Abstract
Commercial gadolinium magnetic resonance imaging (MRI) contrast agents are limited by low relaxivity (r1) and coordination to only a single water molecule (q = 1). Consequently, gram quantities of these agents must be injected to obtain sufficient diagnostic contrast. In this study, MRI contrast agents for T1 and T2 relaxivity were synthesized using hydroxypyridinone (HOPO) and terephthalamide (TAM) chelators with mesityl and 1,4,7-triazacyclononane (TACN) capping moieties. When covalently conjugated to a highly biocompatible esteramide dendrimer, T2 relaxation rates up to 52 mM-1s-1 and T1 relaxation rates up to 31 mM-1s-1 per gadolinium are observed under clinically relevant conditions. These values are believed to be brought about by using a dendritic macromolecule to decrease the molecular tumbling time of the small molecule complexes. These agents also show high aqueous solubility and low toxicity in vitro. In this study we report six new compounds: three discrete complexes and three dendrimer conjugates.
Keywords: MRI contrast agent; hydroxypyridinone (HOPO); relaxivity; 1,4,7-triazacyclononane (TACN); terephthalamide (TAM); dendrimer; esteramide (EA) dendrimer; drug delivery; gadolinium
Introduction
Magnetic resonance imaging (MRI) utilizes paramagnetic Gd(III) contrast agents (CA's) to distinguish background tissue from regions of diagnostic interest. MRI CA's provide clarity in images of cancer, atherosclerosis, stroke, and other abnormalities, revealing their exact location and size. Currently, ten Gd(III) CA's are approved for clinical use worldwide: Gd-DTPA (Gd-diethylene triamine pentaacetic acid, Gadopentetate), Gd-DOTA (Gd-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid, Gadoterate) and their derivatives. These agents have suboptimal water exchange rates (i.e. slower than the Larmour frequency of clinical MRI imagers ideal for this class of agent) and bind only a single water molecule in the inner metal coordination sphere (q = 1). These parameters contribute to low relaxivity (r1 is a function of T1 relaxation time and r2 is a function of T2 relaxation time; measured in mM-1s-1), a determinant of the length of water or proton exchange times. For Gd-DOTA, r1 = 3.0 mM-1s-1 and r2 = 3.9 mM-1s-1, therefore, gram quantities of these agents must be administered in order to obtain an adequate image contrast (1-4).
According to the Solomon-Bloembergen-Morgan (SBM) Equations (5-7), relaxivity can be increased by having shorter water exchange times (τm), a slower tumbling rate (1/τr), and a greater number of inner-sphere water molecules (q). Current MRI contrast agent prototypes improve on all three of these parameters as compared to Gd-DTPA and Gd-DOTA families of clinically approved commercial agents (8-9).
Previous research in the Raymond group has developed MRI contrast agents for T1 applications with fast water exchange (τm) and higher q values (10-11). In 2007, the first q = 3 Gd(III) contrast agents with fast water exchange and high thermodynamic stability were developed, though the use of the 1,4,7-Triazacyclononane (TACN) cap and hexadentate hydroxypyridinone (HOPO) ligands (12, Figure 1).
Figure 1.
Previously developed hexadentate oxygen donor Gd(III)MRI contrast agents with fast water exchange and high q values (12). Left: Gd-TACN-tris-(1-Me)-3,2-HOPO (1). Right: Gd-TACN-tris-1,2-HOPO (2).
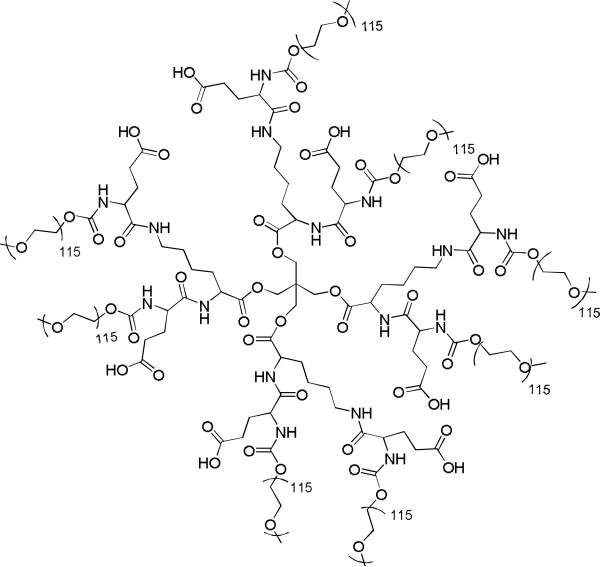
Increasing relaxivity by slowing the molecular tumbling rate (1/τr) has been achieved by conjugation to macromolecules. Increases in molecular weight by addition to a dendrimer (13-15) protein (16-18), nanoparticle (19), or nanodiamond (20) has effectively increased T1 relaxivity in Gd-DOTA, Gd-DTPA, and Gd-HOPO ligand chelator systems. However, this increase in molecular weight will ideally be accompanied by additional mechanisms of excretion, as macromolecules larger than ~40 kDa are not easily cleared from the body. Dendrimers of this size developed in the Fréchet group have been shown to be highly biocompatible and effective macromolecules for drug delivery (21-24), with favorable bioaccumulation and excretion profiles. The structure of these dendrimers can be readily modulated to incorporate slow degradation in vivo, thus facilitating excretion as exemplified by the esteramide dendrimers used for this study (25, Figure 2). In addition to increasing the relaxivity of the gadolinium, the dendrimer can also provide high water solubility, protection from premature enzymatic degradation, and longer blood residence times, leading to an overall dramatic improvement in the pharmacokinetic profile of the agent.
Figure 2.
Previously reported esteramide dendrimer (EA dendrimer, 3) developed for drug delivery. The architecture is designed for stability for the duration of a complete MRI scan with biodegradability as an eventual excretion pathway (25).
Excretion profiles are of importance in gadolinium based CAs because a disorder known as Nephrogenic Systemic Fibrosis (NSF) has been associated with renal deficient patients receiving acyclic Gd(III) contrast agents. In these patients lacking a proper excretion mechanism due to severe kidney dysfunction, small molecule contrast agents not excreted within several days or weeks can dissociate gadolinium, leaving the toxic ligands and free Gd(III) ions in the body. This Gd(III) has a high affinity for Ca(II) ion channels and has been associated with a thickening of organs and skin (1). NSF is currently incurable, although preventative measures and efforts to couple therapy with dialysis therapy have been explored (26). Novel contrast agents with higher relaxivity per-Gd would require injection of a much lower dose, which coupled to stronger binding of the gadolinium by the ligand, would contribute to enhanced patient safety. Additionally, because water soluble macromolecules below 40 kDa tend to be excreted well from the body and do not passively cross cell membranes, covalent attachment of strongly binding chelates to dendrimers and similar macromolecules may allow for easier excretion of Gd(III) than that observed with free small molecule chelates.
Much attention is paid to T1 relaxivity, or positive mode imaging, as this is the main mode of imaging currently in use. T1 contrast is inherently easier to improve as tissue has a slower T1 relaxation rate than T2 under physiological conditions. However, contrast agents that are versatile for both T1 and T2 relaxivity would be potentially be useful for improving scans taken in either imaging mode. Gd(III) contrast agents with highly desirable attributes according to the SBM Equation also exhibit high T2 relaxivity values, and some have acceptable r2/r1 values to enhance T2 images as well. High T2 relaxivity contrast agents are used for negative mode imaging, preferred for imaging softer tissue. Currently, clinical T2 image enhancement is achieved through paramagnetic Fe(III) agents (27), however, a single versatile agent such as current extracellular Gd(III) agents provide patients with fewer opportunities for adverse medication reactions and clinical issues.
Results/Discussion
Complex Synthesis & Characterization
Previously synthesized TACN-capped HOPO ligands lacked a synthetic handle to which a dendritic macromolecule could be attached (12). A novel TACN capped complex was synthesized with two (1-Me)-3,2-HOPO ligands and a functionalized terephthalamide (TAM) ligand, for a hexadentate all-oxygen donor Gd(III) complex (see Supporting Information for experimental detail). Gd-TACN-bis(1-Me)-3,2-HOPO-TAM-ethylamine was conjugated via an amide bond to the esteramide dendrimer, which can theoretically yield up to eight complexes per dendrimer (Scheme 1). In the case of TACN capped ligands such as 4, actual loading was typically significantly lower than the theortical limit. Since small molecule complex 4 was different than the tris-(1-Me)-3,2-HOPO ligands previously described (Werner et al. 2007), an analogue of this with an ethyl functionality, Gd-TACN-bis(1-Me)-3,2-HOPO-TAM-ethyl, 5, was synthesized for relaxivity and cytotoxicity comparison to the novel complex and conjugated macromolecule (Figure 3). Table 1 shows the relaxivity measurements for these small-molecule complex and dendrimer conjugated complexes.
Scheme 1.
Conjugation of the Gd-TACN-bis-(1-Me)-3,2-HOPO-TAM-ethylamine complex to the EA dendrimer (6). Conjugations to other ligands were carried out under similar conditions.
Figure 3.

Novel TACN capped complexes to increase T1 and T2 relaxivity. Gd-TACN-trisHOPO (12) has a q = 3 Gd-TACN-bis-(1-Me)-3,2-HOPO-TAM-ethylamine (left, 4) was conjugated to the esteramide dendrimer and Gd-TACN-bis-(1-Me)-3,2-HOPO-TAM-ethyl (right, 5) was used as a small molecule analogue to compare relaxivity and cytotoxicity.
Table 1.
Small molecule and macromolecular per Gd(III) r1 and r2 relaxivity data at 60 MHz and 37 °C.
| Complex | r1 complex (mM-1s-1) | r1 Complex + Dendrimer (mM-1s-1) | r2 Complex (mM-1s-1) | r2 Complex + Dendrimer (mM-1s-1) |
|---|---|---|---|---|
| Mn-DPDP (Teslascan™) (27) | 1.88*at 20 mHz | -- | 2.18 *at 20 mHz | -- |
| Gd-DTPA (Magnevists™) (1) | 3.3 ± 0.3 | -- | 3.9 | -- |
| Gd-DOTA (Dotarem™) (1) | 3.0 ± 0.3 | -- | 3.5 | -- |
| Gd-TACN-N1 (4) | 9.89 ± 0.03 | 31.0 ± 0.4 | 16 ± 0.5 | 52 ± 2 |
| Gd-Mes-N1 (7) | 9.3 ± 0.2* | 19.6 ± 0.4 | 16.0 ± 0.2 | 29.2 ± 0.3 |
| Gd-Mes-N3 (8) | 7.2 ± 0.2* | 18.7 ± 0.3 | 12.6 ± 0.4 | 23.6 ± 0.3 |
| Gd-TACN-ethyl (5) | 7.27 ± 0.09 | -- | 11.2 ± 0.2 | -- |
| Gd-Mes-Asp- Asp2-12OH (9) | 14.8 ± 0.4 | -- | 17.4 ± 0.1 | -- |
| Gd-Mes-PEG450 (10) | 12.01 ± 0.05 | -- | 21.3 ± 0.3 | -- |
Previously only reported at 298K and 20 MHz (11.9 for Mes-N1 and 9.9 Mes-N3).
Data listed novel to this study (Werner et al. 2009).
Previously synthesized mesityl-capped HOPO ligands had high q values of 2-3, but lacked the solubility (<100 μM in water) required for clinical use. Gd-Mesbis(1-Me)-3,2-HOPO-TAM-ethylamine and Gd-Mes-bis(1-Me)-3,2-HOPO-TAM-N3 (Figure 4, 9) were conjugated to the esteramide dendrimer as above with the expectation that the PEGylated dendritic macromolecule would suitably solubilize them. Not only did this impart improved aqueous solubility to the complexes, but also substantially increased the per gadolinium r1 and r2 relaxivities over those of the small molecules. (Table 1). Furthermore, while the TACN capped ligands above typically exhibited low (12-13%) loading on the dendrimer according to ICP, mesityl capped ligands 7 and 8 were attached to the dendrimer with an average of seven and six Gd(III) complexes per dendrimer, respectively (See Supporting Information).
Figure 4.
Gd-Mes-bis(1-Me)-3,2-HOPO-TAM-ethylamine (left, 7) and Gd-Mesbis(1-Me)-3,2-HOPO-TAM-ethylamine-bisethylamine (right, 8) were originally synthesized to capitalize on high relaxivity stemming from a higher q value. However, these compounds were not suitable for MRI contrast agents due to their low solubility (<10 μM in water) and subsequent aggregation (9). Upon conjugation to the esteramide dendrimer, they become suitably soluble and have high relaxivities.
Additionally, a mesityl complex with a PEG450 (9) was compared with a novel branched mesityl complex, Gd-Mes-bis(1-Me)-3,2-HOPO-TAM-Asp-Asp2-12OH (Figure 5). With the TREN capped system, the branched dendrimer had a larger T1 relaxivity and it was sought to compare both the T1 and T2 relaxivities of these higher q coordination compounds. Although solubility of these complexes was increased significantly (> 1 mM) over their small molecule counterparts, the large doses required for MRI imaging would still exclude these complexes due to solubility.
Figure 5.
Mesityl capped ligands with branched dendrimer (Gd-Mes-bis-(1-Me)-3,2-HOPO-TAM-Asp-Asp2-12OH, left, 9) and PEGylated dendrimer (Gd-Mes-bis-(1-Me)-3,2-HOPO-TAM-PEG450, right, 10). The branched dendrimer uses its rigidity to achieve a larger T1 relaxivity and the PEGylated dendrimer uses its much larger surface area to achieve a larger T2 relaxivity.
T1 & T2 Relaxivity
Due to their coordination of two or three water molecules rather than one, the small molecule complexes show T1 relaxivity values two to three times higher than that of commercial contrast agents (7-9 mM-1s-1, Table 1). The T2 relaxivities were relatively high for small molecules as well. The outer-sphere water exchange, which is a larger factor in T2 than in T1 (28-29), favors interaction with the TACN cap over the hydrophobic mesityl cap. The r2/r1 ratio (or T2 relaxivity to T1 relaxivity), is also important (Table 2). The small molecule contrast agents outperform the commercial contrast agents in this aspect as well.
Table 2.
Relative ratios of r2 relaxation rates compared with r1 relaxation rates.
| Complex | r2/r1 |
|---|---|
| Mn-DPDP | 1.16 |
| Gd-DTPA | 1.18 |
| Gd-DOTA | 1.17 |
| Gd-TACN-N1 | 1.61 |
| Gd-TACN-N1 + EA dendrimer | 1.68 |
| Gd-Mes-N1 | 1.72 |
| Gd-Mes-N1 + EA Dendrimer | 1.49 |
| Gd-Mes-N3 | 1.75 |
| Gd-Mes-N3 + EA Dendrimer | 1.26 |
| Gd-TACN-ethyl | 1.54 |
| Gd-Mes-Asp-Asp-12OH | 1.18 |
| Gd-Mes-PEG450 | 1.78 |
The mesityl capped HOPO complexes show how relaxivity changes as the molecular weight and the shape of molecule are modified. The Gd-Mesityl-PEG450 complex has a similar molecular weight to that of the branched dendrimer Gd-Mes-Asp-Asp2-12OH. However despite their chelation and structural similarities, both their T1 and T2 relaxivities vary significantly differ from each other (Table 1). While the more dendritic branched mesityl (Asp-Asp2-12OH) had a larger T1 relaxivity than the small molecule , the mesityl with a PEG450 chain obtained a much larger increase in its T2 relaxivity and therefore exhibits a much larger r2/r1 relaxivity ratio (Table 2). This was attributed to the much larger surface area of the PEG450 molecule as compared to the ASP-ASP2-12OH molecule. The larger surface area allowed for many more outer sphere solvent interactions, significantly increasing the r2 relaxivity. The branched dendrimer slows the tumbling of the gadolinium complex through its high molecular weight and steric restrictions, but coordinates fewer outer sphere water molecules due to its much lower hydrodynamic volume, leading to a larger increase in T1 relaxivity than T2.
Both the Gd-TACN capped complex and the Gd-Mesityl capped complexes were conjugated to the esteramide dendrimer (taScheme 1). The esteramide dendrimer has eight complex loading sites in its core which are sterically hindered enough to slow the local motion of the Gd(III) along with slowing the rotational correlation time. This hindrance of molecular motion may explain why the T1 and T2 relaxivities per Gd(III) are substantially increased by this conjugation (Table 1).
Gd-TACN-bis(1-Me)-3,2-HOPO-TAM-ethylamine esteramide conjugate has a per Gd(III) relaxivity of 31 (mM-1s-1) for r1 and 52 (mM-1s-1) for r2 for a r2/r1 of 1.68. The T1 relaxivity is about ten times that of the commercial contrast agents at physiological conditions (37 °C) and clinically relevant field strength (60 MHz). Although the relaxivity is much larger, it is done at the expense of increasing molecular weight by 40-50 fold. This is a limitation of this conjugate, as about a fivefold higher mass dose would be required clinically. The relaxivity values of the Gdmesityl complexes increased upon conjugation to the dendrimer, however, not as dramatically. However, upon conjugation to the esteramide dendrimer, these complexes, as well as those described above, obtained high solubility (> 50 mM) in water and exhibited no or minimal aggregation as measured by Dynamic Light Scattering (DLS, see Supporting Information). This is notable data for other potential MRI contrast agents, which exhibit high relaxivity through high q values and fast water exchange, but lack the required solubility under physiological conditions.
Cytotoxicity
Cytotoxicity of these compounds was conducted so that animal studies may be pursued in the future. Since the dendrimer has a murine in vivo excretion time of 48-72 h (25), Gd(III) complex-dendrimer conjugates must be evaluated over longer durations than small molecule cytotoxicity testing. Complexes and dendritic macromolecule conjugates exhibit no cytotoxicity (see Supporting Information) up to 25 μM over 72 hours using HeLa cells.
Conclusions
The relaxivity, solubility, and biocompatibility of several MRI contrast agents have been significantly enhanced in this study by covalent attachment to polymeric and dendrimer carriers, and novel small molecule T1 and T2 MRI contrast agents with fast water exchange and high q values have been synthesized and evaluated for biological suitability. Small molecules attached to either branched or linear dendritic macromolecules have been evaluated to show T1 and T2 relaxivity, solubility and suitability increases. An esteramide dendrimer was conjugated via peptide bond to q = 2-3 MRI contrast agents to slow molecular tumbling time and significantly increase T1 and T2 relaxivity under physiologically relevant conditions. Altering systemic parameters increased relaxivity and biocompatibility significantly in q = 2-3 systems. As a result of much higher per-Gd(III) relaxivity, less agent would be required, decreasing patient exposure to potentially toxic Gd(III) ions. These agents also show substantially improved relaxivity and solubility over the small molecule complexes, making them attractive candidates for further exploration as next generation contrast agents.
Acknowledgements
The authors acknowledge NIH Grant R01 EB 002047, NIH Grant HL069832, Dr. Sylvie L. Pailloux, Dr. Ankona Datta, and Dr. Eric J. Werner, Adam D. Hill, and Dr. Christopher M. Andolina.
Literature
- 1.Port M, Idee JM, Medina C, Robic C, Sabatou M, Corot C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals. 2008;21:469–490. doi: 10.1007/s10534-008-9135-x. [DOI] [PubMed] [Google Scholar]
- 2.Major JL, Meade TJ. Bioresponsive, cell-penetrating and multimeric MR contrast agents. Accounts. Chem. Res. 2009;42:893–903. doi: 10.1021/ar800245h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caravan P. Protein-targeted gadolinium-based magnetic resonance imaging (MRI) contrast agents: design and mechanism of action. Accounts. Chem. Res. 2009;42:851–862. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 4.De Leon-Rodriguez LM, Lubag AJM, Malloy CR, Martinez GV, Gillies RJ, Sherry AD. Responsive MRI Agents for Sensing Metabolism in Vivo. Accounts. Chem. Res. 42:948–957. doi: 10.1021/ar800237f. 20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon I, Bloembergen N. Nuclear magnetic interactions in the HF molecule. J. Chem. Phys. 1956;25:261. [Google Scholar]
- 6.Bloembergen N. Proton relaxation times in paramagnetic solutions. J. Chem. Phys. 1957;27:572. [Google Scholar]
- 7.Bloembergen N, Morgan LO. Proton relaxation times in paramagnetic solutions. Effects of electron spin relaxation. J. Chem. Phys. 1961;34:842. [Google Scholar]
- 8.Pierre VC, Botta M, Aime S, Raymond KN. Substituent Effects on Gd(III)-based MRI contrast agents: optimizing the stability and selectivity of the complex and the number of coordinated water molecules. Inorg. Chem. 2006;45:8355–8364. doi: 10.1021/ic061262q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner EJ, Botta M, Aime S, Raymond KN. Effect of a mesitylene-based ligand cap on the relaxometric properties of Gd(III) hydroxypyridonate MRI contrast agents. Contrast Media Mole Imaging. 2009;4:220–229. doi: 10.1002/cmmi.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raymond KN, Pierre VC. Next generation, high relaxivity MRI agents. Bioconj. Chem. 2005;16:3–8. doi: 10.1021/bc049817y. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Franklin SJ, Wisenhunt DW, Jr, Raymond KN. Gadolinium complex of tris[93-hydroxy-1-methyl-2-oxo-1,2-didehydropyridine-4-carboxamido)ethyl]-amine: a new class of gadolinium magnetic resonance relaxation agents. J. Am. Chem. Soc. 1995;117:7245–7246. [Google Scholar]
- 12.Werner EJ, Avedano S, Botta M, Hay BP, Moore EG, Aime S, Raymond KN. Highly soluble tris-hydroxypyridonate Gd(III) complexes with increased hydration number, fast water exchange, slow electronic relaxation, and high relaxivity. J. Am. Chem. Soc. 1870–1871;2007:129. doi: 10.1021/ja068026z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiener EC, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DW, Lauterbur PC. Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents. Magn. Reson. Med. 1994;31:1–8. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]
- 14.Wiener EC, Konda S, Shadron A, Brechbiel MW, Gansow O. Targeting dendrimer-chelates to tumors and tumor cells expressing the high-affinity folate receptor. Invest. Radiol. 1997;32:748–754. doi: 10.1097/00004424-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Pierre VC, Botta M, Raymond KN. Dendrimeric gadolinium chelate with fast water exchange and high relaxivity at high magnetic field strength. J. Am. Chem. Soc. 2005;127:504–505. doi: 10.1021/ja045263y. [DOI] [PubMed] [Google Scholar]
- 16.Datta A, Raymond KN. Gd-hydroxypyridinone (HOPO)-based high-relaxivity magnetic resonance imaging (MRI) contrast agents. Accounts. Chem. Res. 2009;42:938–947. doi: 10.1021/ar800250h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooker JM, Datta A, Botta M, Raymond KN, Francis MB. Magnetic resonance contrast agents from viral capsid shells: a comparison of exterior and interior cargo strategies. Nano Lett. 2007;7:2207–2210. doi: 10.1021/nl070512c. [DOI] [PubMed] [Google Scholar]
- 18.Werner EJ, Datta A, Jocher CJ, Raymond KN. High-relaxivity MRI contrast agents: where coordination chemistry meets medical imaging. Angew. Chem. Intl. Ed. 2008;47:8568–8580. doi: 10.1002/anie.200800212. [DOI] [PubMed] [Google Scholar]
- 19.Song Y, Xu X, MacRenaris KW, Zhang X-Q, Mirkin CA, Meade TJ. Multimodal gadolinium-enriched DNA-gold nanoparticle conjugates for cellular imaging. Angew. Chem. Intl. Ed. 2009;48:9143–9147. doi: 10.1002/anie.200904666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manus LM, Mastarone DJ, Waters EA, Zhang X-Q, Schultz-Sikma EA, MacRenaris KW, Ho D, Meade TJ. Gd(III)-nanodiamond conjugates for MRI contrast enhancement. Nano. Lett. 2010;10:484–489. doi: 10.1021/nl903264h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihre HR, Padilla De Jesús O, Szoka FC, Jr., Fréchet JMJ. Polyester Dendritic Systems for Drug Delivery Applications: Design, Synthesis and Characterization. Bioconj. Chem. 2002;13:443–52. doi: 10.1021/bc010102u. [DOI] [PubMed] [Google Scholar]
- 22.Padilla De Jesús O, Ihre HR, Gagne L, Fréchet JMJ, Szoka FC., Jr. Polyester Dendritic Systems for Drug Delivery Applications: In Vitro and In Vivo Evaluation. Bioconj. Chem. 2002;13:453–61. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 23.Fox ME, Szoka FC, Frechet JMJ. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Accounts. Chem. Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fréchet JMJ, Dy EE, Szoka FC. A Single Dose of Doxorubcin-functionalized Bow-Tie Dendrimer Cures Mice Bearing C-26 Colon Carcinomas. Proc. Nat. Acad. Soc. USA. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Poll DG, Kieler-Ferguson HM, Floyd WC, Guillaudeu SJ, Jerger K, Szoka FC, Frechet JM. Design, synthesis, and biological evaluation of a robust, biodegradable dendrimer. Bioconj. Chem. 2010;21:764–773. doi: 10.1021/bc900553n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yantasee W, Fryxell GE, Porter GA, Pattamakomsan K, Sukwarotwat V, Chouyyok W, Koonsiripaiboon V, Xu J, Raymond KN. Novel sorbents for removal of gadolinium-based contrast agents in sorbent dialysis and hemoperfusion: preventive approaches to nephrogenic systemic fibrosis. Nanomed. 2010;6:1–8. doi: 10.1016/j.nano.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babes L, Denizot B, Tanguy G, Le Jeune JJ, Jallet P. Synthesis of iron oxide nanoparticles used as MRI contrast agents: a parametric study. J. Colloid and Interface Sci. 1999;212:478–482. doi: 10.1006/jcis.1998.6053. [DOI] [PubMed] [Google Scholar]
- 28.Gillis P, Moiny F, Brooks RA. On T(2)-shortening by strongly magnetized spheres: a partial refocusing model. Magn. Reson. Med. 2002;47:257–263. doi: 10.1002/mrm.10059. [DOI] [PubMed] [Google Scholar]
- 29.Brooks RA, Moiny F, Gillis P. On T2-shortening by weakly magnetized particles: the chemical exchange model. Magn. Reson. Med. 2001;45:1014–1020. doi: 10.1002/mrm.1135. [DOI] [PubMed] [Google Scholar]