is unclear.
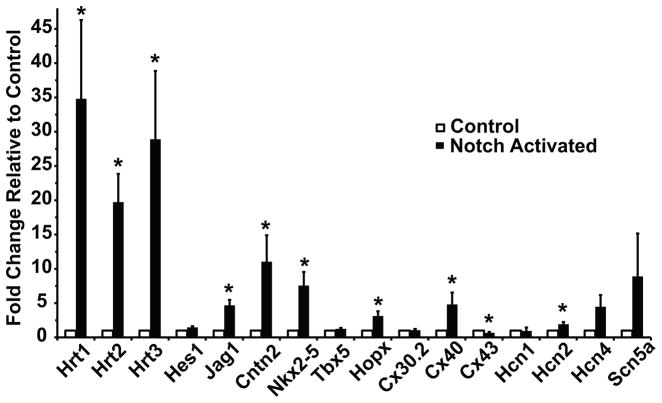
Primary mouse ventricular cardiomyocytes from perinatal pups were isolated and infected with the ICNX adenovirus expressing constitutively active Notch1 intracellular domain (N1ICD) or control GFP adenovirus. After forty-eight hours in culture, Notch activation robustly up-regulates the known Notch targets Hrt1, Hrt2, Hrt3 and Jag1 (Figure 3). Cntn2 is robustly up-regulated more than 10-fold with transient Notch activation (Figure 3). Nkx2-5 and its downstream target Hopx, a gene expressed in the His-Purkinje system,22 are significantly up-regulated. Of the connexin isoforms, the His-Purkinje enriched Cx40 is increased, while the predominantly chamber myocardial Cx43 is decreased and nodal specific Cx30.2 is unchanged. Notch activation results in a significant up-regulation of the pacemaker channel gene Hcn2, which is enriched within the bundle branches of the conduction system,23 and a trend toward increased expression of Hcn4 and the His-Purkinje enriched Scn5a. Globally, the transcriptional changes induced by Notch activation represent a phenotypic transition toward a His-Purkinje conduction cell phenotype. Many of the effects of transient Notch expression in 14.5 dpc ventricular cardiomyocytes are similar to the transcriptional changes seen in newborn ventricular cardiomyocytes (Supplemental Figure 7).
Figure 3.
Transient Notch Activation Converts the Transcriptional Profile of Newborn Cardiomyocytes to a Conduction-like Phenotype. RT-qPCR analysis of induced gene expression changes for canonical Notch target genes and conduction system-enriched genes after activation of Notch signaling (black bars) for 48 hours in cultured perinatal ventricular cardiomyocytes. All fold changes are normalized to control cells infected with a GFP-expressing adenovirus (white bars). Notch activation up-regulates known Notch targets, as well as Purkinje fiber-enriched transcripts. n=7 replicates. Data are expressed as mean ± SEM. Group comparison was performed using a Student’s unpaired 2-tailed t-test. *p<0.05
Notch Converts the Cellular Electrophysiology of Newborn Ventricular Cardiomyocytes to a Purkinje-like Phenotype
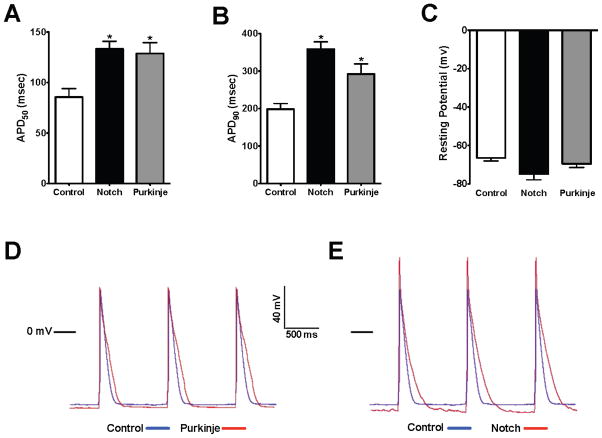
To determine whether transient Notch activation can alter the cellular electrophysiology of newborn cardiomyocytes, we performed current clamp studies on ventricular cardiomyocytes derived from Cntn2-EGFP mice, where the effects of transient Notch activation on cellular electrophysiology can be compared with that of endogenous Purkinje cells. In comparison with control Cntn2-EGFP negative (Cntn2-EGFP−) ventricular cardiomyocytes, Cntn2-EGFP+ Purkinje cells exhibited a significant action potential prolongation (APD50 Cntn2-EGFP− 85 ms vs Cntn2-EGFP+ 129 ms, p=0.006) as well as a slight hyperpolarization of the resting membrane potential (Figure 4A-D). Adenoviral-mediated expression of N1ICD resulted in Cntn2-EGFP−cells becoming more “Purkinje-like” based on action potential prolongation at 50% and 90% repolarization, as well as changes in the action potential morphology (APD50 133 ms, p=0.0004 when compared with control, Figure 4A–C, E). Electrophysiologic changes occurred in all newborn cardiomyocytes in response to Notch activation. Taken together with the gene expression changes, this suggests that Notch activation is capable of reprogramming newborn cardiomyocytes to a conduction cell-like phenotype.
Figure 4.
Notch Converts the Cellular Electrophysiology of Newborn Ventricular Cardiomyocytes to a Purkinje-like Phenotype. Comparison of the action potential duration (APD) at 50% (A) or 90% (B) repolarization in newborn cardiomyocytes isolated from Cntn2-EGFP mice, which identifies endogenous Purkinje fibers. EGFP− chamber cardiomyocytes (white bars, n=9) have a significantly shorter APD50 and APD90 when compared with EGFP+ Purkinje cells (grey bars, n=8). Notch activation in EGFP− chamber cardiomyocytes (black bars, n=10) results in a significant prolongation of both the APD50 and APD90 towards a Purkinje-like phenotype. (C) The resting membrane potential was not significantly different between chamber cardiomyocytes, Purkinje cells, and Notch activated cells though there was slight membrane hyperpolarization in Purkinje cells and with Notch activation. (D) Superimposed action potentials from a control lacZ-infected (blue) and EGFP+ Purkinje cell (red). (E) Superimposed action potentials from a control lacZ-infected (blue) and Notch activated cell (red) demonstrate Notch-induced changes in the action potential morphology similar to those in Purkinje cells. Group comparison was performed using a one-way ANOVA and Newman-Keuls test for post-hoc analysis. *p<0.05
Notch-Mediated Reprogramming is Mediated Through Canonical Signaling
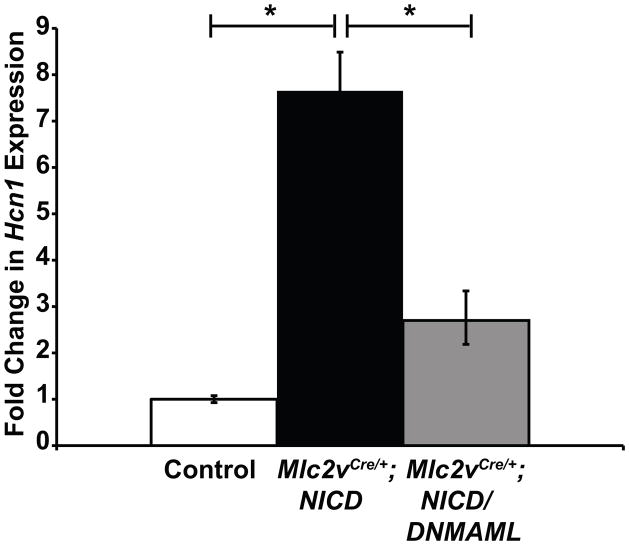
Recent data suggests that in addition to canonical CSL (CBF-1, suppressor of hairless, Lag-1; or RBPj-kappa) -dependent Notch signaling, non-canonical Notch signaling plays important roles in development and disease, including its role in modulation of the Wnt signaling pathway.24 To address the mechanism of Notch-induced conduction system reprogramming, we inactivated canonical Notch signaling in Notch activated mice by inhibiting activation of genes downstream of Mastermind-like1 protein using a dominant-negative approach (Mlc2vCre/+; NICD/DNMAML). Inactivation of canonical signaling by DNMAML rescues ectopic up-regulation of expression of the pacemaker channel gene Hcn1 (7.7 fold upregulation in Mlc2vCre/+; NICD vs 2.7 fold upregulation in Mlc2vCre/+; NICD/DNMAML, Figure 5, p=0.02). For comparison, levels of the known canonical Notch target Hes1 are decreased to a similar degree in the presence of DNMAML (Supplemental Figure 8). We previously have examined a cohort of over fifty Notch activated mice and found that 100% have ventricular preexcitation by 3 weeks of age. In contrast, only 7/11 Mlc2vCre/+; NICD/DNMAML mice are preexcited, while 12/12 littermate Mlc2vCre/+; NICD mice are preexcited by four weeks of age (Chi square p=0.027). These results suggest that the effects of Notch activation to promote a conduction-like phenotype are at least partially MAML dependent and therefore mediated by canonical Notch signaling.
Figure 5.
Reprogramming to a Conduction-like Phenotype in Vivo is Dependent on Canonical Notch Signaling. (A) RT-qPCR analysis of Hcn1 gene expression changes within the mid-ventricular region of Notch activated hearts, with and without downstream inactivation of canonical Notch signaling via DNMAML (Mlc2vCre/+; NICD versus Mlc2vCre/+; NICD/DNMAML). Hcn1 levels are nearly 8-fold upregulated with Notch activation, however in the presence of DNMAML, Hcn1 levels are significantly reduced. This suggests that the effects of Notch activation to promote a conduction-like phenotype are largely MAML-dependent and therefore mediated by canonical Notch signaling. Control mice are NICD or NICD/DNMAML littermates. n=9 control, n=8 Mlc2vCre/+; NICD, n=6 Mlc2vCre/+; NICD/DNMAML. Data are expressed as mean ± SEM. Group comparison was performed using a one-way ANOVA and Tukey-Kramer test for post-hoc analysis. *p<0.05
Discussion
The results presented here demonstrate that Notch can modify the transcriptome and cellular electrophysiology of cardiac myocytes to resemble cells of the specialized conduction system. These findings are relevant to the better understanding of how to control cardiovascular progenitor cell differentiation and how to engineer regenerative cardiac tissues.
Various approaches have been suggested for the generation of a biological pacemaker. These have included the manipulation ex-vivo of human embryonic stem cell or induced pluripotent stem cells to become pacemaker cells, followed by implantation into diseased hearts. However, many important barriers remain to be overcome with a cell therapy approach, including cell-cell coupling between injected cells and native myocardium. A second approach involves gene therapy. For example, adenoviral-mediated delivery of Hcn2 channels, which contribute to the “funny current”, has been performed in dogs, and this type of biological pacemaker compared favorably with that of electronic units in the same animal (25–27 and reviewed in 1). A third approach, supported by the results reported in this manuscript, is to directly reprogram existing cardiomyocytes in vivo to a conduction-like phenotype. Results from Cx40-Cre lineage analysis suggest that the differentiation step to the ventricular conduction system has definitively occurred by 16.5 dpc in the mouse4. However, it is encouraging that our findings indicate that plasticity of mature myocytes to adopt a conduction-like phenotype persists later in life than previously appreciated. We have clearly demonstrated the ability to reprogram a small percentage of cells in vivo to Purkinje-like cells using a single factor. Thus, although further characterization of the effects of Notch will need to be undertaken prior to meeting a bar for convincing therapeutic value, this study suggests the possibility that cellular reprogramming strategies may be a viable strategy. It will be interesting to determine whether adenovirally-delivered Notch, either alone or in combination with other factors, may enhance the development of conduction tissue from adult myocardium and whether this effect is stable once the Notch signal is turned off.
Amongst the genes up-regulated by Notch activation in vivo are Nkx2-5 and Tbx5, which have been previously implicated in development and function of the conduction system.11, 28–30,31 Like Notch, Nkx2-5 and Tbx5 each function to regulate cardiac morphogenesis and cellular electrophysiology. Our ex-vivo experiments using myocytes isolated at various ages indicate that Notch can activate Nkx2-5 at all times tested, while up-regulation of Tbx5 was restricted to prenatal stages. Future studies will address the mechanism for differential responsiveness under specific developmental contexts.
The development of cardiac preexcitation resembling Wolff-Parkinson-White syndrome in Notch activated mice15 is likely to result from the combined effects of several distinct Notch functions, including effects on morphogenesis and cellular electrophysiology. Mispatterning of the AV boundary likely contributes to the abnormal presence of myocardium traversing between atrium and ventricle. However, the ability of Notch to alter cellular electrophysiology, and specifically to promote a Purkinje fiber-like phenotype in ventricular cardiomyocytes, may be equally significant for altering the electrical conducting properties of accessory pathway tissue, which is required for a bypass tract to become functional and for the animal to manifest preexcitation. In Notch activated newborn hearts, and in neonatal human hearts32, isolated strands of cardiomyocytes are observed in the absence of electrical preexcitaton; these remnants of AV canal myocardium are not electrically active. In Notch activated mice, as in some humans, preexcitation emerges only later in life, perhaps due to Notch-mediated changes in cellular electrophysiology of bypass myocardium. Tbx2 and PRKAG2, genes implicated in preexcitation syndromes, also regulate the expression and/or function of ion channel genes such as Scn5a.33, 34 Thus, changes in cellular electrophysiology may play an integral role in the development of preexcitation syndromes, antegrade conduction properties of accessory pathways, and risk of sudden cardiac death. Future studies will aim to determine the individual currents regulated by Notch, and to clarify the degree to which the role of specific currents and ion channel functions are conserved between mouse and human in specialized conduction tissue.
Microdeletions of Bone Morphogenetic Protein-2 (BMP-2) have recently been associated with a syndrome of WPW together with Alagille syndrome.35 JAGGED1, known to be implicated in Alagille syndrome, is located 3.8 MB centromeric from BMP-2, raising the question of whether altered Notch signaling contributes to the observed WPW phenotype in this human syndrome. Almost one-third of WPW patients develop atrial fibrillation at a young age, which often resolves with ablation of the bypass tracts. An intriguing hypothesis is whether Notch pathway induced alterations in ion channel expression within the atrium, or near the atrial insertion of the accessory pathway, may play a role in the genesis of atrial fibrillation in humans.
While our in vitro studies demonstrate an effect of activating Notch on a large percentage of infected cardiomyocytes, the effect in vivo is more modest. In ventricular myocardium, there are cells that shift their phenotype to become similar to Purkinje cells as evidenced by upregulation of both Cntn2 and Cx40 and a characteristic action potential morphology, though there are also cells that can best be described as transitioning towards a Purkinje phenotype in response to Notch activation, as well as non-responders (Supplemental Figure 4). The identification of additional signals that augment or restrict the degree of conversion will require further study. In Notch-activated hearts, we observed spatially-restricted non-homogenous up-regulation of Cntn2 and phenotypic variability in cellular electrophysiology. Possible explanations include additional combinatorial signals, either cooperative or inhibitory, which contribute to the competency of ventricular cardiomyocytes to respond to activated Notch in vivo. Alternatively, there may be a stronger bias against conduction system reprogramming inherent in a subset of cardiomyocytes, which is overcome by higher amounts of activated Notch provided by adenoviral delivery in the in vitro system. One future approach to address these possibilities might be to inject activated Notch into neonatal rodent ventricles, an approach that has been employed previously (Palatinus et al. Am J Phys., 2011). Whether Notch is also required during conduction system development, apart from its previously demonstrated effects on the AV node15 or whether alternative signaling pathways can instruct cardiomyocytes to become conduction cells in the absence of Notch signaling, is not entirely clear. However, Notch-regulated genes appear to be important for patterning the ventricular conduction system as evidenced by postnatal patterning defects observed with CCS-LacZ.
Notch signaling controls many facets of development, postnatal homeostasis and disease in many organ systems. The results presented here reveal a novel function for Notch signaling to influence cardiomyocyte cell fate decisions, and future investigations will further probe the interactions between Notch and other signaling pathways known to orchestrate conduction system lineage decisions in the heart.
Supplementary Material
Conduction disorders are a significant cause of morbidity and mortality, often requiring implantation of electronic pacemakers to coordinate activation of the heart. Though pacemaker technology is quite advanced, there are important limitations to electronic devices when compared with native conduction tissues. Cellular based replacement strategies and regenerative approaches for the treatment of several cardiac diseases, including heart failure, are currently being tested in animals and humans. One suchstrategy involves reprogramming, or converting one cell type into another cell type. We provide evidence that a single factor, Notch, is capable of reprogramming cardiomyocytes into Purkinje-like conduction cells both in vivo and in vitro. Notch activation produces changes in gene expression and cellular electrophysiology in cardiomyocytes as late as the perinatal period. The ability to manipulate cardiomyocyte cell fate toward cells of the conduction lineage may enable development of therapies to replace or augment electronic pacemakers in individuals with conduction disorders.
Acknowledgments
We thank Warren Pear for providing the ICNX virus. We thank Ashley Cohen, Feiyan Liu, Jie Zhang and Fang-Yu Liu for technical support.
Funding Sources: Stacey Rentschler, M.D., Ph.D., holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. A.Y. was a research fellow supported by the Sarnoff Cardiovascular Research Foundation. VVP is an Innovative Researcher of the AHA. Supported by K08 HL107449 from NHLBI to S.R., R01 HL105734 to VVP, R01 HL105983 and NYSTEM N08G-132 to GIF, U01 HL100405, RO1 HL095634, and the AHA-Jon Holden DeHaan Myogenesis Center to JAE.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Rosen MR, Robinson RB, Brink PR, Cohen IS. The road to biological pacing. Nat Rev Cardiol. 2011;8:656–666. doi: 10.1038/nrcardio.2011.120. [DOI] [PubMed] [Google Scholar]
- 2.Gourdie RG, Mima T, Thompson RP, Mikawa T. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development. 1995;121:1423–1431. doi: 10.1242/dev.121.5.1423. [DOI] [PubMed] [Google Scholar]
- 3.Cheng G, Litchenberg WH, Cole GJ, Mikawa T, Thompson RP, Gourdie RG. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development. 1999;126:5041–5049. doi: 10.1242/dev.126.22.5041. [DOI] [PubMed] [Google Scholar]
- 4.Miquerol L, Moreno-Rascon N, Beyer S, Dupays L, Meilhac SM, Buckingham ME, Franco D, Kelly RG. Biphasic development of the mammalian ventricular conduction system. Circ Res. 2010;107:153–161. doi: 10.1161/CIRCRESAHA.110.218156. [DOI] [PubMed] [Google Scholar]
- 5.Gourdie RG, Wei Y, Kim D, Klatt SC, Mikawa T. Endothelin-induced conversion of embryonic heart muscle cells into impulse-conducting Purkinje fibers. Proc Natl Acad Sci U S A. 1998;95:6815–6818. doi: 10.1073/pnas.95.12.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–786. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiese C, Grieskamp T, Airik R, Mommersteeg MT, Gardiwal A, de Gier-de Vries C, Schuster-Gossler K, Moorman AF, Kispert A, Christoffels VM. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ Res. 2009;104:388–397. doi: 10.1161/CIRCRESAHA.108.187062. [DOI] [PubMed] [Google Scholar]
- 9.Bakker ML, Boukens BJ, Mommersteeg MT, Brons JF, Wakker V, Moorman AF, Christoffels VM. Transcription factor Tbx3 is required for the specification of the atrioventricular conduction system. Circ Res. 2008;102:1340–1349. doi: 10.1161/CIRCRESAHA.107.169565. [DOI] [PubMed] [Google Scholar]
- 10.Hoogaars WM, Engel A, Brons JF, Verkerk AO, de Lange FJ, Wong LY, Bakker ML, Clout DE, Wakker V, Barnett P, Ravesloot JH, Moorman AF, Verheijck EE, Christoffels VM. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007;21:1098–1112. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jay PY, Harris BS, Maguire CT, Buerger A, Wakimoto H, Tanaka M, Kupershmidt S, Roden DM, Schultheiss TM, O’Brien TX, Gourdie RG, Berul CI, Izumo S. Nkx2–5 mutation causes anatomic hypoplasia of the cardiac conduction system. J Clin Invest. 2004;113:1130–1137. doi: 10.1172/JCI19846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meysen S, Marger L, Hewett KW, Jarry-Guichard T, Agarkova I, Chauvin JP, Perriard JC, Izumo S, Gourdie RG, Mangoni ME, Nargeot J, Gros D, Miquerol L. Nkx2.5 cell-autonomous gene function is required for the postnatal formation of the peripheral ventricular conduction system. Dev Biol. 2007;303:740–753. doi: 10.1016/j.ydbio.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 13.Moskowitz IP, Kim JB, Moore ML, Wolf CM, Peterson MA, Shendure J, Nobrega MA, Yokota Y, Berul C, Izumo S, Seidman JG, Seidman CE. A molecular pathway including Id2, Tbx5, and Nkx2–5 required for cardiac conduction system development. Cell. 2007;129:1365–1376. doi: 10.1016/j.cell.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Milan DJ, Giokas AC, Serluca FC, Peterson RT, MacRae CA. Notch1b and neuregulin are required for specification of central cardiac conduction tissue. Development. 2006;133:1125–1132. doi: 10.1242/dev.02279. [DOI] [PubMed] [Google Scholar]
- 15.Rentschler S, Harris BS, Kuznekoff L, Jain R, Manderfield L, Lu MM, Morley GE, Patel VV, Epstein JA. Notch signaling regulates murine atrioventricular conduction and the formation of accessory pathways. J Clin Invest. 2011;121:525–533. doi: 10.1172/JCI44470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rentschler S, Vaidya DM, Tamaddon H, Degenhardt K, Sassoon D, Morley GE, Jalife J, Fishman GI. Visualization and functional characterization of the developing murine cardiac conduction system. Development. 2001;128:1785–1792. doi: 10.1242/dev.128.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallante BA, Giovannone S, Fang-Yu L, Zhang J, Liu N, Kang G, Dun W, Boyden PA, Fishman GI. Contactin-2 expression in the cardiac Purkinje fiber network. Circ Arrhythm Electrophysiol. 2010;3:186–194. doi: 10.1161/CIRCEP.109.928820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aster JC, Xu L, Karnell FG, Patriub V, Pui JC, Pear WS. Essential roles for ankyrin repeat and transactivation domains in induction of T-cell leukemia by notch1. Mol Cell Biol. 2000;20:7505–7515. doi: 10.1128/mcb.20.20.7505-7515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985;249:H1056–1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- 21.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 22.Ismat FA, Zhang M, Kook H, Huang B, Zhou R, Ferrari VA, Epstein JA, Patel VV. Homeobox protein Hop functions in the adult cardiac conduction system. Circ Res. 2005;96:898–903. doi: 10.1161/01.RES.0000163108.47258.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann S, Layh B, Ludwig A. Novel insights into the distribution of cardiac HCN channels: An expression study in the mouse heart. J Mol Cell Cardiol. 2011;51:997–1006. doi: 10.1016/j.yjmcc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, Srivastava D. Notch post-translationally regulates beta-catenin protein in stem and progenitor cells. Nat Cell Biol. 2011;13:1244–1251. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu J, Plotnikov AN, Danilo P, Jr, Shlapakova I, Cohen IS, Robinson RB, Rosen MR. Expression and function of a biological pacemaker in canine heart. Circulation. 2003;107:1106–1109. doi: 10.1161/01.cir.0000059939.97249.2c. [DOI] [PubMed] [Google Scholar]
- 26.Plotnikov AN, Sosunov EA, Qu J, Shlapakova IN, Anyukhovsky EP, Liu L, Janse MJ, Brink PR, Cohen IS, Robinson RB, Danilo P, Jr, Rosen MR. Biological pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circulation. 2004;109:506–512. doi: 10.1161/01.CIR.0000114527.10764.CC. [DOI] [PubMed] [Google Scholar]
- 27.Bucchi A, Plotnikov AN, Shlapakova I, Danilo P, Jr, Kryukova Y, Qu J, Lu Z, Liu H, Pan Z, Potapova I, KenKnight B, Girouard S, Cohen IS, Brink PR, Robinson RB, Rosen MR. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation. 2006;114:992–999. doi: 10.1161/CIRCULATIONAHA.106.617613. [DOI] [PubMed] [Google Scholar]
- 28.Pashmforoush M, Lu JT, Chen H, Amand TS, Kondo R, Pradervand S, Evans SM, Clark B, Feramisco JR, Giles W, Ho SY, Benson DW, Silberbach M, Shou W, Chien KR. Nkx2–5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117:373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- 29.Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 30.Briggs LE, Takeda M, Cuadra AE, Wakimoto H, Marks MH, Walker AJ, Seki T, Oh SP, Lu JT, Sumners C, Raizada MK, Horikoshi N, Weinberg EO, Yasui K, Ikeda Y, Chien KR, Kasahara H. Perinatal loss of Nkx2–5 results in rapid conduction and contraction defects. Circ Res. 2008;103:580–590. doi: 10.1161/CIRCRESAHA.108.171835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munshi NV, McAnally J, Bezprozvannaya S, Berry JM, Richardson JA, Hill JA, Olson EN. Cx30.2 enhancer analysis identifies Gata4 as a novel regulator of atrioventricular delay. Development. 2009;136:2665–2674. doi: 10.1242/dev.038562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahurij ND, Gittenberger-De Groot AC, Kolditz DP, Bokenkamp R, Schalij MJ, Poelmann RE, Blom NA. Accessory atrioventricular myocardial connections in the developing human heart: relevance for perinatal supraventricular tachycardias. Circulation. 2008;117:2850–2858. doi: 10.1161/CIRCULATIONAHA.107.756288. [DOI] [PubMed] [Google Scholar]
- 33.Light PE, Wallace CH, Dyck JR. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation. 2003;107:1962–1965. doi: 10.1161/01.CIR.0000069269.60167.02. [DOI] [PubMed] [Google Scholar]
- 34.Aanhaanen WT, Boukens BJ, Sizarov A, Wakker V, de Gier-de Vries C, van Ginneken AC, Moorman AF, Coronel R, Christoffels VM. Defective Tbx2-dependent patterning of the atrioventricular canal myocardium causes accessory pathway formation in mice. J Clin Invest. 2011;121:534–544. doi: 10.1172/JCI44350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Gloan L, Pichon O, Isidor B, Boceno M, Rival JM, David A, Le Caignec C. A 8.26Mb deletion in 6q16 and a 4.95Mb deletion in 20p12 including JAG1 and BMP2 in a patient with Alagille syndrome and Wolff-Parkinson-White syndrome. Eur J Med Genet. 2008;51:651–657. doi: 10.1016/j.ejmg.2008.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.