Abstract
Little is known about health care use in the cognitive impairment, not dementia (CIND) subpopulation. Using a cohort of 7,130 persons aged 71 years or over from the Health and Retirement Survey we compared mean and total health care use from 2002–2008 for those with no cognitive impairment [CI], CIND, or dementia in 2002.
Cognitive status was determined using a validated method based on self or proxy interview measures. Health care use was also based on self or proxy reports.
Based on the HRS, the CIND subpopulation in 2002 was 5.3 million; or 23% of the total population 71 years of age or over. Mean hospital nights was similar and mean nursing home nights was less in persons with CIND compared to persons with dementia. The CIND subpopulation, however, had more total hospital and nursing home nights; 71,000 total hospital nights and 223,000 total nursing home nights versus 32,000 hospital nights and 138,000 nursing home nights in the dementia subpopulation.
A relatively large population and high health care use result in a large health care impact of the CIND subpopulation.
Keywords: Older Adults, Cognitive Impairment, Health Services Use
Introduction
With most nations projected to experience an increase in the proportion of their population that is aged, future dementia care and cost burdens are a significant concern.1 Globally, the number of persons with dementia is expected to triple by 2050.2 Alzheimer’s disease is the most common type of dementia and accounts for 60% to 80% of all cases in the U.S.3 Persons with Alzheimer’s or other dementias are three times more likely to be hospitalized and nearly nine times more likely have a nursing home stay than are persons without these conditions.3 It is estimated that patients with dementia accrue more than three times the Medicare expenditures of patients without dementia.4 Thus, dementia has a large impact on the use and cost of health care services.
Because of the important personal, family, and social impact of dementia, there is great interest in interventions that could prevent or delay the onset of this disease.5 This interest has led to greater awareness of earlier stages of cognitive impairment such as cognitive impairment, not dementia (CIND). CIND is defined as cognitive impairment that represents a decline from prior functioning but does not reach a level that meets diagnostic criteria for dementia.6 Operational definitions of CIND and mild cognitive impairment (MCI) have converged recently and CIND and MCI identify substantially the same individuals.7 The prevalence of CIND in persons 71 years or older population has been estimated to be over 20% or 5 million older adults in the U.S.,8 which is 60% greater than the prevalence of dementia.9 Early evidence suggests that the physical and emotional strain of caring for persons with CIND may be similar to that of the strain of caring for persons with dementia.10 Although a rich literature documents the excess use of health care associated with dementia11, little is known about the impact of CIND on health care use.
We are aware of no nationally representative reports characterizing the health care impact of this large group of older adults who suffer from cognitive impairment but do not meet criteria for dementia. We used nationally representative data to compare home health, hospital, and nursing home use over a six year period among older adults with no cognitive impairment, CIND, or dementia in 2002. We also provide estimates of the number of persons with no cognitive impairment, CIND, or dementia in 2002 within six age by gender categories and mean and total hospital and nursing home nights for the period 2002 to 2008 by 2002 cognitive status within the age by gender subpopulations. We present data on both health care use and population size so that we may estimate health care impact—a product of rates of use and population size—across the no CI, CIND, and dementia subpopulations. . Six years of follow-up allow differential death and conversion to or reversion from CIND or dementia across the subpopulations to factor into the estimates of health care impact.
Methods
Data for the analyses came from the Health and Retirement Study (HRS) 2002, 2004, 2006, and 2008 interviews. The HRS is an ongoing nationally representative study. The sample was constructed from a multi-stage national area probability sample with oversamples of black and Hispanic persons and residents of Florida. Project-supplied sampling weights can be used to create nationally representative estimates. For our project, we focused on the 7,130 respondents who were aged 71 years or over at the 2002 interview and who either self-responded (6,040) or were represented by a proxy respondent (n=1,090). Interviews were conducted face-to-face or over the telephone.
Over the years, HRS subsamples have been selected to test new data collection modules or gather timely data. Between 2001 and 2003, the Aging, Demographics, and Memory Study (ADAMS) was conducted on an HRS sub-sample. To create the sub-sample, 1,770 HRS respondents were selected using stratified random sampling with strata based on five levels of a self or proxy cognitive measure taken from the HRS. Of these, 856 persons aged 71 years or over completed all in-home measures and neuropsychiatric assessments. The ADAMS sub-sample has been used to validate the HRS index (a set of measures used to determine cognitive status in the full HRS sample).
ADAMS Cognitive Status Determination
A consensus panel comprised of neuropsychologists, neurologists, geropsychiatrists, and internists reviewed information collected during the in-home assessments and assigned a diagnosis.8 Dementia in the ADAMS was diagnosed based on guidelines from the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition12 and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.13 CIND is a definition that has evolved over the past 20 years7 and the investigators of the ADAMS have been among the primary developers of this definition.8 The ADAMS defined CIND as cognitive and functional impairment that did not meet diagnostic criteria for dementia. The ADAMS consensus panel assigned persons with CIND into 12 diagnostic subcategories; the most prevalent subcategories were prodromal Alzheimer’s disease, impairment due to medical conditions, stroke, and vascular cognitive impairment without dementia. The relative prevalence of these subcategories is consistent with data from a regional sample of African-Americans.7 A more complete description of CIND and its subcategories is provided in Unverzagt et al, 20077 and in Plassman et al, 2008.8 A complete description of the ADAMS sampling strategy and assessment are available in Langa et al, 2005.14
HRS Cognitive Status Determination
Neuropsychological and diagnostic assessments were completed only on the ADAMS subsample. In order to use the much larger HRS sample to study health service use and outcomes by cognitive status we rely on cutpoints based on scales used in the core HRS interviews.9 We have used the only HRS cognitive status method available for classifying all HRS participants into no CI, CIND, or dementia groups. The predictive value for dementia has been shown to be greatest from scores that rely on cognitive performance in multiple domains.1 In one cohort study with neuropsychological testing for dementia at 6-year follow-up, multiple combinations of baseline performance scores and subjective complaints were shown to predict dementia equally well.1 Notably, short-term memory, working memory, and speed of processing scores were used. The HRS cognitive status index uses measures from each of these domains. To determine cognitive status in self-respondents, a modified Telephone Interview for Cognitive Status (TICS) was used. The HRS-modified TICS is a 27-point cognitive index developed from three interviewer-administered items that represent short-term memory, working memory, and speed of processing.9 The 27-point cognitive index includes: 1) an immediate and delayed 10-noun free recall test to measure short-term memory (0 to 20 points possible); 2) a serial seven subtraction test to measure working memory (0 to 5 points); and 3) a counting backwards test to measure speed of mental processing (0 to 2 points). The HRS-modified TICS cognitive index was validated by comparing the distribution of normal, cognitive impairment, no dementia (CIND), and dementia cases based on the ADAMS diagnostic assessments to those based on several possible cut points on the 27-point modified TICS. This validity assessment was only possible among persons who completed both the ADAMS substudy and the 2002 HRS interview. The best cut points showed prevalence estimates that were within 0.5%, 0.9%, and 1.4% of normal, CIND, and dementia prevalence in the ADAMS subsample. Using this approach, a score of 12 or above indicates normal cognitive function, 7 to 11 CIND, and 6 or less dementia.
To determine cognitive status in those using a proxy-respondent, proxy assessments of memory and instrumental activity of daily living (IADL) limitations on five IADLs, and interviewer assessment of cognitive impairment were used.9 Proxy assessment of memory was scored 0 to 4 (excellent to poor) and interviewer assessment of cognitive impairment was scored 0 to 2 (no cognitive impairment, may have cognitive impairment, and has cognitive impairment). These scores were added to the total number of five IADLs in which the proxy reported limitation to achieve a score range from 0 to 11. Cutpoints of 0 to 2 (no cognitive impairment), 3 to 5 (CIND), and 6 to 11 (dementia) are within 2.1, 1.5, and 0.6 percentage points, respectively, of prevalence estimates based on the ADAMS substudy diagnostic assessment. Our categorization of HRS respondents as no CI, CIND, or dementia (e.g., Tables 2 and 3) is based on the HRS cognitive status index for self- and proxy-respondents described above.
Table 2.
Comparison of Sociodemographic Characteristics and Chronic Illness among Persons 71 Years or Over with No Cognitive Impairment (CI), Cognitive impairment, not dementia (CIND), and Dementia in the 2002 Health and Retirement Study (N=7,130).
| Total Sample (N=7,130) |
No CI (n=4,400) |
CIND (n=1,674) |
Dementia (n=1,056) |
P-value for difference* |
|
|---|---|---|---|---|---|
| Age, mean (SD) | 79.2 (6.2) | 77.7 (5.3) | 80.5 (6.4) | 83.5 (7.0) | <.0001 |
| 71–79, % | 56.5 | 66.4 | 47.0 | 30.3 | <.0001 |
| 80–89, % | 36.1 | 30.5 | 43.0 | 48.9 | |
| 90 or over, % | 7.3 | 3.1 | 10.0 | 20.8 | |
| Gender | |||||
| Female, % | 59.2 | 58.2 | 58.4 | 64.8 | .0003 |
| Ethnicity | |||||
| Hispanic, % | 6.5 | 4.0 | 10.3 | 11.3 | <.0001 |
| Non-Hispanic Black, % | 11.0 | 6.4 | 15.9 | 22.6 | |
| Non-Hispanic White, % | 81.1 | 88.3 | 72.3 | 64.5 | |
| Non-Hispanic Other, % | 1.4 | 1.3 | 1.5 | 1.6 | |
| Education | |||||
| Years in school, mean (SD) | 11.6 (3.6) | 12.6 (2.9) | 10.5 (3.7) | 9.1 (4.1) | <.0001 |
| Chronic Conditions | |||||
| Hypertension, % | 59.6 | 58.2 | 62.5 | 60.8 | .0057 |
| Diabetes, % | 18.5 | 16.6 | 21.7 | 21.4 | <.0001 |
| Heart disease not CHF, % | 29.7 | 29.0 | 31.6 | 29.9 | .1431 |
| Congestive heart failure, % | 5.6 | 4.0 | 6.7 | 10.9 | <.0001 |
| Chronic lung disease, % | 10.8 | 10.4 | 11.5 | 10.9 | .4373 |
| Arthritis, % | 67.8 | 67.0 | 69.4 | 68.7 | .1642 |
| Cancer not skin, % | 18.2 | 19.0 | 17.4 | 15.9 | .0428 |
| Stroke, % | 10.9 | 6.8 | 13.6 | 23.5 | <.0001 |
p-values show significance comparing baseline characteristics across the three dementia status groups; they were obtained from a general linear model for continuous variables (age and education) and a chi-square test for categorical variables.
Table 3.
Unadjusted, Unweighted Home Health Care, Hospital Stays, and Nursing Home Stays 2002 to 2008 for Older Adults with No Cognitive Impairment (CI), Cognitive Impairment, Not Dementia (CIND), and Dementia at the 2002 Health and Retirement Study.
| Total Sample (n=7,130) |
No CI (n=4,400) |
CIND (n=1,674) |
Dementia (n=1,056) |
|
|---|---|---|---|---|
| Died 2002–2008, % | 35.7 | 23.6 | 43.9 | 73.2 |
| Non-death attrition 2004, % | 4.2 | 3.8 | 4.7 | 5.8 |
| Non-death attrition 2006, % | 5.7 | 5.3 | 6.7 | 6.4 |
| Non-death attrition 2008, % | 7.1 | 6.8 | 7.8 | 8.1 |
| Any Home Health Care, % | 34 | 30 | 40 | 43 |
| Overnight Hospital Stay, % | 68 | 66 | 72 | 72 |
| Distinct stays, mean (SD) | 2.1 (3.0) | 2.0 (2.6) | 2.4 (3.5) | 2.4 (3.3) |
| Total nights, mean (SD) | 11.3 (23.1) | 10.3 (22.4) | 13.2 (24.5) | 12.5 (23.7) |
| Length of stay, mean (SD) | 5.8 (10.2) | 5.5 (8.2) | 6.4 (9.5) | 6.2 (16.6) |
| Overnight Nursing Home, % | 24 | 18 | 29 | 40 |
| Distinct stays, mean (SD) | 0.48 (2.4) | 0.31 (1.7) | 0.55 (1.5) | 1.1 (4.9) |
| Total nights, mean (SD) | 30.0 (123.3) | 17.2 (85.4) | 41.1 (144.2) | 67.4 (196) |
| Length of stay, mean (SD) | 80.4 (143.6) | 63.3 (119.0) | 86.3 (149.6) | 108.5 (174.5) |
In an another validity assessment of the HRS index, Crimmins et al (2011) showed that among HRS self-respondents, 24% of those diagnosed as demented in the ADAMS were classified as demented and 46% were classified as CIND using the HRS index. Similarly, 25% of self-respondents diagnosed with CIND in the ADAMS were classified as CIND using the HRS index; 64% were misclassified as no CI. Among HRS self-respondents with no CI based on the ADAMS, 88% were classified as such using the HRS index. For HRS participants with a proxy-respondent, 82% diagnosed with dementia by the ADAMS diagnostic criteria were correctly classified using the HRS index and 51% of participants with CIND were correctly classified. Among those with CIND based on the ADAMS, 25% were misclassified as no CI and 23% as dementia using the HRS index. For those with a proxy-respondent and classified as no CI based on the ADAMS, 75% were classified as such using the HRS index with the remainder having been classified as CIND.
Health Service Use Variables
Information on health service use was obtained from the self, proxy, or exit interview. The exit interview was a proxy interview completed for persons who died in the interim between surveys. Thus, health care use in the final year of life is included in our health service use estimates. Home health care use was defined as “a medically trained person coming to your home to help you.” For hospital use, the number of different times the respondent was a patient overnight in a hospital over the past two years and the total (across all unique hospital admissions) number of hospital nights were asked. For nursing home the question was “In the last two years have you been a patient overnight in a nursing home, convalescent home, or other long-term health care facility?” The number of different times the respondent was a patient in a nursing home (including any current stay) and the total (across all unique stays) number of nights in a nursing home over the past two years were asked. For our analyses, we combined data from the 2004, 2006, and 2008 interviews to determine health service use over the six-year period 2002 to 2008.
Additional Variables
Death was determined from the National Death Index and provided in the HRS public-use data. Age, gender, ethnicity, and education were self or proxy reported. For chronic illnesses, presence or absence of particular chronic diseases was based on self or proxy reports that a doctor had told the respondent that he/she has the condition. Chronic conditions available in the HRS include congestive heart failure (CHF), heart disease other than CHF, hypertension, lung disease such as chronic bronchitis or emphysema, diabetes, cancer (other than skin), stroke, and arthritis.
Analysis
We first provide descriptive data on the assessments and subjective reports that make-up the cognitive status determination (see Table 1). In Table 2 we provide descriptive data on sociodemographic and chronic illness by no cognitive impairment, CIND, and dementia in 2002. We then present 6-year (2002 to 2008) rates of death and use of home health care, hospital, and nursing home by cognitive status (Table 3). Figures 1 and 2 provide weighted mean and total, respectively, hospital and nursing home nights for age by gender by cognitive status subpopulations. Both mean and total nights take into account multiple stays over the 2002–2008 period; total nights was calculated by multiplying the mean of a subpopulation by the number of persons in that subpopulation. Data were weighted to the 2002 U.S. population by using HRS supplied sampling weights.
Table 1.
Self- and Proxy-Respondent Scoring and Prevalence of No Cognitive Impairment, Cognitive Impairment, Not Dementia (CIND), and Dementia, 2002 Health and Retirement Study (N=7,130).
| Mean (SD) | Prevalence (n) | |
|---|---|---|
| Self-Respondent (n=6,040) | ||
| Short-term memory (0 to 20) | 8.4 (3.5) | |
| Working memory (0 to 5) | 3.3 (1.8) | |
| Speed of processing (0 to 2) | 1.9 (0.5) | |
| Total score (0 to 27), note: high score is better | 13.6 (4.6) | |
| No cognitive impairment (12 to 27) | 68.4 (4133) | |
| CIND (7 to 11) | 23.9 (1446) | |
| Dementia (0 to 6) | 7.6 (461) | |
| Proxy-Respondent (n=1,090) | ||
| IADL limitations (0 to 5) | 2.4 (2.1) | |
| Proxy memory assessment (0 to 4) | 2.7 (1.2) | |
| Interviewer cognitive impairment assessment (0 to 2) | 1.1 (0.9) | |
| Total score (0 to 11), note: high score is bad | 6.2 (3.7) | |
| No cognitive impairment (0 to 2) | 24.5 (267) | |
| CIND (3 to 5) | 20.9 (228) | |
| Dementia (6 to 11) | 54.6 (595) |
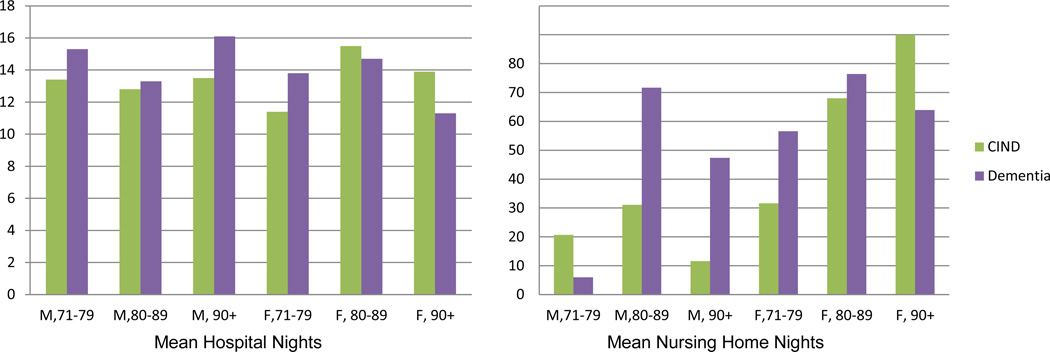
Figure 1.
Weighted Mean Hospital and Nursing Home Nights per Individual from 2002–2008 within 2002 Age by Gender by CIND/Dementia Subpopulations.
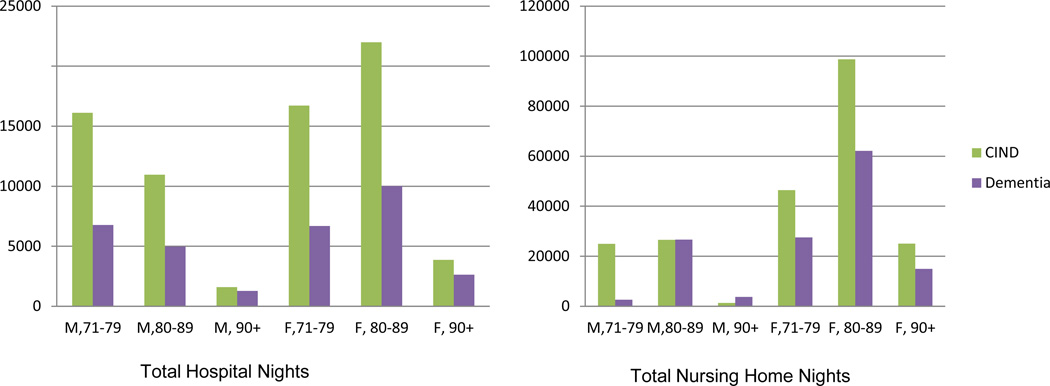
Figure 2.
Cumulative Hospital and Nursing Home Nights from 2002–2008 within 2002 Age by Gender by CIND/Dementia Subpopulations for the U.S. (000).
We conducted several sensitivity analyses. First, we have shown data for a six year window but we also explored a two year window. Second, we explored rates of conversion over time from CIND to no CI and from CIND to dementia. Third, to explore whether demographic or health differences between the groups drive health service use differences, we ran a generalized linear model with a negative binomial distribution to model the mean number of hospital and nursing home nights while adjusting for age, chronic conditions(listed in Table 2), and years of education. Odds ratios were obtained by exponentiating model coefficients. Finally, to gauge the validity of the health services use by dementia status data on the HRS index for the full HRS sample, we replicated the HRS analyses using the final primary diagnosis available in the ADAMS. This analysis is only possible in the ADAMS subsample with complete data on our other variables of interest (N=773).
Results
Main Results
Of the 7,130 respondents aged 71 years or over in 2002, 85 percent were self- and 15 percent were proxy-respondents. Table 1 shows the mean and standard deviations for the items included in the total cognitive status score separately for the self- and proxy-respondents. Based on the no cognitive impairment, CIND, and dementia cutoffs established for the total score, 24 percent of self-respondents had CIND and 21 percent of proxy-respondents had CIND. Dementia, however, was far more prevalent in the proxy- compared to self-respondents at 55 percent and 8 percent, respectively.
Table 2 shows the mean age for the total sample to be 79 years. The mean ages of the respondents with CIND and dementia were higher compared to those with no cognitive impairment. For the sample as a whole, 59 percent was female but a higher proportion of those with dementia was female (65 percent). Also, for the sample overall, 7 percent was Hispanic and 11 percent non-Hispanic black but for those with CIND or dementia a higher proportion was minority—10 and 11 percent Hispanic and 16 and 23 percent non-Hispanic black, respectively. Mean years of schooling was less for those with CIND or dementia than for those with no cognitive impairment. The prevalence of several self-reported chronic illnesses differed across no cognitive impairment, CIND, and dementia groups including diabetes, congestive heart failure, and stroke.
Almost 36 percent of the total sample died between baseline and the 2008 interview as shown in Table 3. Death was more common for those with dementia and 73 percent of those with dementia had died by the 2008 follow-up; whereas 24 percent and 44 percent had died among those with no cognitive impairment and CIND, respectively. We defined non-death attrition as persons with no death index or interview data at a particular follow-up. When compared to the no cognitive impairment group, non-death attrition was 20 to 30 percent more prevalent in the CIND and dementia groups.
Health care use data over the six-year observation period unadjusted for attrition show that about one-third of the sample used home health services at some point, two-thirds had a hospital stay, and one fourth a nursing home stay. These rates are greater among those with impaired cognition, as expected. Home health use was reported by 40 percent with CIND and 43 percent with dementia. The percent with a hospital stay, total nights in a hospital over the six-year period, and mean length of stay differed little between the CIND and dementia groups. Nursing home stays were less common in the CIND group where 29 percent reported a stay compared to 40 percent of the dementia group. Total nursing home nights was also less in the CIND group; 41 for the CIND compared to 67 for the dementia group. Nursing home mean length of stay was also lower in the CIND group at 86 days compared to 109 days for the dementia group.
Figure 1 shows weighted mean hospital and nursing home nights. With the exception of the over 80 female subpopulation, mean hospital nights was modestly lower for the CIND as compared to the dementia subpopulation. In the case of nursing home nights, the mean was greater in the case of CIND for the 71 to 79 male subpopulation and the 90 or over female population. In all other age by gender groups, however, mean nursing home nights was considerably less in the CIND as compared to dementia subpopulation.
Although not shown, in the 2002 population aged 71 years or over there were fewer men than women and the population in both genders declined with increasing age group. As noted in the introduction, the CIND subpopulation was larger than the dementia subpopulation and this was true within each age by gender category as well. Figure 2 gives a graphic of the total hospital and nursing home nights by CIND or dementia status within age by gender subpopulations. In the case of hospital nights, the CIND subpopulation had nearly twice as many nights in comparison to the dementia subpopulation in each age by gender group except men 90 years or over. And, excepting men over 80 years of age, the CIND population exceeded the dementia population in nursing home nights as well; considerably so in the case of the 71 to 79 year old male and female populations. A table containing the 95 percent confidence intervals for the 2002 total U.S. population aged 71 years or over and for the cognitive status subpopulations within six age by gender groups as well as the weighted six-year total nights in hospital and nursing home for these subpopulations is available from the corresponding author on request.
Sensitivity Analysis Results
Sensitivity analyses showed, first, that the pattern of health service use across CIND and dementia populations in the two year period 2002–2004 was consistent with that observed in the six year period 2002–2008. Second, conversion from CIND to no CI (22–35%) was slightly more common than conversion from CIND to dementia (13–18%) in each two year period. Third, multivariate models with adjustments for age, chronic conditions(listed in Table 2), and years of education showed that, for mean hospital nights, the CIND group had a higher adjusted mean number of hospitalizations than the no CI group (odds ratio 1.16) but the dementia group did not (odds ratio 1.07). In the case of mean nursing home nights, both the CIND and dementia group had a higher adjusted mean than the no CI group, odds ratios of 2.2 and 3.3, respectively. Finally, our re-estimation of health services use based on the ADAMS diagnostic assessment available in the ADAMS subsample did not change our conclusions based on the HRS full sample. Estimates for the no CI and dementia groups were remarkably similar (within a few percentage points) for percent with any home health care, overnight hospital stay, and overnight nursing home. However, health services use in the CIND group was greater by 10 percentage points (any home health and overnight hospital) and 5 percentage points (overnight nursing home) in the ADAMS subsample.
Discussion
We have used nationally representative data weighted to the U.S. population to show health care use over a six year period for the no CI, CIND, and dementia subpopulations. Based on the HRS, the CIND subpopulation 71 years of age or over in 2002 was 5.3 million; or 23% of the total population 71 years of age or over. This prevalence resulted in a relatively large impact of the CIND subpopulation on total care nights; 71,000 hospital nights and 223,000 nursing home nights. This compares to 32,000 hospital nights and 138,000 nursing home nights in the dementia subpopulation. The female CIND subpopulation in particular had relatively large numbers; approaching 45,000 hospital nights and 170,000 nursing home nights.
The data presented do not diminish the importance of dementia. Our interests were in presenting health service use over a period of time for a cohort of persons with CIND. To put the numbers in perspective, we compared them to persons with no CI and to those with dementia. As noted, persons with dementia were far more likely to die (73%) in the period than were persons with CIND (40%) indicating the illness severity of dementia. We did not adjust for attrition due to death since our objective was to show the total health service use of the cohorts over a period of time.
There are several limitations with this report. First, we have relied upon a cognitive status score derived from measures available in the HRS and this score does not represent an official diagnosis. Data from the ADAMS substudy of the HRS, however, indicate that our health service use estimates are very close for no CI and dementia and conservative for CIND.
Also, we relied upon self-reported health service use. Data from a public hospital system indicated that older adults underreported health service use, including hospitalizations15 and, similarly, data from a large health maintenance organization showed that older adults underreported health service use although hospital nights was less underreported than outpatient visits.16 A systematic review found that the accuracy of self-report health service use varies by age and cognitive status.17 Thus, we would expect that not only are the data that we presented underestimates of use but that respondents with CIND and dementia were more errant in their reporting than respondents with no cognitive impairment and that respondents with dementia were more errant in their reporting than the respondents with CIND. However, proxy respondents were far more prevalent in the dementia group than in the CIND group perhaps offsetting the effect of bias from self-respondents with dementia. Proxy interviews have been found in one study to result in valid information for chronic illness18 but we are not aware of a study of the validity of proxy interviews for health service use. Finally, the ADAMS diagnostic criteria used for validation in this report are nearly a decade old at the time of this writing. Although there have been debates about changing the diagnostic criteria, [e.g.,19] these have not yet been implemented and it is unclear what effect these might have on our estimates of health care impact.
With increasing attention and awareness of the impact of the CIND subpopulation, more resources may be directed at this group. Interventions are being translated that may improve cognition and prevent or delay CIND and perhaps then prevent progression to dementia. If a method of delaying CIND progression by even six years were introduced, the number of people affected with dementia by 2050 would be reduced by 38 percent.20 At present, there are no disease-modifying treatments for CIND but cognitive training has been shown to improve cognitive abilities with improvements maintained to two years.21 Similarly, physical exercise is emerging as a promising intervention for preservation of cognitive function.22 And, there are emerging plausible mechanisms by which cognitive and physical training may be synergistic in their beneficial effects on cognition.23 Pursuit of these and other efforts may ultimately reduce the health care impact of CIND and dementia.
Acknowledgments
This work was supported by National Institute on Aging (P30 AG024967) and National Institute on Aging (AG031222). The Health and Retirement Study (HRS) is sponsored by the National Institute on Aging (U01 AG009740) and is performed at the Institute for Social Research, University of Michigan. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All persons who contributed significantly to this work are listed as authors and the authors have no conflicts of interest regarding this work.
References
- 1.Baars MA, van Boxtel MP, Dijkstra JB, et al. Predictive value of mild cognitive impairment for dementia. The influence of case definition and age. Dement Geriatr Cogn Disord. 2009;27(2):173–181. doi: 10.1159/000200465. [DOI] [PubMed] [Google Scholar]
- 2.International. AsD. World Alzheimer Report 2010. The Global Economic Impact of Dementia. London, UK: 2010. [Google Scholar]
- 3.Alzheimer's Association. 2012 Alzheimer's Disease Facts and Figures. Chicago, IL: 2010. [Google Scholar]
- 4.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health, U S, Department of Health and Human Services. Preventing Alzheimer’s Disease and Cognitive Decline. 2010 [Google Scholar]
- 6.Plassman BL, Langa KM, McCammon RJ, et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011 doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unverzagt FW, Sujuan G, Lane KA, et al. Mild cognitive dysfunction: an epidemiological perspective with an emphasis on African Americans. J Geriatr Psychiatry Neurol. 2007;20(4):215–226. doi: 10.1177/0891988707308804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher GG, Franks MM, Plassman BL, et al. Caring for individuals with dementia and cognitive impairment, not dementia: findings from the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59(3):488–494. doi: 10.1111/j.1532-5415.2010.03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu CW, N S, Torgan R, et al. Logintudinal study of effects of patient characteristics on direct costs in Alzheimer's disease. Neurology. 2006;67(6):998–1005. doi: 10.1212/01.wnl.0000230160.13272.1b. [DOI] [PubMed] [Google Scholar]
- 12.APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 13.APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 14.Langa KM, Plassman BL, Wallace RB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25(4):181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 15.Wallihan DB, Stump TE, Callahan CM. Accuracy of self-reported health services use and patterns of care among urban older adults. Med Care. 1999;37(7):662–670. doi: 10.1097/00005650-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Cronan TA, Walen HR. Accuracy of self-reported healthcare use in patients with osteoarthritis. J Rheumatol. 2002;29(10):2181–2184. [PubMed] [Google Scholar]
- 17.Bhandari A, Wagner T. Self-reported utilization of health care services: improving measurement and accuracy. Med Care Res Rev. 2006;63(2):217–235. doi: 10.1177/1077558705285298. [DOI] [PubMed] [Google Scholar]
- 18.Klinkenberg M, Smit JH, Deeg DJH, Willems DL, Onwuteaka-Philipsen BD, van der Wal G. Proxy reporting in after-death interviews: the use of proxy respondents in retrospective assessment of chronic diseases and symptom burden in the terminal phase of life. Palliat Med. 2003;17(2):191–201. doi: 10.1191/0269216303pm661oa. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RS, Weir DR, Leurgans SE, et al. Sources of variability in estimates of the prevalence of Alzheimer's disease in the United States. Alzheimers Dement. 2011;7(1):74–79. doi: 10.1016/j.jalz.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sloane PD, Zimmerman S, Suchindran C, et al. The public health impact of Alzheimer's disease, 2000–2050: potential implication of treatment advances. Annu Rev Public Health. 2002;23:213–231. doi: 10.1146/annurev.publhealth.23.100901.140525. [DOI] [PubMed] [Google Scholar]
- 21.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101(4):1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 23.Kempermann G, Fabel K, Ehninger D, et al. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;4:189. doi: 10.3389/fnins.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]