Abstract
Background: The Western diet increases risk of metabolic disease.
Objective: We determined whether lowering the ratio of saturated fatty acids to monounsaturated fatty acids in the Western diet would affect physical activity and energy expenditure.
Design: With the use of a balanced design, 2 cohorts of 18 and 14 young adults were enrolled in separate randomized, double-masked, crossover trials that compared a 3-wk high–palmitic acid diet (HPA; similar to the Western diet fat composition) to a low–palmitic acid and high–oleic acid diet (HOA; similar to the Mediterranean diet fat composition). All foods were provided by the investigators, and the palmitic acid (PA):oleic acid (OA) ratio was manipulated by adding different oil blends to the same foods. In both cohorts, we assessed physical activity (monitored continuously by using accelerometry) and resting energy expenditure (REE). To gain insight into a possible mood disturbance that might explain changes in physical activity, the Profile of Mood States (POMS) was administered in cohort 2.
Results: Physical activity was higher during the HOA than during the HPA in 15 of 17 subjects in cohort 1 (P = 0.008) (mean: 12% higher; P = 0.003) and in 12 of 12 subjects in the second, confirmatory cohort (P = 0.005) (mean: 15% higher; P = 0.003). When the HOA was compared with the HPA, REE measured during the fed state was 3% higher for cohort 1 (P < 0.01), and REE was 4.5% higher in the fasted state for cohort 2 (P = 0.04). POMS testing showed that the anger-hostility score was significantly higher during the HPA (P = 0.007).
Conclusions: The replacement of dietary PA with OA was associated with increased physical activity and REE and less anger. Besides presumed effects on mitochondrial function (increased REE), the dietary PA:OA ratio appears to affect behavior. The second cohort was derived from a study that was registered at clinicaltrials.gov as R01DK082803.
INTRODUCTION
During the past several decades the incidences of obesity and associated health problems, such as type 2 diabetes, have greatly increased (1, 2). This obesity epidemic is especially evident in westernized countries where sedentary lifestyles, the overconsumption of calorically dense foods, and a high saturated fat intake impede optimal health (2, 3). Some data have suggested that an excessive energy intake, not abnormally decreased energy expenditure, is the principal cause of the obesity epidemic (1). However, obesity is fundamentally a disorder of energy-balance regulation, and low resting energy expenditure (REE)4 or sedentary behavior also can contribute to weight gain (4, 5). Despite considerable industry investment in the formulation of pharmaceutical agents that promote energy wasting, efforts in the development of antiobesity drugs continue to fall short (6). Physical activity still remains the safest and most commonly prescribed means of raising energy expenditure.
The 2 most prevalent fatty acids (FAs) in the Western diet are the SFA palmitic acid (PA; 16:0) and the MUFA oleic acid (OA; C18:1), each of which is present in approximately equal amounts as a percentage of dietary energy (SFA: 13.7% energy; MUFA: 11.7%) (7). Although an increased dietary PA:OA ratio has been linked to elevated serum LDL concentrations and ischemic heart disease (8), the widespread and effective use of statin drugs might lessen the concern about lowering SFA intake. However, we have been interested in how SFA compared with MUFA in the diet affects risk of diabetes and obesity (9); our previous studies suggested that lowering the dietary PA:OA ratio increased daily energy requirements for weight maintenance as well as the energy cost of physical activity (10, 11). In this article, we asked whether a shift in the FA composition of the diet might affect physical activity and REE.
Dietary FA may influence physical activity via effects on mood (eg, anger and hostility) (12), but a meta-analysis has also shown that low-intensity exercise improves the self-rated effect (13). Thus, we examined the relation between dietary FA, physical activity behavior, and mood.
SUBJECTS AND METHODS
Subjects, screening, and overall design
Two cohorts of adult volunteers were derived from 2 separate, double-masked, crossover trials that used an identical dietary protocol for the determination of effects of changes in the dietary PA:OA ratio. Each study (cohorts 1 and 2) used a crossover design; subjects were randomly assigned into 2 groups that differed with respect to the treatment order; the trial lasted for 2 treatment periods. For cohort 1, the first volunteer began the study on 30 August 2007; therefore, the study was not registered as a clinical trial. However, cohort 2 was derived from a study that was registered at clinicaltrials.gov as R01DK082803.
The procedures followed for the 2 clinical studies were in accordance with the ethical standards of the relevant institutional committees. Cohort 1 consisted of healthy, sedentary, nonobese healthy men (n = 9) and women (n = 9) aged 18–40 y. Some salient characteristics of these subjects are shown in Table 1. Exclusion criteria included prescription medication, regular aerobic exercise training, dyslipidemia (14), evidence of type 2 diabetes (15), and women with abnormal ovulation. In addition, subjects were excluded who manifested an abnormally high value (>4.65) for the HOMA-IR (16). Subjects in cohort 1 participated in a trial in which comprehensive transcriptional and metabolomic profiling was used to assess the effects of dietary FA composition on metabolic outcomes, such as insulin sensitivity (17).
TABLE 1.
Demographic and metabolic characteristics1
| Cohort 1 |
Cohort 2 |
|||||
| M | W | Lean M | Obese M | Lean W | Obese W | |
| Age (y) | 28.9 ± 2.5 | 30.0 ± 2.2 | 30.5 ± 2.8 | 38.0 ± 0.5 | 24.1 ± 1.1 | 26.8 ± 4.6 |
| Body weight (kg) | 77.9 ± 3.6 | 66.9 ± 4.0 | 74.9 ± 9.6 | 87.6 ± 12.5 | 61.1 ± 2.6 | 115.7 ± 27.8 |
| Height (cm) | 180.8 ± 2.3 | 171.1 ± 1.3 | 176.6 ± 4.1 | 174.8 ± 8.0 | 169.0 ± 2.1 | 169.1 ± 2.8 |
| BMI (kg/m2) | 23.8 ± 0.9 | 22.8 ± 1.3 | 23.7 ± 2.2 | 28.5 ± 1.52 | 21.4 ± 0.9 | 40.2 ± 8.4 |
| Abdominal circumference (cm) | 81.0 ± 3.8 | 80.6 ± 3.9 | 84.3 ± 5.1 | 94.4 ± 6.7 | 76.6 ± 2.8 | 107.8 ± 14.2 |
| Body fat (%)3 | 17.8 ± 2.7 | 30.6 ± 3.1 | 22.0 ± 2.8 | 32.2 ± 0.7 | 27.3 ± 2.2 | 49.1 ± 0.2 |
| FFM (kg) | 64.4 ± 3.0 | 46.5 ± 1.5 | 59.5 ± 7.8 | 59.4 ± 8.2 | 44.5 ± 1.8 | 59.5 ± 13.9 |
| TC (mmol/L) | 3.7 ± 0.2 | 3.9 ± 0.3 | 4.9 ± 0.2 | 5.1 ± 0.0 | 4.3 ± 0.5 | 5.0 ± 0.8 |
| LDL (mmol/L) | 2.1 ± 0.2 | 2.0 ± 0.2 | 2.8 ± 0.2 | 3.2 ± 0.2 | 2.3 ± 0.5 | 3.3 ± 0.7 |
| HDL (mmol/L) | 1.3 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.2 | 1.1 ± 0.2 | 1.7 ± 0.3 | 1.1 ± 0.0 |
| LDL:HDL ratio | 1.7 ± 0.3 | 1.2 ± 0.1 | 1.7 ± 0.2 | 3.0 ± 0.5 | 1.6 ± 0.5 | 3.1 ± 0.6 |
| TAG (mmol/L) | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 | 1.6 ± 0.9 | 0.8 ± 0.1 | 1.4 ± 0.3 |
| Fasting insulin (pmol/L) | 18.9 ± 3.6 | 24.2 ± 5.1 | 27.1 ± 4.7 | 53.8 ± 24.0 | 26.0 ± 2.3 | 87.5 ± 6.3 |
| Fasting glucose (mmol/L) | 4.4 ± 0.1 | 4.3 ± 0.1 | 4.5 ± 0.2 | 4.9 ± 0.2 | 4.2 ± 0.1 | 4.3 ± 0.0 |
| HOMA-IR | 0.5 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.2 | 1.6 ± 0.7 | 0.7 ± 0.1 | 2.4 ± 0.2 |
| ALT (U/L) | 30.4 ± 5.3 | 22.6 ± 1.5 | 29.8 ± 3.2 | 33.0 ± 2.0 | 24.8 ± 6.5 | 29.0 ± 2.0 |
| Hemoglobin (g/dL) | 14.4 ± 0.4 | 12.8 ± 0.5 | 14.9 ± 0.1 | 14.2 ± 0.4 | 12.6 ± 0.2 | 13.1 ± 0.3 |
| NEFAs (mmol/L) | 0.4 ± 0.1 | 0.3 ± 0.1 | ND | ND | ND | ND |
All values are means ± SEMs. n = 18 in cohort 1 and n = 14 in cohort 2 (includes subjects with physical activity, Profile of Mood States, or resting energy-expenditure data presented in Results and described in Subjects and Methods). There were 2 obese men and 2 obese women in cohort 2. ALT, serum concentration of alanine aminotransferase; FFM, fat-free mass; HDL, serum concentration of HDL cholesterol; LDL, serum concentration of LDL cholesterol; ND, nondetermined; NEFA, nonesterified fatty acid; TAG, serum concentration of triacylglycerol (triglycerides); TC, serum total cholesterol concentration.
See Subjects and Methods for an explanation of why one man with BMI (in kg/m2) >25 but <30 was included in our obese group because his percentage of body fat was exceptionally high for his BMI.
Data were obtained at the time of screening except for the percentage of body fat, FFM, and NEFAs, which were obtained at the end of the 1-wk baseline diet.
To confirm results from the first cohort, we used data collected from cohort 2 (Table 1). This cohort was comprised of 12 subjects (6 men and 6 women) with physical activity measurements; in one of these subjects, REE was not measured because of technical issues. Mood data were collected for the last 10 subjects studied. We were able to study mood only in 2 additional subjects (for a total of 6 men and 6 women with mood data). Thus, a total of 14 individual subjects contributed to data on physical activity, mood, and/or REE for cohort 2.
The recruitment strategy for cohort 2 was identical to that for cohort 1, except for 2 factors. First, in contrast to in cohort 1, we allowed women in cohort 2 who were receiving hormonal contraception. Second, subjects were recruited in 2 ranges of BMI (in kg/m2) of 18–25 and ≥30. However, after an initial clinical evaluation, we evaluated body composition during screening in 2 male subjects with respective BMIs of 31.7 and 27.0; these men were reassigned respectively to the lean and obese categories after their BMI was corrected for an empirical relation between BMI and percentage of body fat (18). On this basis, there were 2 of 6 men and 2 of 6 women who were judged to be obese. Cohort 2 participated in a trial designed in part to investigate how dietary FA composition affects palmitate metabolism.
Diets
Detailed screening of cohort 1 indicated a habitual intake of 37% of kilocalories from total fat, 14.5% of kilocalories from saturated fat, and 12% of kilocalories from monounsaturated fat, which was consistent with the usual American diet (7). The habitual diet of cohort 2 was similar at 35.8% of kilocalories from total fat, 12.0% of kilocalories from saturated fat, and 13.0% of kilocalories from monounsaturated fat. All subjects in both cohorts ingested solid-food diets until after completion of physical activity measurements (as well as mood assessment in cohort 2). All foods and beverages, except water, were provided to subjects at an energy intake sufficient to maintain body weight. After screening, a low-fat and low-PA baseline-control diet was fed for 7 d (protein: 19.7% of kilocalories; carbohydrate: 51.6% of kilocalories; fat: 28.4% of kilocalories; PA: 5.3% of kilocalories; and OA: 15.9% of kilocalories) (14). Subjects participated in a crossover study of 3-wk diet periods (separated by another 1 wk of the control diet) that consisted of a diet that resembled the habitual diet and was a high–palmitic acid diet (HPA; similar to Western diet fat composition) (fat: 40.4% of kilocalories; PA: 16.0% of kilocalories; and OA: 16.2% of kilocalories) or a low-PA and high–oleic acid diet (HOA; similar to the Mediterranean diet fat composition) (fat: 40.1% of kilocalories; PA: 2.4% of kilocalories; and OA: 28.8% of kilocalories) (Table 2). For all 3 diets, the FA composition was varied by adding oil blends to the 6 precisely formulated meals that composed the control diet and the 9 meals that composed experimental diets.
TABLE 2.
Composition of experimental diets1
| HPA | HOA | |
| Percentage of kcal | ||
| Protein | 16.8 | 16.89 |
| Carbohydrate | 42.85 | 43.51 |
| Fat | 40.45 | 40.13 |
| Fatty acid profile (g/100 g) | ||
| Palmitic | 40.29 | 4.59 |
| Oleic | 39.95 | 74.8 |
| Linoleic | 10.37 | 14.29 |
| Stearic | 4.22 | 2.81 |
| α-Linolenic | 0.18 | 0.14 |
| Myristic | 0.97 | 0 |
| Palmitoleic | 0.18 | 0.12 |
| Eicosapentaenoic | 0 | 0 |
| Docosahexaenoic | 0 | 0 |
| Arachidonic | 0 | 0 |
| Percentage of kcal | ||
| 12:0 | 0 | 0 |
| 14:0 | 0.42 | 0.05 |
| 16:0 | 16.02 | 2.37 |
| 18:0 | 1.8 | 1.27 |
| 18:1 | 16.23 | 28.75 |
| 18:3 | 0.16 | 0.19 |
| 18:2 | 4.97 | 6.36 |
HOA, high–oleic acid diet; HPA, high–palmitic acid diet.
Foods, including chicken and turkey (the only sources of meat), were all very low in fat. Thus, FAs were mainly provided by vegetable-oil blends appropriate to each diet (Natural Oils International Inc). The HPA and HOA otherwise contained the exact same foods with a 3-d rotating menu. These oils, at room temperature, were mixed with foods that had been warmed; thus, these oils were not used for cooking. The oil blend for the control diet consisted of palm oil (36.9%), high-oleic sunflower oil (19.3%), and hazelnut oil (43.8%). The HPA oil blend consisted of palm oil (89%), peanut oil (6.75%), and virgin olive oil (4.25%), and the HOA blend consisted only of hazelnut oil. Except for the virgin olive oil used only in the HPA, all natural oils were first extracted (eg, centrifugation) and refined [alkaline refining (crude oil treated with alkali to separate impurities from triglycerides) and filtering to remove impurities such as seed fragments]. The HOA and HPA had identical, low glycemic loads (10.7; average of 3 d of menus) (19, 20).
Diets were prescribed in a random order, stratified by sex, and separated by a 1-wk period of the baseline-control diet. All foods and drinks, except water, were provided by the General Clinical Research Center (GCRC), and body-energy balances were maintained throughout the study as previously described (21). For cohort 1, 10 of 17 subjects ingested the HPA first, and in cohort 2, equal numbers of subjects ingested the HPA or HOA first. Thus, boredom or fatigue from the study was not weighted toward the HPA because fewer subjects in the combined cohorts ingested the HPA last. However, the diet order was considered in the statistical analysis.
Subjects in the first cohort ate breakfast in the GCRC from Sunday to Friday, but most subjects chose to eat their 2 remaining meals each day at home. Subjects in the second cohort ate breakfast in the GCRC from Monday to Friday and at home on Saturday and Sunday. Each meal was packaged and ready to be reheated by using either an oven or microwave. Because the foods, themselves, were practically devoid of fat, subjects also were given containers of oil to add to each meal after it had been reheated. Subjects also were given instructions regarding convenient ways to add the oils to various food items on the menu. Each day, subjects completed and signed a questionnaire that attested to their having eaten all of the food (and food oil) and to not having consumed any food or drink, except water, not on the menu. On Sunday (cohort 1) or Monday (cohort 2), volunteers completed questionnaires pertaining to the previous 1 or 2 d. All food and oil containers were inspected each day to be sure all food and oil was consumed. Subjects were given instructions to use spatulas that were provided to help scrape all oil from its container but, ultimately, were instructed to lick the oil container to finally empty it. Occasionally, there was evidence of incomplete food or oil consumption or a subject would admit to the ingestion, usually inadvertently, of, eg, a cookie or mint. However, any consistent noncompliance was grounds for removal from the study. Fortunately, only one subject left the study on day 3 because of an aversion to the food (ie, a dislike of fat-free cottage cheese). Because completely following the diet was a dichotomous issue, and subjects were queried each day about this, we did not use a detailed diet history during the study. Because the food was provided ready to eat to subjects, except for the reheating of the nonfat component, and food and oil containers were returned for daily inspection, there was no need to use photography, eg, to show what food was offered to the subject or was not eaten. For the first cohort, the average number of days when food was returned during the HPA and HOA was 1.33 and 1.67 d, respectively, and the average daily consumption of oil for the HPA and HOA as a percentage of the total oil administered (127.8 and 127.6 g/d, respectively) was 99.9% and 99.2%, respectively.
Assessment of physical activity
Because both cohorts participated in studies in which discrete outcomes were being assessed for the entire diet period, we wanted an integrated measurement of physical activity over this same period. Thus, data were derived from recordings during days 3–7 of the control diet and days 2–21 of each experimental diet for cohort 1 and during days 3–6 of the control diet and days 2–18 of each experimental diet for cohort 2. For cohort 1, because of the dysfunction of the activity monitor, data presented in this article concern only 8 men. For both cohorts, physical activity (counts · min−1 · d−1) was assessed daily during nonsleeping hours by using an activity monitor that was worn at the hip (belt at the waist) (ActiGraph GT1M; ActiGraph).
The activity monitor is a small, electromechanical device that records acceleration and deceleration of movement. The activity monitor records these accelerations and decelerations as activity counts and provides data for different intensities of physical activity. These counts are linearly related to the intensity of the movement performed by the participant and are summed over a set period of time (epoch). Because activity monitors do not rely on self-report, they provide an objective measure of physical activity, which correlates with total and activity-related energy expenditure measured by using the doubly labeled water technique (22).
The reliability of this activity monitor increases when subjects wear them ≥3 compared with 1 d (23). The reported intradevice and interdevice reliabilities of this activity monitor are 2.9% and 3.5%, with a mechanical shaker used to induce movement (24). The actual use of this particular monitor, which is worn at the hip, has been shown to account for ∼76–79% of the variability in activity-related energy expenditure, which is measured by using a respiratory room calorimeter with positive predictive values for sedentary, light, moderate, and vigorous activities of 80%, 66%, 69%, and 74%, respectively (25).
With the use of the framework of Treuth et al (26), we also analyzed minutes spent and average physical activity at sedentary, light, or moderate-vigorous physical activity levels (<100, 101–2999, and ≥3000 counts · min−1 · d−1, respectively) for cohort 1 only. One of the definitions of the word sedentary is sitting. Because we showed data related to the sedentary range of activity, we note that sedentary activities included resting and watching TV in the research by Treuth et al (26).
Indirect calorimetry
For cohort 1, on day 20 of each experimental diet period (HPA and HOA), after an evening meal at 1800 (one-third of the daily energy intake), we carried out indirect calorimetry overnight in both fed and fasted states, as previously described (10). Except for bedside bathroom privileges, subjects remained in bed for the duration of the indirect calorimetry study. Measurements of oxygen consumption (˙O2), and carbon dioxide production (˙CO2) (Vmax SPECTRA 29; Sensor Medics Corp) were used for 20 min each time at 60-min intervals postmeal for 7 h (fed state) (1740, 1900, 2000, 2100, 2200, 2300, 2400, and 0100) and at 120-min intervals until 11 h postmeal (fasted state) (0300 and 0500) (10). Urine was collected during the entire interval, and protein oxidation was estimated from the rate of urea nitrogen for both the fed (1720–0120) period and the fasted period (0120–0720) (10). REE and substrate utilization were calculated according to standard procedures by using urine urea nitrogen measurements as estimates of protein oxidation (10, 27, 28) and Weir's equation (29) as follows:
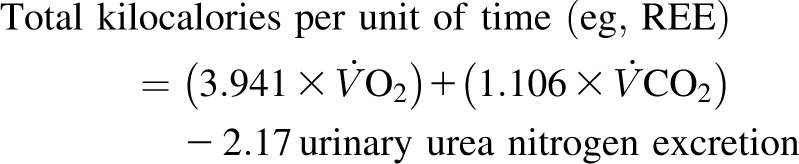 |
For cohort 2, indirect calorimetry in the fed state was carried out on day 21 of each experimental diet as part of a [1-13C]-acetate recovery study to correct for labeled carbon dioxide recovery during palmitate tracer studies on the 2 d before the acetate test. The protocol consisted of a 9-h administration (every 20 min) of a liquid diet that was formulated on the basis of either experimental diet (each dose was one-twenty-seventh or 3.7% of the daily energy intake). Fed measurements were obtained at 360 and 420 min after the initiation of feedings; subjects were allowed to rest in bed or sit in a chair between indirect calorimetry measurements, which, however, were conducted in the supine position. One measurement of REE (50 min) in the fasted state was made 10.3 h after the last 20-min dose of formula (0500–0550).
Assessment of mood (cohort 2 only)
On day 4 of the control diet and day 12 or 13 of experimental diets, subjects were asked to complete the Profile of Mood States (POMS) (30), which is a self-rating questionnaire. Subscale scores for 5 negative mood states (tension-anxiety, depression-dejection, anger-hostility, fatigue-inertia, and confusion-bewilderment states) and one positive mood state (vigor-activity) were calculated. A total mood disturbance (TMD) score was calculated by subtracting the one positive mood state from the sum of the 5 negative states.
Body composition
On the first day of the baseline diet, body composition was assessed by using dual-energy X-ray absorptiometry (GE Lunar Prodigy Densitometer, version 5.6)
Metabolite assays
The FA composition of serum phospholipids was analyzed by using recently described methods (17, 21). Nonesterified FAs in serum were assessed by using capillary gas chromatography–mass spectrometry (31). Serum concentrations of total cholesterol, HDL cholesterol, and triacylglycerols (triglycerides) were measured at the Clinical Chemistry Laboratory at Fletcher Allen Health Care, which is an affiliate of the University of Vermont, by using a colorimetric method (Vitros 5.1 FS Chemistry System; Ortho-Clinical Diagnostics). LDL cholesterol was calculated. The following measurements were also determined at the Clinical Chemistry Laboratory at Fletcher Allen Health Care (methods): serum glucose (by using colorimetric reflectance spectrophotometry); insulin (by using a chemiluminescent immunoassay), hemoglobin (by using an automated cell counter), and serum concentrations of alanine aminotransferase (by using rate reflectance spectrophotometry).
Statistics
All data are expressed as means ± SEMs. Analyses were performed with SAS software (version 9.2; SAS Institute Inc). Each study (cohorts 1 and 2) used a crossover design; subjects were randomly assigned into 2 groups that differed with respect to treatment order, and the trial lasted for 2 treatment periods. Diet effects were analyzed by using a repeated-measures ANOVA, including sequence and treatment effects, with the baseline value as a covariate. In addition, Wilcoxon's signed-rank test was used to examine potential diet-associated increases (decreases) in variables of interest, without regard to the magnitude of the increase (decrease).
RESULTS
Body weight
For both cohorts, there were no diet effects on body-weight changes during either experimental diet.
Serum phosphatidylcholine
To determine whether diets provided to subjects had their presumed effects on body lipids, we examined the diet effect on the PA:OA ratio for serum phosphatidylcholine in the fasted state. For both cohorts, every subject exhibited a higher PA:OA ratio during the HPA than HOA (P ≤ 0.001 for the diet effect by using Wilcoxon's signed-rank test for cohorts 1 and 2). In addition, as reported previously (17), in cohort 1, the mean value for the ratio was higher during the HPA than HOA (3.13 ± 0.10 compared with 1.49 ± 0.08, respectively; P < 0.01); similar results were shown for cohort 2 (3.62 ± 0.17 compared with 1.94 ± 0.14, respectively; P < 0.0001).
Physical activity
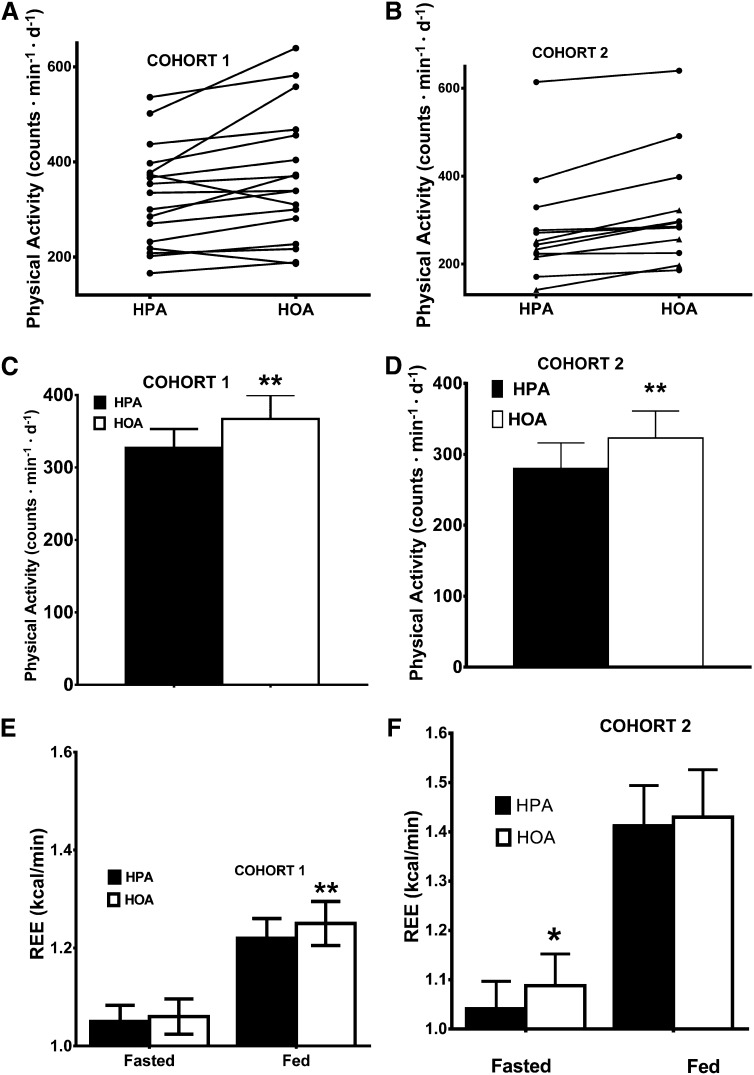
As an index of physical activity behavior, we monitored physical movement by using accelerometry. Physical activity was significantly higher during the HOA in 15 or 17 subjects in cohort 1 (P = 0.008) (Figure 1A) and in all subjects in cohort 2 (P = 0.005) (Figure 1B). For cohorts 1 and 2, mean physical activity was 12% (P = 0.01) and 15% (P = 0.003) higher with the HOA than HPA, respectively; differences between means (HOA – HPA) for the 2 cohorts were almost identical at 40 and 43 counts · min−1 · d−1, respectively (Figure 2, C and D). As noted in Subjects and Methods, for cohort 1, we explored whether diet affected the amount of time per day spent at different levels of physical activity, but no such effect was detected (26).
FIGURE 1.
Physical activity and REE. A and B: Effects of diet condition on individual changes in physical activity during the 2 experimental diets. Diet effects were analyzed by using Wilcoxon's signed-rank test in cohorts 1 (n = 17; P = 0. 008) (A) and 2 (n = 12; P = 0.005) (B). Lean subjects are designated by circles, and obese subjects are designated by triangles. Effects of diet condition on average (mean ± SEM) physical activity were calculated in cohorts 1 (HPA: 327 ± 26 counts · min−1 · d−1; HOA: 367 ± 33 counts · min−1 · d−1) (C) and 2 (HPA: 280 ± 36 counts · min−1 · d−1; HOA: 323 ± 38 counts · min−1 · d−1) (D). Diet effects were analyzed by using a repeated-measures ANOVA, including sequence and treatment effects, with the baseline value as a covariate. Effects of diets on mean (±SEM) REE (kcal/min) in the fasted and fed state were calculated in cohorts 1 (n = 18; HPA: fasted, 1.05 ± 0.03 kcal/min; fed, 1.22 ± 0.04 kcal/min; HOA: fasted, 1.06 ± 0.04 kcal/min; fed, 1.25 ± 0.04 kcal/min) (E) and 2 (n = 11; HPA: fasted, 1.04 ± 0.06 kcal/min; fed, 1.41 ± 0.08 kcal/min; HOA: fasted, 1.09 ± 0.06 kcal/min; fed, 1.43 ± 0.10 kcal/min) (F). *,**Diet effect, *P ≤ 0.05, **P ≤ 0.01. HOA, high–oleic acid diet; HPA, high–palmitic acid diet; REE, resting energy expenditure.
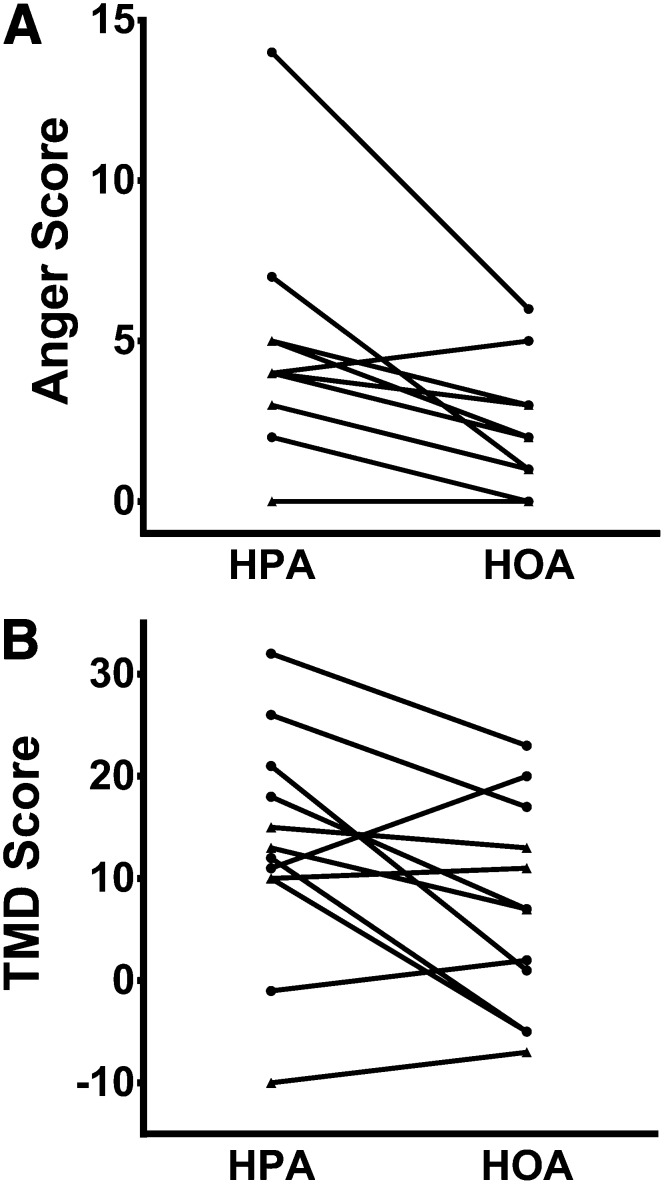
FIGURE 2.
Changes in anger-hostility and TMD scores during the HPA and HOA. Diet effects were analyzed by using Wilcoxon's signed-rank test. A: Ten of 12 men and women exhibited a lower anger-hostility score with the HOA (P = 0.005). Two pairs of subjects showed identical, anger-hostility scores with both the HPA and HOA (5 and 2 for 2 subjects and 4 and 2 for 2 subjects, respectively), which resulted in 2 pairs of overlapping points. Thus, only 10 distinct data points are visible, although all data points were included in the statistical analysis. B: Eight of 12 men and women exhibited a lower TMD score with the HOA (P = 0.06). Two subjects (one lean subject and one obese subject) had a score of 10 with the HPA; we labeled this data point for the obese subject. Lean subjects are designated by circles, and obese subjects are designated by triangles. HOA, high–oleic acid diet; HPA, high–palmitic acid diet; TMD, total mood disturbance.
REE
For cohort 1, the HOA was associated with a 3% higher average REE measured in the fed state (P < 0.01) (Figure 1E). For cohort 2, there was no diet difference in REE in the fed state (1.3% higher during the HOA), but in the fasted state, REE was 4.5% greater during the HOA (P = 0.04) (Figure 1 F).
For the assessment of effects of diets on REE within cohorts, we did not correct for fat-free mass during the diet to avoid bias as a result of possible effects of the diets on body composition (17). However, because, as shown in Figure 1, especially values for REE in the fed state were higher in cohort 2 (daytime measurements) than cohort 1 (supine, evening measurements), we compared the 2 cohorts by expressing REE per fat-free mass. On this basis, there was no difference between cohorts in REE in the fasted state or diet change (HOA – HPA) in REE during either the fed or fasted state. However, for the fed state, values for cohort 2 were significantly higher than those for cohort 1 during either the HOA or HPA (P = 0.012 and P < 0.001, respectively). The history and physical examination and laboratory measurements used at screening and during the dietary trial did not detect subjects who manifested malabsorption, but we did not collect feces during the 2 diets to quantify the excretion of fat. However, our extensive lipidomic analysis performed in cohort 1 subjects suggested that the diets were well absorbed because the PA and OA content of blood and muscle lipids increased to an extent commensurate with the 3-wk diet and single meals (17).
POMS
After the results on physical activity from cohort 1 became available, we studied cohort 2 to explore whether the composition of dietary FA might affect mood. Ten of 12 men and women (cohort 2) exhibited a lower anger-hostility score with the HOA (P = 0.005) (Figure 2A). Eight of 12 men and women exhibited a lower TMD score with the HOA (P = 0.06) (Figure 2B). The mean score on the anger-hostility subscale was significantly lower (P = 0.007) during the HOA than HPA, but there also was a trend for a lower mean TMD score during the HOA (P = 0.096) (Table 3). This study had >80% power to detect a doubling of scores for individual scales of the POMS as significant, with a type I error rate of 5%. We showed no significant correlations between the change in any mood score, including the anger-hostility or TMD score (HOA – HPA) and change in physical activity. In addition, there was no correlation between the change in physical activity and any mood score assessed on the baseline diet.
TABLE 3.
Results from the POMS questionnaire1
| HPA | HOA | P | |
| Tension-anxiety | 3.1 ± 0.7 | 3.3 ± 0.7 | 0.400 |
| Depression-dejection | 4.6 ± 1.0 | 2.7 ± 0.5 | 0.132 |
| Anger-hostility | 4.7 ± 1.0 | 2.2 ± 0.5 | 0.007 |
| Fatigue-inertia | 5.3 ± 1.0 | 3.7 ± 0.9 | 0.330 |
| Confusion-bewilderment | 3.6 ± 0.4 | 2.9 ± 0.5 | 0.441 |
| Vigor-activity | 8.2 ± 1.1 | 7.8 ± 1.1 | 0.801 |
| Total mood disturbance | 13.1 ± 3.2 | 7.0 ± 2.9 | 0.096 |
All values are means ± SEMs of POMS raw scores (n = 6 men and n = 6 women). The total mood disturbance score was calculated by subtracting the one positive mood state from the sum of the 5 negative states. Diet effects were analyzed by using a repeated-measures ANOVA, including sequence and treatment effects, with the baseline value as a covariate. HOA, high–oleic acid diet; HPA, high–palmitic acid diet; POMS, Profile of Mood States.
DISCUSSION
The alteration of the dietary SFA:MUFA ratio in the same individuals affected physical activity and mood and to a mild extent REE also. Our study design could not determine whether these outcomes were primarily determined by increased dietary OA per se or decreased PA. Despite obvious environmental triggers, such as advertising, that promote excessive eating or less physical activity (1, 32–34) as well as potentially innate differences in REE (4), cognition also plays a role in behaviors relevant to physical activity and food selection and consumption (35). We showed that 27 of 29 subjects in the 2 cohorts increased their physical activity during the low-SFA diet, which resulted in mean 12–15% increases in physical activity compared with the habitual, high-SFA diet. These results seemingly conflict with our previous observation that the variation of the dietary PA:OA ratio did not affect physical activity (10). However, in the earlier study, physical activity was monitored for only 1 wk by using the wrist position for the accelerometer, and the dietary FA composition was manipulated by using liquid diets without a crossover design. Although the observed increments in physical activity could have had a substantial effect on energy balance if not compensated by changes in food intake, we observed no significant changes in body weight (as was our design). Moreover, in our recent article (17), we did not report a correlation between diet change in physical activity and change in insulin sensitivity. However, our study pointed to the possibility that dietary SFA compared with MUFA may affect brain function (cognition) because both physical activity behavior and mood were differentially affected by the diets.
A persuasive argument has been made that an excessive dietary energy intake, not physical activity, is a major contributor to obesity (1). However, Levine et al (5) reported that obese compared with nonobese people differed in the time per day that they stood compared with sat, and these posture differences may affect mortality risk (36). Although there is no clear evidence that the intake of either total FA or SFA has been temporally associated with the epidemic of obesity, the latter is a risk factor for type 2 diabetes (37). Although more than one-half of Americans ingest more SFA than has been recommended for optimal health (7), more physically fit individuals tend to eat lower amounts of SFA (38). In the current study, we showed that, in 2 separate cohorts, the lowering of the PA:OA ratio of the diet caused modest increases in REE, but the effect was detected only under conditions when subjects rested quietly in bed (fed state in cohort 1 and fasted state in cohort 2). We also acknowledge that, in our previous, parallel-group trial, there was no trend toward a diet-induced difference (10). Both these previous data (10) and the differing effects of the diets on REE in the 2 cohorts of the current study emphasized that interindividual variation and differences in experimental design may alter subtle dietary effects on REE. Because individuals spend ∼17 h/d eating or within 7 h of eating (the thermic effect of food), the increment in REE with the HOA for cohort 1 accounted for ∼20 kcal/d. However, the biological relevance of the REE findings likely relates to a proof of concept that these diets might have subtle differential effects on mitochondrial function because REE was perturbed during strict resting conditions. We reported that the HPA was associated with a higher serum concentration of ceramide (17), and the inhibition of muscle synthesis of ceramide has been associated with decreased REE (39). There is also evidence that OA may increase mitochondrial uncoupling, especially after exercise training (40).
It is generally assumed that higher, executive-type brain function mediates the desire to exercise per se (35). In this regard, Sartorius et al (41) recently reported that feeding mice a high-PA diet prevented the stimulation of locomotor activity otherwise caused by the intracerebroventricular application of insulin seen in mice fed a low-PA and high-OA diet. Sartorius et al (41) also studied the effect of diets supplemented with either canola oil (low PA and high OA) or dairy fat (high PA) in humans and showed that the high-PA diet caused a decrease in hippocampal and cortical activity, which was assessed by using functional magnetic resonance imaging techniques. Studies that have used functional magnetic resonance imaging also have shown that patients with metabolic syndrome have an abnormal cerebrovascular response to a cognitive challenge (42), which implies a link between nutritional status and brain function. Although the progress toward a mechanistic understanding of these findings will require additional investigation, it is tempting to speculate that the FA composition of the diet might affect neural circuitries that influence physical activity. Relevant to this hypothesis, changes in the dietary FA composition can affect the lipid composition of the brain, which is accompanied by alterations in signaling and inflammation in the hypothalamus (43). Subjects were not told the identity of the 2 study oils, and the investigators were very careful not to know the order of the diets in each subject. Subjects displayed no curiosity about which diet they consumed; anecdotally, when asked, subjects did not seem to know which diet they were ingesting. However, it is possible that physical differences between oils (eg, the melting point) might have altered behavior in some way.
Our studies with the use of the POMS instrument were intended to discern if consumption of the HPA was associated with depression or lethargy, which we thought, a priori, might induce sedentary behavior. However, instead, we showed that the anger-hostility score was higher during the HPA, and there was also a trend for an increase in the total mood disturbance during this same diet. At present, we do not know if diet-related effects on mood, in turn, induced changes in physical activity or whether changes in physical activity affected mood, but we showed no correlations between changes in mood scores on the POMS and changes in physical activity. On the basis of the literature, it was plausible that the reduced anger-hostility score with the HOA was actually a consequence of increased physical activity on that diet; a walking program in female college students was shown to reduce the anger-hostility score measured by using the POMS (44). However, in vitro studies have linked PA (but not OA) to increased secretion of TNF-α (45, 46), and in turn, TNF-α has been linked to feelings of anger and hostility (47, 48). Thus, the cellular PA:OA ratio also may be directly related to feelings of anger because of the secretion of TNF-α by peripheral blood monocytes or, perhaps, synthesis within the brain, which would imply a central inflammatory process (43, 49).
In conclusion, data from 2 rigorously controlled, crossover studies suggest that lowering the saturated fat content of a typical Western diet by replacing PA with OA increased daily physical activity and REE and also affected mood, particularly anger and hostility. Because posture can contribute to differences in energy expenditure in lean compared with obese adults (5), these results might have relevance to an individual's propensity for weight gain. Our findings suggest that a high consumption of PA might dampen motivation for physical activity. In the face of environmental, sociologic, and cultural pressures that discourage obligatory physical activity, even small changes in posture and lifestyle activity could influence energy balance and body habitus. We surmise that studies designed to examine the role of specific dietary FAs in the regulation of physical activity patterns are worthy of additional consideration. Furthermore, we speculate that our studies of physical activity and mood raise the possibility that the type of fat we eat may alter cognitive function.
Acknowledgments
We thank our subjects for their patience and hard work in enduring our rigorous protocols. We also are very grateful to the staff of the University of Vermont General Clinical Research Center for dietary, nursing, and informatics support.
The authors’ responsibilities were as follows—CLK and DMM: designed the research; CLK, KIC, CLT, DBE, TRK, and DMM: conducted the research; JYB: analyzed data; CLK, JYB, CLT, JAD, and DMM: wrote the manuscript; and CLK: had primary responsibility for the final content of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: FA, fatty acid; GCRC, General Clinical Research Center; HOA, high–oleic acid diet; HPA, high–palmitic acid diet; OA, oleic acid; PA, palmitic acid; POMS, Profile of Mood States; REE, resting energy expenditure; TMD, total mood disturbance.
REFERENCES
- 1.Swinburn BA, Sacks G, Lo SK, Westerterp KR, Rush EC, Rosenbaum M, Luke A, Schoeller DA, DeLany JP, Butte NF, et al. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am J Clin Nutr 2009;89:1723–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev 2012;13:659–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berglund L, Lefevre M, Ginsberg HN, Kris-Etherton PM, Elmer PJ, Stewart PW, Ershow A, Pearson TA, Dennis BH, Roheim PS, et al. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am J Clin Nutr 2007;86:1611–20 [DOI] [PubMed] [Google Scholar]
- 4.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WGH, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med 1988;318:467–72 [DOI] [PubMed] [Google Scholar]
- 5.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science 2005;307:584–6 [DOI] [PubMed] [Google Scholar]
- 6.Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol 2010;6:578–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 2011;123:e18–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astrup A, Dyerberg J, Elwood P, Hermansen K, Hu FB, Jakobsen MU, Kok FJ, Krauss RM, Lecerf JM, Legrand P, et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr 2011;93:684–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kien CL. Dietary interventions for metabolic syndrome: role of modifying dietary fats. Curr Diab Rep 2009;9:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kien CL, Bunn JY, Ugrasbul F. Increasing dietary palmitic acid decreases fat oxidation and daily energy expenditure (PMCID: PMC1314972). Am J Clin Nutr 2005;82:320–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Børsheim E, Kien CL, Pearl WM. Differential effects of dietary intake of palmitic acid and oleic acid on oxygen consumption during and after exercise. Metabolism 2006;55:1215–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontani G, Corradeschi F, Felici A, Alfatti F, Bugarini R, Fiaschi AI, Cerretani D, Montorfano G, Rizzo AM, Berra B. Blood profiles, body fat and mood state in healthy subjects on different diets supplemented with Omega-3 polyunsaturated fatty acids. Eur J Clin Invest 2005;35:499–507 [DOI] [PubMed] [Google Scholar]
- 13.Reed J, Ones DS. The effect of acute aerobic exercise on positive activated affect: a meta-analysis. Psychol Sport Exerc 2006;7:477–514 [Google Scholar]
- 14.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(suppl 1):S64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern SE, Williams K, Ferrannini E, Defronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 2005;54:333–9 [DOI] [PubMed] [Google Scholar]
- 17.Kien CL, Bunn JY, Poynter ME, Stevens R, Bain J, Ikayeva O, Fukagawa NK, Champagne CM, Crain KI, Koves TR, et al. A lipidomics analysis of the relationship between dietary fatty acid composition and insulin sensitivity in young adults. Diabetes (Epub ahead of print 13 December 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 2000;72:694–701 [DOI] [PubMed] [Google Scholar]
- 19.Louie JC, Flood V, Turner N, Everingham C, Gwynn J. Methodology for adding glycemic index values to 24-hour recalls. Nutrition 2011;27:59–64 [DOI] [PubMed] [Google Scholar]
- 20.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56 [DOI] [PubMed] [Google Scholar]
- 21.Kien CL, Everingham KI, Stevens D, Fukagawa NK, Muoio DM. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity (Silver Spring) 2011;19:305–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekelund U, Sjostrom M, Yngve A, Poortvliet E, Nilsson A, Froberg K, Wedderkopp N, Westerterp K. Physical activity assessed by activity monitor and doubly labeled water in children. Med Sci Sports Exerc 2001;33:275–81 [DOI] [PubMed] [Google Scholar]
- 23.Janz KF, Witt J, Mahoney LT. The stability of children's physical activity as measured by accelerometry and self-report. Med Sci Sports Exerc 1995;27:1326–32 [PubMed] [Google Scholar]
- 24.Silva P, Mota J, Esliger D, Welk G. Technical reliability assessment of the Actigraph GT1M Accelerometer. Meas Phys Educ Exerc Sci 2010;14:79–91 [Google Scholar]
- 25.Puyau MR, Adolph AL, Vohra FA, Zakeri I, Butte NF. Prediction of activity energy expenditure using accelerometers in children. Med Sci Sports Exerc 2004;36:1625–31 [PubMed] [Google Scholar]
- 26.Treuth MS, Schmitz K, Catellier DJ, McMurray RG, Murray DM, Almeida MJ, Going S, Norman JE, Pate R. Defining accelerometer thresholds for activity intensities in adolescent girls. Med Sci Sports Exerc 2004;36:1259–66 [PMC free article] [PubMed] [Google Scholar]
- 27.Garrow JS. Amsterdam, Netherlands: North-Holland Publishing Co, 1974 [Google Scholar]
- 28.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–34 [DOI] [PubMed] [Google Scholar]
- 29.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNair DM, Lorr M, Droppleman LF. Profile of Mood State manual (revised). San Diego, CA: Educational and Industrial Testing Service, 1992 [Google Scholar]
- 31.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ello-Martin JA, Ledikwe JH, Rolls BJ. The influence of food portion size and energy density on energy intake: implications for weight management. Am J Clin Nutr 2005;82:236S–41S [DOI] [PubMed] [Google Scholar]
- 33.Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr 2004;79:6–16 [DOI] [PubMed] [Google Scholar]
- 34.Hruschka DJ. Do economic constraints on food choice make people fat? A critical review of two hypotheses for the poverty-obesity paradox. Am J Hum Biol 2012;24:277–85 [DOI] [PubMed] [Google Scholar]
- 35.McAuley E, Mullen SP, Szabo AN, White SM, Wojcicki TR, Mailey EL, Gothe NP, Olson EA, Voss M, Erickson K, et al. Self-regulatory processes and exercise adherence in older adults: executive function and self-efficacy effects. Am J Prev Med 2011;41:284–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katzmarzyk PT, Lee IM. Sedentary behaviour and life expectancy in the USA: a cause-deleted life table analysis. BMJ Open 2012;2:e000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salmerón J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019–26 [DOI] [PubMed] [Google Scholar]
- 38.Brodney S, McPherson RS, Carpenter RS, Welten D, Blair SN. Nutrient intake of physically fit and unfit men and women. Med Sci Sports Exerc 2001;33:459–67 [DOI] [PubMed] [Google Scholar]
- 39.Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM, et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 2010;59:2453–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonkonogi M, Krook A, Walsh B, Sahlin K. Endurance training increases stimulation of uncoupling of skeletal muscle mitochondria in humans by non-esterified fatty acids: an uncoupling-protein-mediated effect? Biochem J 2000;351:805–10 [PMC free article] [PubMed] [Google Scholar]
- 41.Sartorius T, Ketterer C, Kullmann S, Balzer M, Rotermund C, Binder S, Hallschmid M, Machann J, Schick F, Somoza V, et al. Monounsaturated fatty acids prevent the aversive effects of obesity on locomotion, brain activity, and sleep behavior. Diabetes 2012;61:1669–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoth KF, Gonzales MM, Tarumi T, Miles SC, Tanaka H, Haley AP. Functional MR imaging evidence of altered functional activation in metabolic syndrome. AJNR Am J Neuroradiol 2011;32:541–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE 2012;7:e30571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakuragi S, Sugiyama Y. Effects of daily walking on subjective symptoms, mood and autonomic nervous function. J Physiol Anthropol 2006;25:281–9 [DOI] [PubMed] [Google Scholar]
- 45.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coll T, Eyre E, Rodriguez-Calvo R, Palomer X, Sanchez RM, Merlos M, Laguna JC, Vazquez-Carrera M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 2008;283:11107–16 [DOI] [PubMed] [Google Scholar]
- 47.Suarez EC, Lewis JG, Kuhn C. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain Behav Immun 2002;16:675–84 [DOI] [PubMed] [Google Scholar]
- 48.Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Labile anger during interferon alfa treatment is associated with a polymorphism in tumor necrosis factor alpha. Clin Neuropharmacol 2010;33:191–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soliman ML, Puig KL, Combs CK, Rosenberger TA. Acetate reduces microglia inflammatory signaling in vitro. J Neurochem 2012;123:555–67 [DOI] [PMC free article] [PubMed] [Google Scholar]