Abstract
Background: Vitamin C (ascorbate) is likely to be essential for skeletal muscle structure and function via its role as an enzyme cofactor for collagen and carnitine biosynthesis. Vitamin C may also protect these metabolically active cells from oxidative stress.
Objective: We investigated the bioavailability of vitamin C to human skeletal muscle in relation to dietary intake and plasma concentrations and compared this relation with ascorbate uptake by leukocytes.
Design: Thirty-six nonsmoking men were randomly assigned to receive 6 wk of 0.5 or 2 kiwifruit/d, an outstanding dietary source of vitamin C. Fasting blood samples were drawn weekly, and 24-h urine and leukocyte samples were collected before intervention, after intervention, and after washout. Needle biopsies of skeletal muscle (vastus lateralis) were carried out before and after intervention.
Results: Baseline vastus lateralis ascorbate concentrations were ∼16 nmol/g tissue. After intervention with 0.5 or 2 kiwifruit/d, these concentrations increased ∼3.5-fold to 53 and 61 nmol/g, respectively. There was no significant difference between the responses of the 2 groups. Mononuclear cell and neutrophil ascorbate concentrations increased only ∼1.5- and ∼2-fold, respectively. Muscle ascorbate concentrations were highly correlated (P < 0.001) with dietary intake (R = 0.61) and plasma concentrations (R = 0.75) in the range from 5 to 80 μmol/L.
Conclusions: Human skeletal muscle is highly responsive to vitamin C intake and plasma concentrations and exhibits a greater relative uptake of ascorbate than leukocytes. Thus, muscle appears to comprise a relatively labile pool of ascorbate and is likely to be prone to ascorbate depletion with inadequate dietary intake. This trial was registered at the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) as ACTRN12611000162910.
INTRODUCTION
Vitamin C (ascorbate) is an essential micronutrient with many important biological functions. Ascorbate is a cofactor for a variety of metalloenzymes that are necessary for the biosynthesis of collagen, carnitine, neurotransmitters, and peptide hormones as well as the regulation of transcription factors such as hypoxia-inducible factor-1 (1–3). Ascorbate also acts as a potent water-soluble antioxidant, with the ability to scavenge a wide variety of reactive oxygen and nitrogen species and regenerate other small molecule antioxidants from their respective radicals (4). Humans have lost the ability to synthesize ascorbate from glucose because of mutations in the gene encoding l-gulono-γ-lactone oxidase, which is the terminal enzyme in the ascorbate biosynthetic pathway (5). Therefore, an adequate and regular dietary intake is essential to prevent hypovitaminosis C and the potentially fatal deficiency disease scurvy (6). Clinical manifestations of scurvy include blood vessel fragility and bleeding, which result in petechial and other hemorrhages, skin changes that result in follicular hyperkeratosis, impaired wound healing, gum swelling and bleeding, joint pain and effusions, anemia, weakness, and fatigue (6–8). Many of these symptoms are attributed to the role of ascorbate in collagen and carnitine biosynthesis (9).
Plasma ascorbate status reflects recent dietary intake, whereas leukocyte concentrations are thought to more closely reflect tissue stores (10, 11). However, whether leukocytes are an accurate model for the ascorbate status of other tissues is uncertain. Our previous study with vitamin C–deficient Gulo knockout mice indicated that the maximal tissue uptake within different organs occurred at varying doses of ascorbate (12). Thus, the relative uptake of ascorbate by leukocytes may not be representative for all tissues or organs. Although ascorbate has previously been measured in skeletal muscle obtained at autopsy (13), to our knowledge, no studies have investigated the bioavailability of vitamin C to human skeletal muscle. Skeletal muscle contains relatively low concentrations of ascorbate compared with in other organs (13), but because of the large amount of skeletal muscle present in the body, it has been estimated to comprise ≤67% of the total body vitamin C (10) and, thus, represents the major pool of vitamin C in the body. The accumulation of ascorbate in muscle tissue is thought to protect these metabolically active cells against oxidative stress (14). Therefore, vitamin C is likely to be essential for both skeletal muscle structure and function because of its dual role as an antioxidant and as an enzyme cofactor for collagen and carnitine biosynthesis (15, 16).
Overall, it is likely that muscle tissue has a high requirement for and turnover of vitamin C. Therefore, the aims of the current study were to investigate the bioavailability of vitamin C to human skeletal muscle in relation to dietary intake and plasma concentrations and to compare this relation to the uptake of ascorbate by peripheral blood leukocytes. Zespri gold kiwifruit (Actinidia chinensis var. Hort 16A), which are an outstanding dietary source of vitamin C (17), were used as the intervention in this study. Our previous human study in which we investigated the bioavailability of vitamin C from gold kiwifruit indicated that the consumption of 0.5 kiwifruit/d resulted in a significant increase of plasma ascorbate concentrations in marginally deficient individuals, whereas the consumption of 2 kiwifruit/d was required to saturate the plasma, as shown by a significant increase in the urinary ascorbate excretion at this dose (18). In the current study, we supplemented participants with 0.5 or 2 kiwifruit/d for 6 wk and measured ascorbate concentrations in plasma, urine, leukocytes, and skeletal muscle (vastus lateralis) before and after intervention.
SUBJECTS AND METHODS
Participants
This study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures that involved human participants were approved by the Upper South Regional Ethics Committee (URA/11/02/003). The study was registered at the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au; ACTRN12611000162910).
Nonsmoking men aged 18–35 y were recruited from local tertiary institutes, and 134 subjects underwent a screening interview to determine their eligibility for the study. Exclusion criteria included being a recent smoker (within the past 1 y), having an allergy or intolerance to kiwifruit, consumption of vitamin C–containing supplements (within the past 3 mo), taking prescription medication (within the past 3 mo), excessive alcohol consumption (>21 standard drinks/wk), high fruit and vegetable consumption (>5 servings/d), having diabetes or bleeding disorders, and fainting because of a fear of needles. Anthropometric measures were carried out to determine BMI (in kg/m2), and a fasting venous blood sample was drawn to determine plasma ascorbate concentrations.
Sample-size calculations indicated that at 80% power and with α = 0.05, a sample size of 15 participants per intervention group would detect a minimum difference of 10 μmol ascorbate/L as determined by using data derived from our vitamin C bioavailability study (18). To allow for potential withdrawal because of the length of the study, 36 nonsmoking participants with below-average plasma ascorbate concentrations were enrolled by the study coordinator and provided signed informed consent.
Two healthy men volunteered to undergo muscle biopsies to act as a comparator group. Their anthropometric data were BMI of 24 and 27 and ages of 31 and 39 y.
Study design
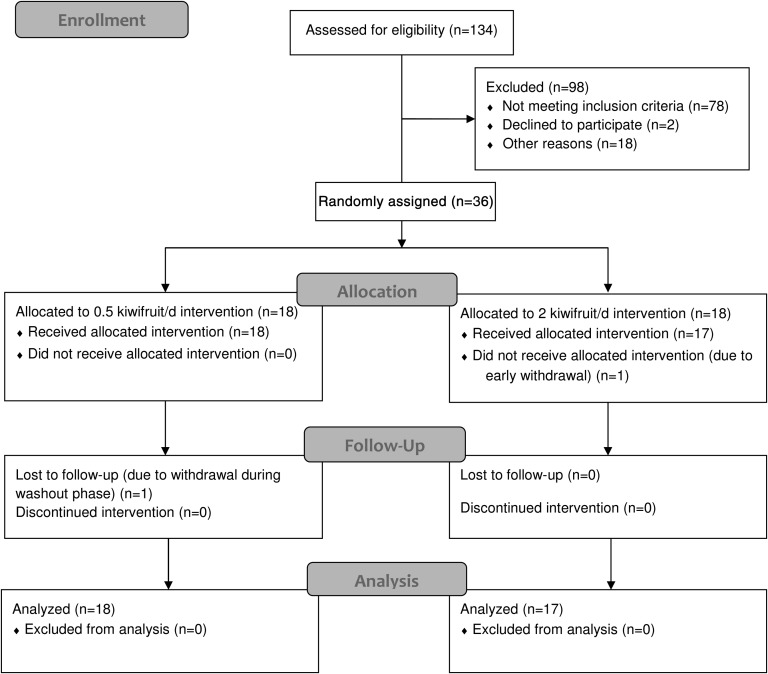
The study used a parallel-arm design (Figure 1), and participants were randomly assigned by the study coordinator into a low-dose group (0.5 kiwifruit/d) or a high-dose group (2 kiwifruit/d) by using a random-numbers chart. Blocking was used to account for the range in prestudy plasma ascorbate concentrations. The study was carried out in a phase I clinical trials unit and comprised a lead-in phase of 5 wk, an intervention phase of 6 wk, and a washout phase of 4 wk. Initially, participants were encouraged to reduce their dietary vitamin C intake by avoiding the consumption of juice and other vitamin C–fortified beverages and by substituting high–vitamin C–containing foods with low–vitamin C–containing foods. Fasting venous blood samples were drawn weekly throughout the study to monitor participant plasma ascorbate concentrations derived from normal daily diets and the intervention. Twenty-four–hour urine and extra blood for leukocyte isolations were collected at baseline (week 5), after intervention (week 11), and after washout (week 15). Muscle biopsies were carried out before and after intervention. Participants also completed 7-d food and beverage records on 4 occasions (at the beginning of the study, before intervention, after intervention, and after washout) to monitor their dietary vitamin C intakes.
FIGURE 1.
Study design. Parallel arms comprised 0.5 or 2 kiwifruit/d for 6 wk.
Intervention
Gold kiwifruit (Actinidia chinensis var. Hort. 16A) were provided by Zespri International Ltd and stored at ≤4°C. Participants were provided with sufficient kiwifruit each week to consume 0.5 or 2 kiwifruit /d. The ascorbate content of the kiwifruit was determined from an extract of the flesh that was measured by using HPLC with electrochemical detection (18). This method indicated that the fruit contained 116 ± 10 mg ascorbate/100 g (n = 5). Participants were asked not to consume the skins, and on the basis of the amount of fruit ingested, the amount of vitamin C consumed was calculated to be ∼53 mg for one-half of a kiwifruit or ∼212 mg for 2 kiwifruit.
Sample collection and processing
Plasma and urine
Peripheral blood (4 mL) was collected into evacuated tubes containing K3-EDTA and kept on ice at all times (19). Samples were centrifuged at 4°C to pellet cells, and the plasma was collected and kept on ice for the extraction of ascorbate. Urine was collected over 24 h into collection bottles containing K2-EDTA (final concentration: 100 μmol/L) (20). Plasma and urine samples were treated with an equal volume of ice-cold 0.54-mol/L HPLC-grade perchloric acid with diethylene triamine pentaacetic acid (DTPA)4 (100 μmol/L) to precipitate the protein (21). The perchloric acid and DTPA extracts were centrifuged, and the deproteinated supernatant fluids were stored at −80°C until HPLC analysis.
Mononuclear leukocytes and neutrophils
Peripheral blood was collected into BD Vacutainer Cell Preparation Tubes (Becton, Dickinson and Co) that contained sodium heparin and kept at room temperature. Tubes were centrifuged in a horizontal rotor for 30 min at 1800 relative centrifugal force without brake. After centrifugation, the layer above the gel that contained the mononuclear leukocytes was removed, and cells were washed with phosphate-buffered saline and finally suspended in Hanks balanced salt solution (HBSS). After the first centrifugation step previously described, neutrophils and erythrocytes were extracted from below the gel layer. Erythrocytes were removed by using dextran sedimentation and hypotonic lysis (22), and neutrophils were suspended in HBSS. Isolated leukocytes were counted by using a hemocytometer, standardized for cell number, suspended in HBSS, and an equal volume of ice-cold 0.54-mol/L perchloric acid/DTPA solution was added to precipitate the protein (21). Deproteinated supernatant fluids were stored at −80°C.
Muscle tissue
Needle biopsies were performed by an experienced plastic surgeon. Local anesthetic (2 mL 1% lignocaine with adrenaline 1:200,000) was injected into the subcutaneous fat of the anterolateral midthigh. A Quick-Core biopsy needle (14 gauge; 6 cm long with a 20-mm throw; Cook Medical Inc) was inserted into the vastus lateralis muscle and retrieved a small piece of tissue (13.5 ± 8.2 mg; n = 52). Fascia, if present, was removed, and the muscle tissue sample was placed into a preweighed Eppendorf tube (Eppendorf International) on ice, the weight of the tissue was determined, and the sample was frozen at −80°C. Our previous studies have shown that ascorbate in intact frozen tissue remains stable for many months (12, 23). Immediately before HPLC analysis, the frozen muscle tissue sample was homogenized for 60 s in 50 μL ice-cold phosphate-buffered saline with a Dounce ground glass pestle, and an equal volume of ice-cold 0.54-mol/L HPLC-grade perchloric acid/DTPA solution was added (21). Deproteinated supernatant fluids were stored at −80°C.
Analysis of ascorbate by HPLC
The ascorbate content of the kiwifruit, plasma, urine, leukocytes, and muscle tissue was analyzed by using reverse-phase HPLC with a Synergi 4 micron Hydro-RP 80-A column (Phenomenex NZ Ltd) and an ESA coulochem II electrochemical detector (ESA Inc) as described previously (18). The plasma ascorbate content was expressed as micromoles per liter, the urinary ascorbate content was expressed as micromoles per 24 h, the leukocyte ascorbate content was expressed as nanomoles per 108 cells, and the muscle tissue ascorbate content was expressed as nanomoles per gram of wet weight.
Analysis of food and beverage records
The number of servings of fruit and vegetables consumed by each participant was estimated from 7-d food and beverage records as described previously (18). The vitamin C content of the consumed foods and beverages were estimated with Diet Cruncher software (version 1.6; Way Down South Software) and the 2006 New Zealand FOODfiles Food Composition Database (New Zealand Institute for Plant and Food Research Ltd).
Statistical analysis
Data are represented as either means ± SDs or means ± SEMs as indicated. Differences between paired and unpaired data were determined by using the 2-tailed t test, and P ≤ 0.05 was considered significant. Linear regression analysis (Pearson's correlations) and ANOVA with Fisher's pairwise multiple-comparison procedures were carried out with SigmaStat software (version 11; Systat Software Inc).
RESULTS
Screening phase
A total of 134 young men were screened for this study. Thirty-six subjects, who had below-average plasma ascorbate concentrations and also satisfied other inclusion exclusion criteria, were enrolled. These individuals were randomly assigned to either a low-dose group (0.5 kiwifruit/d; n = 18) or high-dose group (2 kiwifruit/d; n = 18). One of the participants who enrolled in the high-dose group withdrew early in the study and was not used in the data analysis. Characteristics of screened and enrolled individuals are shown in Table 1. There were no significant differences between the 2 intervention groups, although both intervention groups had significantly lower plasma ascorbate concentrations than the screened group (P < 0.001). The average ± SD fasting plasma ascorbate concentration for screened individuals was 48 ± 16 μmol/L, with a range of 3 to 92 μmol/L, whereas average ± SD fasting plasma ascorbate concentrations for enrolled groups (low and high dose) were 34 ± 10 and 35 ± 7 μmol/L, respectively, with a range from 15 to 45 μmol/L.
TABLE 1.
Characteristics of the individuals screened and enrolled in the study1
| Screened group (n = 134) | 0.5-kiwifruit/d group (n = 18) | 2-kiwifruit/d group (n = 17) | |
| Age (y) | 21 ± 3 | 22 ± 4 | 22 ± 3 |
| Weight (kg) | 81 ± 16 | 89 ± 23 | 81 ± 15 |
| Height (cm) | 182 ± 7 | 181 ± 7 | 181 ± 7 |
| BMI (kg/m2) | 24 ± 4 | 27 ± 6* | 25 ± 4 |
| Ascorbate (μmol/L) | 48 ± 16 | 34 ± 10** | 35 ± 7** |
All values are means ± SDs. There were no significant differences between the 2 intervention groups. *,**For intervention groups compared with screened group (unpaired t test): *P < 0.05, ** P < 0.001.
Dietary intake of vitamin C
An analysis of food and beverage records at baseline indicated a mean intake of <3 servings fruit and vegetables/d and a mean intake of <30 mg vitamin C/d (Table 2). The addition of 0.5 kiwifruit to the daily diet of the low-intervention group did not alter their baseline fruit and vegetable intake. However, this addition significantly increased their daily vitamin C intake from 29 to 73 mg/d (Table 2). The addition of 2 kiwifruit to the daily diet of the high-intervention group significantly increased their fruit and vegetable intake from 3 to 5 servings/d and their daily vitamin C intake >7-fold to 214 mg/d (Table 2). After the 4-wk washout period, the vitamin C intake of participants had returned to baseline intakes.
TABLE 2.
Dietary intake of vitamin C in 0.5- and 2-kiwifruit/d groups1
| 0.5 kiwifruit/d |
2 kiwifruit/d |
||||||
| Baseline | Intervention | Washout | Baseline | Intervention | Washout | Between-group intervention P | |
| Fruit and vegetables (servings/d) | 2.9 ± 0.3 (18) | 3.4 ± 0.4 (17) | 2.6 ± 0.3 (15) | 2.7 ± 0.3 (17) | 4.8 ± 0.3* (17) | 2.7 ± 0.3 (16) | 0.001 |
| Vitamin C (mg/d) | 28.6 ± 3.1 (18) | 73.4 ± 4.2* (17) | 31.4 ± 5.1 (15) | 29.1 ± 3.1 (17) | 214 ± 4.5* (17) | 28.8 ± 4.3 (16) | <0.0001 |
All values are means ± SEMs; n in parentheses. For 0.5- compared with 2-kiwifruit/d groups after intervention, P values were determined by using the unpaired t test. *For intervention compared with baseline, P < 0.0001 (paired t test).
Ascorbate status of plasma and urine
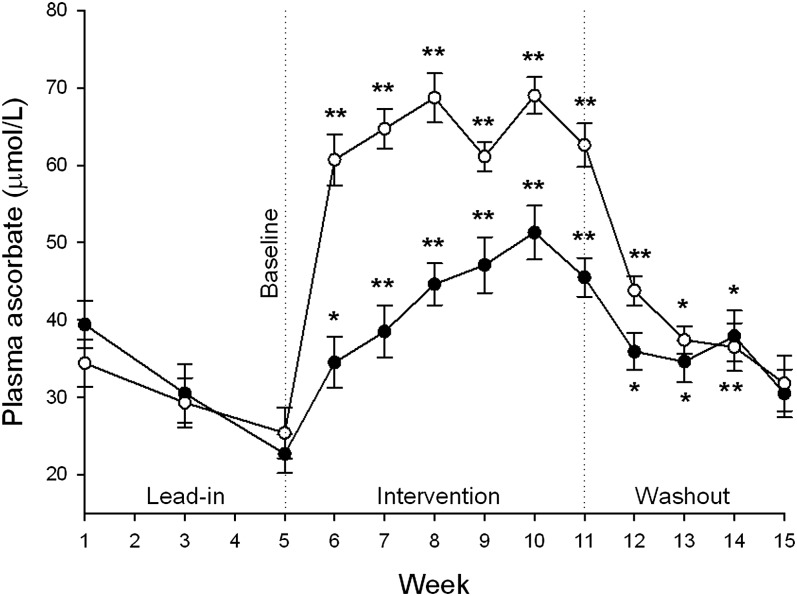
At baseline, the low-dose group had a mean plasma ascorbate concentration of 23 μmol/L and this increased to 46 μmol/L after 6 wk of intervention (Figure 2). The high-dose group had a baseline mean concentration of 25 μmol/L, and after intervention, this concentration increased to 63 μmol/L (Figure 2), which was close to saturating (ie, did not increase further after additional vitamin C intake) (18). Plasma ascorbate concentrations of the 2 groups were statistically different from each other within the first week of intervention, and this difference was maintained for the entire 6 wk of intervention (P < 0.001). At the end of the 4-wk washout period, plasma ascorbate concentrations for both high- and low-dose groups had decreased and were not different from baseline concentrations (Figure 2).
FIGURE 2.
Mean ± SEM plasma ascorbate concentrations in the 0.5-kiwifruit/d (•; n = 18) and 2-kiwifruit/d (○; n = 17) groups during the lead-in, intervention, and washout phases of the study. Significant differences (P < 0.001) were observed between the 2 interventions from weeks 6 to 11; no differences were observed during the washout phase. *,**For comparison with baseline (week 5; 2-factor ANOVA with Fisher's pairwise multiple-comparison procedure): *P < 0.01, **P < 0.001.
After 6 wk of intervention, the urinary excretion of ascorbate had increased 2-fold in the low–kiwifruit-dose group and 15-fold in the high-dose group (Table 3), which suggested that the higher intake of kiwifruit resulted in plasma concentrations close to saturation (18).
TABLE 3.
Increase in ascorbate concentrations of fluids, cells, and tissue after supplementation with 0.5 or 2 kiwifruit/d for 6 wk1
| 0.5 kiwifruit/d |
2 kiwifruit/d |
||||||
| Baseline | Intervention | Washout | Baseline | Intervention | Washout | Between-group intervention P | |
| Plasma (μmol/L) | 22.7 ± 2.5 (17) | 45.5 ± 2.5*** (18) | 37.9 ± 3.3. (15) | 25.4 ± 3.3 (16) | 62.6 ± 2.8*** (17) | 31.8 ± 3.6 (17) | <0.0001 |
| Urine (μmol/24 h) | 32.3 ± 8.1 (18) | 70.5 ± 17.0* (17) | 64.7 ± 29.8 (16) | 32.2 ± 11.2 (17) | 485.0 ± 69.9*** (17) | 64.3 ± 25.1 (17) | <0.0001 |
| Mononuclear cells (nmol/108 cells) | 60.5 ± 6.1 (18) | 90.9 ± 6.1** (18) | 75.2 ± 5.2 (17) | 58.4 ± 5.6 (17) | 91.0 ± 6.2** (16) | 71.5 ± 5.0 (17) | 0.998 |
| Neutrophils (nmol/108 cells) | 13.7 ± 2.4 (18) | 30.4 ± 2.5*** (18) | 24.8 ± 1.7*** (17) | 16.2 ± 3.3 (17) | 33.7 ± 2.9** (17) | 24.7 ± 1.4* (17) | 0.393 |
| Skeletal muscle (nmol/g)2 | 15.1 ± 2.5 (17) | 52.8 ± 5.0*** (18) | ND | 17.1 ± 3.5 (17) | 60.8 ± 5.5*** (17) | ND | 0.284 |
All values are means ± SEMs; n in parentheses. For 0.5- compared with 2-kiwifruit/d groups after intervention, P values were determined by using the unpaired t test. *,**,***For intervention compared with baseline (paired t tests): *P < 0.05, **P < 0.01, ***P < 0.001. ND, not determined.
To convert muscle ascorbate values from nanomoles per gram of tissue to micrograms per 100 mg of tissue, multiply by 0.0199.
Ascorbate status of leukocytes and muscle tissue
Baseline ascorbate concentrations of mononuclear cells and neutrophils are shown in Table 3. After 6 wk of intervention, mononuclear cell ascorbate concentrations increased ∼1.5-fold for both the low–kiwifruit-dose group (P = 0.004) and the high–kiwifruit-dose group (P = 0.004), whereas neutrophil ascorbate concentrations increased >2-fold (low-dose group: P = 0.0002; high-dose group: P = 0.003; Table 3). There were no significant differences in leukocyte ascorbate concentrations between the 2 intervention groups, which indicated that leukocytes were saturating with the low kiwifruit intake.
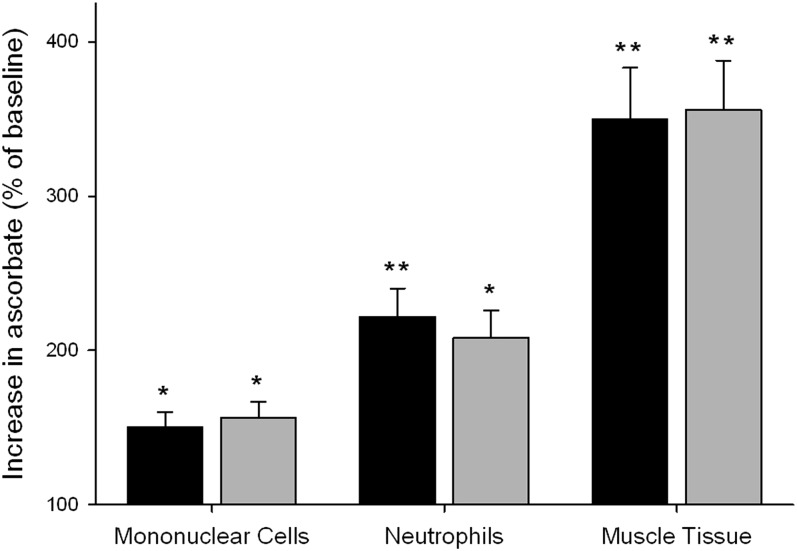
Baseline mean muscle tissue ascorbate concentrations were ∼16 nmol/g tissue (Table 3), with a range from 1.0 to 43.2 nmol/g tissue. After 6 wk of intervention, there was a ∼3.5-fold increase in mean muscle tissue ascorbate concentrations to 53 and 61 nmol/g tissue in the low- and high-dose groups, respectively (P < 0.0001; Table 3). Thus, muscle tissue appeared to have a significantly greater relative uptake of ascorbate than both mononuclear cells and neutrophils (Figure 3). There was no difference in muscle tissue ascorbate concentrations between the 2 intervention groups, which suggested that muscle tissue ascorbate concentrations were saturating with the low kiwifruit intake.
FIGURE 3.
Mean ± SEM relative increases in ascorbate concentrations in peripheral blood mononuclear cells, neutrophils, and skeletal muscle tissue after supplementation with 0.5 kiwifruit/d (n = 18; black bars) or 2 kiwifruit/d (n = 18; gray bars). Baseline values are shown in Table 3. *,**For intervention compared with baseline (paired t test): *P < 0.01, **P < 0.001.
Two healthy male volunteers with saturating plasma ascorbate concentrations (74.6 ± 2.0 μmol/L) were recruited to compare with our participants. The muscle tissue ascorbate concentrations of the volunteers were 60.3 ± 13.6 nmol/g tissue, which compared well with those of study participants after intervention (Table 3).
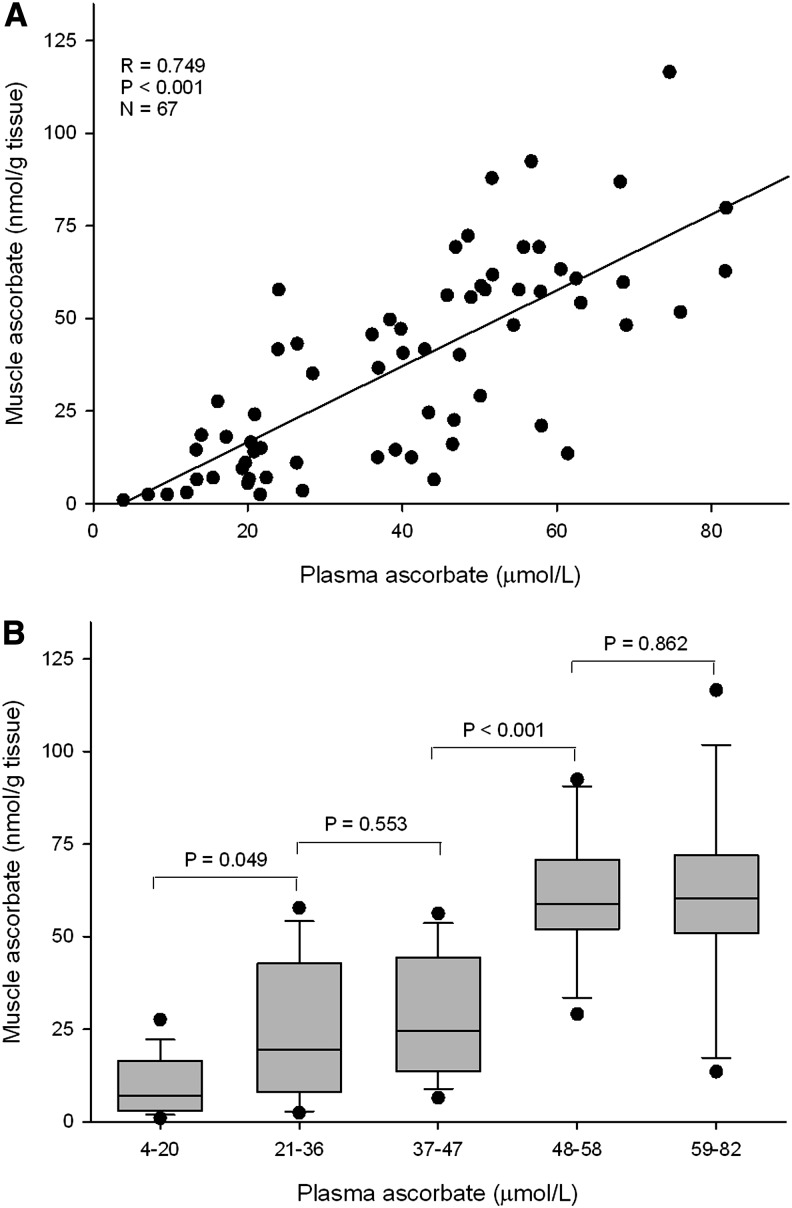
Correlations of muscle ascorbate status with vitamin C intake and plasma and leukocyte concentrations were investigated by using linear regression analysis. Strong correlations were observed between muscle ascorbate status and vitamin C intake (R = 0.61, P < 0.001; Table 4) and plasma concentrations in the range of 5–80 μmol/L (R = 0.75, P < 0.001; Table 4, Figure 4A). These correlations were comparable with that of plasma ascorbate concentrations with dietary intake (R = 0.71, P < 0.001) and were greater than those exhibited for neutrophils and mononuclear cells (Table 4). Analysis of muscle ascorbate on the basis of quintiles of plasma ascorbate showed significantly lower muscle ascorbate for plasma concentrations <21 μmol/L, which provided a mean muscle ascorbate concentration of 11 nmol/g. Second and third quintiles had comparable mean muscle ascorbate of 25 and 29 nmol/g. Notably, a significant increase in muscle ascorbate was observed at plasma concentrations >47 μmol/L, with mean muscle ascorbate concentrations ∼60 nmol/g (Figure 4B).
TABLE 4.
Correlation coefficients for dietary intake of vitamin C and concentrations of ascorbate in plasma, muscle tissue, neutrophils, and mononuclear cells1
| Dietary intake |
Plasma |
Muscle |
|||||||
| R | P | n2 | R | P | n | R | P | n | |
| Plasma | 0.707 | <0.001 | 98 | — | — | — | — | — | — |
| Muscle | 0.613 | <0.001 | 68 | 0.749 | <0.001 | 67 | — | — | — |
| Neutrophils | 0.417 | <0.001 | 100 | 0.432 | <0.001 | 102 | 0.571 | <0.001 | 69 |
| Mononuclear cells | 0.353 | <0.001 | 99 | 0.460 | <0.001 | 101 | 0.436 | <0.001 | 68 |
R and P values were determined by using linear regression analysis (Pearson's correlations).
n, number of samples analyzed.
FIGURE 4.
Correlation of muscle tissue ascorbate status with plasma ascorbate concentrations (A) and muscle tissue ascorbate status relative to quintiles of plasma ascorbate concentrations (B). Box plots show medians with 25th and 75th percentiles as boundaries, whiskers are for the 10th and 90th percentiles, and symbols indicate outliers. For trend across quintiles, P < 0.001 (1-factor ANOVA with Fisher's pairwise multiple-comparison procedure) (n = 67).
Because skeletal muscle ascorbate appears to be highly correlated with plasma ascorbate, it is likely that the variability observed in participant baseline muscle ascorbate (as previously indicated) was due to the variability observed in participant baseline plasma ascorbate (range: 3–61 μmol/L).
DISCUSSION
To our knowledge, our study provides novel data on the effects of vitamin C depletion and dietary supplementation on human skeletal muscle tissue ascorbate status. At baseline, mean plasma ascorbate concentrations of study participants were marginally deficient (24), and their skeletal muscle tissue ascorbate concentrations were similarly low, with a ∼3.5-fold uptake of ascorbate by muscle tissue observed after intervention. This uptake was significantly greater than the relative uptake of ascorbate by mononuclear cells and neutrophils, which suggestes that muscle is a more-labile compartment of whole-body vitamin C. Although kiwifruit was used as the source of vitamin C in this study, we (Carr et al, unpublished observations, June–August 2012) and other authors (25, 26) have shown no differences in the comparative bioavailability of synthetic and fruit-derived vitamin C.
We also investigated the previously unreported relation between human skeletal muscle ascorbate status and vitamin C intake and plasma concentrations. Plasma ascorbate is generally thought to reflect recent dietary intake, whereas tissue status and, in particular, leukocyte ascorbate concentrations are thought to be more indicative of the total body status (11). We showed skeletal muscle ascorbate concentrations were strongly correlated with dietary intake and plasma ascorbate status. The correlations between leukocytes and plasma ascorbate concentrations were weaker, which suggested that muscle is more responsive to changes in circulating ascorbate concentrations than are leukocytes. This effect may reflect either a higher turnover of ascorbate in metabolically active skeletal muscle or differences in uptake between muscle cells and leukocytes.
Ascorbate is transported into muscle cells primarily via sodium-dependent vitamin C transporter 2 (SVCT2), which is a high-affinity, low-capacity transporter (27–30). Although neutrophils express SVCT2 (31) when their respiratory burst is activated, they primarily transport dehydroascorbic acid (the oxidized form of ascorbate) via the glucose transporters, followed by the intracellular reduction to ascorbate (32). Thus, the difference in relative uptake of ascorbate between muscle cells and leukocytes could be due to differences in expression concentrations of SVCT2 in each cell type.
Because muscle tissue appears to comprise a significantly more labile vitamin C pool than leukocytes, it is likely to be prone to depletion when vitamin C intake is inadequate. This inadequacy could have a number of implications. Skeletal muscle fibers are greatly dependent on fatty acid oxidation for their energy requirements, and carnitine plays a vital role in the regulation of skeletal muscle fuel selection and physiologic function (33, 34). Indeed, skeletal muscle contains ∼98% of the body's total store of carnitine (16, 33), and ascorbate is required for its synthesis through its action as a cofactor for the metalloenzymes trimethyllysine hydroxylase and γ-butyrobetaine hydroxylase (35). Carnitine deficiency has been reported in human skeletal muscle, and deficient biosynthesis has been implicated in some cases (34, 36, 37). The effect of vitamin C deficiency on human skeletal muscle carnitine concentrations has not been explored but has been shown to result in a ≤50% reduction in skeletal muscle carnitine in guinea pigs (38–41). Hence, it is possible that muscle concentrations could be similarly decreased in humans. Reduced muscle carnitine is associated with muscle weakness (42) and may be associated with the symptom of fatigue observed in vitamin C–deficient individuals (43).
It is well established that reactive oxygen species are generated in skeletal muscle during exercise, and this generation has been associated with muscle damage and inflammation (44, 45). Although a number of studies have shown that antioxidants such as ascorbate can attenuate the oxidative stress associated with exercise, whether this effect is desirable remains to be elucidated (46). Instead, there may be beneficial adaptive responses to elevated concentrations of reactive oxygen species, such as the increased activity of antioxidant enzymes, upregulation of redox-sensitive gene expression, and increased mitochondrial biogenesis (46). As such, high-dose vitamin C supplementation is not recommended for athletes who are undergoing endurance exercise training; however, an adequate dietary intake of the vitamin is likely to be required for normal muscle function (46, 47).
On the basis of the dietary analysis of our participants, it appears that a mean intake of ∼70 mg vitamin C/d is sufficient to saturate muscle tissue, whereas an intake of ∼30 mg/d is clearly inadequate. High nondietary intakes of vitamin C do not appear necessary to replenish normal muscle tissue, and the dietary intakes recommended in the United States and Canada (75 mg vitamin C/d for women and 90 mg vitamin C/d for men) should be sufficient. In contrast, the British and Australasian recommended dietary vitamin C intakes for adults of 40 and 45 mg/d, respectively, may not be sufficient to saturate muscle tissue, and additional studies need to be carried out to establish this.
Because of potential inaccuracies of dietary intake questionnaires and the variability between vitamin C intake and plasma concentrations in different individuals, due to genetic polymorphisms, smoking status and illness, plasma ascorbate concentrations are generally considered a more accurate marker of vitamin C status (48). This relation has been confirmed by our study, whereby we observed a higher correlation between plasma ascorbate concentrations and muscle status than that between vitamin C intake and muscle status. When plasma ascorbate was separated into quintiles, we observed significantly lower muscle ascorbate at plasma concentrations <21 μmol/L and significantly higher muscle ascorbate at plasma concentrations >47 μmol/L. Lykkesfeldt and Poulsen (24) suggested that plasma ascorbate concentrations between 23 and 50 μmol/L should be considered suboptimal, and concentrations <23 μmol/L should be considered deficient, and our muscle data supported this premise. Thus, plasma ascorbate concentrations ≥50 μmol/L should be aimed for to optimize muscle tissue status. However, even at plasma ascorbate concentrations ≥60 μmol and greater, some individuals presented with relatively low muscle ascorbate concentrations. The reason for this variability is not certain and did not appear to be related to BMI (data not shown).
An early study by Schaus (13) determined that pectoral muscle obtained from 63 individuals at autopsy contained 3.3 μg vitamin C/100 mg wet weight. This concentration is higher than that observed in our postintervention male participants and 2 healthy male volunteers (ie, ∼1.2 μg ascorbate/100 mg wet weight), but likely reflected differences in methodology (49). Schaus (13) observed higher vitamin C concentrations in the myocardium and pituitary of women; however, Schaus (13) reported no significant differences between men and women for pectoral muscle or the cerebral cortex. Therefore, because of sex differences that are known to influence vitamin C status, more studies are needed to confirm if skeletal muscle ascorbate concentrations differ between men and women.
Overall, we have shown that skeletal muscle ascorbate exhibits a strong association with vitamin C intake and plasma concentrations, which indicated that muscle tissue is very responsive to recent vitamin C intake and, thus, represents a relatively labile pool. This premise was supported by an animal study that showed a mean ascorbate transit time <18 h in skeletal muscle (50). Because of the accessibility and ease of sampling, leukocytes are often used as a model for tissue ascorbate status. However, it is apparent from our study that the uptake of ascorbate into mononuclear cells and neutrophils significantly underestimates the relative uptake of ascorbate into muscle tissue. Because of the multiple potential functions of ascorbate within muscle tissue, a daily vitamin C intake that provides plasma ascorbate concentrations ≥50 μmol/L should be consumed to maintain an optimal skeletal muscle ascorbate status.
Acknowledgments
We express our gratitude to the young men who participated in this study, many of whom showed great dedication and perseverance. We acknowledge Maria Webb and Heather Webb for assistance with recruitment, Wathsala Kumarasinghe for the diet analysis, Jo Kepple for the use of the Primorus Clinical Trials Unit, and Lynley Drummond for consultation on the study design.
The authors’ responsibilities were as follows—MCMV and ACC: designed the study; ACC, SMB, and JWS: conducted the study; ACC: performed the statistical analysis; ACC and JMP: wrote the manuscript; MCMV: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: DTPA, diethylene triamine pentaacetic acid; HBSS, Hanks balanced salt solution; SVCT2, sodium-dependent vitamin C transporter 2.
REFERENCES
- 1.Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr 1986;6:365–406 [DOI] [PubMed] [Google Scholar]
- 2.Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol 2008;4:152–6 [DOI] [PubMed] [Google Scholar]
- 3.Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 2002;1569:1–9 [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 1999;13:1007–24 [DOI] [PubMed] [Google Scholar]
- 5.Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J Biol Chem 1994;269:13685–8 [PubMed] [Google Scholar]
- 6.Krebs HA. The Sheffield experiment on the vitamin C requirement of human adults. Proc Nutr Soc 1953;12:237–46 [Google Scholar]
- 7.Hodges RE, Baker EM, Hood J, Sauberlich HE, March SC. Experimental scurvy in man. Am J Clin Nutr 1969;22:535–48 [DOI] [PubMed] [Google Scholar]
- 8.Hodges RE, Hood J, Canham JE, Sauberlich HE, Baker EM. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr 1971;24:432–43 [DOI] [PubMed] [Google Scholar]
- 9.Fain O. Musculoskeletal manifestations of scurvy. Joint Bone Spine 2005;72:124–8 [DOI] [PubMed] [Google Scholar]
- 10.Omaye ST, Schaus EE, Kutnink MA, Hawkes WC. Measurement of vitamin C in blood components by high-performance liquid chromatography. Implication in assessing vitamin C status. Ann N Y Acad Sci 1987;498:389–401 [DOI] [PubMed] [Google Scholar]
- 11.Jacob RA. Assessment of human vitamin C status. J Nutr 1990;120(suppl 11):1480–5 [DOI] [PubMed] [Google Scholar]
- 12.Vissers MCM, Bozonet SM, Pearson JF, Braithwaite LJ. Dietary ascorbate affects steady state tissue levels in vitamin C-deficient mice: tissue deficiency after sub-optimal intake and superior bioavailability from a food source (kiwifruit). Am J Clin Nutr 2011;93:292–301 [DOI] [PubMed] [Google Scholar]
- 13.Schaus R. The ascorbic acid content of human pituitary, cerebral cortex, heart, and skeletal muscle and its relation to age. Am J Clin Nutr 1957;5:39–41 [DOI] [PubMed] [Google Scholar]
- 14.Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 2008;34:347–55 [DOI] [PubMed] [Google Scholar]
- 15.Franceschi RT. The role of ascorbic acid in mesenchymal differentiation. Nutr Rev 1992;50:65–70 [DOI] [PubMed] [Google Scholar]
- 16.Rebouche CJ. Ascorbic acid and carnitine biosynthesis. Am J Clin Nutr 1991;54(suppl):1147S–52S [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama I, Yamashita Y, Yamanaka M, Shimohashi A, Fukuda T, Oota T. Varietal difference in vitamin C content in the fruit of kiwifruit and other actinidia species. J Agric Food Chem 2004;52:5472–5 [DOI] [PubMed] [Google Scholar]
- 18.Carr AC, Pullar JM, Moran S, Vissers MCM. Bioavailability of vitamin C from kiwifruit in non-smoking males: determination of ‘healthy’ and ‘optimal’ intakes. J Nutr Sci 2012;1:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lykkesfeldt J. Ascorbate and dehydroascorbic acid as biomarkers of oxidative stress: validity of clinical data depends on vacutainer system used. Nutr Res 2012;32:66–9 [DOI] [PubMed] [Google Scholar]
- 20.Chalmers AH, Cowley DM, McWhinney BC. Stability of ascorbate in urine: relevance to analyses for ascorbate and oxalate. Clin Chem 1985;31:1703–5 [PubMed] [Google Scholar]
- 21.Lee W, Hamernyik P, Hutchinson M, Raisys VA, Labbe RF. Ascorbic acid in lymphocytes: cell preparation and liquid-chromatographic assay. Clin Chem 1982;28:2165–9 [PubMed] [Google Scholar]
- 22.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 1968;97:77–89 [PubMed] [Google Scholar]
- 23.Kuiper C, Molenaar IG, Dachs GU, Currie MJ, Sykes PH, Vissers MC. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res 2010;70:5749–58 [DOI] [PubMed] [Google Scholar]
- 24.Lykkesfeldt J, Poulsen HE. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr 2010;103:1251–9 [DOI] [PubMed] [Google Scholar]
- 25.Nelson EW, Streiff RR, Cerda JJ. Comparative bioavailability of folate and vitamin C from a synthetic and a natural source. Am J Clin Nutr 1975;28:1014–9 [DOI] [PubMed] [Google Scholar]
- 26.Bates CJ, Jones KS, Bluck LJ. Stable isotope-labelled vitamin C as a probe for vitamin C absorption by human subjects. Br J Nutr 2004;91:699–705 [DOI] [PubMed] [Google Scholar]
- 27.Savini I, Catani MV, Duranti G, Ceci R, Sabatini S, Avigliano L. Vitamin C homeostasis in skeletal muscle cells. Free Radic Biol Med 2005;38:898–907 [DOI] [PubMed] [Google Scholar]
- 28.Savini I, Rossi A, Catani MV, Ceci R, Avigliano L. Redox regulation of vitamin C transporter SVCT2 in C2C12 myotubes. Biochem Biophys Res Commun 2007;361:385–90 [DOI] [PubMed] [Google Scholar]
- 29.Kuo SM, MacLean ME, McCormick K, Wilson JX. Gender and sodium-ascorbate transporter isoforms determine ascorbate concentrations in mice. J Nutr 2004;134:2216–21 [DOI] [PubMed] [Google Scholar]
- 30.Low M, Sandoval D, Aviles E, Perez F, Nualart F, Henriquez JP. The ascorbic acid transporter SVCT2 is expressed in slow-twitch skeletal muscle fibres. Histochem Cell Biol 2009;131:565–74 [DOI] [PubMed] [Google Scholar]
- 31.Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, Kirk KL, Levine M. 6-Bromo-6-deoxy-L-ascorbic acid: an ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J Biol Chem 2005;280:5211–20 [DOI] [PubMed] [Google Scholar]
- 32.Washko PW, Wang Y, Levine M. Ascorbic acid recycling in human neutrophils. J Biol Chem 1993;268:15531–5 [PubMed] [Google Scholar]
- 33.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 2007;581:431–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karpati G, Carpenter S, Engel AG, Watters G, Allen J, Rothman S, Klassen G, Mamer OA. The syndrome of systemic carnitine deficiency. Clinical, morphologic, biochemical, and pathophysiologic features. Neurology 1975;25:16–24 [DOI] [PubMed] [Google Scholar]
- 35.Hulse JD, Ellis SR, Henderson LM. Carnitine biosynthesis. beta-Hydroxylation of trimethyllysine by an alpha-ketoglutarate-dependent mitochondrial dioxygenase. J Biol Chem 1978;253:1654–9 [PubMed] [Google Scholar]
- 36.Engel AG, Angelini C. Carnitine deficiency of human skeletal muscle with associated lipid storage myopathy: a new syndrome. Science 1973;179:899–902 [DOI] [PubMed] [Google Scholar]
- 37.Vielhaber S, Feistner H, Weis J, Kreuder J, Sailer M, Schroder JM, Kunz WS. Primary carnitine deficiency: adult onset lipid storage myopathy with a mild clinical course. J Clin Neurosci 2004;11:919–24 [DOI] [PubMed] [Google Scholar]
- 38.Dunn WA, Rettura G, Seifter E, Englard S. Carnitine biosynthesis from gamma-butyrobetaine and from exogenous protein-bound 6-N-trimethyl-L-lysine by the perfused guinea pig liver. Effect of ascorbate deficiency on the in situ activity of gamma-butyrobetaine hydroxylase. J Biol Chem 1984;259:10764–70 [PubMed] [Google Scholar]
- 39.Hughes RE, Hurley RJ, Jones E. Dietary ascorbic acid and muscle carnitine (beta-OH-gamma-(trimethylamino) butyric acid) in guinea-pigs. Br J Nutr 1980;43:385–7 [DOI] [PubMed] [Google Scholar]
- 40.Nelson PJ, Pruitt RE, Henderson LL, Jenness R, Henderson LM. Effect of ascorbic acid deficiency on the in vivo synthesis of carnitine. Biochim Biophys Acta 1981;672:123–7 [DOI] [PubMed] [Google Scholar]
- 41.Thoma WJ, Henderson LM. Effect of vitamin C deficiency on hydroxylation of trimethylaminobutyrate to carnitine in the guinea pig. Biochim Biophys Acta 1984;797:136–9 [DOI] [PubMed] [Google Scholar]
- 42.Johnston CS, Swan PD, Corte C. Substrate utilization and work efficiency during submaximal exercise in vitamin C depleted-repleted adults. Int J Vitam Nutr Res 1999;69:41–4 [DOI] [PubMed] [Google Scholar]
- 43.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 1996;93:3704–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol 2007;102:1664–70 [DOI] [PubMed] [Google Scholar]
- 45.Dekkers JC, van Doornen LJ, Kemper HC. The role of antioxidant vitamins and enzymes in the prevention of exercise-induced muscle damage. Sports Med 1996;21:213–38 [DOI] [PubMed] [Google Scholar]
- 46.Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med 2011;41:1043–69 [DOI] [PubMed] [Google Scholar]
- 47.Braakhuis AJ. Effect of vitamin C supplements on physical performance. Curr Sports Med Rep 2012;11:180–4 [DOI] [PubMed] [Google Scholar]
- 48.Dehghan M, Akhtar-Danesh N, McMillan CR, Thabane L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr J 2007;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Washko PW, Welch RW, Dhariwal KR, Wang Y, Levine M. Ascorbic acid and dehydroascorbic acid analyses in biological samples. Anal Biochem 1992;204:1–14 [DOI] [PubMed] [Google Scholar]
- 50.Toutain PL, Bechu D, Hidiroglou M. Ascorbic acid disposition kinetics in the plasma and tissues of calves. Am J Physiol 1997;273:R1585–97 [DOI] [PubMed] [Google Scholar]