Abstract
Non-shivering thermogenesis in brown adipose tissue (BAT) plays an important role in thermoregulatory cold-defense and, through its metabolic consumption of energy reserves to produce heat, can affect the long-term regulation of adiposity. An orexinergic pathway from the perifornical lateral hypothalamus (PeF/LH) to the rostral raphe pallidus (rRPa) has been demonstrated to increase the gain of the excitatory drives to medullary sympathetic premotor neurons controlling BAT sympathetic outflow and BAT thermogenesis. With this background, we consider neural mechanisms that could underlie orexin’s modulation of the excitability of BAT sympathetic premotor neurons in rRPa and the potential role of altered BAT thermogenesis in pathological conditions associated with the absence of the central orexin system. Overall, these new data enhance our understanding of the role of central orexin in regulating body temperature and energy homeostasis and provide further insight into the neurochemical regulation of BAT thermogenesis and metabolism.
Keywords: endocannabinoid, narcolepsy, obesity, stress, ultradian rhythm
Introduction
Orexins (hypocretins) are neuropeptides synthesized by neurons in the perifornical region of the lateral hypothalamus (PeF-LH)1,2 that have widespread projections that position the orexin system to influence a variety of behaviors and physiological functions including sleep-wake states and stress-arousal responses,3,4 as well as the neuroendocrine, metabolic and autonomic variables engaged during these states.5-8 Loss of orexin neurons from the PeF-LH leads to the disordered sleep patterns of narcolepsy and is often accompanied by lethargy and impairments in metabolic homeostasis, including a high risk for obesity9,10 and for altered thermoregulation.11
Brown adipose tissue (BAT) is a unique mammalian metabolic furnace that is under the direct control of the sympathetic nervous system12 and produces heat through the uncoupling of its mitochondrial oxidative phosphorylation.13 BAT thermogenesis plays a significant role in the maintenance of body temperature and BAT energy consumption contributes to body weight regulation, the importance of which has been recently reinforced with the demonstration of metabolically significant BAT depots in humans and the discovery of a reduced BAT activity in obese persons.14-16 Since mammalian, including human, BAT thermogenesis contributes to thermoregulatory and metabolic homeostasis and is altered during the behavioral and state changes with which changes in orexin are also associated, a connection between the orexin system and the central circuits that influence the sympathetic outflow to BAT has been sought.
Establishing a Connection between Orexin Neurotransmission and Central Regulation of BAT
In the study by Tupone and colleagues,17 anatomical tracing and orexin immunohistochemical localization were combined with in vivo electrophysiological techniques to elucidate a central neural pathway through which orexin neurons influence BAT thermogenesis and energy expenditure in rats. Viral retrograde tracing from BAT and cholera toxin retrograde tracing from the rostral raphe pallidus (rRPa) indicated that a population of orexin neurons in the PeF-LH is synaptically connected to BAT via a direct projection from the PeF-LH to the rRPa. The connection from PeF-LH to neurons in the rRPa is important because the rRPa contains sympathetic premotor neurons whose excitatory drive to BAT sympathetic preganglionic neurons in the thoracic spinal cord determines the sympathetic outflow to BAT and, in turn, the level of BAT metabolism and thermogenesis.
In anesthetized, paralyzed and artificially ventilated rats whose core temperature was maintained below 37°C, such that there was a low level of ongoing BAT sympathetic nerve activity (SNA), nanoinjection of orexin-A into the rRPa produced large and sustained increases in BAT sympathetic outflow, in BAT thermogenesis and in heart rate. Activation of neurons in the PeF-LH with nanoinjection of n-methyl-D-aspartate (NMDA) also markedly enhanced BAT SNA and BAT thermogenesis over a long time course. In contrast, in rats that were warmed slightly to core temperatures at which the BAT sympathetic nerve was quiescent, neither nanoinjection of orexin in rRPa nor direct, NMDA-mediated activation of PeF-LH neurons, including those containing orexin, increased BAT SNA or BAT temperature. These results indicate the ability of orexin released from the terminals of orexin neurons in PeF-LH directly into the rRPa to produce a strong potentiation of ongoing BAT SNA, BAT thermogenesis and BAT energy expenditure.
Orexin in rRPa Potentiates the Excitatory Drive to BAT Sympathetic Premotor Neurons
The potentiation of BAT SNA by orexin in the rRPa may be viewed as an increase in the gain of the excitatory inputs to BAT sympathetic premotor neurons in the rRPa—when orexin is released in the rRPa, the level of discharge of BAT sympathetic premotor neurons due to their excitatory inputs is augmented with respect to that in the absence of orexin. On the other hand, if the excitatory drive to BAT sympathetic premotor neurons is low (or the inhibitory inputs are sufficiently high), as in the case of a warm core temperature greater than ~37°C, then orexin in rRPa is not capable of augmenting the discharge of BAT sympathetic premotor neurons to a level that results in activity on the sympathetic nerve to BAT. The findings that cooling-evoked (i.e., thermoregulatory) sympathetic outflow to BAT is dependent on glutamate receptor activation in the rRPa18 and that blockade of local GABAA receptors in the rRPa with bicuculline elicits a potent increase in BAT SNA19 suggest basic mechanisms that could each contribute to the ability of orexin in the rRPa to increase the gain of the excitatory drive to BAT sympathetic premotor neurons and thereby facilitate ongoing sympathetic activity to BAT and BAT thermogenesis.
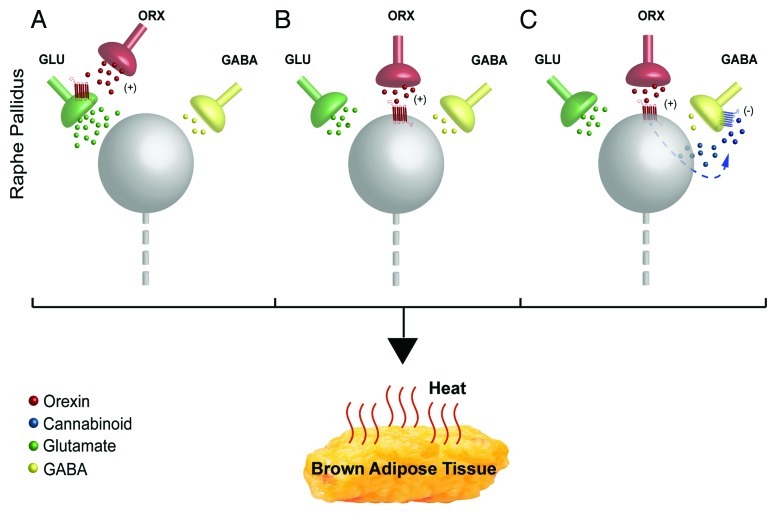
First, orexin could bind to orexin receptors on sympathetic premotor neurons (Fig. 1B), including serotonergic neurons,20,21 in rRPa to alter their responsiveness to excitatory synaptic inputs. Orexin has a potent effect at postsynaptic receptors, acting through G-protein coupled receptors to increase cytosolic calcium levels22 and orexin receptors have been localized on neurons in the rRPa,23 but there has been no further characterization of the medullary neurons expressing the orexin receptor. We have demonstrated that activation of serotonergic receptors in the spinal cord increases the gain of the spinal thermogenic network by potentiating glutamatergic excitation of BAT sympathetic preganglionic neurons.24,25 This result raises the interesting possibility that the BAT excitatory effect of orexin release in the rRPa could reflect a selective stimulation of local serotonergic BAT sympathetic premotor neurons producing a spinal serotonergic potentiation of descending glutamatergic drive to BAT sympathetic preganglionic neurons. Postsynaptic effects of orexin could also be mediated by orexin receptors on local interneurons in the rRPa area that, in turn, affect the activity of BAT sympathetic premotor neurons. Alternatively, orexin could act at orexin receptors on presynaptic terminals (Fig. 1A) to reduce GABA release or increase glutamate release onto BAT sympathetic premotor neurons in rRPa. In this regard, orexin increased the frequency of glutamatergic miniature postsynaptic potentials in the presence of TTX.22 Interestingly, orexin release in rRPa could increase BAT sympathetic premotor neuron activity by reducing GABA release through an endocannabinoid-mediated retrograde neurotransmission (Fig. 1C) as demonstrated to mediate orexin’s antinociceptive effect within the periaqueductal gray.26 Orexin could presynaptically potentiate glutamate release onto BAT sympathetic premotor neurons in the rRPa, similar to mechanisms suggested for the potentiation of masseter muscle tone with microinjection of orexin into the trigeminal nucleus.27 However, in the latter experiments, as in ours, if there is a strong dependence on glutamate receptor activation for excitatory transmission through the nucleus in which orexin is being injected, identifying a presynaptic, orexin receptor-mediated mechanism for glutamate release would require in vitro approaches rather than simply demonstrating a reduction in the excitatory effects of orexin by glutamate receptor blockade in vivo.
Figure 1. Potential synaptic mechanisms underlying the orexin-evoked increase in activity of sympathetic premotor neurons for BAT in rostral raphe pallidus (rRPa). (A) Orexin could bind to presynaptic orexin receptors to augment the ongoing release of glutamate onto BAT sympathetic premotor neurons (gray sphere). (B) Orexin could act at postsynaptic orexin receptors on BAT sympathetic premotor neurons to increase their excitability, thereby augmenting their discharge evoked by active glutamatergic inputs. (C) Orexin binding to postsynaptic orexin receptors could stimulate synthesis of endocannabinoid, which would increase the activity of the BAT sympathetic premotor neurons in rRPa by acting retrogradely to inhibit a tonic GABA release from presynaptic terminals.
Potential Physiological Sequelae of the Central Orexin Influence on BAT Thermogenesis
Orexin may play a role in the regulation of the ultradian rhythm of BAT thermogenesis28,29 which is characterized in rodents by increases in BAT temperature every ~1–2 h during the awake period of the ultradian sleep/wake cycle.28,30 Supporting this possibility, the activity of orexinergic neurons and the levels of orexin in the extracellular fluid oscillate with the ultradian sleep/wake cycle, with higher indices of orexin activity during the waking state31-33 and ventricular administration of orexin increases BAT temperature.34 Although an ultradian rhythm in BAT temperature in orexin-null mice has not been assessed, the ultradian sleep/wake cycle of these mice is disrupted, with shorter wakefulness periods than wild-type mice.30,35 We postulate that the ultradian increases in BAT temperature are mediated by the periodic release of orexin in the rRPa, resulting in increases in the gain of the BAT thermogenic pathway that increases body and brain temperatures. Although the functional implications of orexin’s ability to increase the gain of the thermogenic neurotransmission in the rRPa remain untested, the close correlation of BAT thermogenesis and the resulting increase in body temperature during periods of wakefulness suggests that BAT thermogenesis may contribute to an enhanced metabolism during periods of wakefulness or arousal that require enhanced performance. Conversely, low levels of thermogenesis during sleep states may act to conserve metabolic resources during a behavioral state in which energy stores are not being replenished. Indeed, by modulating the gain of BAT excitatory neurotransmission in the rRPa, the degree of orexin receptor activation in the rRPa should have a significant influence on the consumption of energy stores in white adipose tissue and thus on the regulation of body weight.
Narcolepsy is the neurological disorder attributable to reduced orexin neurotransmission36-38 and, although principally characterized by altered sleep/wake cycles, narcolepsy is also associated with obesity in human patients,9,39-43 despite a reduced caloric intake44 and a normal total physical activity compared either to healthy control subjects45 or to patients with idiopathic hypersomnia.9 Mice that lack orexin neurons also gain more weight than wild-type controls despite reduced food intake; however, a decrease in spontaneous motor activity likely also contributes to their weight gain.10 Conversely, augmented orexin activity prevents diet-induced obesity and could contribute to a lean phenotype.46 The excess weight gain in orexin-null mice is attributable to impaired thermogenesis in BAT.47 Although the potential role for diminished BAT activation in the increased incidence of obesity in narcoleptic patients has not been tested, this hypothesis would be consistent with the recent demonstrations of an inverse relationship between the activity of BAT and body mass index in adult humans14-16 and with the overall reduction in BAT energy consumption expected in the absence of orexin.17
Handling and a variety of other stressful situations for rodents elicit increases in core temperature to which BAT thermogenesis may contribute.48,49 Ablation of orexin neurons, but not the absence of orexin per se, reduces the increase in core body temperature evoked by repeated handling stress, indicating that orexin-containing neurons release neurotransmitters other than orexin (e.g., dynorphin or glutamate, which are normally co-expressed in orexin neurons50,51) to elicit stress-evoked increases in body temperature.52 However, orexin, likely derived from the placenta, is required for the development and differentiation of BAT and systemic orexin administration during gestation in orexin-null dams rescues this developmental defect in the newborn pups.47 Thus, the genotype of the dam could determine the development of BAT in the offspring, requiring cautious interpretation of phenotypic data related to BAT thermogenesis from genetically-driven ablation and knockout models of orexin-producing cells. Considering the potential for differences in the development of BAT to contribute to altered body temperature responses, BAT thermogenic competence and UCP-1 expression levels (see refs. 47 vs. 50) must be rigorously assessed.
Summary
An orexinergic input to the rRPa has been demonstrated to potentiate the excitatory drives to medullary sympathetic premotor neurons controlling BAT sympathetic outflow and BAT thermogenesis. These results provide a potential mechanism contributing to the disrupted regulation of body temperature, energy metabolism and body weight in the absence (narcolepsy) or dysregulation of orexin secretion.
Glossary
Abbreviations:
- BAT
brown adipose tissue
- NMDA
n-methyl-D-aspartate
- PeF/LH
perifornical lateral hypothalamus
- rRPa
rostral raphe pallidus
- SNA
sympathetic nerve activity
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/19736
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31:6518–26. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai T, Mieda M, Tsujino N. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–61. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuwaki T. Orexin links emotional stress to autonomic functions. Auton Neurosci. 2011;161:20–7. doi: 10.1016/j.autneu.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Smith MS, True C, Grove KL. The neuroendocrine basis of lactation-induced suppression of GnRH: role of kisspeptin and leptin. Brain Res. 2010;1364:139–52. doi: 10.1016/j.brainres.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dampney RAL, Horiuchi J, Carrive P. Blockade of orexin receptors differentially modulates sympathetic and respiratory responses to hypothalamic activation in rats. Autonom Neurosci. 2011;163:114. doi: 10.1016/j.autneu.2011.05.206. [DOI] [Google Scholar]
- 8.Luong L, Carrive P. Orexin microinjection in the medullary raphe increases heart rate and arterial pressure but does not reduce tail skin blood flow in the awake rat. Neuroscience. 2012;202:209–17. doi: 10.1016/j.neuroscience.2011.11.073. [DOI] [PubMed] [Google Scholar]
- 9.Kok SW, Overeem S, Visscher TL, Lammers GJ, Seidell JC, Pijl H, et al. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res. 2003;11:1147–54. doi: 10.1038/oby.2003.156. [DOI] [PubMed] [Google Scholar]
- 10.Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/S0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 11.Plazzi G, Moghadam KK, Maggi LS, Donadio V, Vetrugno R, Liguori R, et al. Autonomic disturbances in narcolepsy. Sleep Med Rev. 2011;15:187–96. doi: 10.1016/j.smrv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137–49. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 14.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 16.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6:e17247. doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;31:15944–55. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–36. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol. 1999;276:R290–7. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- 20.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–26. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Matsumura K, Hübschle T, Nakamura Y, Hioki H, Fujiyama F, et al. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–80. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–71. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003;991:84–95. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–37. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madden CJ, Morrison SF. Brown adipose tissue sympathetic nerve activity is potentiated by activation of 5-hydroxytryptamine (5-HT)1A/5-HT7 receptors in the rat spinal cord. Neuropharmacology. 2008;54:487–96. doi: 10.1016/j.neuropharm.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, et al. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci. 2011;31:14600–10. doi: 10.1523/JNEUROSCI.2671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peever JH, Lai YY, Siegel JM. Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol. 2003;89:2591–600. doi: 10.1152/jn.00968.2002. [DOI] [PubMed] [Google Scholar]
- 28.Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, et al. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest-activity cycle. Neuroscience. 2009;164:849–61. doi: 10.1016/j.neuroscience.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol. 2007;581:649–63. doi: 10.1113/jphysiol.2007.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mochizuki T, Klerman EB, Sakurai T, Scammell TE. Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R533–40. doi: 10.1152/ajpregu.00887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–70. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–81. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 33.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–62. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monda M, Viggiano A, De Luca V. Paradoxical [correction of parodoxical] effect of orexin A: hypophagia induced by hyperthermia. Brain Res. 2003;961:220–8. doi: 10.1016/S0006-8993(02)03953-7. [DOI] [PubMed] [Google Scholar]
- 35.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/S0092-8674(00)81973-X. [DOI] [PubMed] [Google Scholar]
- 37.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/S0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 38.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M, Kanbayashi T, Sugiura T, Inoue Y. Relationship between clinical characteristics of narcolepsy and CSF orexin-A levels. J Sleep Res. 2011;20:45–9. doi: 10.1111/j.1365-2869.2010.00870.x. [DOI] [PubMed] [Google Scholar]
- 40.Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur Arch Psychiatry Clin Neurosci. 2001;251:85–9. doi: 10.1007/s004060170057. [DOI] [PubMed] [Google Scholar]
- 41.Nishino S, Ripley B, Overeem S, Nevsimalova S, Lammers GJ, Vankova J, et al. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol. 2001;50:381–8. doi: 10.1002/ana.1130. [DOI] [PubMed] [Google Scholar]
- 42.Poli F, Plazzi G, Di Dalmazi G, Ribichini D, Vicennati V, Pizza F, et al. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;32:1491–7. doi: 10.1093/sleep/32.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuld A, Hebebrand J, Geller F, Pollmächer T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–5. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 44.Lammers GJ, Pijl H, Iestra J, Langius JA, Buunk G, Meinders AE. Spontaneous food choice in narcolepsy. Sleep. 1996;19:75–6. doi: 10.1093/sleep/19.1.75. [DOI] [PubMed] [Google Scholar]
- 45.Middelkoop HA, Lammers GJ, Van Hilten BJ, Ruwhof C, Pijl H, Kamphuisen HA. Circadian distribution of motor activity and immobility in narcolepsy: assessment with continuous motor activity monitoring. Psychophysiology. 1995;32:286–91. doi: 10.1111/j.1469-8986.1995.tb02957.x. [DOI] [PubMed] [Google Scholar]
- 46.Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14:478–90. doi: 10.1016/j.cmet.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Ootsuka Y, Blessing WW, Nalivaiko E. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress. 2008;11:125–33. doi: 10.1080/10253890701638303. [DOI] [PubMed] [Google Scholar]
- 49.Shibata H, Nagasaka T. Role of sympathetic nervous system in immobilization- and cold-induced brown adipose tissue thermogenesis in rats. Jpn J Physiol. 1984;34:103–11. doi: 10.2170/jjphysiol.34.103. [DOI] [PubMed] [Google Scholar]
- 50.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, et al. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol. 2010;588:4117–29. doi: 10.1113/jphysiol.2010.195099. [DOI] [PMC free article] [PubMed] [Google Scholar]