Abstract
It can be argued that adolescents’ decision making is biased more by motivational factors than by cognitively driven calculations of outcome probabilities. Brain-based models, derived from structural and functional neuroimaging perspectives to account for this bias, have focused on purported differences in rates of development of motivational and regulatory-control systems. This article proposes a neurochemically based framework for understanding adolescents’ behavioral biases_and suggests that there should be an increased focus on the dopaminergic substrates of incentive motivation, which increases into adolescence and decreases thereafter. The article also discusses the manner in which this increase interacts with executive control systems in affecting self-regulation.
Keywords: adolescence, reward, cognitive control, dopamine, development
In recent years, adolescent behavior has been scrutinized from developmental, neuroscientific, and public health perspectives. One goal of this scrutiny has been to investigate decision-making processes, their mechanistic underpinnings, and differential engagement across contexts. Brain-based theories emphasize dynamics between subcortically centered neural systems that promote motivation and prefrontally guided executive networks that mediate control processes (Casey, Jones, & Hare, 2008; Ernst, Pine, & Hardin, 2006; Steinberg, 2010). Extant models (Casey et al., 2008) hypothesize differences in rates of functional development between networks.
One implication of this approach is that if the prefrontal executive control system could more rapidly catch up to the subcortical limbic-striatal systems (hypothesized to developmentally stabilize at earlier ages), behavior might be better regulated. Thus, there is an implicit assumption that the control system can handle any demand imposed upon it. However, systems that mediate working memory and other control functions can become overloaded due to capacity constraints (Callicott et al., 1999; Yun, Krystal, & Mathalon, 2010) or depleted when loads exceed an optimal range (Baumeister, Brazlavsky, Muraven, & Tice, 1998), with negative impacts on efficiency. When evaluating successes or failures of self-regulation, one must consider not only the individual’s capacity but also the level of demand at a given instance. The primary thesis of this discussion is that during adolescence, the capacity for control is adultlike, but demand is high, exceeding levels typically present during other developmental stages. This high demand, which we believe to be normative, taxes the control system and impedes self-regulation. We propose that the source of the high demand lies in a dopamine-driven developmental increase in incentive motivation, which serves a number of adaptive functions. Our goal here is to suggest that the mechanistic underpinnings of this increase should be a focus of investigation, which would represent a paradigm shift given the field’s focus on control capacities in explaining adolescent behavior.
An accumulation of evidence suggests an increase in incentive motivation from childhood to adolescence and a decline from adolescence into adulthood (Luciana, Wahlstrom, Porter, & Collins, 2012; Steinberg, 2010). Few brain-based developmental processes can account for this quadratic pattern. We have presented mechanistic accounts of how this patterning may be modulated by normative developmental changes in dopamine activity (Luciana et al., 2012; Wahlstrom, White, Collins, & Luciana, 2010). Here, we will briefly outline evidence for this increase, describing how adolescent-specific changes in the dopamine system could account for it. Implications for self-regulatory capacity are discussed in terms of the impact of increased incentive motivation on the control system’s load burden in contexts where prospects for reward are high.
INCENTIVE MOTIVATION DEFINED
Incentive motivation (IM) refers to the energizing of instrumental behavior—that is, to the vigor and rate of responding--by anticipated reward acquisition (Depue & Collins, 1999; Niv, Daw, Joel, & Dayan, 2007). High-incentive states are characterized by positive attentional biases, approach-related motor behavior, and subjective states of anticipatory engagement, especially under novel conditions (Depue & Collins, 1999; Segerstrom, 2001; Vollstadt-Klein, Loeber, Richter, Kirsch, Bach et al., 2011). Approach to, rather than avoidance of, novelty reflects positive motivation (Beckmann, Marusich, Gipson, & Bardo, 2011; Powell, Geyer, Gallagher, & Paulus, 2004). Importantly, this motivation serves a critical adaptive function, given that novel contexts serve as learning environments for immediate reward acquisition and long-term goal formation. IM is distinct from sensation-seeking in that the latter construct is predominantly arousal-based and valence-free (Zuckerman, 1994).
IM is neurally mediated by circuitry that includes the midbrain tegmental region, the striatum, core limbic regions such as the extended amygdala, the anterior cingulate, and medial regions of the orbitofrontal cortex (Depue & Collins, 1999). Numerous animal, human pharmacological, and molecular genetic studies indicate that dopamine (DA) modulates activity within this circuitry to promote IM (Depue & Collins, 1999; Koob & Volkow, 2010). Moreover, variations in human DA function underlie individual differences in IM-related traits (Dreher, Kohn, Kolanchana, Weinberger, & Berman, 2009; Wacker, Chavanon, & Stemmler, 2006; Zald, Cowan, Ricccardi, Baldwin, Ansari, Shelby, et al., 2008).
The Value of an Adolescent Increase in Incentive Motivation
An adolescent-limited increase in IM is a positive development that may confer evolutionary advantages and that serves ultimately--through the promotion of learning--to encourage a sense of agency as youth make the transition to adult independence (Spear, 2000; Wahlstrom et al., 2010). Given that young adolescents lack experience, there needs to be a means of assuring exposure to unfamiliar contexts so that outcomes associated with consolidated learning will become available to guide future behavior. Thus, heightened IM serves as a starting point to direct behavior toward novel contexts. Then, with repeated experience in such contexts (e.g., in the course of instrumental learning), adolescents can develop expectations regarding reward value and calculate risk/benefit trade-offs based on this experience.
CHANGES IN INCENTIVE MOTIVATION FROM CHILDHOOD TO ADULTHOOD
Substance use begins in adolescence (Eaton, Kann, Kinchen, Shanklin, Ross, et al., 2010), as does sexual experimentation (Hawes, Wellings, & Stephenson, 2010). The pursuit of friendships with new groups and explorations of novel clothing, foods, music, and spiritual beliefs increase (Czikszentmihalyi, Larson, & Prescott, 1977; King & Roeser, 2009; Zillman & Gan, 1997). Openness to experience, relative to other major personality traits, increases dramatically between ages 12 to 16 (McCrae et al., 2002), driven primarily by an increased appreciation of aesthetics and alternative value systems. Within the confines of their controlled environments, adolescent animals are similarly drawn to novelty (Douglas, Varlinskaya & Spear, 2003; Stansfield & Kirstein, 2006).
Moreover, human adolescents self-report greater reward responsiveness than do adults (Urosevic, Collins, Muetzel, Lim, & Luciana, 2012. Self-reported sensation-seeking, incorporating aspects of IM, increases from early to middle adolescence before declining into adulthood, as evidenced by cross-sectional (Steinberg et al., 2010) and longitudinal (Harden & Tucker-Drob, 2011) work. Laboratory paradigms indicate adolescents’ preferences for immediate versus long-term rewards (Olson, Collins, Hooper, Muetzel, Lim, & Luciana, 2009; Steinberg, Graham, O’Brien, Woolard, Cauffman, & Banich, 2009).
Examinations of probabilistic learning from positive and negative feedback suggest age-related changes in the processing of predicted rewards (Cohen, Asarnow, Sabb, Bilder, Bookheimer, Knowlton, & Poldrack 2010). Functional MRI studies provide limited supportive evidence that, relative to children, adolescents show increased responses in regions of the brain’s reward system when they anticipate or receive rewards or view rewarding stimuli (Cohen et al., 2010; Galvan, Hare, Parra, Penn, Voss, et al., 2006; Somerville, Hare, & Casey, 2010; van Leijenhorst, Zannoli, VanMeel, Westerberg, Rombouts, & Crone, 2010). This literature is complicated, though, by paradigmatic differences across studies and failures to find age-related differences, as well as by some observed effects in the nonpredicted direction (Bjork, Knutson, Fong, Caggiano, Bennett, & Homer, 2004; Bjork, Smith, Chen, & Hommer, 2010; May, Delgado, Dahl, Stenger, Ryan, Fiez, & Carter, 2004).
Finally, IM declines after adolescence, as indicated by animal evidence (Spear, 2000), as well as by behavioral self-reports (Harden & Tucker-Drob, 2011; Steinberg et al., 2009; Urosevic et al., 2012 and by the aforementioned fMRI studies, which demonstrate decreased activity in reward structures, such as the nucleus accumbens, when adults anticipate rewards and when they receive feedback about wins versus losses. Together, these lines of evidence support the idea that a quadratic function may exist on behavioral and biological levels with respect to incentive motivated behavior between childhood and adulthood.
Adolescent Changes in the Dopamine System
A role for dopamine in the facilitation of IM is strongly supported (Berridge & Robinson, 1998; Depue & Collins, 1999; Schultz, Tremblay, & Hollerman, 2000). Against the backdrop of changes in cortical structure that occur during adolescence (Asato, Terwilliger, Woo, & Luna, 2010; Gogtay, Giedd, Lusk, Hayashi, Greenstein, et al., 2004; Schmithorst & Yuan, 2010), as well as during other developmental periods (Bäckman, Nyberg, Lindenberger, Li, & Farde, 2006), neurochemical systems also change. This evidence has been reviewed extensively with respect to dopamine (Ernst et al., 2009; Luciana et al., 2012 Spear, 2011; Wahlstrom et al., 2010).
During adolescence, there are peaks in DA tissue concentrations (Andersen, Dumont, & Teicher, 1997), alterations in DA transporter density (Coulter, Happe, & Murrin, 1996; Moll et al., 2000), and changes in densities of both D1 and D2 receptor subtypes in the striatum and prefrontal cortex, which decline from levels observed in early puberty (Andersen, Thompson, Krenzel, & Teicher, 2002; Seeman, Bzowej, Guan, Bergeron, Becker, & Reynolds, 1987; Tarazi, Tomasini, & Baldessarini, 1998).
An important feature of dopamine activity concerns its cells’ modes of firing, termed tonic and phasic (Grace & Bunney, 1984a,b). Tonic (basal) firing represents the summated firing rate of all DA neurons that are active at a given time. It is slow, low-amplitude, and regular in rate, yielding relatively low extracellular dopamine concentrations. Extracellular dopamine is further regulated by dopamine transporter activity and reflects amounts of the transmitter that have dispersed from release sites and from postsynaptic targets. Tonic DA levels are largely independent of environmental triggers and may index an individual’s level of incentive-related drive (Niv et al, 2007; Willuhn, Wanat, Clark, & Phillips, 2010).
Conversely, phasic firing is characterized by high-amplitude bursts of activity, resulting in high synaptic DA concentrations. Phasic firing occurs in response to salient events, particularly during reward learning (Schulz et al., 2000, Willuhn, et al. 2010). A differential between tonic and phasic signal amplitudes (a high signal-to-noise ratio or a low noise-to-signal ratio) permits an organism to detect the phasic signal. If tonic levels are high, environmental cues must be salient (high in reward value) to promote learning. Phasic bursts are large when an organism encounters novel rewarding stimuli (Schultz et al., 2000) or when cues during learning indicate the possibility of a valued reward when reward delivery is uncertain. When reward delivery is fully predictable from experience, phasic firing declines, indicating that learning is established (Schultz et al., 2000).
Importantly, then, there are distinct substrates within the dopamine system for the generation of IM outside of learning and then for the influence of this motivational state on learned behavior. Interactions between the tonic and phasic modes are not well understood. One view is that there is a reciprocal relationship whereby phasic activity is high when tonic activity is low (Goto, Otani, & Grace, 2007). Another is that the modes enable each other. For instance, repeated phasic firings during learning could summate to elevate tonic levels (Niv et al., 2007).
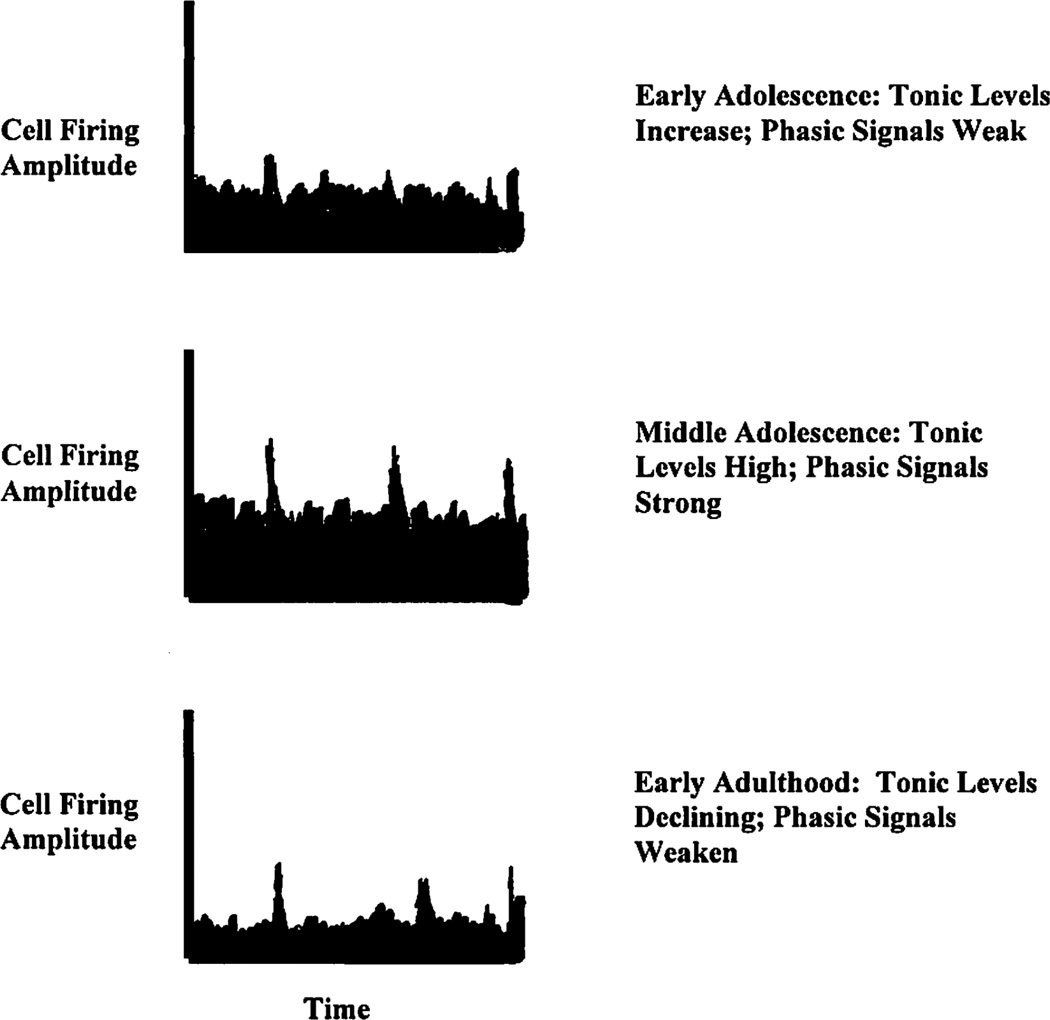
Perhaps adolescence is characterized by such enabling (see Figure 1). We hypothesize that tonic DA, triggered by genetic regulatory factors, increases during early adolescence to establish high levels of motivated approach. In the context of this elevated tonic level, learning signals (phasic signals of reward) are weakly or inconsistently detected (Robinson, Zitzman, Smith, & Spear, 2011; see Figure 1, top). In general, the elevated tone implies that rewards will need to be high in magnitude and that learning experiences will need to be particularly salient (in generating burst signals) to achieve optimal cue-reward associations and to subsequently guide behavior. Thus, adolescents may experience high levels of drive, but in the context of an increased need for strong environmental stimulation to direct learning. As would be expected as a function of the increase in IM, they are drawn to both low- and high-magnitude rewards, but it is only highly salient rewards that generate effective learning signals.
Figure 1.
Illustration of proposed changes in tonic versus phasic dopamine signaling through adolescence. Tonic signals increase, reflecting increases in incentive motivation and enabling approach toward incentive contexts. As discussed in the text, phasic signals must be increasingly strong to be discriminated against the background tonic level. In adulthood, tonic levels return to the intraindividual baseline, and weaker phasic signals are able to influence reward-related learning.
With the pursuit of more salient rewards, phasic signals are increasingly amplified (Figure 1, middle). The adaptive effects of this amplification on behavior will be enabled by increasingly well-established executive abilities mediated by the continued development of frontostriatal connectivity (Asato et al., 2010; Gogtay et al., 2004). For instance, when the medial orbitofrontal cortex receives outcome-related signals during reward learning, a calculation of the outcome’s expected value occurs (Tobler et al., 2007). This calculation allows the individual to make contingency-based decisions. Thus, as the prefrontal cortex attains full maturation in its own right late in adolescence, the individual is best able to benefit, as a function of newly gained experience, from the neurochemically generated learning signals (phasic DA responses).
Finally, as learning is consolidated with respect to external sources of positive reinforcement, phasic dopamine signals decline in amplitude (Figure 1, bottom). This overall decline may serve, over time, to dampen the system’s tonic background state, contributing to a relative (but still normative) decrease in IM in adulthood. In adulthood, more moderate phasic signals may be sufficient to guide behavior given lower background tonic levels.
Supportive evidence for this proposed interplay between tonic and phasic modes of DA activity and effects on behavior relies entirely on animal and computational models (Niv et al., 2007; Willuhn, 2010). Developmental theory has not been incorporated into these approaches. Accordingly, this account is speculative.
IMPLICATIONS FOR EXECUTIVE CONTROL AND THE ACHIEVEMENT OF SELF-REGULATION
If increases in tonic DA activity during adolescence result in increased IM, there are important implications for executive control and, ultimately, self-regulation. Through interactions with prefrontal circuitry, increased tonic DA alters the efficiency and flexibility of control processes (Goto et al., 2007). Executive control refers generally to processes such as working memory, inhibitory control, and flexibility that can be adaptively engaged to meet the demands of particular contexts. Self-regulation is broader in scope, defined in terms of the ability to achieve control over motivational impulses (Blair & Diamond, 2008). That is, self-regulation involves both the recruitment of executive control processes (available cognitive resources) and the extent to which, in any given context, internal states are actually regulated, ignored, and/or perhaps even recruited (see Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010, for example) to accomplish an adaptive goal. Thus, self-regulation is the adaptive management of a given situation’s executive load.
If arousal is high or if one is fatigued or preoccupied, then the executive burden is elevated (e.g., Killgore, 2010; Yerkes & Dodson, 1908) and regulation may be difficult. When the combination of external and internal demands is low, then regulation should be relatively effortless. Importantly, these relations hold regardless of maturation level. Even those with a fully developed (i.e., structurally intact and fully interconnected) executive control system might experience regulatory failures if information-processing demands are sufficiently high or if executive resources are depleted (Baumeister et al.,1998).
When evaluating typical adolescent behavior, the field has quite reasonably attributed self-regulatory failures to prefrontal deficiencies (e.g., Hwang et al., 2010; Hooper et al., 2004) even though executive capacity is, for the most part, adultlike (Conklin et al. 2007; Crone & van der Molen, 2004; Luna et al. 2004). Indeed, it was recently demonstrated that roughly 50% of a large sample of typically developing youth in midadolescence performed as well as adults on a composite measure of cognitive capacity (Steinberg et al., 2009). But as individuals are required to manage increasing quantities of information, the age at which that amount of information can be effectively managed increases (Luciana et al., 2005), supporting the idea that the executive control system is taxed under high demand and that the ability to meet increasing demand increases with age. Adolescents’ behaviors in motivational and psychosocial contexts often fall short of what would be expected on the basis of their otherwise strong levels of ability or available resources (Hooper et al., 2004; Steinberg et al., 2009).
Rather than focusing on the brain’s executive capacity as a source of difficulty for the typical adolescent, perhaps the research emphasis should shift toward understanding the sources of demands on the executive system. Our view is that an increase in IM occurs during adolescence, which places a high demand on the executive system, adding considerably to the system’s burden, and influencing the extent to which self-regulation can be consistently achieved.
Comprehensive validation of this model relies on assessing tonic and phasic aspects of DA neurotransmission within adolescence as experience consolidates. If tonic levels of dopamine underlie incentive-reward motivation, and if such levels increase through adolescence, there should be evidence of increased DA release, decreased DA transporter activity, increased extracellular DA concentrations, and/or decreased autoreceptor regulation (Willuhn et al., 2010; Goto et al., 2007). The variance in overall tonic function exerted by each of these parameters of the system has yet to be determined. As adolescence progresses, we might expect age-related progressions in the behavioral utility of phasic DA responses (as in Cohen et al., 2010) that occur in concert with tonic shifts. Testing these various components of the dopamine system during typical human development represents a major empirical challenge.
CONCLUSIONS, CRITICAL QUESTIONS, AND NEXT STEPS
In the field of adolescent brain development, a prominent goal over the past decade has been to characterize adolescents’ executive abilities. That work has been important in providing data in support of the continuing development of executive skills into the 20s, as well as the continuing development of the neural structures and connections that putatively support such development. A core motive for this research has been to address the issue of adolescent decision making and to consider ways in which substance abuse and other negative outcomes might be prevented. This research has revealed that adolescents can be enormously competent in their levels of executive function, but that self-regulation falters under conditions of high stress or high demand. Here we argue that the field has reached the point where a paradigm shift is needed. Rather than characterizing such situations as executive failures due to an inability to recruit sufficient cognitive resources, it should be recognized that the resources exist but that demands on the system are high.
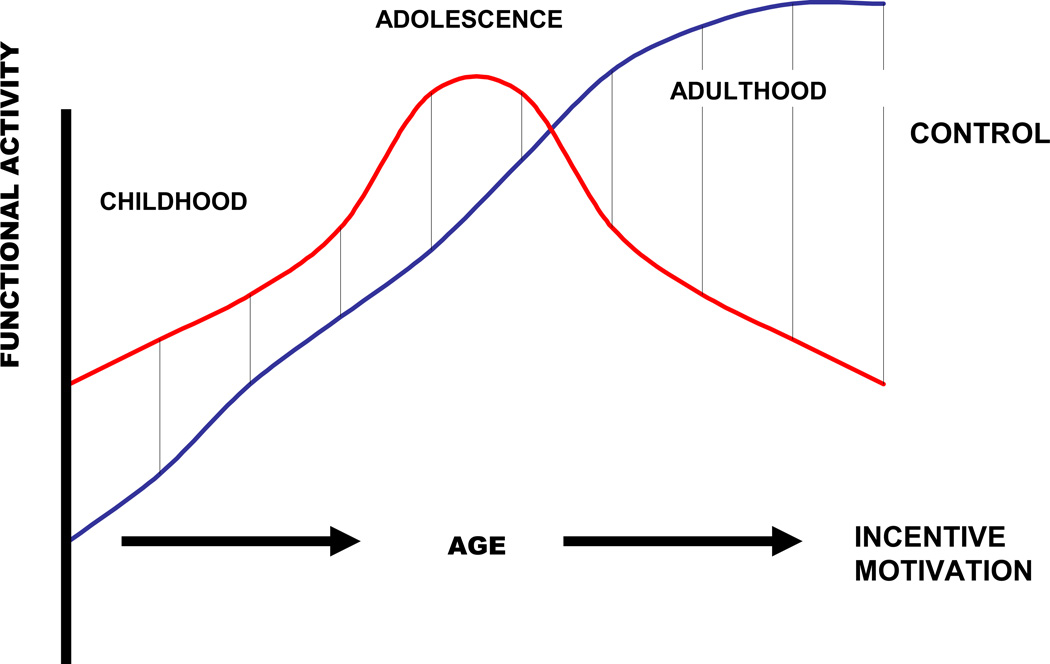
Thus, the context of such falterings should be scrutinized. Under novel conditions, the executive burden may become too strong for adolescents to manage. Critically, the same regulatory failures would occur in adulthood if the same level of incentive motivation were present. However, this is rarely the case (on average, in the typically functioning person) due to a general age-related decline in the activity of the incentive system due to declines in dopamine signaling. Thus, a quadratic function may exist with respect to incentive motivation, while a linear function may exist with respect to the development of executive control. The intersection of these functions, depicted in Figure 2, determines behavioral biases in childhood, adolescence, and adulthood, respectively. As indicated in the figure, behavior is biased toward successful control when incentive motivational drive is relatively low.
Figure 2.
Proposed interplay between executive control and incentive motivation from childhood into young adulthood. When the red line exceeds the blue line, the potential exists for the executive control system to be overloaded beyond its capacity.
Importantly, biologically determined trait-based individual differences very likely interact with these age-based trends to determine developmental outcomes (Wahlstrom et al. 2010). To assist those at high risk for psychopathology that involves reward systems, there may be a need to focus less on executive control and cognitive capacity and more on the neurochemical substrates of drive states such as incentive motivation. These substrates are potentially broader in scope and more dynamic than those emphasized within the field thus far. Moreover, that focus should incorporate the full range of development from childhood into adulthood. The inverted-U-shaped trajectory described in Figure 2 is not convincingly explained by structural brain changes, or by increasing cortical-subcortical or even whole-brain connectivity. It is potentially explained by neurochemical changes.
This framework is important in terms of the roles that parents and society play in allocating privileges to teens. A broad societal challenge is to devise ways in which adolescents can indulge their increased drive toward motor exploration and exploitation of rewarding contexts, given the potential for many adaptive consequences, without creating or perpetuating opportunities for danger or neural compromise. Substance use, for instance, affects the dopamine system’s neuroplasticity in ways that can alter subsequent development (Koob & Volkow, 2010).
A limitation of this model is, of course, that it describes the intersection of only one form of motivation (positive incentive) with executive control capacity, holding constant other sources of motivation (i.e., punishment sensitivity) that most certainly have an impact on behavior (Ernst et al., 2006). A complete account of adolescent behavior cannot be achieved without inclusion of a broader range of motivations and their interactions with regulatory control systems.
In terms of directions for further research, dopamine systems have not been directly tested in human adolescents even though the technology exists to facilitate such an assessment.. That technology includes pharmacological challenge approaches, as well as positron emission tomography (PET) imaging. Given the preponderance of animal evidence in support of neurochemical change as a substrate for adolescent-limited increases in incentive motivation, perhaps current limitations on conducting such studies in minors should be relaxed, but with appropriate regulatory monitoring, to allow the dopamine system to be directly examined in adolescents as it has been in older adults as well as patient populations (c.f., Backman et al., 2006). Until then, computational modeling of the interplay between tonic and phasic levels of dopamine (cf., Niv et al., 2007), as well as imaging genetics (Hariri, Drabant, & Weinberger, 2006) of developmental groups, might also be informative, using behavioral indices that represent each mode of action (i.e., reward prediction error versus effort-based response vigor) as guides.
To conclude, the perspective advanced here is that the field may have reached a necessary turning point in terms of which brain-behavior associations should be targets of investigation in the attempt to achieve a complete understanding of typical adolescent behavior.
Acknowledgments
The preparation of this paper was supported by grant DA017843 awarded by the National Institute on Drug Abuse to M. Luciana.
References
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+)-7-OH-DPAT. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1997;356:173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: A DTI Study. Cerebral Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience and Biobehavioral Reviews. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behavioural Brain Research. 2011;216:159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research: Brain Research Reviews. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Homer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. The Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, Adults and Rewards: Comparing Motivational Neurocircuitry Recruitment Using fMRI. PLoS ONE. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology. 2008;20:899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cerebral Cortex. 1999;9(1):20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;Volume: 13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin HH, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: Behavioral evidence of protracted frontal lobe development. Developmental Neuropsychology. 2007;31(1):103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, Murrin LC. Postnatal development of the dopamine transporter: a quantitative autoradiographic study. Developmental Brain Research. 1996;92:172–181. doi: 10.1016/0165-3806(96)00004-1. [DOI] [PubMed] [Google Scholar]
- Crone EA, Van der Molen MW. Developmental changes in real-life decision-making: performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Developmental Neuropsychology. 2004;25:251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. Journal of Youth and Adolescence. 1977;6:281–294. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana P, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, et al. Youth Risk Behavior Surveillance—United States, 2009. Morbidity and Mortality Weekly Report. 2010;59:SS-5. [PubMed] [Google Scholar]
- Ernst M, Pine D, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Galván A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in Adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 1984a;4(11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J. Neurosci. 1984b;4(11):2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropsychopharmacology. 2007;53(5):583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59(10):888–897. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Harden KP, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsivity during adolescence: Further evidence for a dual systems model. Developmental Psychology. 2011;47:739–746. doi: 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- Hawes Z, Wellings K, Stephenson J. First heterosexual intercourse in the United Kingdom: A review of the literature. Journal of Sex Research. 2010;47(2&3):137–152. doi: 10.1080/00224490903509399. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: Implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. Journal of Neuroscience. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- King PD, Roeser RW. Religion and spirituality in adolescent development. In: Lerner RM, Steinberg LS, editors. Handbook of Adolescent Psychology. 3rd Edition. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- Koob GF, Volkow N. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luciana M, Wahlstrom D, Porter JN, Collins PF. Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Developmental Psychology. 2012;Vol 48(3):844–861. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5) doi: 10.1111/j.1467-8624.2004.00745.x. 13571372. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT, Jr, Terracciano A, Parker WD, Mills CJ, De Fruyt F, Mervielde I. Personality trait development from age 12 to age 18: longitudinal, cross-sectional, and cross-cultural analyses. J. Pers. Soc. Psychol. 2002;83(6):1456–1468. [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Developmental Brain Research. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: Opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in adolescents: a diffusion tensor imaging study. Journal of Cognitive Neuroscience. 2009;21(7):1401–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Geyer MA, Gallagher D, Paulus MP. The balance between approach and avoidance behaviors in a novel object exploration paradigm in mice. Behavioural Brain Research. 2004;152:341–349. doi: 10.1016/j.bbr.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Zitzman DL, Smith KJ, Spear LP. Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience. 2011;176:296–307. doi: 10.1016/j.neuroscience.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan WY. White matter development during adolescence as shown by diffusion MRI. Brain & Cognition. 2010;72(1):16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10(3):272–283. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Optimism and attentional bias for negative and positive stimuli. Personality and Social Psychology Bulletin. 2001;27(10):1334–1343. [Google Scholar]
- Somerville LH, Hare TA, Casey BJ. Frontostriatal maturation predicts behavioral regulation failures to appetitive cues in adolescence. J. Cogn. Neuroscience. 2010;23(9):2103–2114. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Reward, aversions and affect in adolescence: Emerging controversies across laboratory, animal and human data. Developmental Cognitive Neuroscience. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Developmental Psychobiology. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparisons with D2-like receptors. Developmental Brain Research. 1998;110:227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schulz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. Journal of Neurophysiology. 2007;97:1621–1632. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel RM, Lim KO, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental Psychology. 2012 doi: 10.1037/a0027502. advance on-line publication: [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, Hermann D, Mann K, Kiefer F. Validating incentive salience with functional magnetic resonance imaging: Association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addiction Biology. 2011 doi: 10.1111/j.1369-1600.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- Wacker J, Chavanon M, Stemmler G. Investigating the dopaminergic basis of extraversion in humans: A multilevel approach. Journal of Personality and Social Psychology. 2006;91(1):171–187. doi: 10.1037/0022-3514.91.1.171. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Collins PF, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: Behavioral implications and issues in assessment. Brain & Cognition. 2010;72:146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark MJ, Phillips PEM. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. In: Self DW, Staley JK, editors. Behavioral Neuroscience of Drug Addiction, Current Topics in Behavioral Neurosciences. Vol. 3. 2010. pp. 29–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18:459–482. [Google Scholar]
- Yun RJ, Krystal JH, Mathalon DH. Working memory overload: fronto-limbic interactions and effects on subsequent working memory function. Brain Imaging Behav. 2010;4(1):96–108. doi: 10.1007/s11682-010-9089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain dopamine receptor availability Is inversely associated with novelty-seeking traits in humans. The Journal of Neuroscience. 2008;28(53):14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillmann D, Gan S. Musical taste in adolescence. In: Hargreaves DJ, North A, editors. The social psychology of music. Oxford, England UK: Oxford University Press; 1997. pp. 161–187. [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge: Cambridge University Press; 1994. [Google Scholar]