Abstract
Background: Inflammation underlies the etiology of colorectal cancer (CRC). Hyperhomocysteinemia is associated with inflammation and may be a risk marker for CRC. Cysteine is a metabolic product of homocysteine and a precursor of the antioxidant glutathione. It is unknown whether cysteine is associated with CRC.
Objective: The objective was to assess the associations between homocysteine and cysteine and CRC incidence in postmenopausal women.
Design: Associations between homocysteine and cysteine and incident CRC in the Women's Health Initiative observational cohort were assessed by using a nested case-control design. Cases and controls (n = 988/group) were matched for age (mean ± SD age: 67 ± 7 y), ethnicity (85.2% white, 8.9% black, 2.2% Hispanic/Latina, and 3.6% other), hysterectomy status, and date of blood draw. Homocysteine and cysteine were measured by HPLC with postcolumn fluorimetric detection.
Results: Multivariate-adjusted ORs (95% CIs) for CRC were 1.46 (1.05, 2.04) for the highest quartile of homocysteine (>9.85 μmol/L) compared with the lowest quartile (≤6.74 μmol/L) (P = 0.02) and 0.57 (0.40, 0.82) for the highest quartile of cysteine (>309 μmol/L) compared with the lowest quartile (≤260 μmol/L) (P = 0.01). The association with homocysteine was significant for proximal colon tumors (P = 0.008) but not for distal or rectal tumors, whereas the association with cysteine was significant for rectal tumors (P = 0.02), borderline for proximal tumors (P = 0.06), and not significant for distal tumors. The associations with both homocysteine and cysteine were significant for localized tumors (P ≤ 0.01) but not for metastases.
Conclusion: High plasma homocysteine is associated with increased risk of CRC, whereas high cysteine is associated with decreased risk. This trial was registered at clinicaltrials.gov as NCT 00000611.
INTRODUCTION
Chronic inflammation of the large intestine and rectum is a major etiologic factor underlying the development of colorectal cancer (CRC)4 (1). This inflammation may be the consequence of infectious agents or inflammatory bowel disease (IBD; an independent risk factor for CRC) (2) or may result as a localized response to tissue stressors, including premalignant lesions (adenomatous polyps) (1). Importantly, metabolic conditions that promote systemic or localized inflammation, such as nutritional deficiencies, could accentuate the inflammatory response within the large intestine and rectum, and thus increase the risk of CRC.
Deficiency of the B vitamin folate is a risk factor for CRC (3) and also causes increased plasma concentration of the sulfur amino acid homocysteine. Hyperhomocysteinemia is an established independent risk factor for vascular disease (4), possibly through inflammatory mechanisms (5, 6). Whether hyperhomocysteinemia plays a role in promoting the development or progression of CRC is less clear. Hyperhomocysteinemia is highly prevalent in patients with IBD (7, 8) and may be directly associated with inflammation or may be due to decreased absorption or increased requirements for folate and other nutrients [vitamins B-12, B-6, and B-2 (riboflavin)] required for one-carbon and homocysteine metabolism. In support of the former, homocysteine induces an inflammatory response in cultures of human intestinal microvascular endothelial cells (9). Thus, there may be a link between folate and CRC through homocysteine.
Similar to homocysteine, cysteine has been shown to be a risk factor for vascular disease (10), but very little is known about the relation between cysteine and CRC. Cysteine concentrations are typically correlated with homocysteine, and elevations in both cysteine and homocysteine occur in renal disease. Cysteine may be a biomarker for CRC as an indicator of B vitamin status or as an indicator of oxidative stress that may accompany inflammatory conditions.
Several prospective studies have examined the associations between homocysteine and colorectal adenoma recurrence and between homocysteine and CRC. In 3 of 4 prospective cohort studies, elevated homocysteine was correlated with increased adenoma recurrence (11–14). In contrast, no significant associations have been found between homocysteine and risk of CRC (15–18). Only one study has investigated whether cysteine is related to CRC risk. A trend toward decreased risk with higher cysteine concentrations was observed but did not reach significance (18).
We are investigating biomarkers of one-carbon metabolism and inflammation and their influence on risk of CRC in a nested, case-control study within the Women's Health Initiative Observational Study (WHI-OS) (19, 20). Here, we report on the prospective associations between homocysteine and cysteine and CRC risk.
SUBJECTS AND METHODS
Study population
The WHI-OS prospective cohort consists of 93,676 women who were enrolled at 40 US clinical institutions between 1993 and 1998. The study design and baseline description of the cohort have been described (19, 20). Baseline eligibility requirements included postmenopausal status, age between 50 and 79 y, and low likelihood of loss to follow-up within 3 y due to relocation or death resulting from a preexisting medical condition. For this retrospective, nested, case-control analysis of CRC risk, all centrally or locally (if no central adjudication) confirmed invasive CRCs were selected as cases as of 24 April 2008. Cases and controls were excluded if they had no available biospecimens [plasma, serum, red blood cells (RBCs), and DNA]. Risk-set sampling was used to select controls from within the WHI-OS cohort who were free of any type of CRC, invasive or noninvasive, at the time of case diagnosis. Cases and controls were matched on the basis of age (±3 y), race-ethnicity, enrollment date (±1 y), hysterectomy status, history of CRC, and time of blood draw (±6 mo). All incident cases were included regardless of disease stage at time of diagnosis. The analyses in this report are based on 988 incident cases of CRC and 988 matched controls. The study was approved by the human subjects review boards at the Fred Hutchinson Cancer Research Center where the Women's Health Initiative Clinical Coordinating Center is located and at all 40 clinical centers at which participants were recruited for the WHI-OS study. Additional institutional review board approval was obtained at the University of California, Davis, and the German Cancer Research Center where analyte measurements and data analyses were conducted. Written informed consent was obtained from all participants.
Demographic and health data collection
Demographic characteristics, including age, race, education, and household income, and health-related characteristics, including medical history, use of postmenopausal hormones and other medications, physical activity (min/wk), and smoking history, were recorded at baseline entry into the study by using standardized questionnaires (19). Height and weight were measured and BMI was calculated by using the equation BMI = weight (kg)/height (m)2. Changes in health status and clinical outcomes were reported through annual self-administered questionnaires provided to each participant and during a clinic visit occurring 3 y after entry into the study. CRC cases were first reported by participant self-report and confirmed by trained physician adjudicators by using medical records and the International Classification of Diseases for Oncology, second edition, codes (http://seer.cancer.gov). Adenocarcinoma cases were classified as “proximal” if they were located in the cecum (code C180), ascending colon (C182), hepatic flexure (C183), transverse colon (C184), or splenic flexure (C185); “distal” if they were located in the descending colon (C186) or sigmoid colon (C187); and “rectal” (C199, C209). The classifications of the tumors followed the National Cancer Institute Surveillance Epidemiology and End Results criteria (http://seer.cancer.gov).
Blood sample processing and analysis
Participants were instructed to fast at least 12 h before undergoing phlebotomy. Blood samples were collected at baseline and were kept at 4°C for up to 1 h before centrifugation. Plasma and serum were collected and stored at −70°C until analysis. Total plasma homocysteine and cysteine were determined by HPLC with postcolumn fluorescence detection (21), RBC folate and plasma vitamin B-12 by radioassay (SimulTRAC; MP Biomedicals), plasma pyridoxal-5′-phosphate (PLP) by HPLC with fluorescence detection (22), and plasma creatinine by the Jaffe rate reaction method (DxC Instrument; Beckman Coulter). Interassay CVs for each of the assays were as follows: homocysteine, 6.5%; cysteine, 7.1%; RBC folate, 10.2%; vitamin B-12, 6.2%; PLP, 5.9%; and creatinine, 4.1%.
Statistical analyses
Baseline characteristics of CRC cases and controls were compared by t tests (for continuous variables) and chi-square tests (for categorical variables). Homocysteine and cysteine concentrations were categorized into quartiles on the basis of the distribution in the non-CRC controls. Conditional logistic regression was used to estimate ORs and 95% CIs for all cases of CRC combined and for categories of CRC (tumor site: proximal, distal, and rectal; stage: local/regional and metastatic) among quartiles of homocysteine and cysteine, with the lowest quartiles of both variables serving as the reference groups. OR and 95% CI determinations were made in sequential models with the first model adjusted only for age (continuous) and subsequent multivariate models adjusted for baseline variables selected a priori on the basis of knowledge of known CRC risk factors, including BMI (in kg/m2; <25, 25–30, >30–35, >35), past medical history of colonoscopy (yes or no), smoking status (never, past, or current), leisure physical activity (0–180, >180–705, or >705 min/wk), postmenopausal hormone use (never, past, or current), and concentrations of RBC folate, plasma vitamin B-12, plasma PLP, and plasma creatinine. Tests of linear trend were conducted by using the Wald test across increasing quartiles of homocysteine and cysteine with median values of each quartile modeled as a single continuous variable. Significance was defined as P < 0.05, and all statistical tests were 2-sided. Analyses were conducted by using SAS (version 9.2; SAS Institute Inc).
RESULTS
Baseline characteristics of the CRC cases and controls are presented in Table 1. The CRC group had a higher mean BMI, a greater number of pack-years of smoking, and fewer minutes per week of moderate to strenuous physical activity than the control group. The CRC cases were less likely to have had a previous colonoscopy but more likely to have had a colon polyp removed. The mean ± SD time from baseline to CRC diagnosis was 5.2 ± 3.1 y. The majority of incident tumors among the cases were proximal (58.3%), followed by distal (21.3%) and rectal (18.6%). Most of the tumors were moderately or well differentiated (69.8%), with approximately one-fifth that were poorly differentiated. Metastatic disease was observed in 12.8% of the cases.
TABLE 1.
Characteristics of CRC cases and controls1
| Cases |
Controls |
||||
| Characteristics | n | Value | n | Value | P value |
| Age | 988 | 67 ± 7 | 988 | 67 ± 7 | 0.50 |
| 50–54 y | 58 | 5.9 | 55 | 5.6 | 0.96 |
| 55–59 y | 105 | 10.6 | 104 | 10.5 | |
| 60–64 y | 190 | 19.2 | 192 | 19.4 | |
| 65–69 y | 260 | 26.3 | 245 | 24.8 | |
| 70–74 y | 230 | 23.3 | 246 | 24.9 | |
| 75–79 y | 145 | 14.7 | 146 | 14.8 | |
| BMI2 | 976 | 28.1 ± 6.0 | 978 | 27.1 ± 5.9 | 0.0001 |
| <25.0 kg/m2 | 342 | 35.1 | 394 | 40.3 | 0.002 |
| 25.0–29.9 kg/m2 | 331 | 33.9 | 355 | 36.3 | |
| 30–34.9 kg/m2 | 187 | 19.2 | 143 | 14.6 | |
| ≥35.0 kg/m2 | 116 | 11.8 | 86 | 8.8 | |
| Ethnicity (several categories) | 988 | 100 | 988 | 100 | 1.0 |
| White | 842 | 85.2 | 842 | 85.2 | |
| Black or African American | 88 | 8.9 | 88 | 8.9 | |
| Other3 | 58 | 5.9 | 58 | 5.9 | |
| Family income | 950 | 100 | 940 | 100 | 0.08 |
| <$34,999 | 457 | 48.1 | 421 | 44.8 | |
| $35,000–$74,999 | 343 | 36.1 | 329 | 35.0 | |
| ≥$75,000 | 125 | 13.1 | 162 | 17.2 | |
| Don't know | 25 | 2.6 | 28 | 3.0 | |
| Education (high school or less) | 195 | 19.9 | 223 | 22.8 | 0.12 |
| Residence location (US region) | 988 | 100 | 988 | 100 | 0.42 |
| Northeast | 255 | 25.8 | 227 | 22.3 | |
| South | 223 | 22.6 | 240 | 24.3 | |
| Midwest | 228 | 23.1 | 222 | 22.5 | |
| West | 282 | 28.5 | 299 | 30.3 | |
| Smoking (pack-years) | 951 | 13.0 ± 21.7 | 951 | 8.9 ± 17.0 | <0.0001 |
| Moderate or strenuous activity (min/wk) | 975 | 95.9 ± 135.9 | 979 | 109.1 ± 143.4 | 0.04 |
| Family history of CRC (yes) | 192 | 21 | 163 | 18 | 0.10 |
| History of colonoscopy or sigmoidoscopy (yes) | 508 | 52 | 589 | 60 | 0.0003 |
| History of colon polyp removal (yes) | 123 | 25 | 105 | 18 | 0.008 |
| Tumor location4 | 990 | 100 | — | — | |
| Proximal | 577 | 58.3 | — | — | |
| Distal | 211 | 21.3 | — | — | |
| Rectal | 184 | 18.6 | — | — | |
| Overlapping/unknown | 18 | 1.8 | — | — | |
| Tumor grade4 | 990 | 100 | — | — | |
| Well differentiated | 75 | 7.6 | — | — | |
| Moderately differentiated | 616 | 62.2 | — | — | |
| Poorly differentiated | 202 | 20.4 | — | — | |
| Anaplastic | 12 | 1.2 | — | — | |
| Unknown/not determined | 85 | 8.6 | — | — | |
| Tumor stage (SEER staging)4 | 989 | 100 | — | — | |
| Localized | 431 | 43.6 | — | — | |
| Regional | 409 | 41.3 | — | — | |
| Distant | 127 | 12.8 | — | — | |
| Unknown/not determined | 22 | 2.2 | — | — | |
| Homocysteine | 966 | 9.42 ± 1.315 | 966 | 9.12 ± 1.285 | 0.04 |
| ≤6.74 μmol/L | 227 | 23.5 | 257 | 26.6 | |
| >6.74–7.85 μmol/L | 235 | 24.3 | 253 | 26.2 | |
| >7.85–9.85 μmol/L | 238 | 24.6 | 243 | 25.1 | |
| >9.85 μmol/L | 267 | 27.6 | 214 | 22.1 | |
| Cysteine | 967 | 283.9 ± 1.15 | 967 | 284.0 ± 1.15 | 0.39 |
| ≤260.0 μmol/L | 247 | 25.5 | 224 | 23.2 | |
| >260–282.0 μmol/L | 235 | 24.3 | 258 | 26.7 | |
| >282.0–309.0 μmol/L | 250 | 25.9 | 236 | 24.4 | |
| >309.0 μmol/L | 235 | 24.3 | 249 | 25.8 | |
All values in the “Value” column are means ± SDs or percentages. Differences between cases and controls were compared by t tests (continuous variables) and chi-square tests (categorical variables). CRC, colorectal cancer; SEER, Surveillance Epidemiology and End Results.
Measured at baseline.
Hispanic, Asian or Pacific Islander, American Indian or Alaskan Native, or missing.
Two individuals are listed as having both colon cancer and CRC, so information on both tumors is reported here.
Values are geometric means ± SDs.
Homocysteine was higher in the cases compared with the controls (geometric mean ± SD: 9.42 ± 1.31 compared with 9.12 ± 1.28 μmol/L, P = 0.04), and the cases had a higher proportion of subjects within the upper quartile of homocysteine values (Table 1). No difference in geometric mean cysteine concentrations (283.9 ± 1.1 compared with 284.0 ± 1.1 μmol/L) or in percentage distribution of cysteine concentrations among quartiles was observed between the groups (Table 1).
The risk of incident CRC by quartiles of baseline homocysteine is presented in Table 2. After age was controlled for, a significant trend (P-trend = 0.006) toward increasing ORs for CRC (all tumors combined) was observed from the lowest to highest quartile of homocysteine (OR for the highest compared with the lowest quartile: 1.45; 95% CI: 1.09, 1.92). This significant trend was observed not only for all tumors combined but also for proximal (1.57; 1.09, 2.26; P-trend = 0.005) and local/regional tumors (1.46; 1.08, 1.98; P-trend = 0.008). In multivariate analyses controlled for age, BMI, race-ethnicity, history of colonoscopy, smoking history, weekly physical activity, hormone use, and B vitamin status (RBC folate, vitamin B-12, and PLP), the trends remained significant with little change in the magnitude of the ORs. No significant trends were observed for distal and metastatic tumors in either the age-adjusted or the multivariate analyses.
TABLE 2.
ORs (95% CIs) of CRC associated with baseline homocysteine values1
| Quartiles of homocysteine |
|||||
| Model | 1 (≤6.74 μmol/L) | 2 (>6.74–7.85 μmol/L) | 3 (>7.85–9.85 μmol/L) | 4 (>9.85 μmol/L) | P-trend2 |
| n | 461 | 455 | 537 | 481 | |
| All participants | |||||
| Age-adjusted | 1 | 1.02 (0.78, 1.34) | 1.11 (0.86, 1.45) | 1.45 (1.09, 1.92) | 0.006 |
| Multivariate3 | 1 | 1.06 (0.79, 1.41) | 1.05 (0.79, 1.40) | 1.46 (1.05, 2.04) | 0.02 |
| By tumor site | |||||
| Proximal | |||||
| Age-adjusted | 1 | 0.90 (0.64, 1.27) | 1.04 (0.75, 1.46) | 1.57 (1.09, 2.26) | 0.005 |
| Multivariate3 | 1 | 0.92 (0.63, 1.34) | 1.07 (0.73, 1.55) | 1.72 (1.11, 2.67) | 0.008 |
| Distal | |||||
| Age-adjusted | 1 | 1.42 (0.77, 2.59) | 1.08 (0.62, 1.90) | 1.38 (0.72, 2.64) | 0.50 |
| Multivariate3 | 1 | 1.46 (0.73, 2.90) | 1.02 (0.53, 1.94) | 1.28 (0.61, 2.71) | 0.71 |
| Rectal | |||||
| Age-adjusted | 1 | 0.98 (0.51, 1.89) | 1.33 (0.68, 2.59) | 1.15 (0.55, 2.39) | 0.74 |
| Multivariate3 | 1 | 1.12 (0.54, 2.33) | 1.21 (0.55, 2.65) | 1.08 (0.45, 2.57) | 0.93 |
| By stage | |||||
| Local/regional | |||||
| Age-adjusted | 1 | 1.01 (0.76, 1.35) | 1.08 (0.81, 1.43) | 1.46 (1.08, 1.98) | 0.008 |
| Multivariate3 | 1 | 1.01 (0.73, 1.38) | 1.04 (0.76, 1.43) | 1.54 (1.07, 2.21) | 0.01 |
| Metastatic | |||||
| Age-adjusted | 1 | 0.86 (0.42, 1.73) | 1.23 (0.63, 2.37) | 1.32 (0.60, 2.90) | 0.38 |
| Multivariate3 | 1 | 1.23 (0.55, 2.73) | 1.36 (0.63, 2.90) | 1.65 (0.63, 4.32) | 0.31 |
ORs (95% CIs) for CRC were determined by conditional logistic regression. CRC, colorectal cancer.
Medians for each quartile used in the trend test: quartile 1 = 6.05 μmol/L, quartile 2 = 7.33 μmol/L, quartile 3 = 8.64 μmol/L, quartile 4 = 11.22 μmol/L.
Multivariate analyses were adjusted for age, baseline BMI, ever had colonoscopy, smoking, physical activity (min/wk of moderate or strenuous activity), hormone replacement therapy, red blood cell folate, plasma vitamin B-12, and plasma pyridoxal-5′-phosphate.
The risk of incident CRC by quartiles of baseline cysteine is presented in Table 3. In age-adjusted analyses, no significant trend in CRC risk (all tumors combined) was observed with increasing cysteine quartile [OR (95% CI) for the highest compared with the lowest quartile: 0.88 (0.65, 1.19); P-trend = 0.61]. This finding also was true for proximal distal, local/regional, and metastatic tumors. However, in multivariate analyses, the ORs strengthened and the trend toward decreasing ORs for CRC (all tumors combined) from the lowest to the highest quartile of cysteine was significant [0.57 (0.40, 0.82); P-trend = 0.01]. The trend also was significant for rectal tumors [0.24 (0.09, 0.65); P-trend = 0.02], borderline significant for proximal tumors [0.61 (0.39, 0.97); P-trend = 0.06], and not significant for distal tumors [0.57 (0.24, 1.34); P-trend = 0.34]. The trend was also significant for local/regional tumors [0.51 (0.35, 0.76); P-trend = 0.003] but not for metastases [0.84 (0.32, 2.19); P-trend = 0.68].
TABLE 3.
ORs (95% CIs) of CRC associated with baseline cysteine values1
| Quartiles of cysteine |
|||||
| Model | 1 (≤260 μmol/L) | 2 (>260–282 μmol/L) | 3 (>282–309 μmol/L) | 4 (>309 μmol/L) | P-trend2 |
| n | 500 | 464 | 502 | 468 | |
| All participants | |||||
| Age-adjusted | 1 | 0.88 (0.67, 1.15) | 1.03 (0.78, 1.35) | 0.88 (0.65, 1.19) | 0.61 |
| Multivariate3 | 1 | 0.73 (0.54, 0.98) | 0.86 (0.63, 1.16) | 0.57 (0.40, 0.82) | 0.01 |
| By tumor site | |||||
| Proximal | |||||
| Age-adjusted | 1 | 0.77 (0.54, 1.09) | 0.87 (0.60, 1.25) | 0.89 (0.61, 1.31) | 0.69 |
| Multivariate3 | 1 | 0.67 (0.46, 0.99) | 0.75 (0.50, 1.13) | 0.61 (0.39, 0.97) | 0.06 |
| Distal | |||||
| Age-adjusted | 1 | 1.25 (0.69, 2.27) | 1.40 (0.75, 2.61) | 1.18 (0.60, 2.34) | 0.57 |
| Multivariate3 | 1 | 0.82 (0.41, 1.61) | 1.02 (0.50, 2.08) | 0.57 (0.24, 1.34) | 0.34 |
| Rectal | |||||
| Age-adjusted | 1 | 1.05 (0.54, 2.03) | 1.28 (0.69, 2.36) | 0.53 (0.26, 1.07) | 0.14 |
| Multivariate3 | 1 | 0.70 (0.31, 1.59) | 0.93 (0.43, 1.98) | 0.24 (0.09, 0.65) | 0.02 |
| By stage | |||||
| Local/regional | |||||
| Age-adjusted | 1 | 0.90 (0.68, 1.20) | 1.04 (0.77, 1.40) | 0.84 (0.61, 1.15) | 0.41 |
| Multivariate3 | 1 | 0.73 (0.53, 1.01) | 0.86 (0.62, 1.21) | 0.51 (0.35, 0.76) | 0.003 |
| Metastatic | |||||
| Age-adjusted | 1 | 0.90 (0.43, 1.89) | 0.80 (0.38, 1.68) | 0.85 (0.38, 1.94) | 0.63 |
| Multivariate3 | 1 | 0.92 (0.41, 2.08) | 0.87 (0.37, 2.04) | 0.84 (0.32, 2.19) | 0.68 |
ORs (95% CIs) for CRC were determined by conditional logistic regression. CRC, colorectal cancer.
Medians for each quartile used in the trend test: quartile 1 = 243 μmol/L, quartile 2 = 271 μmol/L, quartile 3 = 295 μmol/L, quartile 4 = 327 μmol/L.
Multivariate analyses were adjusted for age, baseline BMI, ever had colonoscopy, smoking, physical activity (min/wk of moderate or strenuous activity), hormone replacement therapy, red blood cell folate, plasma vitamin B-12, and plasma pyridoxal-5′-phosphate.
Because renal function is a strong determinant of both homocysteine and cysteine, potential confounding of the associations between homocysteine and cysteine and CRC risk by plasma creatinine was assessed. Creatinine values were available for only 404 of the 988 case-control pairs. There was no difference in case/control status between participants with and without creatinine values (data not shown). Separate multivariate analyses were conducted, and the results were compared between those subjects who had creatinine values and those who did not. No significant differences in the magnitude of the ORs or the significance of the trends were observed between the groups with and without creatinine values, indicating that renal function was not an important confounding variable (data not shown).
DISCUSSION
In this nested case-control analysis of the WHI-OS cohort, elevated homocysteine and low cysteine, after confounding variables were controlled for, were associated with increased ORs of incident CRC over an average follow-up period of 5.2 y. The association with elevated homocysteine is significant for all CRC tumors combined and for proximal and local/regional tumors, whereas the association with low cysteine is significant for all tumors combined and for rectal tumors and borderline significant for proximal tumors. This analysis represents the largest study of the associations between homocysteine and cysteine and incident CRC to date.
Four previous studies assessed the longitudinal associations between homocysteine and incident CRC (Table 4). In the Northern Sweden Health and Disease cohort (15), no difference was seen in baseline median homocysteine between incident CRC cases (n = 226) and age- and sex-matched controls (n = 437), and no trend was observed for increased ORs for incident CRC with increasing quintiles of homocysteine over a median 4.2-y follow-up period. In the Alpha-Tocopherol, Beta-Carotene Cancer Prevention study (16), which included only male smokers, no difference was found in median homocysteine between colon cancer cases (n = 152) and age-matched controls (n = 152) or between rectal cancer cases (n = 126) and age-matched controls (n = 126), and no trends were observed in colon or rectal cancer rates with increasing homocysteine quintiles over a 17-y follow-up period. In the Multiethnic Cohort Study (17), which included both men and women, no difference was found in baseline median homocysteine between incident CRC cases (n = 224) and age-, sex-, and race-ethnicity–matched controls (n = 441), and no trend was observed for incident CRC with increasing quartiles of homocysteine (no median follow-up period was reported for this study). Last, in men participating in the Physicians’ Health Study (18), no difference in median homocysteine was noted between CRC cases (n = 197) and age- and smoking status-matched controls (n = 371) after a median 10-y follow-up period.
TABLE 4.
Homocysteine and incident CRC: comparison of studies1
| Study (reference) | Study sample | Outcome2 |
| Northern Sweden Health and Disease cohort (15) | Cases: 94 M/132 FControls: 184 M/253 F | Highest Hcy quintile (>15.1 μmol/L) vs lowest quintile (≤9.7 μmol/L); OR: 1.12 (95% CI: 0.63, 1.99), NS |
| Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (16) | Cases: 275 MControls: 275 M | Highest Hcy quintile (18.8 μmol/L) vs lowest quintile (9.2 μmol/L); OR: 1.22 (95% CI: 0.68, 2.17), NS |
| Multiethnic Cohort Study (17) | Cases: 140 M/84 FControls: 257 M/154 F | Highest Hcy quartile (>11.8 μmol/L) vs lowest quartile (≤7.65 μmol/L); OR: 1.00 (95% CI: 0.56, 1.79), NS |
| Physician's Health Study (18) | Cases: 197 MControls: 371 M | Cases: median Hcy = 11 μmol/LControls: median Hcy = 10 μmol/LNo difference in medians (P = 0.13) |
| Women's Health Initiative observational cohort | Cases: 988 FControls: 988 F | Highest Hcy quartile (>9.85 μmol/L) vs lowest quartile (≤6.74 μmol/L); OR: 1.46 (95% CI: 1.05, 2.04), P = 0.02 |
CRC, colorectal cancer; Hcy, homocysteine.
ORs (95% CIs) are for all forms of CRC and are multivariate adjusted.
It is unclear why the WHI-OS cohort exhibited significant associations between homocysteine and incident CRC, whereas previous studies did not. The sample sizes of cases and controls in the WHI-OS study were much larger than those in the previous studies, and thus there was more power to see associations. However, the range of homocysteine values in the WHI-OS study was lower than in the previous studies (Table 4), with only a portion of subjects in the highest quartile exceeding the upper limit of the reference range (3.8–11.0 μmol/L; UC Davis Medical Center Clinical Laboratory). This finding would be expected to limit the ability to detect significant associations between homocysteine and CRC. Another major difference is that the WHI-OS included only women, whereas the previous studies included only men or both men and women. It is possible that associations between homocysteine and CRC are stronger in women than in men. The previous studies had limited statistical power to assess the relation specifically in women. It should be noted, however, that there is little or no precedent from the cardiovascular disease literature that associations with homocysteine are modified by sex.
The Multiethnic Cohort Study (17) is the only previous study to assess the association between cysteine and incident CRC. In this study, plasma cysteine values were available for only subsamples of the cases (n = 118) and controls (n = 211), and there was no significant difference in the ORs for incident CRC observed among cysteine quartiles. However, there was a nonsignificant trend toward decreasing ORs with increasing cysteine quartiles, which is consistent with the significant trend observed in the WHI-OS cohort.
The longitudinal design of the present study suggests a potential causal relation between homocysteine, cysteine, and CRC. Indeed, there is experimental support for a role of homocysteine in disruption of cellular processes and promotion of inflammation in IBD, a known risk factor for CRC (7). In vitro exposure of human endothelial cells to homocysteine alone and in combination with the inflammatory cytokine TNF-α induces expression of other inflammatory cytokines, including monocyte chemoattractant protein 1 and IL-8 (9, 23), through activation of the nuclear transcription factor κB pathway (24). Increased expression of vascular adhesion molecules, such as vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and plasminogen activator inhibitor-1, is induced by homocysteine as well (9, 25). These factors increase T cell and monocyte adhesion, which contributes to the inflammatory process. Homocysteine also promotes cellular toxicity caused by TNF-α (26). Together, these findings support a role for homocysteine in promoting the inflammatory state that predisposes to initiation and progression of CRC.
There is little evidence for a mechanistic relation between cysteine and inflammation or CRC. Cysteine is a precursor for glutathione, a key intracellular antioxidant. Oxidative stress is an important cause of inflammation and may contribute to the development of CRC. Low intracellular cysteine may lead to low intracellular glutathione and consequently may increase susceptibility to oxidative stress and inflammation. This hypothesis remains to be explored.
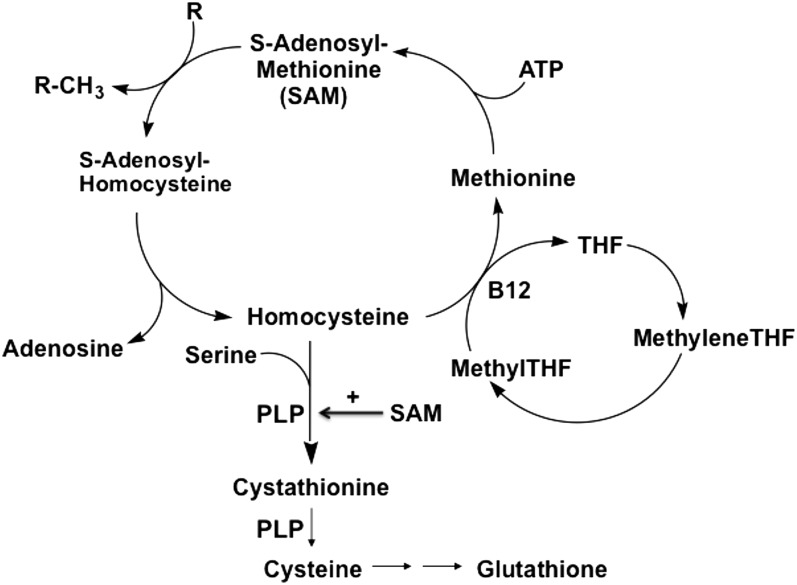
Alternatively, homocysteine and cysteine may be biomarkers of low B vitamin status. As shown in Figure 1, folate, vitamin B-12, and vitamin B-6 participate in the remethylation of homocysteine to form methionine and the catabolism of homocysteine to form cystathionine and then cysteine. Deficiencies of folate and vitamin B-12 cause a primary block in homocysteine remethylation and a secondary block in homocysteine catabolism because of reduced synthesis of S-adenosylmethionine, which is an allosteric activator of the enzyme that initiates homocysteine catabolism, cystathionine β-synthase (27). Because vitamin B-6 (in the form of PLP) serves as a cofactor for the conversion of homocysteine to cystathionine and then cysteine, deficiency of vitamin B-6 directly inhibits homocysteine catabolism (27). Thus, deficiencies of folate, vitamin B-12, and vitamin B-6 are associated with elevated homocysteine and reduced cysteine. Notably, high homocysteine and low cysteine were associated with increased risk of CRC in the WHI-OS cohort. B vitamin deficiencies, particularly folate and vitamin B-6, have been implicated in CRC risk (15–18). Assessments of associations between the B vitamins and CRC risk in the WHI-OS cohort are in progress and will be reported separately. However, it should be noted that the observed associations between homocysteine, cysteine, and CRC were independent of folate, vitamin B-12, and PLP in the multivariate analyses (Tables 2 and 3).
FIGURE 1.
Homocysteine metabolism. Folate, in the form of methylTHF, and vitamin B-12 serve as substrate and cofactor, respectively, in the remethylation of homocysteine to form methionine and SAM. SAM serves as an allosteric activator of cystathionine β-synthase, the enzyme that converts homocysteine to cystathionine. Vitamin B-6, in the form of PLP, serves as a cofactor in the conversion of homocysteine to cystathionine and then to cysteine. Deficiencies of folate, vitamin B-12, and vitamin B-6 cause increased homocysteine and decreased cysteine (27). B12, vitamin B-12; methyleneTHF, methylenetetrahydrofolate; methylTHF, methyltetrahydrofolate; PLP, pyridoxal-5′-phosphate; R, methyl acceptor; R-CH3, methylated acceptor; SAM, S-adenosylmethionine; THF, tetrahydrofolate.
Both homocysteine and cysteine (to a lesser extent) were associated with tumors of the proximal colon. This finding is consistent with a recent finding that high folate concentration in healthy colonic tissue was associated with reduced risk of proximal adenomas but not distal adenomas (28). Proximal colon tumors are often characterized by microsatellite instability, a consequence of DNA damage and inhibition of DNA repair mechanisms. Notably, elevated homocysteine has been associated with increased microsatellite instability in CRC tumors (29). Another characteristic of proximal colon tumors is the CpG island methylator phenotype (CIMP) in which there is a high degree of gene promoter methylation and consequent gene silencing. However, in one study of CRC tumors, no association was observed between homocysteine and CIMP (30). No studies have examined the relations between cysteine and microsatellite instability or CIMP in CRC, and such data were not available in the present study.
Key strengths of this study include physician-adjudicated CRC outcomes and well-standardized specimen and data collection, as well as the large sample size, which allowed for assessments of associations between homocysteine and cysteine and subtypes of CRC. Limitations of this study include the potential for residual confounding by demographic, lifestyle, and other factors that were not collected in the Women's Health Initiative study and therefore not controlled for in the multivariate analyses. Another important limitation is that homocysteine, cysteine, and the B vitamins were only measured at one time point. Single measurements may not be reflective of long-term status and thus may dilute associations with incident CRC.
In conclusion, to our knowledge, this is the largest study to date to assess longitudinal associations between homocysteine, cysteine, and CRC risk. The results indicate that elevated homocysteine and low cysteine are risk factors for incident CRC. It remains to be determined if interventions targeted to decrease homocysteine and increase cysteine concentrations, such as B vitamin supplements, will reduce the risk of CRC.
Acknowledgments
We thank the participants of the Women's Health Initiative for their contributions, as well as the following: the Program Office at the National Heart, Lung, and Blood Institute, Bethesda, MD (Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller); the Clinical Coordinating Center at Fred Hutchinson Cancer Research Center, Seattle, WA (Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg); and the investigators and academic centers (Brigham and Women's Hospital, Harvard Medical School, Boston, MA: JoAnn E Manson; MedStar Health Research Institute/Howard University, Washington, DC: Barbara V Howard; Stanford Prevention Research Center, Stanford, CA: Marcia L Stefanick; The Ohio State University, Columbus, OH: Rebecca Jackson; University of Arizona, Tucson/Phoenix, AZ: Cynthia A Thomson; University at Buffalo, Buffalo, NY: Jean Wactawski-Wende; University of Florida, Gainesville/Jacksonville, FL: Marian Limacher; University of Iowa, Iowa City/Davenport, IA: Robert Wallace; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller; Wake Forest University School of Medicine, Winston-Salem, NC: Sally Shumaker). We also acknowledge the contribution of Katherine Howes for measurements of homocysteine, cysteine, PLP, and RBC folate and Pamela Yang for measurements of plasma folate and vitamin B-12. The WHI program is funded by the National Heart, Lung, and Blood Institute, NIH, US Department of Health and Human Services, through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
The authors’ responsibilities were as follows—CMU: designed and conducted the research; JWM, SAAB, MLN, XS, RG, and CMU: provided essential reagents or provided essential materials; ECB and YZ: analyzed data or performed statistical analysis; JWM: wrote the manuscript; JWM and CMU: had primary responsibility for final content; and all authors: interpreted results and provided critical review of manuscript drafts. All of the authors read and approved the final manuscript. The authors stated no conflicts of interest related to this study.
Footnotes
Abbreviations used: CIMP, CpG island methylator phenotype; CRC, colorectal cancer; IBD, inflammatory bowel disease; PLP, pyridoxal-5′-phosphate; RBC, red blood cell; WHI-OS, Women's Health Initiative Observational Study.
REFERENCES
- 1.Slattery ML, Fitzpatrick FA. Convergence of hormones, inflammation, and energy-related factors: a novel pathway of cancer etiology. Cancer Prev Res (Phila) 2009;2:922–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 2006;6:130–40 [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E. Epidemiological studies of folate and colorectal neoplasia: a review. J Nutr 2002;132:2350S–5S [DOI] [PubMed] [Google Scholar]
- 4.Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med 1998;49:31–62 [DOI] [PubMed] [Google Scholar]
- 5.McCully KS. Chemical pathology of homocysteine. IV. Excitotoxicity, oxidative stress, endothelial dysfunction, and inflammation. Ann Clin Lab Sci 2009;39:219–32 [PubMed] [Google Scholar]
- 6.Shai I, Stampfer MJ, Ma J. Homocysteine as a risk factor for coronary heart diseases and its association with inflammatory biomarkers, lipids and dietary factors. Atherosclerosis 2004;177:375–81 [DOI] [PubMed] [Google Scholar]
- 7.Peyrin-Biroulet L, Rodriguez-Guéant RM, Chamaillard M, Desreumaux P, Xia B, Bronowicki JP, Bigard MA, Guéant JL. Vascular and cellular stress in inflammatory bowel disease: revisiting the role of homocysteine. Am J Gastroenterol 2007;102:1108–15 [DOI] [PubMed] [Google Scholar]
- 8.Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, Laghi Pasini F. Hyperhomocysteinemia, inflammation, and autoimmunity. Autoimmun Rev 2007;6:503–9 [DOI] [PubMed] [Google Scholar]
- 9.Danese S, Sgambato A, Papa A, Scaldaferri F, Pola R, Sans M, Lovecchio M, Gasbarrini G, Cittadini A, Gasbarrini A. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am J Gastroenterol 2005;100:886–95 [DOI] [PubMed] [Google Scholar]
- 10.El-Khairy L, Ueland PM, Refsum H, Graham IM, Vollset SE. Plasma total cysteine as a risk factor for vascular disease: the European Concerted Action Project. Circulation 2001;103:2544–9 [DOI] [PubMed] [Google Scholar]
- 11.Levine AJ, Grau MV, Mott LA, Ueland PM, Baron JA. Baseline plasma total homocysteine and adenoma recurrence: results from a double blind randomized clinical trial of aspirin and folate supplementation. Cancer Epidemiol Biomarkers Prev 2010;19:2541–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez ME, Henning SM, Alberts DS. Folate and colorectal neoplasia: relation between plasma and dietary markers of folate and adenoma recurrence. Am J Clin Nutr 2004;79:691–7 [DOI] [PubMed] [Google Scholar]
- 13.Martínez ME, Giovannucci E, Jiang R, Henning SM, Jacobs ET, Thompson P, Smith-Warner SA, Alberts DS. Folate fortification, plasma folate, homocysteine and colorectal adenoma recurrence. Int J Cancer 2006;119:1440–6 [DOI] [PubMed] [Google Scholar]
- 14.Bobe G, Murphy G, Rogers CJ, Hance KW, Albert PS, Laiyemo AO, Sansbury LB, Lanza E, Schatzkin A, Cross AJ. Serum adiponectin, leptin, C-peptide, homocysteine, and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer Epidemiol Biomarkers Prev 2010;19:1441–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Guelpen B, Hultdin J, Johansson I, Hallmans G, Stenling R, Riboli E, Winkvist A, Palmqvist R. Low folate levels may protect against colorectal cancer. Gut 2006;55:1461–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstein SJ, Albanes D, Selhub J, Graubard B, Lim U, Taylor PR, Virtamo J, Stolzenberg-Solomon R. One-carbon metabolism biomarkers and risk of colon and rectal cancers. Cancer Epidemiol Biomarkers Prev 2008;17:3233–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Marchand L, White KK, Nomura AM, Wilkens LR, Selhub JS, Tiirikainen M, Goodman MT, Murphy SP, Henderson BE, Kolonel LN. Plasma levels of B vitamins and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 2009;18:2195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JE, Li H, Giovannucci E, Lee IM, Selhub J, Stampfer M, Ma J. Prospective study of plasma vitamin B6 and risk of colorectal cancer in men. Cancer Epidemiol Biomarkers Prev 2009;18:1197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- 20.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003;13:S107–21 [DOI] [PubMed] [Google Scholar]
- 21.Gilfix BM, Blank DW, Rosenblatt DS. Novel reductant for determination of total plasma homocysteine. Clin Chem 1997;43:687–8 [PubMed] [Google Scholar]
- 22.Talwar D, Quasim T, McMillan DC, Kinsella J, Williamson C, O'Reilly DS. Optimisation and validation of a sensitive high-performance liquid chromatography assay for routine measurement of pyridoxal 5-phosphate in human plasma and red cells using pre-column semicarbazide derivatisation. J Chromatogr B Analyt Technol Biomed Life Sci 2003;792:333–43 [DOI] [PubMed] [Google Scholar]
- 23.Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation 2001;103:2717–23 [DOI] [PubMed] [Google Scholar]
- 24.Au-Yeung KK, Woo CW, Sung FL, Yip JC, Siow YLOK. Hyperhomocysteinemia activates nuclear factor-kappaB in endothelial cells via oxidative stress. Circ Res 2004;94:28–36 [DOI] [PubMed] [Google Scholar]
- 25.Xu D, Neville R, Finkel T. Homocysteine accelerates endothelial cell senescence. FEBS Lett 2000;470:20–4 [DOI] [PubMed] [Google Scholar]
- 26.Ratter F, Gassner C, Shatrov V, Lehmann V. Modulation of tumor necrosis factor-alpha-mediated cytotoxicity by changes of the cellular methylation state: mechanism and in vivo relevance. Int Immunol 1999;11:519–27 [DOI] [PubMed] [Google Scholar]
- 27.Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr 1992;55:131–8 [DOI] [PubMed] [Google Scholar]
- 28.Flood A, Mason JB, Liu Z, Cash BD, Schatzkin A, Schoenfeld PS, Cross AJ. Concentration of folate in colorectal tissue biopsies predicts prevalence of adenomatous polyps. Gut 2011;60:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen LH, Lindebjerg J, Crüger DG, Brandslund I, Jakobsen A, Kolvraa S, Nielsen JN. Microsatellite instability and the association with plasma homocysteine and thymidylate synthase in colorectal cancer. Cancer Invest 2008;26:583–9 [DOI] [PubMed] [Google Scholar]
- 30.Van Guelpen B, Dahlin AM, Hultdin J, Eklöf V, Johansson I, Henriksson ML, Cullman I, Hallmans G, Palmqvist R. One-carbon metabolism and CpG island methylator phenotype status in incident colorectal cancer: a nested case-referent study. Cancer Causes Control 2010;21:557–66 [DOI] [PubMed] [Google Scholar]