Abstract
Background: Observational studies associate higher intakes of n−3 (omega-3) long-chain polyunsaturated fatty acids (LCPUFAs) during pregnancy with higher gestation duration and birth size. The results of randomized supplementation trials using various n−3 LCPUFA sources and amounts are mixed.
Objective: We tested the hypothesis that 600 mg/d of the n−3 LCPUFA docosahexaenoic acid (DHA) can increase maternal and newborn DHA status, gestation duration, birth weight, and length. Safety was assessed.
Design: This phase III, double-blind, randomized controlled trial was conducted between January 2006 and October 2011. Women (n = 350) consumed capsules (placebo, DHA) from <20 wk of gestation to birth. Blood (enrollment, birth, and cord) was analyzed for red blood cell (RBC) phospholipid DHA. The statistical analysis was intent-to-treat.
Results: Most of the capsules were consumed (76% placebo; 78% DHA); the mean DHA intake for the treated group was 469 mg/d. In comparison with placebo, DHA supplementation resulted in higher maternal and cord RBC-phospholipid-DHA (2.6%; P < 0.001), longer gestation duration (2.9 d; P = 0.041), and greater birth weight (172 g; P = 0.004), length (0.7 cm; P = 0.022), and head circumference (0.5 cm; P = 0.012). In addition, the DHA group had fewer infants born at <34 wk of gestation (P = 0.025) and shorter hospital stays for infants born preterm (40.8 compared with 8.9 d; P = 0.026) than did the placebo group. No safety concerns were identified.
Conclusions: A supplement of 600 mg DHA/d in the last half of gestation resulted in overall greater gestation duration and infant size. A reduction in early preterm and very-low birth weight could be important clinical and public health outcomes of DHA supplementation. This trial was registered at clinicaltrials.gov as NCT00266825.
INTRODUCTION
n−3 Long-chain PUFAs (LCPUFAs)4 are of interest during pregnancy because there is evidence that they can improve pregnancy outcomes, such as gestation duration, and because they are also believed to increase infant growth and enhance short- and long-term development of the offspring. Olson and Joensen (1) first observed that Faroe Islanders, who consume more n−3 LCPUFA–rich seafood than do Danes, had a longer gestation duration and infants with higher birth weights. Many recent clinical studies have supplied DHA in some form to pregnant women and evaluated both pregnancy outcome and/or infant development with mixed results for both types of outcomes (see references 2–4 for recent reviews). Two recent large randomized clinical trials (RCTs) in Mexico and Australia supplied pregnant women with 400 and 800 mg DHA/d, respectively, during the last half of pregnancy. Results from these trials were mixed; the first trial found no increase in gestation or in infant weight, length, or head circumference at birth with supplementation, although supplementation resulted in greater birth weight in infants of women in their first pregnancy (5). The second trial yielded a 1-d increase in gestation duration (from 281 to 282 d) and an increase in birth weight of 67 g. The effects in the latter trial were small but statistically significant (6).
Many variables exist among pregnancy supplementation studies that could explain the variability in observed outcomes. One example is dose; experimental groups were provided with as little as 100 mg DHA/d and as much as 2200 mg DHA/d. In addition, because the sources of supplemental DHA differed among trials, subjects received varying amounts of EPA (3). The trials were also conducted in populations that have variable usual intakes of DHA from food and who have widely differing expected mean birth weights and gestational ages. In the United States, women have a mean gestation of 270.9 d (7), and the mean birth weight of infants is ∼3390 g. Moreover, a decline in US birth weight since 1990 (8) suggests that there is room for improvement in birth weight and gestation duration.
We conducted a phase III, double-blind, placebo-controlled RCT in the Kansas City metropolitan area from 2006 to 2011 to test 2 general hypotheses and to evaluate the safety of DHA supplementation. The results of the first general hypothesis and the safety evaluation are included in this report. We predicted that women who received 600 mg DHA/d would have a higher red blood cell (RBC)-phospholipid-DHA content and a longer gestation duration, and their infants would have greater weight and length at birth. These were our primary outcomes. We also suspected that greater birth size and gestation duration could reduce the number of preterm and low-birth-weight (LBW) children; therefore, we evaluated the frequency of preterm and early preterm birth using low and very low birth weight (VLBW) as secondary outcomes. The results for primary and secondary pregnancy outcomes and safety are reported here. A later report will address the second general study hypothesis that maternal DHA supplementation can enhance development, in particular visual acuity and cognitive function from birth to 18 mo.
SUBJECTS AND METHODS
Study design
We enrolled 350 women in the Kansas City metropolitan area in a phase III, placebo-controlled RCT (NCT00266825) between January 2006 and November 2009. The project was reviewed and approved by the University of Kansas Medical Center Human Subjects Committee (protocol no. 10186). Participants and data collectors were blinded to allocation, as were all investigators until children were 18 mo of age and had completed early cognitive and visual acuity development testing. The sole exception was that the study biostatistician had access to assignment (but not data) to allow the Data Safety and Monitoring Board (DSMB) to evaluate serious adverse events (SAEs). At 18 mo, the study was unblinded to study investigators for analysis; however, personnel collecting data remain blind to study group assignment because this cohort of children will be assessed for cognitive development until they are 6 y of age. The data analysis began in January 2012 after all data to 18 mo had been collected and the data had been checked, entered into our database, and locked. Study subjects were provided with capsules containing DHA or the same number of placebo capsules from the time they enrolled until birth. The study biostatistician (BJG) generated randomization schedules for 2 maternal age groups (16–25.99 and 26–35.99 y), and each sequence of 8 random numbers included 4 assignments per group to stratify by age and treatment. The Investigational Pharmacy personnel assigned women to placebo or DHA based on the age shared by the study personnel. Once a study subject was assigned to treatment, all available data from that subject were recorded and included in statistical analyses according to intent-to-treat design.
Women were eligible for enrollment if they were English-speaking, between 8 and 20 wk of gestation, between 16 and 35.99 y of age, and planning to deliver at a hospital in the Kansas City metropolitan area. Subjects were excluded if they were carrying more than one fetus, had preexisting diabetes mellitus or systolic blood pressure ≥140 mm Hg at enrollment, or had any serious health condition likely to affect the prenatal or postnatal growth and development of their offspring, including cancer, lupus, hepatitis, HIV/AIDS, or a diagnosed alcohol or chemical dependency. As morbid obesity increases obstetrical risk, subjects were also excluded if the initial screening based on their self-reported weight and height suggested a BMI (in kg/m2) ≥40. Three women had a BMI >40 when their height was measured and medical records became available for confirmation of BMI, but they were kept in the study under intent-to-treat guidelines. Women who smoked during pregnancy were not excluded.
Subjects assigned to the treatment group received 3 capsules/d of a marine algae-oil source of DHA (200 mg DHA/capsule, DHASCO; DSM Nutritional Products, formerly Martek Biosciences) from enrollment until birth, whereas those in the placebo group received 3 capsules containing half soybean and half corn oil (also provided by DSM Nutritional Products). The placebo capsules did not contain DHA but did contain α-linolenic acid—a precursor of DHA. The fatty acid composition of the capsules is shown in Table 1. DSM Nutritional Products donated the capsules for the study but had no role in the study design, analysis, interpretation, or dissemination. Both DHA and placebo capsules were orange-flavored to prevent subjects from guessing their oil assignment from eructation. Placebo and DHA capsules each contained 500 mg each of oil; ie, they were half of the size of most over-the-counter fish-oil capsules for greater ease of swallowing.
TABLE 1.
Capsule fatty acid composition1
| Fatty acid profile | Placebo | DHA |
| % by wt | % by wt | |
| Caprylic acid (8:0)2 | 1.5 | None |
| Capric acid (10:0) | 0.9 | 2.1 |
| Lauric acid (12:0) | <0.1 | 5.4 |
| Myristic acid (14:0) | <0.1 | 15.5 |
| Palmitic acid (16:0) | 10.6 | 14.6 |
| Palmitoleic acid (16:1) | <0.1 | 1.6 |
| Stearic acid (18:0) | 4.6 | 0.6 |
| Oleic acid (18:1n−9) | 21.4 | 14.9 |
| Vaccenic acid (18:1n−7) | 1.5 | <0.1 |
| Linoleic acid (18:2n−6) | 51.2 | 0.8 |
| γ-Linolenic acid (18:3n−6) | 0.4 | <0.1 |
| α-Linolenic acid (18:3n−3) | 6.3 | 1.4 |
| Arachidic acid (20:0) | 0.4 | 5.0 |
| Dihomo-γ-linolenic acid (20:3n−6) | None | <0.1 |
| Arachidonic acid (20:4n−6) | None | <0.1 |
| EPA (20:5n−3) | None | <0.1 |
| Eicosenoic acid (20:1n−9) | 0.3 | None |
| Behenic acid (22:0) | 0.5 | None |
| Docosapentaenoic acid (22:5n−3) | None | 0.2 |
| DHA (22:6n−3) | None | 41.5 |
| Lignoceric acid (24:0) | 0.2 | None |
500-mg size 10, orange-flavored oval capsules; analysis provided by DSM Nutritional Products and rounded to the nearest 0.1%.
Fatty acid designation is #carbons:#double bonds:#carbons from the methyl carbon.
The capsules were held at the University of Kansas Medical Center Investigational Pharmacy, and pharmacy personnel supplied the capsules to women in sealed bottles after allocating them to a group. Thereafter, a month's supply of capsules (one bottle) was mailed each month until birth. We instructed subjects to take 3 capsules/d, but if they could not take that many they were encouraged to take as many as they could each day up to the limit of 3. They were also told that it was important to have an accurate assessment of their capsule intake; we provided each subject with a self-addressed stamped envelope to return capsule bottles from the previous month. Returned bottles were sent to the pharmacy with the envelope still sealed, and pharmacy personnel counted the remaining capsules, recorded the number, and destroyed them. The number of returned capsules was documented by the Investigational Pharmacy, and this record was the basis used to calculate capsule intake for each subject at the end of the treatment phase. Subjects received a small financial incentive for returning the partially used or empty capsule bottles. If the capsules were not returned or if more than half were returned to the pharmacy, the subjects were telephoned by study personnel and asked about any problems that they were having in an attempt to proactively resolve problems with compliance.
We obtained complete medical records for mother through pregnancy and 30 d postpartum; and for the infant from birth to 18 mo of age. Expected date of delivery was determined by ultrasound late in the first or early in the second trimester. The recorded expected date of delivery was used to determine gestation duration, even if a later ultrasound indicated a different expected date of delivery. We used birth weight, length, and head circumference from the medical record as primary data. All adverse events from the medical record were recorded by body system and are available as an addendum; SAEs included unanticipated hospitalization or hospitalization after discharge, congenital anomalies, life-threatening illness, and death. SAEs were reported to the institution Human Subjects Committee and to the study Medical Monitor as we became aware of them. These included both pregnancy and postnatal SAEs experienced by mothers and infants for 30 d after the intervention. The DSMB was chaired by a faculty member of the University and included a community obstetrician, a neonatologist (MKG, who also served as the study Medical Monitor), and a member of the University of Kansas Medical Center compliance office as well as the study biostatistician (BJG). The biostatistician could link SAEs to group if requested by the DSMB but did not have access to data until the study was unblinded. The DSMB reviewed all SAEs at 6-mo intervals and had the power to stop the study if any serious safety issues emerged.
Subjects
Subject characteristics were obtained from the medical record and from interviews with study personnel at enrollment (Table 2). The research protocol and informed consent process complied with the Declaration of Helsinki (including the October 1996 amendment) and were approved by the Institutional Review Boards/Human Subjects Committees at the University of Kansas Medical Center (Kansas City, KS); the University of Missouri–Kansas City (Truman Medical Center); and St Luke's Hospital (Kansas City, MO). Most of the subjects were enrolled in clinics associated with these hospitals.
TABLE 2.
Participant characteristics (no significant testing per CONSORT guidelines)1
| Placebo(n = 172) | DHA(n = 178) | |
| Maternal age (y) | 24.8 ± 4.72 | 25.3 ± 4.9 |
| Maternal race (%) | ||
| Black | 46 | 37 |
| Not black | 54 | 63 |
| Maternal ethnicity (%) | ||
| Hispanic | 8 | 8 |
| Not Hispanic | 92 | 92 |
| Gestation at enrollment (d) | 99.6 ± 26.1 | 102.9 ± 25.3 |
| Maternal education (y) | 13.36 ± 2.72 | 13.69 ± 2.67 |
| Maternal PPVT | 99.1 ± 15.8 | 99.7 ± 14.7 |
| Income by zip code ($) | 43,572 ± 19,201 | 45,599 ± 18,136 |
| Prior pregnancy (n) | 1.3 ± 1.4 | 1.2 ± 1.3 |
| Prepregnancy BMI (kg/m2) | 26.6 ± 5.1 | 27.2 ± 5.5 |
| Prepregnancy weight status (%)3 | ||
| Underweight | 1 | 1 |
| Normal | 42 | 41 |
| Overweight | 31 | 30 |
| Obese | 26 | 29 |
| Enrollment to birth gain (kg) | 11.6 ± 5.5 | 12.5 ± 6.0 |
| Low (%) | 32 | 25 |
| Adequate (%) | 28 | 29 |
| Excessive (%) | 39 | 47 |
| History of smoking (%) | 45 | 41 |
| History of smoking (pack-years)4 | 1.72 ± 3.46 | 1.38 ± 2.96 |
| Smoked during pregnancy (%) | 38 | 30 |
| Cigarettes (no./d) | 2.3 ± 5.2 | 1.9 ± 3.9 |
| Time smoked (d) | 51 ± 97 | 40.7 ± 89 |
| Alcohol use before pregnancy (%) | 53 | 52 |
| Alcohol used during pregnancy (%) | 2 | 1 |
| (no. drinks/d) | 0.00 ± 0.02 | 0.02 ± 0.21 |
| RBC-phospholipid-DHA at start (% by wt) | 4.3 ± 1.3 | 4.3 ± 1.1 |
| Prenatal vitamin/mineral supplement (%) | 99 | 97 |
| Iron supplementation (%) | 22 | 24 |
| Voluntary DHA intake from supplements (%) | 15 | 9 |
| Additional DHA intake from supplements (mg/d) | 33 ± 79 | 20 ± 66 |
CONSORT, Consolidated Standards of Reporting Trials; PPVT, Peabody Picture Vocabulary Test; RBC, red blood cell.
Mean ± SD (all such values); determined by using SPSS 18.0 (IBM).
Compared with Institute of Medicine guidelines (8).
Years smoked × packs/d.
Analysis of RBC fatty acids
Subjects provided blood samples at enrollment and on the morning after birth. Blood samples were collected by venipuncture into 5-mL sodium–EDTA tubes (Vacutainer; Becton-Dickinson) and placed on ice immediately. Venous cord blood was obtained at the time of birth. Plasma and RBCs were separated by centrifugation (3000 × g, 10 min; 4°C), frozen, and stored under nitrogen at −80°C until analyzed. Lipids were isolated according to a modification of Folch et al (9), and RBC lipids were fractionated (10) by thin-layer chromatography. RBC phospholipids were transmethylated with boron trifluoride-methanol (11), and the resulting fatty acid methyl esters were separated by using a Varian 3900 gas chromatograph with an SP-2560 capillary column (100 m; Sigma Aldrich) as previously reported (12) and a Star 6.41 Chromatography Workstation for peak integration and analysis. Injector and detector temperatures were programmed at 260°C. The column temperature program for the 41-min column run was as follows: 5 min at 140°C, 4°C increase/min to 240°C, and 240°C for 11 min. Individual peaks were identified by comparison with qualitative standards (PUFA 1 and PUFA 2; Sigma Aldrich), and a weighed standard mixture (Supelco 37 Component FAME mix; Sigma Aldrich) was used to adjust fatty acids for area/weight to calculate a final percentage weight of total fatty acids. RBC-phospholipid-DHA is reported as a percentage of total fatty acids by weight.
Power analyses
An increase in gestation duration of 6 d (from 270 to 276 d) was predicted based on an earlier clinical study (12). Power computations indicated that ∼130 completed patients per group were needed to detect an increase of 6 d in mean gestational age with 97% power, assuming an SD of 13.9 d. An enrollment of 350 was determined by assuming a 25% loss of subjects. We hypothesized higher RBC DHA in women and their infants and higher birth weight, length, and head circumference with DHA supplementation, because these measurements trended higher in an earlier study in which only one-sixth as much DHA was provided. The anticipated sample size gave ∼70% power to detect a mean increase of 150 g in birth weight (from 2975 to 3125 g), assuming an SD of 557 g. No other power analyses were done. The power/sample size calculation and testing for the study hypotheses were based on a 2-sample, one-tailed t test at the α = 0.05 level.
Statistical analyses
SPSS 18.0 statistical software was used for the analysis (IBM). In the first phase of analysis, we tested the mean difference between placebo and DHA-supplemented groups with a 2-sample t test and no covariate for primary efficacy outcomes of gestational age, maternal RBC-phospholipid-DHA, birth weight, and birth length and secondary efficacy outcomes of cord RBC-phospholipid-DHA and head circumference. We tested LBW (<2500 g), VLBW (<1500 g), preterm birth (<37 wk), early preterm birth (<34 wk), preeclampsia, gestational diabetes mellitus, cesarean section, and spontaneous or induced labor as recorded in a hospital record with a one-sided binomial exact tests. One-tailed P values at the α = 0.05 level were considered significant. No adjustment for multiple comparisons was made. All primary and secondary aims used intent-to-treat principles; ie, subjects remained in the group for which they were randomized regardless of DHA consumed. We provide means for placebo and DHA-supplemented groups by race (black compared with nonblack subjects); however, we did not compare them because the study was not designed to determine the effects of race on the primary outcomes. To evaluate days of hospitalization after preterm birth, we used bootstrap procedures assuming unequal variances.
We used multiple linear regression to assess the relations between predictor variables for regression and all of the primary and secondary pregnancy outcomes. Nearly one-half of the subjects were of African descent and more than one-third had smoked for at least some period of time during the pregnancy. The predictor variables represent general classes: study capsule DHA intake, smoking/drinking, and demographics. For exploratory purposes, the most important relation is between the DHA capsule intake (those in the placebo group set to 0) and pregnancy outcomes. Instead of a grouping variable representing placebo control or experimental groups, we used DHA in the form of several predictor variables, depending on their source (ie, DHA capsule intake per week and RBC-phospholipid-DHA at enrollment). For all outcomes, we set the DHA as a predictor variable but then fit all possible subsets of the other predictor variables to explore, for the particular pregnancy outcome, which model is the best. We used a global fit index called adjusted R-squared to determine variables to keep in the final model. After choosing the best subset of variables, it was decided to modify the original protocol and take out prenatal vitamin/mineral supplement as a predictor (98% of participants took them) and replace it with the number of previous pregnancies. A final exploratory analysis investigated the effect of capsule intake on infant outcome as mediated by maternal RBC-phospholipid-DHA. Thus, we correlated maternal and cord blood RBC-phospholipid-DHA with gestational age, birth weight, birth length, and head circumference. Of substantive interest in the regression analysis was that race was one of the predictor variables.
RESULTS
Enrollment and capsule compliance
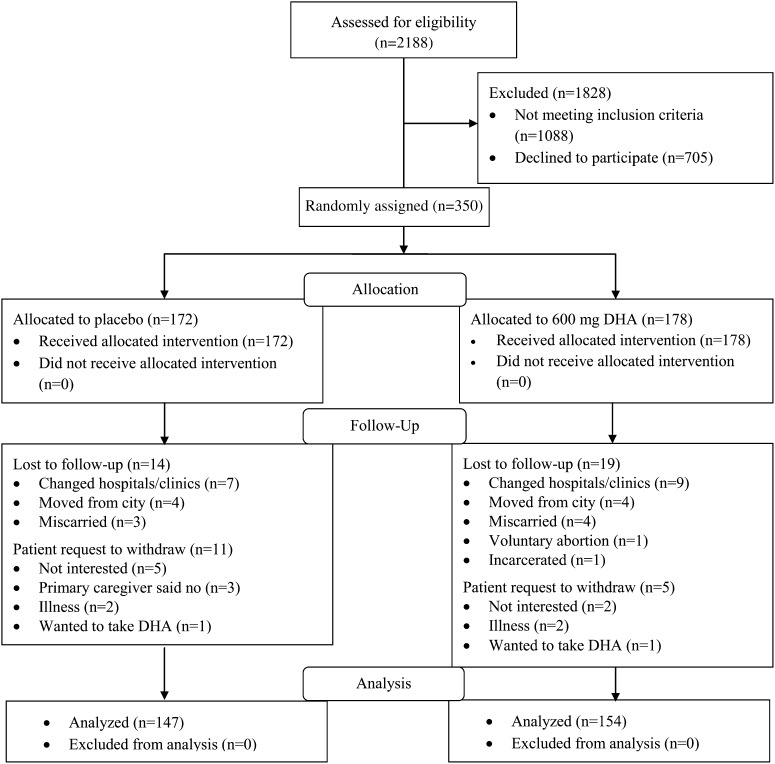
A total of 350 participants were randomized and given capsules (Figure 1). Of these, 172 were randomly assigned to the placebo and 178 to DHA. We obtained birth data for 301 subjects—147 assigned to placebo and 154 assigned to DHA supplementation. The study retention rates at birth were very similar for the 2 groups: placebo (85.5%) and DHA (86.5%). Subject loss was less than anticipated in our sample size calculation. Forty-nine subjects either did not have birth information available or requested to be removed from the study. Capsule compliance was similar for the 2 groups: placebo (76% consumed) and DHA (78% consumed). All data were analyzed regardless of compliance. On the basis of capsule intake and composition, we estimated the mean daily DHA intake from capsules by the DHA group as 469 mg and the mean ± SD study DHA intake from capsules as 74.0 ± 30.4 g.
FIGURE 1.
Consolidated Standards of Reporting Trials flow diagram.
Primary outcomes
RBC-phospholipid-DHA (percentage of total fatty acids by weight) was significantly higher in the DHA-supplemented group at birth and increased significantly from enrollment only in that group (Table 3). Gestational age was also 2.87 d greater, and birth weight and length were higher by 172 g and 0.7 cm, respectively. The effect of DHA supplementation on study primary outcomes was determined for black and nonblack subjects separately, and the results are included in Table 3. We did not compare outcomes between black and nonblack subjects to determine whether they were statistically different.
TABLE 3.
Primary and secondary outcomes1
| Placebo(n = 147) | DHA(n = 154) | P value2 | |
| Primary outcomes | |||
| RBC-phospholipid-DHA (% by wt)3 | |||
| All (n = 301)4 | 4.7 ± 1.3 | 7.3 ± 2.2 | <0.001 |
| Not black (n = 184) | 4.8 ± 1.3 | 7.8 ± 2.1 | |
| Black (n = 117) | 4.7 ± 1.3 | 6.2 ± 2.0 | |
| Gestational age (d) | |||
| All | 272.8 ± 17.0 | 275.7 ± 11.2 | 0.041 |
| Not black | 273.8 ± 14.0 | 275.9 ± 9.9 | |
| Black | 271.4 ± 20.3 | 275.2 ± 13.3 | |
| Birth weight (g) | |||
| All | 3187 ± 602 | 3359 ± 524 | 0.004 |
| Not black | 3282 ± 529 | 3489 ± 456 | |
| Black | 3060 ± 671 | 3110 ± 559 | |
| Birth length (cm) | |||
| All | 49.0 ± 3.4 | 49.7 ± 2.7 | 0.022 |
| Not black | 49.2 ± 2.9 | 50.0 ± 2.6 | |
| Black | 48.6 ± 4.0 | 49.0 ± 2.7 | |
| Secondary outcomes | |||
| Ponderal index5 | 2.7 ± 0.4 | 2.7 ± 0.3 | 0.170 |
| Male (%) | 54 | 49 | NS |
| Cord RBC-phospholipid-DHA (%) | 5.9 ± 1.4 | 7.3 ± 1.8 | 0.001 |
| Head circumference (cm) | 33.7 ± 2.0 | 34.2 ± 1.7 | 0.012 |
| Preterm birth (%)6 | 8.8 | 7.8 | NS |
| Gestation <34 wk (%) | 4.8 | 0.6 | 0.025 |
| Birth weight <2500 g (%) | 9.0 | 3.9 | 0.059 |
| Birth weight <1500 g (%) | 3.4 | 0.0 | 0.026 |
| Admitted to NICU (%) | 8.8 | 8.4 | NS |
| NICU admissions (hospital d) | 38.4 | 7.8 | 0.034 |
| Preterm infants (hospital d) | 40.8 ± 44.0 | 8.9 ± 10.1 | 0.026 |
| C-section birth (%) | 29.9 | 29.9 | NS |
| Labored on own (%) | 60 | 56.5 | NS |
| Preeclampsia (%) | 1.3 | 1.3 | NS |
| Gestational diabetes (%) | 4.1 | 5.6 | NS |
| Capsules (no./wk) | 15.2 ± 5.8 | 15.7 ± 5.6 | NS |
| Study capsule intake (no.) | 354 ± 147 | 370 ± 152 | NS |
NICU, neonatal intensive care unit; RBC, red blood cell.
Mean differences between placebo and DHA-supplemented groups were tested with a 2-sample one-sided t test and no covariate for primary efficacy outcomes and for secondary efficacy outcomes of cord RBC-phospholipid-DHA and head circumference. Other secondary efficacy outcomes were tested with one-sided binomial exact tests. One-tailed P values at α = 0.05 were considered significant. To evaluate days of hospitalization after preterm birth, we used bootstrap procedures assuming unequal variances. All outcomes used intent-to-treat principles.
g/100 g total fatty acids.
The data were not analyzed separately by race.
Weight (g)/length (cm)3 × 100.
Defined as <37 wk of gestation.
Secondary outcomes
Cord RBC-phospholipid-DHA and head circumference were significantly higher in newborns of women assigned to DHA than to placebo. Head circumference was 0.5 cm greater with the intervention (Table 3). Both maternal and cord RBC phospholipid arachidonic acid at birth were lower with DHA supplementation than with placebo (maternal: 12.4 ± 2.1 compared with 13.4 ± 1.8%; cord: 16.1 ± 2.6 compared with 17.0 ± 2.3%), but RBC phospholipid EPA did not differ between the respective groups (maternal: 0.24 ± 0.13 compared with 0.24 ± 0.13%; cord: 0.20 ± 0.24 compared with 0.20 ± 0.14%). There was no suggestion that preeclampsia, gestational diabetes mellitus, or cesarean birth differed between groups. The incidence of preterm birth did not differ between the groups; however, significantly more infants in the placebo group had an early preterm birth (P = 0.025). A trend toward fewer LBW deliveries was not statistically significant (P = 0.059), but there was a significantly lower incidence of VLBW in the DHA-supplemented group (P = 0.026). As might be anticipated from the significant reduction in early preterm/VLBW deliveries in the DHA group, infants born preterm in the DHA group spent significantly fewer days hospitalized after birth (P = 0.026; Table 3).
Predictor analyses
We conducted predictor analyses for the outcomes of RBC-phospholipid-DHA at birth, birth weight, and birth length. Two variables were significant positive predictors of maternal DHA status at birth: maternal DHA status at enrollment (β = 0.372, SE = 0.086, P < 0.001) and DHA intake per week (β = 0.157, SE = 0.011, P < 0.001). Iron supplementation for anemia was associated with lower maternal DHA status (β = −0.594, SE = 0.243, P = 0.015). Cord RBC-phospholipid-DHA was predicted by DHA intake per week (β = 0.090, SE = 0.013, P < 0.001). Statistically significant positive predictors of a higher birth weight included alcohol use before pregnancy (β = 133.2, SE = 58.0, P = 0.022), enrollment BMI (β = 19.3, SE = 6.2, P = 0.002), enrollment to birth weight gain (β = 18.7 SE = 2.6, P < 0.001), and number of previous pregnancies (β = 48.6, SE = 23.1, P = 0.037); significant negative predictors included number of cigarettes smoked during pregnancy (β = −15.7, SE = 7.5, P = 0.036) and black race (β = −241.0, SE =67.7, P < 0.001). Significant positive predictor variables for birth length were alcohol use before pregnancy (β = 0.746, SE = 0.323, P = 0.022), prepregnancy BMI (β = 0.116, SE = 0.035, P = 0.001), and enrollment to birth weight gain (β = 0.088, SE = 0.014, P < 0.001), whereas black race (β = −0.808, SE = 0.396, P = 0.043), cigarettes smoked during pregnancy (β = −0.084, SE = 0.042, P = 0.048), and mg additional DHA intake (Table 2) were negative predictors (β = −0.006, SE = 0.002, P = 0.008).
Correlation of maternal and infant DHA status at birth with birth outcomes
Maternal RBC-phospholipid-DHA was significantly correlated with birth weight and birth head circumference, whereas cord blood RBC-phospholipid-DHA did not correlate significantly with any outcome, although trends for a relation to gestational age and birth weight were observed (P = 0.084 and 0.052, respectively).
Safety evaluation
Phase III trials must include an evaluation of safety. We recorded all SAEs for mother and infant from enrollment until 30 d after birth (Table 4). A complete list of all adverse events is available online (see “Supplemental data” in the online issue). The study did not identify any serious safety concerns related to DHA supplementation for mother or newborn. Common reasons for hospitalization before birth were infection (4 placebo, 6 DHA) and premature rupture of membranes/threatened preterm birth (5 placebo, 1 DHA). One infant in the placebo group died at birth of extreme prematurity, and an additional 13 were admitted to the Neonatal Intensive Care Unit. One infant in the DHA group died in the first week of Tetralogy of Fallot, and 13 were admitted to the neonatal intensive care unit. Ten infants were readmitted to the hospital between discharge and 30 d of age. Seven readmissions were for infection (3 placebo, 4 DHA) (Table 4). We recorded infant hospitalizations between 31 d and 18 mo of age in Table 4, even though safety evaluation typically stops 30 d after the end of the intervention. The major reason for readmission to the hospital after the first month of life was for infection (8 placebo, 5 DHA). Growth failure or metabolic problems were the basis for readmission in an additional 4 children (3 placebo, 1 DHA), and one child in the placebo group was admitted for asthma. One child in the placebo group died of sudden infant death syndrome.
TABLE 4.
Serious adverse events1
| Placebo(n = 147) |
DHA(n = 154) |
|||
| Total n | n | Total n | n | |
| Mother | ||||
| Hospitalization or miscarriage | 18 | — | 18 | — |
| Body | — | 12 | — | 23 |
| Cardiology/blood | — | 3 | — | 2 |
| Gastrointestinal | — | 2 | — | 0 |
| Head/neck/mental | — | 0 | — | 1 |
| Miscarriage | — | 3 | — | 4 |
| Other pregnancy | — | 64 | — | 65 |
| Respiratory | — | 1 | — | 1 |
| Urogenital | — | 2 | — | 2 |
| Postpartum hospitalization | 3 | — | 1 | — |
| Wound healing | — | 2 | — | 0 |
| Mastitis | — | 1 | — | 0 |
| Suicide ideation | — | 0 | — | 1 |
| Infant | ||||
| Hospitalization and death | 31 | — | 26 | — |
| NICU at birth | — | 13 | — | 13 |
| Newborn death | — | 1 | — | 1 |
| Discharge to 30 d | — | 4 | — | 6 |
| 31 d to 18 mo | — | 126 | — | 77 |
| Infant death, SIDS | — | 1 | — | 0 |
| Congenital anomalies | 2 | — | 5 | — |
| Ileal atresia/short gut | — | 1 | — | 0 |
| Hypospadias | — | 1 | — | 1 |
| Cystic fibrosis | — | 0 | — | 1 |
| Tetralogy of Fallot | — | 0 | — | 1 |
| Coronal synostosis | — | 0 | — | 1 |
| Duplicated left ureter | — | 0 | — | 1 |
NICU, neonatal intensive care unit; SIDS, sudden infant death syndrome.
Fever.
One flank pain, one viral syndrome.
Includes 3 premature rupture of membranes, 1 placental abruption, 1 threatened preterm labor, and 1 observation of Rh isoimmunization.
Includes 2 premature rupture of membranes, 1 chorioamnionitis, 1 endomyometritis, 1 high blood pressure, and 1 abnormal Doppler results.
Two were hospitalized twice.
Includes an infant hospitalized twice for cystic fibrosis.
DISCUSSION
We found a significant greater gestation duration and infant size at birth with a 600-mg/d supplement of DHA given to pregnant women from a mean of 101.3 d gestation until birth. DHA supplementation did not affect ponderal index, unlike in a previous small study (13)—indirect evidence that the increases in weight and length with DHA compared with placebo did not alter the proportion of fat and lean mass. The gestation duration was 2.9 d longer with DHA supplementation, similar to the 2.5 d found in the intent-to-treat analysis from an earlier RCT (12) that also included a large number of black women, but agreed well with a recent systematic review that evaluated the effect of marine (fish) oil supplementation in a large number of subjects and concluded that supplementation resulted in a 2.55-d higher gestation duration (4). Birth weight averaged 59.3 g/d higher with the longer gestation. Because infants gain ∼14 g/d in the last week of pregnancy (14), the effect on birth weight appears to be greater than would be expected for the effect on gestation duration.
It has been suggested that the longer gestation duration with fish oil that contains EPA as well as DHA is due to an alteration in the balance of prostaglandins derived from EPA and arachidonic acid (15). Unlike most clinical trials, we provided an algal oil source of DHA and supplemented women with high-DHA eggs compared with ordinary eggs in a previous trial (12). Neither algal oil nor high-DHA eggs provide significant amounts of EPA, in contrast with studies that have used fish oil as a supplement of n−3 LCPUFAs. We found no effect of supplementation with algal oil DHA on RBC phospholipid EPA; however, we did find a small decrease in maternal (and newborn) RBC phospholipid arachidonic acid compared with the placebo; therefore, we cannot rule out a small change in the ratio of arachidonic acid to EPA as the basis for a longer duration of gestation.
The amount of n−3 LCPUFA supplementation (DHA plus EPA) in the extant published trials ranges from 100 mg to 3.3 g/d. A recent trial that provided 900 mg n−3 LCPUFA/d (800 mg DHA/d) (6) found significant increases in gestation duration and birth weight, whereas a trial that provided 400 mg DHA/d did not (5). However, gestation duration was higher with 100 mg DHA/d in an earlier trial (12). Study populations also differed in gestation duration and in birth weight of the placebo group, and these factors may influence response. For example, the placebo groups in 2 large RCTs providing n−3 LCPUFAs during pregnancy reported gestation durations of 279.6 (16) and 281 (6) d in contrast with the mean length of gestation in singleton pregnancies in the United States, which is just <271 d (7). In addition, the control groups in these studies averaged 3618 (16) and 3407 (6) g, whereas those that we studied in the Kansas City Metropolitan area had mean birth weights of 3106 (12) and 3187 g (the current trial). Given the long gestation durations and much higher birth weights in the 2 referenced trials conducted outside of Kansas City, it would be difficult for any intervention to have an effect. That said, one trial (with nearly 1200 subjects per group) found a statistically significant increase in gestation of 1 d and an increase in birth weight of 68 g with an n−3 LCPUFA supplement of 900 mg/d (6). In contrast, we found larger increases in weight with 100 (12) and 600 mg DHA/d; ie, 103 and 172 g, respectively. No RCT has found a reduction in gestation duration or size at birth.
A mean increase in gestation duration and birth weight could reduce the risk of preterm birth and LBW. Several studies have been conducted to determine whether n−3 LCPUFAs can reduce the risk of recurrent preterm birth; however, the results from the 2 large published trials are starkly different. A large 9-country trial in Europe found a reduction in preterm birth and birth before 34 wk of gestation (17), particularly for women who were low fish eaters (18), whereas a large US trial did not (19). In agreement with other studies in healthy pregnant women not selected for prior preterm birth (6, 12), we did not find a reduction in preterm birth with n−3 LCPUFA supplementation; however, we did find a significant reduction in infants with a birth weight <1500 g and in infants born before 34 wk gestation. This latter finding agrees with that of the DHA to Optimize Mother Infant Outcome trial (6). The incidence of early preterm birth in our placebo group was 4.8%, similar to US rates (20), whereas that in the DHA group was only 0.6%. On the basis of our overall results, and in agreement with the results from the DHA to Optimize Mother Infant Outcome trial (6), we speculate that n−3 LCPUFAs may be particularly beneficial in preventing early preterm birth by prolonging what would have been an early preterm birth beyond 34 wk of gestation. We do not know why this happens, but there are some potential causes of early premature birth (higher vascular tone/hypertension or infection), and perhaps DHA affects either vascular tone or inflammatory processes. It is also possible that DHA addresses in part some pathology that underlies the unknown cause of most early premature births and that we can learn from this observation in our study.
A possible limitation of our study was that many prenatal supplements with DHA were introduced to the market between 2006 when the study began and early 2010 when the last subject delivered. We did not exclude women who chose to take a DHA supplement <300 mg/d. Seventeen percent of subjects in the placebo group and 10% of subjects in the DHA group consumed DHA not provided by us. Another possible limitation is that we did not estimate dietary n−3 LCPUFA intakes in the study subjects; however, the biomarker (RBC-phospholipid-DHA) is very responsive to intake and was measured at both enrollment and immediately after birth as an indicator of prior status and study DHA intake. Mean RBC-phospholipid-DHA was low in both groups at enrollment, similar to that in an earlier study from Kansas City (12). It may be considered a limitation that we looked at many secondary variables but did not adjust for multiple comparisons; however, the intent of including secondary outcomes was to determine new plausible hypotheses.
We conclude that supplementing US women with DHA could safely increase mean birth weight and gestational age to numbers that are closer to those found in other developed countries such as Norway (16) and Australia (6). Whether or not this is beneficial to the overall health and development of most infants is open to investigation and, in fact, is another primary aim of the current study. What is suggested by this study, in agreement with results from another large trial (6), is that n−3 LCPUFA supplementation of US pregnant women could reduce the number of infants born before 34 wk of gestation along with the risks to the infant and costs to society of early preterm birth.
Supplementary Material
Acknowledgments
We thank the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1R01 HD047315) and the Office of Dietary Supplements for funding the study; DSM Nutritional Products for donating the DHA and placebo capsules for the study; the University of Kansas DSMB, especially Joan McDowd, who chaired the committee; numerous Dietetics and Nutrition Department MS students who assisted with data collection and entry from 2006 to 2012; the nursing staffs at our participating clinics and hospitals, whose support was essential to recruitment and availability of blood samples; and our subjects, most of whom consented to allow us to study their children until they reach 6 y of age and to evaluate their childhood cognitive performance.
The authors’ responsibilities were as follows—SEC, JC, BJG, and KMG: designed the study; BJG: completed the final statistical analysis and served on the DSMB; SEC and BJG: wrote the manuscript and had primary responsibility for the final content; LAM, EHK, and DJS: set up and coordinated the study and had primary responsibility for the day-to-day management of the study, including recruiting subjects and data collection, entry and management, and supervision of student research assistants; DM and JY: assisted with patient recruitment and consulted on issues related to pregnancy data collection; and MKG: served on the DSMB and as the study Medical Monitor and assisted with interpretation of the fetal and neonatal outcomes. All authors read and approved the final manuscript. SEC has given talks for several companies, including Martek, Mead Johnson Nutrition, and Nestle on results from our studies and the results of others who study the effects of DHA on infant and child outcomes. She is the President of the International Society for the Study of Fatty Acids and Lipids, which has corporate members who produce sources of DHA. JC consults with several companies on developmental measures to assess cognitive development of infants and children. None of the other authors declared a potential conflict of interest.
Footnotes
Abbreviations used: DSMB, Data and Safety Monitoring Board; LBW, low birth weight; LCPUFA, long-chain PUFA; RBC, red blood cell; RCT, randomized clinical trial; SAE, serious adverse event; VLBW, very-low birth weight.
REFERENCES
- 1.Olsen SF, Joensen HD. High liveborn birth weight in the Faroes: a comparison between birth weights in the Faroes and in Denmark. J Epidemiol Community Health 1985;39:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campoy C, Escoano-Margarit V, Anjos T, Szajewska H, Uauy R. Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br J Nutr 2012;107:S85–106 [DOI] [PubMed] [Google Scholar]
- 3.Larqué E, Gil-Sanchez A, Prieto-Sanchez MT, Koletzko B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br J Nutr 2012;107:S77–84 [DOI] [PubMed] [Google Scholar]
- 4. Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by preeclampsia or intrauterine growth restriction. Cochrane Database of Systematic Reviews 2006;3. [DOI] [PubMed]
- 5.Ramakrishnan U, Stein AD, Parra-Cabrera S, Wang M, Imhoff-Kunsch B, Juarez-Marquez S, Rivera J, Martorell R. Effect of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: randomized double-blind, placebo-controlled trial in Mexico. Food Nutr Bull 2010;31:S108–16 [DOI] [PubMed] [Google Scholar]
- 6.Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P. DOMInO Investigative Team Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children. JAMA 2010;304:1675–83 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention QuickStats: mean gestational age, by plurality—United States, 2005. MMWR Morb Mortal Wkly Rep 2008;57:225–52 [Google Scholar]
- 8.Donahue SMA, Kleinman KP, Gillman MW, Oken E. Trends in birth weight and gestational length among singleton term births in the United States: 1990-2005. Obstet Gynecol 2010;115:357–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 10.Zail SS, Pickering A. Fatty acid composition of erythrocytes in hereditary spherocytosis. Br J Haematol 1979;42:399–402 [DOI] [PubMed] [Google Scholar]
- 11.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 1964;5:600–8 [PubMed] [Google Scholar]
- 12.Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol 2003;101:469–79 [DOI] [PubMed] [Google Scholar]
- 13.Courville AB, Harel O, Lammi-Keefe CJ. Consumption of a DHA-containing functional food during pregnancy is associated with lower infant ponderal index and cord plasma insulin concentration. Br J Nutr 2011;106:208–12 [DOI] [PubMed] [Google Scholar]
- 14.Ríos JM. Tufiño-Olivares E, Reza-López S, Sanín LH, Levario-Carrillo M. Birthweight percentiles by gestational age and gender for children in the North of Mexico. Paediatr Perinat Epidemiol 2008;22:188–94 [DOI] [PubMed] [Google Scholar]
- 15.Allen KGD, Harris MA. The role of n−3 fatty acids in gestation and parturition. Exp Biol Med (Maywood) 2001;226:498–506 [DOI] [PubMed] [Google Scholar]
- 16.Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, Ganes T, Drevon CA. Similar effect on infants of n−3 and n−6 fatty acids supplementation to pregnant and lactating women. Pediatrics 2001;108(5):E82. [DOI] [PubMed]
- 17.Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. BJOG 2000;107:382–95 [DOI] [PubMed] [Google Scholar]
- 18.Olsen SF, Osterdal ML, Salvig JD, Weber T, Tabor A, Secher NJ. on behalf of the FOTIP team. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomized clinical trial with fish oil. Eur J Clin Nutr 2007;61:976–85 [DOI] [PubMed] [Google Scholar]
- 19.Harper M, Thom E, Klebanoff M, Thorp J, Jr, Sorokin Y, Varner MW, Wapner RJ, Caritis SN, Iams JD, Carpenter MW, et al. Omega-3 fatty acid supplementation to prevent recurrent preterm birth. Obstet Gynecol 2010;115:234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, Wilson EC. Births: final data for 2009. Natl Vital Stat Rep 2011;60:1–70 [PubMed] [Google Scholar]
- 21.Rasmussen KM, Abrams B, Bodnar LM, Butte NF, Catalano PM, Maria Siega-Riz A. Recommendations for weight gain in pregnancy in the face of the obesity epidemic. Obstet Gynecol 2010;116:1191–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.