Abstract
Patterns describe order which emerges from homogeneity. Complex patterns on the integument are striking because of their visibility throughout an organism's lifespan. Periodic patterning is an effective design because the ensemble of hair or feather follicles (modules) allows the generation of complexity, including regional variations and cyclic regeneration, giving the skin appendages a new lease on life. Spatial patterns include the arrangements of feathers and hairs in specified number, size, and spacing. We explore how a field of equivalent progenitor cells can generate periodically arranged modules based on genetic information, physical-chemical rules and developmental timing. Reconstitution experiments suggest a competitive equilibrium regulated by activators / inhibitors involving Turing reaction-diffusion. Temporal patterns result from oscillating stem cell activities within each module (micro-environment regulation), reflected as growth (anagen) and resting (telogen) phases during the cycling of feather and hair follicles. Stimulating modules with activators initiates the spread of regenerative hair waves, while global inhibitors outside each module (macro-environment) prevent this. Different wave patterns can be simulated by Cellular Automata principles. Hormonal status and seasonal changes can modulate appendage phenotypes, leading to “organ metamorphosis”, with multiple ectodermal organ phenotypes generated from the same precursors. We discuss potential evolutionary novel steps using this module based complexity in several amniote integument organs, exemplified by the spectacular peacock feather pattern. We thus explore the application of the acquired knowledge of patterning in tissue engineering. New hair follicles can be generated after wounding. Hairs and feathers can be reconstituted through self-organization of dissociated progenitor cells.
Introduction
Patterns describe order which emerges from homogeneity. They may appear in spatial arrangement or temporal sequence. They can be repetitive elements which are identical or with variations. Patterns exist in the physical world as well as in living systems. Biological pattern formation is a physical process that was adopted by biological systems during early times in evolution (Fig. 1A). With increasingly complex life forms, periodic patterns became more prevalent, helping organisms adapt to diverse environmental conditions1. Among the myriad of biological contexts in which periodic patterns are found, the ectodermal organs are probably the most conspicuous and intriguing. They often display diverse and delicate patterns that are robust enough to withstand the wear and tear organisms are subject to in their daily interactions with their external environmental. Thus there is significant selection pressure for ectodermal organs to regenerate and to be adaptive. Also, many ectodermal organs are found on the body surface and therefore are most visible. From teeth, to scales, feathers, hairs and pigmentation patterns, these spectacular examples of morphogenesis have long fascinated biologists.
Figure 1.
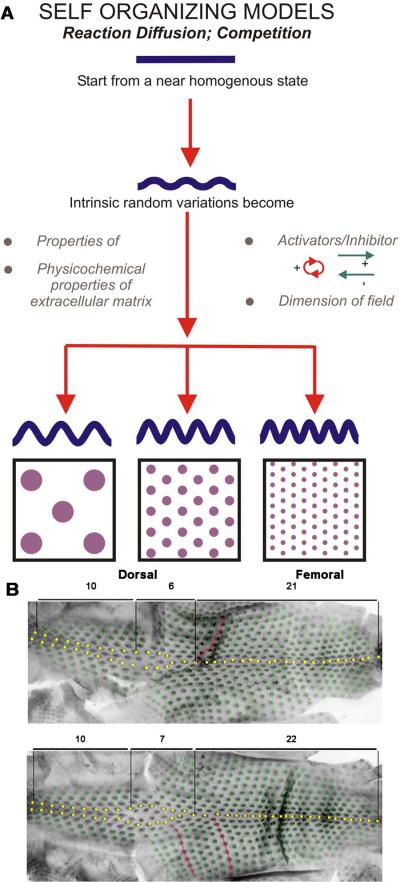
A. Homogeneously distributed cells through random interactions form unstable aggregates. This forms random variations which are amplified above a threshold at which the patterns become set. Distinct patterns are formed by competition between intrinsic factors (properties of the membranes and extracellular matrix), concentrations of activators and inhibitors and the size of the primordia field. From Jiang et al., 2004. B. Feathers from 2 similarly aged chicken embryos demonstrate stochasticity in the placement of feather buds. The feathers show a similar yet non-identical pattern. Numbers of feathers in each region are indicated. Yellow dots highlight feathers along the midline. Green dots highlight subsequent feather buds. Red dots show the relative position of sequential feather buds.
Patterns can emerge both spatially and temporally (Fig. 2A). Patterns that are spatially repetitive have several advantages2. First, having a large number of patterning units allows damaged units to be repaired or replaced without sacrificing overall function3. Second, a population of patterning units can acquire emerging properties that are not easily achieved by a singular unit. For example, numerous hairs form a coat that traps air for endothermy and the array of feathers on a male bird can form a stunning visual pattern to attract a mate. Third, it allows units in different body regions to generate different skin appendage phenotypes which can then be selected to best fit the possible functional needs of that organism. The same set of patterning units can be used to generate distinct phenotypes by changing the characteristic of single units or the rules by which these units interact without requiring an overall redesign. For example, a bird grows flight feathers on the wing but downy feathers on its belly. This results from a combination of periodic patterning and regional specificity (Fig. 2B).
Figure 2.
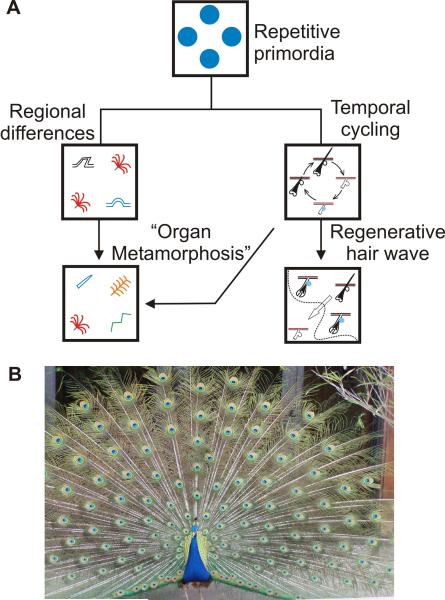
A. Diagram depicting how repetitive primordia in different regions can serve as “modules” and assume different characteristics. Through a process akin to metamorphosis that occurs at the organ level they can develop into different ectodermal organs. Individually they undergo temporal cycling and as a population can form a regenerative wave. B. A peacock shows the stunning complex skin appendage pattern occurring in feather size, shape, arrangement, and pigmentation. These are also sexually dimorphic. Feather patterning uses all the module variation principles discussed here.
There are also advantages to having patterns that are temporally repetitive, i.e., cycling. Instead of continuous renewal, skin appendages undergo repetitive renewal, and in each cycle they regenerate most of the organ. Cyclic molting and regeneration provide the opportunity to discard worn or injured appendages and remake a fully functional new organ4. Cycling also enables the coupling of regeneration among adjacent follicles, making a higher level meta-patterning (or an organization of patterns) possible in a hair follicle population. For example, the regenerative hair wave results from coupling the activation of stem cells in adjacent single hair follicles to become a collective regenerative behavior that spreads over the hair population5, 6. Finally, the regeneration of skin appendages gives the organism an opportunity to make new organ phenotypes that meet the needs of changes in the seasons or at different life stages7. These advantages would not be possible without repetitive patterning that allows one ectodermal organ (skin appendage) to become a population of repetitive organs.
1. HOW DO PERIODIC PATTERNS FORM?
How repetitive patterns form at the cellular and molecular level is of major interest to developmental biologists. In different animals, repetitive patterns may be generated by different mechanisms. In regard to how information is given during pattern formation, in general there are two modes: self-organizing and instructional. In the first mode, physical-chemical rules of interaction dictate how cells can self-organize into periodic patterns6, 8. The molecular clock mechanism in establishing somite segmentation belongs to this category as well9. In the second mode, changes in patterns are effected according to a specific blueprint. The work in the segmentation of Drosophila larvae led many to think that even vertebrate cells were designated with a molecular zip code system directing cells to form patterns. However, if a blueprint is needed, this mode will require a large volume of information. In scenarios where precise placement of repetitive patterns are required, a blue print model offers higher fidelity but self-organization is a more economical design requiring less DNA information.
To explain pattern formation, Alan Turing proposed a reaction-diffusion model. According to this model, periodic patterns can arise from a homogeneous chemical state through interaction between a short range activator and a long range inhibitor (Fig. 1A)10. The activator is autocatalytic (therefore self-amplifying) and can induce the production of the inhibitor. The inhibitor has a longer-range of diffusion and suppresses the activator. The short-range positive feedback enables a system to deviate from the initial homogenous state through minor instabilities, while the long-range negative feedback ensures spacing between activator peaks. These rules are first demonstrated in non-biological systems, most notably chemical reactions11. Theoretical biologists have modified aspects of this model for application to biological patterning and its implication was broadened by the use of an activator / inhibitor concept3, among them hair germ formation3, 12 and feather barb branching13, 14. Most recently, Kondo has developed simulation software, which enables investigators to alter parameters for the RD model to generate many two-dimensional patterns observed in real biological systems, including pigment patterns found on seashells and the skin of the popper fish. More strikingly, the dynamics that are described by mathematical models can be clearly visualized on the skin of zebrafish where pigmentation patterns are locally ablated by a laser and allowed to reform15.
Some researchers address the pattern formation problem by invoking the concept of a pre-pattern. For example, through differential adhesion, cells can sort themselves and form organized cell aggregates with patterns16. While this led to the study of a state before patterns are clear, it did not really solve the problem because it starts with cells with inherently different adhesivity. To go after the origin of pattern formation, we must start from the ground state in which all progenitor cells are equivalent.
2. PERIODIC PATTERNING IN ECTODERMAL ORGANS
2.A. Repetitive patterning during feather morphogenesis
Periodic patterning of feather germs
Avian skin is divided into tracts. Thousands of feathers develop across the avian skin. Within each tract, feathers have similar attributes but may vary and form a gradient of size and shape17. Each developing feather germ within a tract is equidistant from its neighbors, forming a periodic array with each feather germ surrounded by a hexagon of neighboring germs18. This is seen clearly in the spinal tract. Because of the exquisite pattern and sequential formation, this process has led scientists to propose models that the formation of new lateral row buds used the prior medial row formed earlier as a template or mechanical cue19. While the exquisite hexagonal pattern appears to be similar, the exact pattern from one embryo to another is not identical (Fig. 1B), implying that pattern formation is based on stochastic, self-organizing processes rather than a pre-patterned blueprint.
We take this opportunity to clarify some terminology. While functionally the skin is considered as one organ, each hair or feather follicle has also been considered as a “mini-organ” because architecturally it is self-sufficient and separated by inter-follicle spacing. Along this line, the primordium of each feather is called a feather germ when it is flat and a feather bud when it protrudes out of the skin surface.
Restrictive versus de novo molecular expression patterns
We searched for molecules that appear early in the periodic patterning process. In the early developmental stages of skin, prior to feather morphogenesis, certain molecules were expressed at moderate levels throughout the epithelium of the skin (Fig. 3E). Their expression was then up-regulated in regions destined to become feather germs and down-regulated in regions that would become inter-germ. We called this a restrictive expression pattern. Examples of genes that show a restrictive expression pattern include Wnt7a, β-catenin, L-fringe, NCAM, etc20–23. We also saw some genes which showed the reciprocal expression pattern. They are also expressed at a median level all over, but then become increased in the inter-germ and decreased in the bud epithelium. Gremlin24, 25 and Wnt1126 showed this type of restrictive expression pattern. Genes showing the restrictive pattern may be involved in patterning the placement of feather germs and may also be involved in stabilizing the feather bud – interbud border later.
Figure 3.
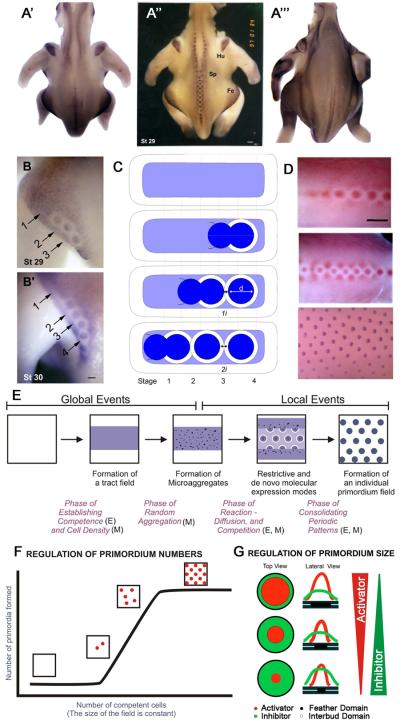
Cellular and molecular events during periodic formation of feather primordia. A-A"'. In situ hybridization for β-catenin demonstrates a restrictive expression pattern. A”. In normal chicken skin development there is moderate staining in each of the feather tracts. As feather buds form, staining increases in the buds and decreases in the surrounding interbud region. The pattern appears normal in scaleless chickens at stage 29 (A') but fails to progress in a restrictive manner at stage 30 (A"'). B. In the femoral tract, the β-catenin-free spacing between buds increases over time. C. Schematic of increasing feather bud spacing during early stages of feather morphogenesis. From Widelitz et al., 2000. D. pERK immunostaining in chicken embryos from stage 29, 32 and 35. From Lin et al., 2009. pERK also demonstrates a restrictive expression pattern. E. Schematic of early acting global and later early events during feather morphogenesis. From Jiang et al., 2004. F. Reconstituting skin explants showed that the size of the feather buds increased with increasing numbers of dissociated mesenchymal fibroblasts. Approximately equal spacing between buds was observed. G. Model depicting how the relative activator / inhibitor activities regulate feather bud size and spacing. From Jiang et al., 1999.
Some genes were not present at the earliest stages of feather germ formation but were induced after the feather germs form. We call this pattern the de novo mode of expression. Genes which showed the de novo mode of expression include sonic hedgehog, Msx2, etc23, 27–29. These genes are involved in stabilizing the boundary of feather germs or later stages of feather bud morphogenesis, but not the initial setting of periodic patterning.
The dynamics of the patterning process from the homogeneous field is best visualized by the expression of the beta-catenin transcript (Fig. 3A). Feather germs begin to form along the midline of the skin and subsequent rows are laid out progressively bilaterally over time. The pattern that forms shows that each feather germ is surrounded by a hexagonal array of neighboring feather germs. As a feather germ (about 200 μm in diameter, with stronger beta-catenin staining) emerges from the homogeneous field, it creates a halo that is negative for beta-catenin (Fig. 3B, B', C). The inhibitory zone, about 200 μm in diameter, is concentric with the feather germ, suggesting that it receives molecular cues from the newly formed feather germ. Most interestingly, in the nascent buds, the feather germ is in direct contact with the inhibitory zone of the previous germ. Then it creates its own inhibitory zone and two feather germs are pushed apart with an inhibitory zone equivalent to the width of the initial feather bud plus that of its neighbor (compare bud 2, 3 in Fig. 3B, B', C). A similar restrictive mode during bud emergence with the creation of a lateral inhibitory zone can be seen in immunostaining for pERK (Fig. 3D;30). The ectodysplasin (eda) receptor (edar) is expressed downstream of beta-catenin31. Experimental mis-expression of Eda activity can alter the size of feather buds but not interfere with the periodic patterning process itself32.
Molecules involved in periodic patterning of feather germs in chicken skin
The formation of feather germs can be explained by the balance of activators and inhibitors. Molecules such as Fibroblast Growth Factors (FGFs)33, 34,35 and Bone Morphogenetic Proteins (BMPs) play a role37–39 in establishing the periodic patterning of feathers. Their specific role can vary in different stages of embryogenesis as well as in different skin regions. In the very early stage when feather tracts are forming, inhibition of BMP sigaling in the mid-ventral apterium can induce ectopic tracts39, but BMP-4 coated beads were also reported to induce additional tracts in the dorsal-scapular semi-apterium40. Once the tracts form, BMP is shown to inhibit periodic patterning of individual buds37, 38. Addition of FGFs in the early stage of patterning can lead to the formation of many small-size feather buds. On the other hand, addition of FGF at later stage leads to the formation of enlarged feather buds34. Once the initial feather germ pattern is set, each germ initiates a lateral inhibitory zone mediated by delta 141 and a bud tip growth zone mediated by Shh27, 42.
More recently, a mutation leading to the up-regulation of BMP12 in the neck of the naked neck chicken was also found to function as an inhibitor of feather formation. Furthermore, retinoic acid expression in the neck skin sensitized this region to the inhibitory effects of BMP1243. However, BMPs do not always serve the role of an inhibitor. BMP7 which signals through the activin rather than BMP receptors44, 45 can stimulate feather germ formation46. BMPs were also reported to serve as activators of feather formation in ventral skin40, 47. This may be due to different functions of BMP in a different context.
Thus tilting the balance of activators and inhibitors can alter the induction of feather tracts or modulate the number and size of feather germs that can form from a homogeneous tract field. The activity of known molecular signalling pathways is context dependent, depending on the timing and location within skin regions. However, the result is consistent with a reaction-diffusion mechanism for feather germ formation38. The function of activator – inhibitor pairs was further tested in vitro using reconstituted dorsal skin explant cultures20. Here, the mesenchyme is separated from the epithelium. The mesenchyme is then dissociated into a single cell suspension before plating at high cell density. The intact epithelium is then layered on top of the mesenchyme and the reconstituted explant is cultured. In this experimental model, all molecular signalling disappears and the cells are reset to a primordial state where each cell is equivalent. Cells randomly migrate in and out of the presumptive feather primordia20, 48 in a competitive equilibrium until stable cell aggregates form. Surprisingly all new buds appeared synchronously under these experimental conditions, thus uncoupling the periodic patterning process from the sequential bud forming process. In another study, feather germs were found to disappear from intact stage 30 chicken embryo skin explants after 12 hours in culture and reformed 6 hours later49. This interesting observation suggests parallel events occur in intact and reconstituted skin explants cultures.
In this in vitro system, one can vary parameters and evaluate what controls the number and size of feather germs. Under these conditions the size of feather germs is constant and the number of feather germs is determined by the mesenchymal cell number. Below a certain threshold of mesenchymal cell number, no feather germs form. Above the threshold, feather buds start to appear randomly. The final hexagonal pattern is the result of highest packaging, not pre-patterned codes (Fig. 3F). To increase the size, one has to alter the activator / inhibitor ratio. Increasing the levels of BMP receptor within the mesenchymal cells reduced the size of feather primordia, while suppressing BMP expression with exogenous Noggin increased feather bud size20.
The molecular gradient itself is not sufficient to establish periodically arranged feather primordia. Cells need to be reorganized in response to chemo-attractant and chemo-repulsive signals30. Treating developing chicken skin with U0126, a MAPK inhibitor, caused individual buds to merge, thus changing spots into stripes. This occurred in a dose- and stage-dependent manner30. Thus, by modifying downstream signalling from the FGF receptor we were able to change the shape as well as the orientation of feather primordia.
The importance of periodic patterning can also be appreciated in scaleless chickens. In this mutant, the beta-catenin expressing tract field forms normally, however, periodic patterning does not occur (Fig. 3A"'). Eventually, feathers fail to form. FGF coated beads are able to rescue this process35, 41, implying the restoration of activator activity can again elicit this periodic patterning process.
Periodic patterning of feather branches
Periodic patterning is again invoked later in feather development during the process of branching morphogenesis. Branching morphogenesis allows for remarkable diversity in feather structures (Sidebar 1). Similar activator / inhibitor principles may be involved in determining the number of barb ridges. Shh and BMP may work as activators and inhibitors (Fig. 5,51, 52), while a Wnt 3a gradient converts the radial symmetrically arranged barb ridges into bilateral symmetry53. This is another example of periodic pattern formation process.
Figure 5.
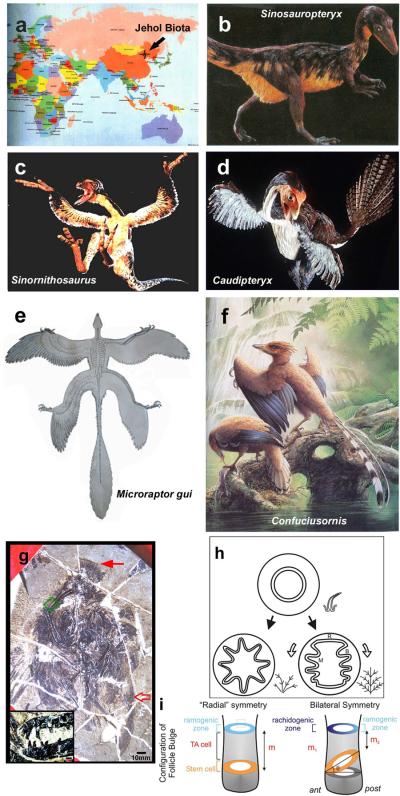
Representatives of feathered dinosaurs and Mesozoic birds from Jehol Biota. a. Map showing the location of the excavated site. b. Sinosauropteryx. c. Sinornithosaurus. d. Caudipteryx. e. Microraptor gui. f. Confuciusornis. Panels b–d reprinted with permission from National Geographic89. Panel e is from Xu et al., 200390. Panel f is from Hou et al., 200391. g. Longirostrornis fossil, primarily in dorsal view. Inset, close up of beak and teeth. h. Model of branching morphogenesis responsible for radial vs bilateral symmetric feathers based on feather cross sections. It shows the barb ridge formation is basically a periodic patterning process. See text for more. i. Schematic depicting the tilting of feather stem cell ring (orange color) leads to the symmetric breaking at ramogenic zone (region where periodic patterning of barb ridges takes place, blue color), thus making radial vs bilateral symmetric feathers.
2.B. Repetitive patterning during hair morphogenesis
In earlier theoretical works, Nagorcka showed how reaction-diffusion can account for many aspects of hair pattern formation13. However, it was not until recently that supporting experimental results started to emerge. During embryogenesis and early post-natal life, the succession of three inductive waves leads to the development of three types of hairs (guard, awl, and zigzag). During hair formation, the factors that promote hair follicle fate include Fgf, Wnt and Eda signals while inhibitory factors include Bmp and Dkk60. The involvement of dynamic interaction between activators and inhibitors in establishing hair patterns during the successive inductive waves are not immediately obvious but can be clearly demonstrated through computer simulation using an R-D based model. Sick and colleagues used a transgenic mouse line that expresses excess Dkk2, a Wnt inhibitor, in the epithelium after the first hair inductive wave to test the proposed involvement of the R-D mechanism in hair patterning. This transgenic mouse line revealed patterns predicted by computer simulation using a Wnt-Dkk-specific reaction-diffusion model61: 1) In addition to blocking the formation of new hair follicles, the excess inhibitor invokes ring-like zones of high activator activity around follicles established during the first induction wave. 2) Given that the pre-existing follicles are insensitive to the activator and inhibitor, this ring-like zone is converted to discreet new follicles surrounding the pre-existing one, thus forming hair clusters. The authors further showed that the ring-like zone of high activator activity is not caused by direct activation of Wnt by Dkk2 in the absence of Krm2, a concern raised by Stark et al.62, because Krm1 is expressed in the epithelium and the Foxn1-Dkk1 mouse exhibited similar phenotypes as the Foxn1-Dkk2 mouse63. These results provide compelling evidence that Wnt-Dkk work as the activator-inhibitor pair in an R-D scheme to set up the inter-follicular space during hair development.
Another piece of evidence comes from analyzing the effect of excess activator. A transgenic mouse line carrying stabilized β-catenin in the basal layer of the epithelium demonstrated precocious follicle formation and an increased number of hair placodes64, 65,64, 65. Most importantly, excess β-catenin leads to elevated mesenchymal Wnt activity, as well as increased BMP2, BMP4 and Dkk1 levels in hair placodes. This is compatible with autocatalysis of the activator and induction of inhibitors by activators that are described in the reaction-diffusion mechanism. Sostdc1, a soluble inhibitor of BMP and modulator of Wnt activity, is also increased in the mutant mouse. The authors proposed a model in which the interplay between Wnts and BMPs are involved in establishing the spacing between hair follicles: epithelial Wnt signal induces mesenchymal Wnt activity. Together, they activate BMPs, which suppress nearby cells through Lef1 and Edar. The boundaries of placodes are further strengthened by the BMP antagonist activity of Sostdc1.
In addition to spacing between hair follicles, hair patterning also involves aligning the hair bulb and shaft so as to achieve the maximal covering efficiency. The collective orientation of the hair follicle is one example of planar cell polarity. It was shown that prenatally, there is a Frizzled 6-dependent event to setup the global hair follicle orientation and postnatally, there is a Frizzled 6-independent mechanism to align neighboring follicles to achieve a more fine-tuned hair pattern66.
Together, these experiments addressed the interaction between activator and inhibitor in an R-D scheme. Proving other features of an R-D mechanism, including the autocatalysis of activator, differential diffusion of activator and inhibitor still remain a challenge to experimental biologists67, 68. It is not surprising that redundancy and extra layers of control mechanisms are built into this putative feedback network. Multiple Wnts, BMPs, DKKs, as well as antagonists of BMPs and DKKs are implicated in this patterning process.
3. EVOLUTION OF COMPLEX INTEGUMENT PATTERNS
3.A. Cyclic temporal patterns: regenerative hair wave in adult animals
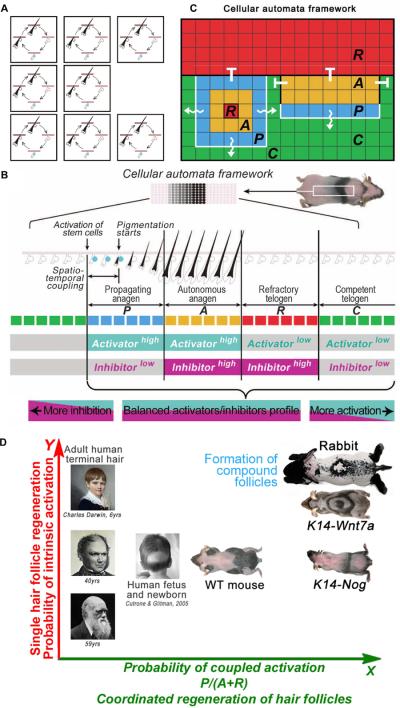
The hair follicle undergoes a cyclic degeneration and regeneration cycle throughout life. The length of growing (anagen) and resting phase (telogen) can determine hair length. A long resting phase can lead to shorter hairs or no hair, i.e., alopecia. We have developed ways to visualize hair stem cell activation over the entire skin in living mice by shaving the hair and examining the initiation of pigmentation as follicles enter anagen. We found cyclic BMP signalling from the subcutaneous adipose layer regulates stem cell activation during hair regeneration (Fig. 4B,5. More molecular analyses showed Wnts serve as activators. These studies revealed that hair growth patterns can be governed by simple rules based on a pair of activator/inhibitor signals. Regeneration in a population of hair follicles spreads like a chain reaction, forming diverse wave patterns6. Mathematical modelling reveals the self-organizing and stochastic nature of this behavior. It emerges at the population level (Fig. 4A), allowing hair regeneration to become a very adaptable trait. These variations are seen among different animal species that have different needs for hair: robust spreading in rabbits, gradual wave-like spreading in mice, and random growth with loss of coupling among follicles in human skin (Fig. 4D). The hair wave can also vary under different physiological conditions of the same individual, such as in puberty, pregnancy and aging.
Figure 4.
A. Schematic illustration of hair cycling in a population of hair follicles. B. A two-dimensional CA model can predict regenerative patterns in a large population of hair SCs. Skin pigmentation patterns result from color changes of many HFs when they collectively cycle through four phases: P (blue)→A (yellow)→R (red)→C (green). Distinct hypothetical activator/inhibitor signaling profiles can be assigned to all four phases. C. Cellular Automata model for the observed hair wave. Wiggly white lines indicate the spreading of the hair wave from hairs in propagating anagen to hairs in competent telogen, whereas hairs in autonomous anagen or propagating anagen cannot initiate the hair cycle in hair follicles that are in refractory telogen. D. Hair follicles may regenerate in response to intrinsic signals which drive each individual follicle or to extrinsic signals which can couple the activation of the hair cycle in neighboring follicles. By modulating the strength of intrinsic stem cell activation (Y axis) and the probability of coupled activation (X axis), different animals or different physiological conditions in the same animal can significantly alter the global dynamics of hair regeneration. As a result, versatile hair growth patterns in rabbits, mice, normal and alopecic human scalps can be explained within the same patterning framework based simply on how hair stem cell activities are “managed”. Panels B, C and D are from Plikus et al., 2011.
Cellular automata models have been used to explain many biological phenomena (Fig. 4C)69, 70. In a general cellular automata model, the field is divided into a number of discrete “cells”, which evolve through a number of time steps, according to a set of rules based on the states of neighboring “cells”. Each “cell” of the model corresponds to an area of the pattern field and information on this area is stored as the “state” of the cell. To simulate the regenerative hair wave, principles of cellular automata are used6. The self-organizing pattern is based on simple rules and probability, so wave patterns under similar activator / inhibitor conditions are similar but non-identical. The spectrum of regenerative hair wave patterns in human (less coupling), mouse, and rabbit (highly coupled) in the space of single follicle cycling (X axis) and follicle coupling (Y axis) can be seen in Fig. 4D.
Change in light/dark cycles produced by the seasonal lengthening and shortening of days or changes in temperature can alter the type of skin appendages. In nature, changes in the length of the light period are translated into changes in the plasma melatonin and prolactin levels which can trigger animals to produce a longer/shorter or whiter/darker coat to improve their chances for survival during a given season71. Now that we know that human and rodent skin and hair follicles are extra-pituitary sites of melatonin synthesis72–74, one wonders to what extent environmental cues (such as the length of the light period) can also affect seasonal changes in skin and skin appendage patterns. A recent work shows hair regeneration is delayed after plucking in mice with a mutation in bmal1, a molecule involved in circadian rhythms75.
3.B. Organ “metamorphosis”: new skin appendage phenotypes in adult animals
The temporal cycling of hair and feather follicles allows each follicle to molt and regenerate a new appendage and also provides an opportunity to alter the appendage phenotype to adapt to seasonal needs or changes in life stages under physiological conditions7. Baby chicks have downy feathers with a similar appearance all over their bodies which mainly function in maintaining warmth and sometimes for maternal bonding. As the bird grows, flight feathers emerge so the growing chicks can leave the nest. Upon puberty, sexual dimorphism becomes apparent76. In crown, tail, saddle and many body regions, feathers grow into specific shapes and lengths for communication purposes: to attract birds of the opposite sex or to scare away birds of the same sex. Some feathers also change their pigmentation patterns.
“Metamorphosis” is a change in the phenotype of an individual that gives the organism a new lease on life to adapt to different environments. Since their genotype is unchanged, mechanisms regulating this expression must be epigenetic. The most dramatic example of metamorphosis is from the aquatic tadpole to a terrestrial frog. In the skin, the small radial symmetric downy feathers in chicks can be replaced by feathers with bilateral symmetry, sometimes growing up to a meter in length, as in the adult peacock (Fig. 2B). The pigmentation of these new feathers can also be highly ornamental compared to their predecessors. These differences take place despite the fact that the cells share the same genome and are produced from the same follicle. What might control this difference in gene expression? We presume that macro-environmental changes have altered the follicle micro-environment resulting in altered gene expression. In this way the follicle stem cells are modulated to form different types of ectodermal organ phenotypes. We see parallels between morphogenetic changes which take place at the organ and organism levels and name the former “organ metamorphosis”. The repetitive nature of ectodermal organs which occurs in space and time enables these changes to take place (Fig. 2A). Spatial redundancy allows some appendage follicles to rest while the general function at the organism level is continued by other follicles. The temporal cycling allows a follicle to re-invent itself with a mechanism that we still do not completely understand – but is likely to involve some hormonal regulation and epigenetic changes.
An obvious example of organ metamorphosis occurs during postnatal chicken development17. Similar changes can also be seen in mammals. In the human, hair follicles in beard, axilla and genital regions are transformed from vellus to terminal hairs during puberty7, 77. In aging, the reverse tends to occur in the scalp of some males, leading to androgenic alopecia. The mechanism controlling how scalp and occipital hairs respond to sex hormones is still unknown but appears to be mediated by differences in dermal papillae which exhibit a varied response to stimulation with androgens or estrogens78–80.
Sexual dimorphism in many mammalian and avian species can be striking. Such differences are generated by the combinatorial use of principles described above (Fig. 2A). During organ regeneration, new phenotypes can be made from the same follicle. Androgenic and estrogenic hormones can then modulate skin appendage stem cells to different modes of morphogenesis and growth. Since these changes are region-specific, the process must also rely on region-specific mechanisms. Thus the repetitive nature of ectodermal organs sets up the modular basis of morphogenesis that allows temporally and spatially complex integument patterns to form. These, in turn, can help animals to be better suited to their environment.
Peacock feathers illustrate the most extraordinary example of biological pattern formation (Fig. 2B). The complex pattern is built through several layers of patterning processes. The downy plumage of a young male peacock chick undergoes molting at sexual maturity. At this time, a much larger feather grows from the same follicle. The nearly concentric pigment pattern on each feather is likely set up by molecular gradients. Within a feather tract, each feather is positioned with regular spacing from its neighbors and grows to a specific length. In other body regions, there are fan shape feathers that show both chemical and physical colors. Further, these processes do not occur in females, whose feathers are short and brownish. The complex pattern here is made possible by the use of modular principles.
3.C. Evolution of complex integument patterns
How do these patterns evolve? We can track these changes back to the origin of feathers at the time of dinosaurs2, 50, 82. The Jehol Biota (120–145 million years ago, spread across Northern China) provides some clues (Fig. 5a). Sinosauropteryx had fuzzy fibers with a short shaft and primitive barbs covering its body. These primitive skin appendages appear to be the same all over the body withoutregional differences (Fig. 5b). The branches may have helped to trap air and prevent heat loss in this possibly endothermic creature (Sidebar 1).
Caudipteryx exhibits bilateral symmetric feathers (Fig. 5d) with well formed feather vanes (pennaceous feathers). Furthermore, regional differences where feathers in the body, forelimb and tail are different, imply functional specialization. This variety of feather phenotypes helps to warm the body while providing communications through its tail and forelimbs. However, the bilaterally symmetric nature of its feathers suggests that it could not fly.
Microraptor gui hadboth fore- and hind-limbs covered with pennaceous feathers (Fig. 5e). Feathers at the distal limb positions had asymmetric vanes, suggesting effective aerodynamic function. Those studying it proposed that it moved by gliding.
Longirostravis (Fig. 5g)83 represents early wading birds. It had feathers with an asymmetric vane in the lateral wing, implying that it was a reasonably good flyer. It also shows specialization of its crown feathers.
Thus a gradual evolution of complex feather patterns from dinosaurs to birds seems to have occurred. First, the primitive branching in Sinosauropteryx was probably selected because effectively provided endothermy. A follicle structure suggests that it was subject to cyclic renewal. Later in evolution, Caudipteryx showed hierarchical branching (rachis, barb and barbule structures), the formation of open vanes and clear regional differences in morphology. Sexual dimorphism is observed in Confucisornis. Thus, the repetitive arrangement and cyclic regenerative nature of integument appendages provide the modules that can be altered in different body regions at different physiological stages to allow an organism to evolve complex integument appendage patterns, which are then selected over evolutionary time to increase survivability in its environment.
4. ENGINEERING OF THE PERIODIC PATTERNING PROCESS
Periodic pattern formation used to be considered as a basic science issue. We now have learned that the ability to initiate this process is also essential for the regeneration of new hairs. First, we ought to differentiate between two major categories of hair regeneration. One is the regeneration of the hair shaft from existing hair follicles. This is the behavior described in the regenerative hair wave section, in which the total number of modules (hair follicles) does not change, but the cyclic growth / resting states change. A Cellular Automata model is effective in describing this behavior and can also suggest parameters that might lead to androgenic alopecia.
The other kind is the regeneration of new modules (new hair follicles). Here reaction-diffusion is involved as described in the periodic patterning session. This, of course, is much more challenging, as it is equivalent to making an organ de novo. However, by opening a large wound (> 1 cm diameter after wound contraction) in the back of a mouse, remarkably, new hair germs formed84. Interesting, the new hairs formed in the center of the wound, about 300 μm away from the wound margin. Repair and regenerative processes may be in competition. A repair response is favored near the wound margin, while cells in the wound center are free to regenerate85. Once the regenerative response is elicited, cells self-organize into periodically arranged hair germs, without a need to implant individual hair follicles as seen in the practice of hair transplantation, or the use of pre-patterned tissue scaffolds. In patients with severe wounds (such as burns), no hairs or other skin appendages are able to regenerate. It remains to be seen whether such a phenomenon can occur in humans with proper management.
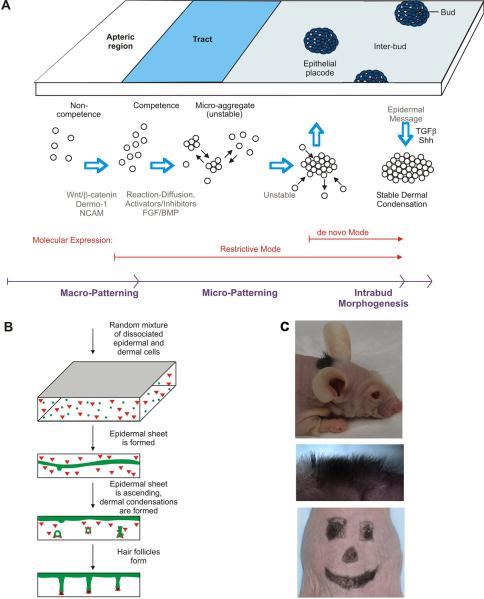
To learn how to generate new skin appendage germs for the purpose of regenerative medicine, we need to reconstitute them from dissociated single cells. We have previously done so for feather germs20; Fig. 6A). Recently, we used a similar process to generate reconstituted new hairs86. While earlier work showed new hair formation, the process can be laborious87 or may frequently form hair cysts88. By mixing dissociated epithelial and mesenchymal cells and allowing them to interact on a flat 3D matrix on the back of a SCID mouse, the reconstituted skin is able to grow hairs that are evenly spaced on a plane and point outwards (Fig. 6B, C).
Figure 6.
A. Schematic summary showing the periodic patterning process during feather morphogenesis. Epithelial – mesenchymal signaling fosters the formation of competent epithelium. Spatially distributed activators and inhibitors of feather formation promote the expression of adhesion molecules which lead to the formation of unstable microaggregates. In these early stages, FGF acts as an activator while BMP acts as an inhibitor. The expression of FGF/pERK later promotes chemotaxis toward a signaling center in this patterning process leading to the formation of stable epithelial placodes and dermal condensations. The placode boundary is unstable at fitow but then becomes stabilized. Adapted from Lin et al., 2009. B. Schematic drawing of the hair reconstitution procedure. Green, epidermal cells; red, dermal cells. Cells are mixed randomly in the three dimensional matrix. Epidermal cells sort themselves out and coalesce to form a layer first near the bottom of the matrix which then rises to the top surface. Dermal cells form condensations adjacent to the epidermis. Hair germs appear periodically and progress to form hair pegs and later, hair follicles. The morphogenetic process occurs between about day 5 to 12 after grafting. C. Reconstituted hair follicles form normally. Using a reasonably stiff matrix to hold multipotential cells, the graft can be cut to specific shapes and sizes for cosmetic applications. Panels B and C are from Lee et al., 2011.
It is important that the number, size and spacing of skin appendage germs are properly regulated. By applying what we learned in periodic pattern formation, we can modulate this process for different applications. For example, with 10 million cells competent to form hairs, we should be able to guide them to form 10 big hairs or 1000 small hairs, depending on the needs of the patients.
Conclusion
The periodic patterning process allows repetitive elements to form with even spacing. Each element then becomes a module that can be modified differently in different regions, forming the basis of region specific skin appendages. Stem cells allow for the cyclic molting and regeneration of each module, forming the basis of physiological regeneration. The combination of these processes and regulation by body hormones make “organ metamorphosis” possible.
We can appreciate that periodic patterning is an efficient design principle which has emerged early in evolution to allow functional redundancy and phenotypic variation. The “module” principle is like using Legos to build different structures, and making the exchange of modular units possible. While many of the regulatory mechanisms remain to be investigated, skin appendage modules serve as a Rosetta stone for us to understand the fundamental principles of patterning morphogenesis.
Basics structure of feathers.
Branching morphogenesis allows for remarkable diversity in feather structures. Feathers develop within tracts on the body surface. Each feather within a tract has common features, although they may differ in size and color; often forming a gradient. For example, ventral body feathers have a larger plumulaceous component, while wing feathers have a larger pennaceous component. The ventral body feathers are symmetric in that the length of the barbs on either side of the central feather backbone (rachis) are of equal length; whereas, flight feathers are bilaterally asymmetric and produce greater lift and aerodynamic stability. Other tracts, such as the tail feathers (rectrices) are used for both flight stability and communication and have different attributes when compared to the ventral body or flight feathers.
Branching can produce radially symmetric downy feathers which provide warmth to young birds. Later in development the downy feathers molt and are replaced by more complex bilaterally symmetric juvenal feathers and then they are replaced later in development by adult feathers. Even in adult feathers, the base often has a downy (plumulaceous) component that continues to provide warmth. A second function that feathers can provide is communication. This is produced by the shape, movement and sexually dimorphic coloring of the feathers and may be used to scare adversaries or to attract a mate. Third, the feathers enable flight which provides access to a totally new environment, the sky. In flight feathers (remiges), neighboring barbs are linked together by barbules. This produces pennaceous wing feathers which can move the air and produce an aerodynamic surface.
Further Reading/Resources.
Chuong CM, Richardson MK. 2009. Special issue on Pattern formation. Int J Dev Biol. Volume 53.
Dhouailly D. edit. 2004. Special issue on Skin Development. Int J Dev Biol. Volume 48
Kondo S, Miura T. 2010. Reaction-diffusion model as a framework for understanding biological pattern formation Science 329:1616–20.
Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition BioEssays : news and reviews in molecular, cellular and developmental biology 2000, 22:753–60.
Newman SA, Bhat R, Mezentseva NV 2009. Cell state switching factors and dynamical patterning modules: complementary mediators of plasticity in development and evolution. J Biosci. 34:553–72.
References
- 1.Chuong CM, Richardson MK. Pattern formation today. The International journal of developmental biology. 2009;53:653–8. doi: 10.1387/ijdb.082594cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, Widelitz R, Jiang TX, Chuong CM. Evo-Devo of amniote integuments and appendages. The International journal of developmental biology. 2004;48:249–70. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. BioEssays : news and reviews in molecular, cellular and developmental biology. 2000;22:753–60. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs E, Merrill BJ, Jamora C, DasGupta R. At the roots of a never-ending cycle. Developmental cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 5.Plikus MV, Chuong CM. Complex Hair Cycle Domain Patterns and Regenerative Hair Waves in Living Rodents. The Journal of investigative dermatology. 2008 doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plikus MV, Baker RE, Chen CC, Fare C, de la Cruz D, Andl T, Maini PK, Millar SE, Widelitz R, Chuong CM. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science (New York, N.Y.) 2011;332:586–9. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuong C-, Randall VA, Widelitz RB, Wu P, Jiang T- Learning regenerative medicine: Physiological regeneration of skin appendages. Physiology. 2011 doi: 10.1152/physiol.00028.2011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christley S, Alber MS, Newman SA. Patterns of mesenchymal condensation in a multiscale, discrete stochastic model. PLoS computational biology. 2007;3:e76. doi: 10.1371/journal.pcbi.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dequeant ML, Glynn E, Gaudenz K, Wahl M, Chen J, Mushegian A, Pourquie O. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science (New York, N.Y.) 2006;314:1595–8. doi: 10.1126/science.1133141. [DOI] [PubMed] [Google Scholar]
- 10.Turing AM. The chemical basis of morphogenesis. 1952;237:37–72. doi: 10.1007/BF02459572. [DOI] [PubMed] [Google Scholar]
- 11.Vanag VK, Epstein IR. Pattern formation mechanisms in reaction-diffusion systems. The International journal of developmental biology. 2009;53:673–81. doi: 10.1387/ijdb.072484vv. [DOI] [PubMed] [Google Scholar]
- 12.Murray JD, Myerscough MR. Pigmentation pattern formation on snakes. Journal of theoretical biology. 1991;149:339–60. doi: 10.1016/s0022-5193(05)80310-8. [DOI] [PubMed] [Google Scholar]
- 13.Nagorcka BN, Mooney JR. From stripes to spots: prepatterns which can be produced in the skin by a reaction-diffusion system. IMA journal of mathematics applied in medicine and biology. 1992;9:249–67. doi: 10.1093/imammb/9.4.249. [DOI] [PubMed] [Google Scholar]
- 14.Maini PK, Baker RE, Chuong CM. Developmental biology. The Turing model comes of molecular age. Science (New York, N.Y.) 2006;314:1397–8. doi: 10.1126/science.1136396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondo S, Miura T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science (New York, N.Y.) 2010;329:1616–20. doi: 10.1126/science.1179047. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg MS, Poole TJ. Strategies for specifying form and pattern: adhesion-guided multicellular assembly Philosophical transactions of the Royal Society of London.Series B. Biological sciences. 1981;295:451–60. doi: 10.1098/rstb.1981.0153. [DOI] [PubMed] [Google Scholar]
- 17.Lucas P, Stettenheim A. Agriculture Handbook. US Department of Agriculture; Washington, DC: 1972. Avian Anatomy. Integument. [Google Scholar]
- 18.Sengel P. Morphogenesis of Skin. Cambridge University Press; Cambridge: 1976. [Google Scholar]
- 19.Oster GF, Murray JD, Harris AK. Mechanical aspects of mesenchymal morphogenesis. Journal of embryology and experimental morphology. 1983;78:83–125. [PubMed] [Google Scholar]
- 20.Jiang TX, Jung HS, Widelitz RB, Chuong CM. Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development (Cambridge, England) 1999;126:4997–5009. doi: 10.1242/dev.126.22.4997. [DOI] [PubMed] [Google Scholar]
- 21.Widelitz RB, Jiang TX, Chen CW, Stott NS, Jung HS, Chuong CM. Wnt-7a in feather morphogenesis: involvement of anterior-posterior asymmetry and proximal-distal elongation demonstrated with an in vitro reconstitution model. Development (Cambridge, England) 1999;126:2577–87. doi: 10.1242/dev.126.12.2577. [DOI] [PubMed] [Google Scholar]
- 22.Chen CW, Chuong CM. Dynamic expression of lunatic fringe during feather morphogenesis: a switch from medial-lateral to anterior-posterior asymmetry. Mechanisms of development. 2000;91:351–4. doi: 10.1016/s0925-4773(99)00285-3. [DOI] [PubMed] [Google Scholar]
- 23.Widelitz RB, Jiang TX, Lu J, Chuong CM. Beta-Catenin in Epithelial Morphogenesis: Conversion of Part of Avian Foot Scales into Feather Buds with a Mutated Beta-Catenin. Developmental biology. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- 24.Ohyama A, Saito F, Ohuchi H, Noji S. Differential expression of two BMP antagonists, gremlin and Follistatin, during development of the chick feather bud. Mechanisms of development. 2001;100:331–3. doi: 10.1016/s0925-4773(00)00525-6. [DOI] [PubMed] [Google Scholar]
- 25.Bardot B, Lecoin L, Fliniaux I, Huillard E, Marx M, Viallet JP. Drm/Gremlin, a BMP antagonist, defines the interbud region during feather development. The International journal of developmental biology. 2004;48:149–56. [PubMed] [Google Scholar]
- 26.Chang CH, Jiang TX, Lin CM, Burrus LW, Chuong CM, Widelitz R. Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mechanisms of development. 2004;121:157–71. doi: 10.1016/j.mod.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ting-Berreth SA, Chuong CM. Sonic Hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death Developmental dynamics : an official publication of the. American Association of Anatomists. 1996;207:157–70. doi: 10.1002/(SICI)1097-0177(199610)207:2<157::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Noveen A, Jiang TX, Ting-Berreth SA, Chuong CM. Homeobox genes Msx-1 and Msx-2 are associated with induction and growth of skin appendages. The Journal of investigative dermatology. 1995;104:711–9. doi: 10.1111/1523-1747.ep12606960. [DOI] [PubMed] [Google Scholar]
- 29.Noramly S, Freeman A, Morgan BA. Beta-Catenin Signaling can Initiate Feather Bud Development. Development (Cambridge, England) 1999;126:3509–21. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- 30.Lin CM, Jiang TX, Baker RE, Maini PK, Widelitz RB, Chuong CM. Spots and stripes: pleomorphic patterning of stem cells via p-ERK-dependent cell chemotaxis shown by feather morphogenesis and mathematical simulation. Developmental biology. 2009;334:369–82. doi: 10.1016/j.ydbio.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houghton L, Lindon C, Morgan BA. The ectodysplasin pathway in feather tract development. Development (Cambridge, England) 2005;132:863–72. doi: 10.1242/dev.01651. [DOI] [PubMed] [Google Scholar]
- 32.Drew CF, Lin CM, Jiang TX, Blunt G, Mou C, Chuong CM, Headon DJ. The Edar subfamily in feather placode formation. Developmental biology. 2007;305:232–45. doi: 10.1016/j.ydbio.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song HK, Lee SH, Goetinck PF. FGF-2 signaling is sufficient to induce dermal condensations during feather development. Developmental dynamics : an official publication of the American Association of Anatomists. 2004;231:741–9. doi: 10.1002/dvdy.20243. [DOI] [PubMed] [Google Scholar]
- 34.Widelitz RB, Jiang TX, Noveen A, Chen CW, Chuong CM. FGF induces new feather buds from developing avian skin. The Journal of investigative dermatology. 1996;107:797–803. doi: 10.1111/1523-1747.ep12330553. [DOI] [PubMed] [Google Scholar]
- 35.Song H, Wang Y, Goetinck PF. Fibroblast growth factor 2 can replace ectodermal signaling for feather development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10246–9. doi: 10.1073/pnas.93.19.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Botchkarev VA. Bone morphogenetic proteins and their antagonists in skin and hair follicle biology. The Journal of investigative dermatology. 2003;120:36–47. doi: 10.1046/j.1523-1747.2003.12002.x. [DOI] [PubMed] [Google Scholar]
- 37.Noramly S, Morgan BA. BMPs mediate lateral inhibition at successive stages in feather tract development. Development (Cambridge, England) 1998;125:3775–87. doi: 10.1242/dev.125.19.3775. [DOI] [PubMed] [Google Scholar]
- 38.Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, Tickle C, Wolpert L, Chuong CM. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Developmental biology. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- 39.Fliniaux I, Viallet JP, Dhouailly D. Signaling dynamics of feather tract formation from the chick somatopleure. Development (Cambridge, England) 2004;131:3955–66. doi: 10.1242/dev.01263. [DOI] [PubMed] [Google Scholar]
- 40.Scaal M, Prols F, Fuchtbauer EM, Patel K, Hornik C, Kohler T, Christ B, Brand-Saberi B. BMPs induce dermal markers and ectopic feather tracts. Mechanisms of development. 2002;110:51–60. doi: 10.1016/s0925-4773(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 41.Viallet JP, Prin F, Olivera-Martinez I, Hirsinger E, Pourquie O, Dhouailly D. Chick Delta-1 gene expression and the formation of the feather primordia. Mechanisms of development. 1998;72:159–68. doi: 10.1016/s0925-4773(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 42.Morgan BA, Orkin RW, Noramly S, Perez A. Stage-specific effects of sonic hedgehog expression in the epidermis. Developmental biology. 1998;201:1–12. doi: 10.1006/dbio.1998.8969. [DOI] [PubMed] [Google Scholar]
- 43.Mou C, Pitel F, Gourichon D, Vignoles F, Tzika A, Tato P, Yu L, Burt DW, Bed'hom B, Tixier-Boichard M, et al. Cryptic patterning of avian skin confers a developmental facility for loss of neck feathering. PLoS biology. 2011;9:e1001028. doi: 10.1371/journal.pbio.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyazono K. Signal transduction by bone morphogenetic protein receptors: functional roles of Smad proteins. Bone. 1999;25:91–3. doi: 10.1016/s8756-3282(99)00113-1. [DOI] [PubMed] [Google Scholar]
- 45.Sebald W, Mueller TD. The interaction of BMP-7 and ActRII implicates a new mode of receptor assembly. Trends in biochemical sciences. 2003;28:518–21. doi: 10.1016/j.tibs.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Michon F, Forest L, Collomb E, Demongeot J, Dhouailly D. BMP2 and BMP7 play antagonistic roles in feather induction. Development (Cambridge, England) 2008;135:2797–805. doi: 10.1242/dev.018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fliniaux I, Viallet JP, Dhouailly D. Ventral vs. dorsal chick dermal progenitor specification. The International journal of developmental biology. 2004;48:103–6. [PubMed] [Google Scholar]
- 48.Serras F, Fraser S, Chuong CM. Asymmetric patterns of gap junctional communication in developing chicken skin. Development (Cambridge, England) 1993;119:85–96. doi: 10.1242/dev.119.Supplement.85. [DOI] [PubMed] [Google Scholar]
- 49.Michon F, Charveron M, Dhouailly D. Dermal condensation formation in the chick embryo: requirement for integrin engagement and subsequent stabilization by a possibl notch/integrin interaction. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:755–68. doi: 10.1002/dvdy.21080. [DOI] [PubMed] [Google Scholar]
- 50.Chuong CM, Homberger DG. Development and evolution of the amniote integument: current landscape and future horizon. Journal of experimental zoology.Part B.Molecular and developmental evolution. 2003;298:1–11. doi: 10.1002/jez.b.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11734–9. doi: 10.1073/pnas.0500781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang CH, Yu M, Wu P, Jiang TX, Yu HS, Widelitz RB, Chuong CM. Sculpting skin appendages out of epidermal layers via temporally and spatially regulated apoptotic events. The Journal of investigative dermatology. 2004;122:1348–55. doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yue Z, Jiang TX, Widelitz RB, Chuong CM. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:951–5. doi: 10.1073/pnas.0506894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin CM, Jiang TX, Widelitz RB, Chuong CM. Molecular signaling in feather morphogenesis. Current opinion in cell biology. 2006;18:730–41. doi: 10.1016/j.ceb.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu M, Yue Z, Wu P, Wu DY, Mayer JA, Medina M, Widelitz RB, Jiang TX, Chuong CM. The biology of feather follicles. The International journal of developmental biology. 2004;48:181–91. doi: 10.1387/ijdb.031776my. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang TX, Tuan TL, Wu P, Widelitz RB, Chuong CM. From buds to follicles: Matrix metalloproteinases in developmental tissue remodeling during feather morphogenesis. Differentiation; research in biological diversity. 2011;81:307–14. doi: 10.1016/j.diff.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–12. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haake AR, Konig G, Sawyer RH. Avian feather development: relationships between morphogenesis and keratinization. Developmental biology. 1984;106:406–13. doi: 10.1016/0012-1606(84)90240-9. [DOI] [PubMed] [Google Scholar]
- 59.Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. The Journal of experimental zoology. 2002;294:160–76. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- 60.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin Nature reviews. Molecular cell biology. 2009;10:207–17. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science (New York, N.Y.) 2006;314:1447–50. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- 62.Stark J, Andl T, Millar SE. Hairy math: insights into hair-follicle spacing and orientation. Cell. 2007;128:17–20. doi: 10.1016/j.cell.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 63.Schlake T, Sick S. Canonical WNT signalling controls hair follicle spacing. Cell adhesion & migration. 2007;1:149–51. doi: 10.4161/cam.1.3.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narhi K, Jarvinen E, Birchmeier W, Taketo MM, Mikkola ML, Thesleff I. Sustained epithelial beta-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development (Cambridge, England) 2008;135:1019–28. doi: 10.1242/dev.016550. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A, et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development (Cambridge, England) 2008;135:2161–72. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Chang H, Nathans J. When whorls collide: the development of hair patterns in frizzled 6 mutant mice. Development (Cambridge, England) 2010;137:4091–9. doi: 10.1242/dev.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang TX, Widelitz RB, Shen WM, Will P, Wu DY, Lin CM, Jung HS, Chuong CM. Integument pattern formation involves genetic and epigenetic controls: feather arrays simulated by digital hormone models. The International journal of developmental biology. 2004;48:117–35. doi: 10.1387/ijdb.041788tj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maini PK, Baker RE, Chuong CM. Developmental biology. The Turing model comes of molecular age. Science (New York, N.Y.) 2006;314:1397–8. doi: 10.1126/science.1136396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolfram S. A New Kind of Science. Wolfram Media; Champaigne, Illiniois: 2002. [Google Scholar]
- 70.Deutsch A, Dormann S, Maini PK. Cellular Automaton Modeling of Biological Pattern Formation. Birkhauser Boston; New York: 2004. [Google Scholar]
- 71.Rose J, Oldfield J, Stormshak F. Apparent role of melatonin and prolactin in initiating winter fur growth in mink. General and comparative endocrinology. 1987;65:212–5. doi: 10.1016/0016-6480(87)90168-7. [DOI] [PubMed] [Google Scholar]
- 72.Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, Slugocki G, McNulty J, Kauser S, Tobin DJ, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:896–8. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- 73.Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. Journal of cellular physiology. 2003;196:144–53. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- 74.Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, Memezawa A, Bettermann A, Aiba S, Carlberg C, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1710–2. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- 75.Lin KK, Kumar V, Geyfman M, Chudova D, Ihler AT, Smyth P, Paus R, Takahashi JS, Andersen B. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS genetics. 2009;5:e1000573. doi: 10.1371/journal.pgen.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mayer JA, Chuong CM, Widelitz R. Rooster feathering, androgenic alopecia, and hormone-dependent tumor growth: what is in common? Differentiation; research in biological diversity. 2004;72:474–88. doi: 10.1111/j.1432-0436.2004.07209003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wheeler MD. Physical changes of puberty. Endocrinology and metabolism clinics of North America. 1991;20:1–14. [PubMed] [Google Scholar]
- 78.Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1967–9. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- 79.Randall VA, Hibberts NA, Thornton MJ, Merrick AE, Hamada K, Kato S, Jenner TJ, de Oliveira I, Messenger AG. Do androgens influence hair growth by altering the paracrine factors secreted by dermal papilla cells? European journal of dermatology : EJD. 2001;11:315–20. [PubMed] [Google Scholar]
- 80.Conrad F, Ohnemus U, Bodo E, Biro T, Tychsen B, Gerstmayer B, Bosio A, Schmidt-Rose T, Altgilbers S, Bettermann A, et al. Substantial sex-dependent differences in the response of human scalp hair follicles to estrogen stimulation in vitro advocate gender tailored management of female versus male pattern balding. The journal of investigative dermatology.Symposium proceedings / the Society for Investigative Dermatology, Inc.[and] European Society for Dermatological Research. 2005;10:243–6. doi: 10.1111/j.1087-0024.2005.10115.x. [DOI] [PubMed] [Google Scholar]
- 81.Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, Jiang TX, Hou L. Adaptation to the sky: Defining the feather with integument fossils from mesozoic China and experimental evidence from molecular laboratories. Journal of experimental zoology.Part B.Molecular and developmental evolution. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prum RO, Brush AH. The evolutionary origin and diversification of feathers. The Quarterly review of biology. 2002;77:261–95. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- 83.Hou L, Chiappe LM, Zhang F, Chuong CM. New Early Cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds Die. Naturwissenschaften. 2004;91:22–5. doi: 10.1007/s00114-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–20. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 85.Chuong CM. Regenerative biology: new hair from healing wounds. Nature. 2007;447:265–6. doi: 10.1038/447265a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee LF, Jiang TX, Garner W, Chuong CM. A simplified procedure to reconstitute hair producing skin. Tiss. Eng. Part C. 2011;17:1–10. doi: 10.1089/ten.tec.2010.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nature protocols. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng Y, Du X, Wang W, Boucher M, Parimoo S, Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. The Journal of investigative dermatology. 2005;124:867–76. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
- 89.Ackerman J. Dinosaurs take wing. New fossil finds from China provide clues to the origin of birds. National Geographic. 1998;194:74–99. [Google Scholar]
- 90.Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X. Four-winged dinosaurs from China. Nature. 2003;421:335–40. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- 91.Hou L, Chuong C-, Yang A, et al. Fossil Birds of China. Yunnan Science and Technology; China: 2003. [Google Scholar]