Abstract
Purpose
To review 15 years of activities in ovarian tissue cryobanking from medical database files, including patient indications, histological evaluation and clinical characteristics.
Methods
Retrospective longitudinal analysis of data from an ovarian tissue bank in an academic hospital. Five hundred and eighty-two patients had their ovarian tissue cryobanked between April 1997 and January 2012. Analysis of cryobanking database: precryopreservation patient characteristics, indications and safety issues, laboratory files and postcryopreservation clinical data.
Results
Of the 582 patients who had their ovarian tissue cryopreserved, 106 patients donated for research purposes and 476 patients for fertility preservation and long-term cryopreservation. Clinical data analysis of the 476 patients revealed a mean age at the time of cryopreservation of 23 ± 8.5 years (range: 9 months – 39 years), with 96.2 % of subjects aged ≤35 years (n = 458). Among 391 cases of malignant disease, hematological malignancies (39.9 %, n = 156) and breast cancer (21.7 %, n = 85) were the two main indications. At histology, malignant cells were found in ovarian tissue from leukemia patients (n = 3) and non-Hodgkin’s lymphoma patients (n = 2). Eleven patients underwent autotransplantation, resulting in 5 live births and 1 ongoing pregnancy.
Conclusion
This is the largest and most comprehensive study to describe and analyze indications and clinical patient characteristics before and after ovarian tissue cryopreservation. The procedure is safe, easy and promising. The database concept is a useful tool in patient selection for autotransplantation.
Keywords: Chemotherapy, Cancer, Premature ovarian failure, Ovarian tissue cryopreservation, Database, Malignant cells, Cryobank
Introduction
In the United States, previous reports estimated the incidence of all types of cancer to be around 700,000 cases/year among the female population. Pooling all types together, 2 % of women aged under 40 years will suffer from cancer. Although cancer remains the second cause of death in women below 40 years of age, its mortality rate is decreasing year upon year thanks to advances in early diagnosis and treatment [28].
However, surviving a malignant disease can leave the patient with late side effects like endocrine disorders and premature ovarian failure (POF), resulting in infertility and psychological distress [40]. It was reported that about one in every six survivors will experience POF [30]. The risk of fertility impairment increases with patient age and is associated with the type, dose and duration of chemotherapy and radiotherapy administered (for review, see [15]). One of the most potentially gonadotoxic agents is cyclophosphamide, often used in lymphoma treatments and prior to bone marrow transplantation (BMT) [36, 61]. For these reasons, fertility preservation has become a priority for patients requiring aggressive treatment for cancer or benign disease [15, 31].
To preserve fertility when gonadotoxic treatment is planned, ovarian tissue cryopreservation (OTC) is proposed to prepubertal girls, patients without a partner or patients unable to undergo ovarian stimulation due to lack of time. Promising outcomes have been reported after orthotopic reimplantation in the pelvic cavity. The first human birth obtained from frozen-thawed ovarian tissue transplantation was achieved by our team in 2004 [14]. Since then, 22 human births have ensued after orthotopic transplantation procedures. The pregnancy rate after autografting of cryopreserved tissue to orthotopic sites is estimated to be about 30 % [4, 8, 10, 18–21, 35, 41, 44, 45, 48, 51, 56–59]. This is only an estimate, as the precise denominator is so far unknown.
In our department, young patients at risk of POF have been offered the possibility to cryobank ovarian tissue since 1997. To allow traceability as required by EU directives for tissue banking, we set up a computerized database. In addition to meeting the requirements of these directives, it also has the advantage of being a simple tool for data analysis in retrospective studies, for updating registers and for patient selection and analysis before autotransplantation.
This article describes our 15 years of experience in OTC, from patient selection prior to OTC to postcryopreservation and autotransplantation outcomes.
Materials and methods
Ovarian tissue cryopreservation procedure
Patient enrolment for OTC
Since 1997, both oncological and non-oncological patients have been referred to our department to discuss possible fertility preservation options. Ovarian tissue cryobanking was approved by the Institutional Review Board of the Université Catholique de Louvain. Every patient situation was evaluated individually according to age, partner status, and time before starting chemotherapy, and the discussion was constantly adapted taking into account worldwide advances and promising results of new techniques. As far as we are concerned, when patients are referred to us to discuss fertility preservation, we are inclined to propose it even when the risk of POF is estimated to be low or medium. Jadoul et al. reported the largest series of ovarian tissue cryopreservation in girls under 16 years of age. When classified according to Wallace’s list of risk factors for fertility impairment [61], 14 patients were found to be at low risk, 32 at medium risk and 4 at high risk of POF. But Jadoul et al. demonstrated that it is difficult to give the patient or her parents an accurate assessment of the risk to fertility, as disease evolution is never completely predictable. Indeed, more or less 20 % of girls changed categories from low/medium to high risk because BMT was needed or because further gonadotoxic drugs were required for disease recurrence. Moreover, persistent ovarian function does not exclude the risk of subsequent POF [26]. In all cases, patients gave their informed consent when aged over 18 years. In case of minors, written consent was sought from parents or guardians. According to the patient situation and latest worldwide results, oral information was given about gonadotoxic treatment side effects and the different methods available to preserve fertility, with their advantages, drawbacks and potential risks. This way, free and enlightened consent was systematically obtained. The consent form also informed patients that tissue would not be used after 42 years of age and, in this case or if the patient should die, it may be used for research purposes.
Human immunodeficiency virus (HIV), hepatitis B and C and syphilis serologies all need to be negative as a prerequisite for OTC in our department. In addition, on the day of cryobanking, nucleic acid testing/polymerase chain reaction (NAT PCR) are performed for HIV and hepatitis B and C as a legal obligation before long-term ovarian tissue banking, to avoid potential cross-contamination during storage. While awaiting PCR results, the frozen fragments are kept in quarantine in a separate tank.
Freezing procedure
Ovarian tissue collection and cryobanking were performed according to the standard procedure applied in our center [17]. Biopsy samples were immediately transferred from the operating room to the clean room in Universal IVF Medium (Medicult ref 1030, Vreeland, the Netherlands) at 4 °C. The entire ovarian tissue treatment procedure was carried out in the clean room under a laminar flow hood, on a cold plate maintained at 2-8 °C. The biopsy was transferred to Leibovitz medium (Gibco ref 31415–029, Paisley, Scotland, UK). The medullar part was removed using surgical scissors and the ovarian cortical tissue was dissected into small strips of 5-6x2x1mm and large strips of 10x5x1mm. These fragments were suspended in cryoprotective medium (Leibovitz medium supplemented with 4 mg/mL of human serum albumin [CAF-DCF, Neder-Over-Heembeek, Belgium] and 1.5 mmol dimethyl sulfoxide [CryoSure-DMSO, Wak-Chemie Medical GmH, Steinbach, Germany]) in precooled cryogenic vials (Nunc ref 177280, NordicCell, Copenhagen, Denmark). The cryovials were cooled in a programmable freezer (Kryo 10, Series III; Planer, Sunbury-on-Thames, UK) with the following program: (1) cooled from 0 °C to −8 °C at −2 °C/min; (2) seeded manually by touching the cryotubes with forceps prechilled in liquid nitrogen; (3) cooled to −40 °C at −0.3 °C/min; (4) cooled to −150 °C at −30 °C/min; and (5) transferred to liquid nitrogen (−196 °C). The cryovials were then inserted into Cryoflex tubing (Nunc) to ensure double sealing for long-term storage in liquid nitrogen.
For thawing, the cryogenic vials containing the fragments were thawed at room temperature for two minutes and immersed in a water bath at 37 °C for another two minutes, then washed three times for five minutes in Leibovitz medium.
For each patient, one cortical strip (4x1x1 mm) was directly fixed in formalin for microscopic examination by an experienced pathologist to analyze tissue histology, follicular density and presence of malignant cells.
Database development
Item description
To comply with recent European regulations (Directive 2004/23/EC), a specific computerized database was created to track all patients undergoing OTC. The database was designed using Microsoft Office Access (Microsoft Windows, 2007) software and divided into three patient files containing (i) precryopreservation clinical characteristics, (ii) laboratory data and (iii) postcryopreservation clinical characteristics. Entries recorded in each file are listed in Fig. 1a. This article will describe the computerized database related only to ovarian tissue storage.
Fig. 1.
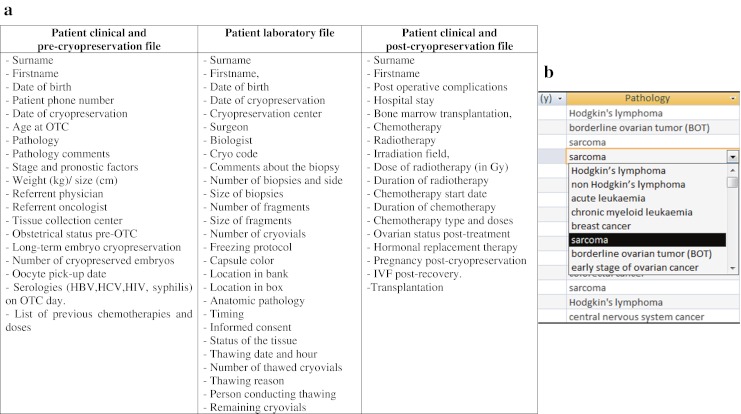
a Ovarian tissue cryopreservation database design. b Example of pre-encoded items to be selected in the OTC database, here in the context of pathology
For some fields, selection of pre-encoded items facilitated the encoding of data (Fig. 1b), e.g.
Pathology: Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, acute leukemia, chronic myeloid leukemia, breast cancer, sarcoma, borderline ovarian tumor (BOT), early-stage ovarian cancer, colorectal cancer, cervical cancer, central nervous system (CNS) cancer, benign hematologic disease, systemic disease, benign ovarian cyst, endometriosis, family history of early menopause, other.
Biologist: XX, Xx, xX.
Surgeon: XX, Xx, xX.
Tissue status: quarantined, released, destroyed.
Number of biopsies and side of collection: 1 right biopsy, 1 left biopsy, 1 right biopsy + 1 left biopsy, right biopsies, left biopsies, right biopsies + left biopsies, 1 right biopsy + left biopsies, right biopsies + 1 left biopsy, 1 left whole ovary, 1 right whole ovary.
Reason for thawing: transplantation, histology, quality control, research, death, rejected after quarantine.
For other fields, completion of pre-filled items also facilitated data registration, e.g.
Biopsy size: right: cm/cm // left: cm/cm.
Data collection and encoding
For patients who underwent OTC prior to the establishment of the computerized database, clinical data were obtained from medical records and, if necessary, by contacting referring physicians and outpatient medical centers, where appropriate. Moreover, for hematological diseases, a double-check was systematically carried out by contacting the oncologists. Laboratory fields were thus filled in retrospectively with available laboratory data. Since 2007, however, for all new patients undergoing OTC, database items have been prospectively completed. Moreover, the database design is regularly updated to comply with amendments to European directives. In order to respect patient data protection as stated in our consent forms, access to the database is secured by two passwords and restricted to members of the medical and laboratory staff involved in ovarian tissue banking.
Database analysis
The Microsoft Office Access program allowed us to create requests in order to select useful data. Furthermore, data filters were used to enable greater depth of analysis. For this study, selected criteria in our requests and data filters were age, date of cryopreservation, pathology, precryopreservation obstetric status, precryopreservation and postcryopreservation gonadotoxic treatment, and precryopreservation anatomic pathology.
For numerical data (e.g. age), standard deviation (SD) and relative risk (RR) were calculated using the SPSS program.
Results
Clinical characteristics of patients
Between April 1997 and January 2012, 582 patients underwent OTC at the Cliniques Universitaires St Luc (Université Catholique de Louvain), 476 for clinical purposes and 106 for research. Analysis of data presented in this article is focused on the clinical indications for fertility preservation.
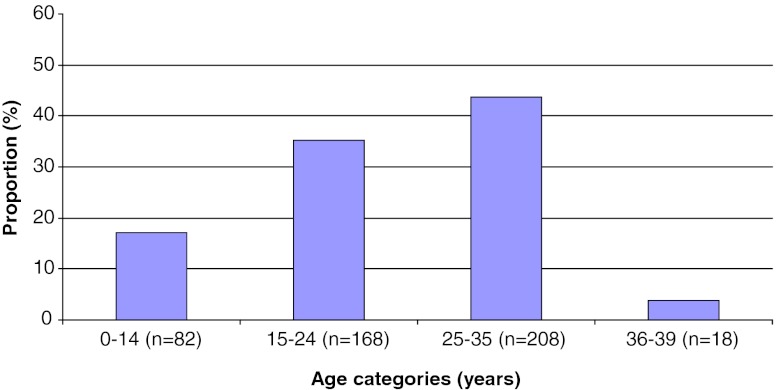
The mean age of patients at the time of cryopreservation was 23.0 ± 8.5 years (range: 9 months – 39 years). Most patients (96.2 %, n = 458) were ≤ 35 years of age. Categorization by age group (Fig. 2) revealed that 17.2 % of patients were aged between 9 months and 14 years (n = 82), 35.3 % between 15 and 24 years (n = 168), 43.7 % between 25 and 35 years (n = 208), and 3.8 % between 36 and 39 years (n = 18) at the time of OTC.
Fig. 2.
Analysis of the preclinical file of the database. Percentage of patients undergoing OTC (n = 476) by age group (in years) – 52.5 % of patients were ≤ 24 years of age and 96.2 % ≤ 35 years
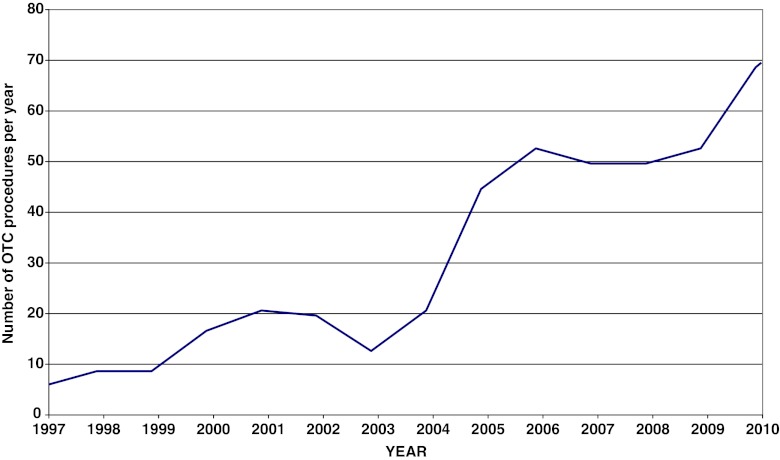
As detailed in Fig. 3, between 1997 and 2004, 13.9 ± 8.5 OTC procedures were carried out every year in our department, while by 2005, that figure had climbed substantially, reaching an average of 52.1 ± 6.8 per year in the period 2005–2011. In 2010 alone, 68 patients were offered OTC in our department.
Fig. 3.
Number of OTC procedures by year in our department
Table 1 shows that among the 476 patients in our series, 87 % (n = 414) were nulliparous at the time of OTC and 13 % (n = 62) had already given birth, with (n = 18) or without (n = 44) a history of miscarriage. In addition to OTC, cryopreserved embryos were also obtained from 28 patients with a partner in whom chemotherapy could be delayed in order to allow ovarian stimulation. In such cases, OTC was always performed prior to ovarian stimulation for IVF and embryo freezing.
Table 1.
Obstetrical status (n = 476) – 87 % of patients were nulliparous at the time of OTC. P number of deliveries, G number of gestations
| Total (100 %; N = 476) | G0 | G1 | G2 | G3 |
|---|---|---|---|---|
| P0 (87 %; N = 414) | N = 386 | N = 23 | N = 4 | N = 1 |
| P1 (10.7 %; N = 51) | N = 33 | N = 15 | N = 3 | |
| P2 (2.1 %; N = 10) | N = 9 | N = 1 | ||
| P3 (0.2 %; N = 1) | N = 1 |
Indications
Among the 476 patients who had their ovarian tissue cryopreserved, 391 underwent the procedure for malignant indications and 85 for benign indications.
Malignant diseases
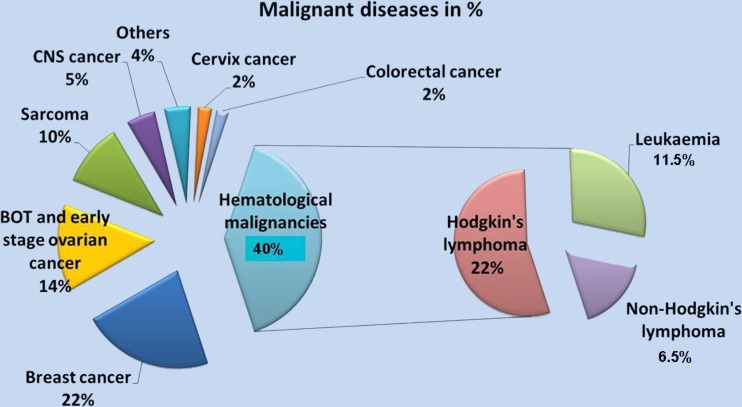
In the series of 391 patients requiring OTC for malignant disease, the two main indications were hematological malignancy (39.9 %, n = 156) and breast cancer (21.7 %, n = 85) (Fig. 4). Among the hematological malignancies, Hodgkin’s lymphoma (n = 85), non-Hodgkin’s lymphoma (n = 26) and leukemia (acute leukemia n = 39; chronic myeloid leukemia n = 6) represented respectively 21.7 %, 6.5 % and 11.5 % of all malignant indications. Other recorded malignant indications were borderline ovarian tumors (BOT) and early-stage ovarian cancer (14.3 %, n = 56), sarcoma (10.5 %, n = 41), central nervous system (CNS) cancer (4.9 %, n = 19), cervical cancer (2.3 %, n = 9), colorectal cancer (2 %, n = 8), and more rarely encountered malignant indications (4.4 %, n = 17), such as endometrial cancer (n = 1), ear/nose/throat cancer (n = 2), germinoma (n = 4), stomach cancer (n = 1), pancreatic cancer (n = 1), lung cancer (n = 1), renal cancer (n = 1), hydatiform mole (n = 3), nephroblastoma (n = 1) and carcinoma of unknown origin (n = 2).
Fig. 4.
Malignant diseases up to January 2012 in our department (n = 391). Hematological malignancies (40 %) and breast cancers (22 %) represent the two main indications for OTC
Benign diseases
Among the 85 patients undergoing OTC for benign disease because of the high risk of POF, recurrent benign ovarian cysts (43.5 %, n = 37), severe endometriosis (16.5 %, n = 14) and systemic diseases requiring gonadotoxic chemotherapy (11.8 %, n = 10) were the most frequent indications. Others were family history of early menopause (9.4 %, n = 8), Turner syndrome (3.5 %, n = 3), galactosemia (1.2 %, n = 1) and benign hematologic diseases requiring BMT (14.1 %, n = 12), namely sickle cell disease (n = 5), thalassemia major (n = 4), medullar aplasia (n = 2), and Kostmann syndrome (n = 1).
Patients with the youngest mean age at OTC were those treated for benign hematological disease (10.4 ± 8.3 years [1–20 years]).
Histological evaluation
Follicle count
A sample of tissue was routinely sent for anatomopathological analysis at the time of cryopreservation to determine follicle count. In patients who have received previous chemotherapy, we now ask an ovarian reserve evaluation by FSH on day 3 and AMH dosage in order to discuss with the patient the OTC indication.
Follicles were not found in ovarian tissue biopsies in 20 of the 476 patients, including 5 of the 15 patients with endometriosis (33 %), 4 of the 54 patients with BOT or early-stage ovarian cancer (7.4 %), 1 of the 3 patients with Turner syndrome (33 %), and 1 of the 7 patients with a family history of early menopause (14 %). The only patient with galactosemia in our series also failed to demonstrate the presence of any follicles in her ovarian cortex. For the remaining patients (n = 8), tissue sent for histological analysis was medullar tissue or a non-representative piece of cortex (small size 1x1mm, or cystic tissue).
Four hundred and forty-two patients (93 %) had never been exposed to any gonadotoxic treatment before OTC was carried out, but 34 (7 %) had already undergone chemotherapy prior to OTC. Among these 34 subjects, 13 had a history of acute leukemia, with 2 patients having already received alkylating agents. Most of the others had also been previously treated with non-alkylating agents. All these patients had undergone only 1 or 2 regimens of chemotherapy. In such cases, we inform patients that the ovarian reserve could already have been depleted and that oocyte quality may be affected [11]. However, the RR of not finding follicles in ovarian tissue samples from these patients was not significantly different from that of patients not receiving previous chemotherapy. Indeed, follicles were observed in all but two subjects (n = 32/34).
Presence of malignant cells
Malignant cells were detected at histological evaluation in 5 patients (2 with non-Hodgkin’s lymphoma and 3 with leukemia). While the risk is low, it nevertheless exists and thus warrants further investigation.
In 18 patients with acute or chronic lymphoid leukemia showing no malignant invasion of their ovarian tissue at histology, we performed PCR and xenografting on their frozen-thawed ovarian cortex. Seven of the 12 acute leukemia patients and 2 of the 6 chronic sufferers presented with positive ovarian tissue at PCR analysis (total of 9 positives out of 18), and 4 xenografted mice developed leukemic masses after 6 months, as previously reported [13].
Postcryopreservation clinical outcome
Biopsy retrieval surgery
None of the 476 patients experienced any serious surgical complications such as bowel, nerve or vascular injury, thromboembolism or cardiorespiratory distress. Minor complications such as urinary tract infection may of course occur, as for any type of laparoscopy, even diagnostic laparoscopy.
Sixty-four percent of patients were discharged the same day, while 23 % were kept for one night, mainly for preoperative check-ups associated with the primary disease. Thirteen percent of patients were hospitalized for more than 72 h because they underwent OTC at the same time as another surgical procedure (breast surgery in particular).
Reimplantation of cryopreserved ovarian tissue
Before autotransplantation, hormonal status, risk of recurrence and overall survival are always assessed in collaboration with oncologists.
Since 2003 in our department, frozen-thawed ovarian tissue has been transplanted to 11 patients with a range of diseases, including a history of malignant hematological disease (Hodgkin’s lymphoma [n = 4] and non-Hodgkin’s lymphoma [n = 1]), neuroectodermal tumor of the right orbit (n = 1), cervical neoplasia (n = 1), Wegener’s disease (n = 1), sickle cell anemia (n = 1) and bilateral ovariectomy for benign disease (n = 2). At the time of OTC, the mean age was 23.8 years (19–30 years).
Thus, eleven of our patients have already undergone ovarian tissue autotransplantation, resulting so far in five deliveries and 1 ongoing pregnancy (4 spontaneous pregnancies and 2 pregnancies after in vitro fertilization). Autotransplantation procedures, outcomes and live births were recently thoroughly detailed and reviewed [17, 19, 20, 59].
In our series, autotransplantation procedures were carried out between 42 and 86 months after OTC. Each patient was grafted with approximately 1/3 to 1/2 of her stored ovarian tissue, allowing the transplantation procedure to be repeated in the event of the patient reverting to menopausal status.
Discussion
Incidence of indications and comparison with other reports
In this article, we describe the design and outcomes of one of the largest and most comprehensive existing ovarian tissue cryobank databases. Indeed, we report a series of 476 patients undergoing OTC for fertility preservation in our department, a number similar to that reported by Rosendahl et al. [46]. Our two main indications are hematological malignancy (39.9 %, n = 156) and breast cancer (21.7 %, n = 85). As detailed in Table 2, age and indications are in the same range as those recorded by other ovarian tissue banks. Rosendahl et al. [46], Oktay and Oktem [39]. Mayerhofer et al. [32], Demeestere et al. [9], Sanchez et al. [50] and Meirow et al. [33] have all reported series including patients with hematological malignancies or breast cancer, but smaller patient numbers.
Table 2.
Series of OTC among malignant disease indications
| Reference | N | Mean age ± SD [range] (in years) | Hematological malignancies | Breast cancer | Other | |||
|---|---|---|---|---|---|---|---|---|
| GLOBAL POPULATION SERIES | Present study | N = 391 | 23.17 | 39.9 % | 21.7 % | 38.4 % | ||
| HL | NHL | Leukemia | ||||||
| 22 % | 6.5 % | 11.5 % | ||||||
| [46, 47] | N = 386 | 25.9 [0.5–39.8] | 27 % | 28 % | 45 % | |||
| [39] | N = 59 | 26 [4–44] | 46 % | 22 % | 32 % | |||
| [32] | N = 78 | 27 [14–41] | 50 % | 25.6 % | 24.4 % | |||
| HL | NHL | Leukemia | ||||||
| 28 % | 0 % | 22 % | ||||||
| [9] | N = 133 | ND | 29 % | 34 % | 37 % | |||
| [50] | N = 200 | 28.2 [11–39] | 20 % (HL) | 55 % | 25 % | |||
| [33] | N = 56 | 24 ± 5.5 | 100 % | 0 % | 0 % | |||
| HL | NHL | Leukemia | ||||||
| 59 % | 25 % | 16 % | ||||||
| PREPUBERTAL POPULATION SERIES | [25] | N = 48 | 10.4 [0.8–15.8] | 56 % | 0 % | 44 % | ||
| HL | NHL | Leukemia | ||||||
| 8 % | 19 % | 29 % | ||||||
| [5] | N = 57 | 14.4 [8–19.8] | 0 % | 0 % | 100 % | |||
| [42] | N = 45 | 6.1 [0.8–15.8] | 13 % | 0 % | 87 % | |||
| [37] | N = 20 | 11.5 [3–15] | 25 % | 0 % | 75 % | |||
ND not determined, HL Hodgkin’s lymphoma, NHL non-Hodgkin’s lymphoma
There are, however, particular issues relating to very young girls that need to be specifically addressed. Acute leukemia, Turner syndrome, benign hematological diseases, sarcomas and CNS cancers are known to occur more frequently in prepubertal girls, as we can see in our prepubertal indications. Embryo and oocyte cryopreservation clearly cannot be proposed in this instance, so OTC is the only available means of preserving fertility in these young patients [22, 25]. Differences were found between the present study and our previous study [25] on prepubertal indications, and also studies by Poirot et al. [42], Borgström et al. [5] and Michaeli et al. [37], who reported large series of prepubertal girls undergoing OTC (Table 2). Indeed, Poirot et al. [42] reported 45 patients with various malignant diseases, while Borgström et al. [5] reported 57 patients suffering exclusively from Turner syndrome. Michaeli et al. [37] also recently reported a series of 20 prepubertal patients (Table 2). Nevertheless, it is difficult to draw conclusions about the indications, as the incidence of one pathology or another is highly dependent on the referral system, and oncologists have a great responsibility in the implementation of these programs. Girls with Turner syndrome are the most difficult to counsel adequately. In these patients, evaluation of the follicular reserve and oocyte quality can be extremely problematic. The limitations are explained, but unless oocyte depletion can be clearly proven, we will not deny these patients OTC if they wish to go ahead with the procedure.
Mean age at the time of OTC remains similar among the different centers (between 26 and 28 years), while the upper age limit can reach 44 years. In our department, the mean age is 23 years, with a maximum age limit of 39 years. Most patients in our series were younger than 35 years. During our first years of cryobanking, a few older patients were included but the upper age limit was later set at 35 years. Indeed, analysis of success rates after ovarian autotransplantation suggests increased pregnancy rates in younger patients [19]. As ovarian cortex freeze-thawing and transplantation is accompanied by follicle loss [15], older patients with lower oocyte reserves may benefit more from oocyte or embryo cryopreservation. Concerning parity, the majority of our patients were nulliparous, but in case of high risk of POF, we do not believe that access to fertility preservation should be denied to women already having children.
Cryopreservation should not be reserved solely for women with malignant disease either, but also when hematopoietic stem cell transplantation (HSCT) is indicated for non-malignant disorders [16]. Other benign diseases, such as recurrent ovarian endometriosis or recurrent ovarian mucinous cysts, are also indications for OTC, even if these indications have become questionable in the light of recent studies. Severe endometriosis is known to be associated with decreased oocyte quality [6] and lower serum anti-Müllerian hormone (AMH) concentrations [55]. This corroborates one of our studies in which endometriotic ovarian cysts were described as a possible cause of reduced ovarian reserve in women with endometriosis [29]. In our study, no follicles were found in ovarian tissue in 33 % of patients with endometriosis (n = 5/15). For these reasons, the value of OTC in case of endometriosis is still a matter of debate.
Cryopreservation procedure
This procedure was initially derived from the protocol designed by Gosden et al. [24] for sheep ovarian tissue, yielding live births. It was validated by our group for human ovarian tissue, first experimentally using a murine xenotransplantation model [38], and then clinically by obtaining the first pregnancy after transplantation of cryopreserved ovarian tissue [14]. So far, slow-freezing using DMSO with or without sucrose [8, 10, 14, 35, 41, 44], propanediol/sucrose [56] or ethylene glycol/sucrose [4] as cryoprotectants are the methods of choice for cryobanking of human ovarian tissue, as these procedures have been shown to restore fertility. Despite working very hard on other protocols (vitrification) in experimental models [3], we have not gathered enough scientific data to modify the slow-freezing protocol.
Postcryopreservation follow-up
No surgical complications were observed after the OTC procedure in our department. This laparoscopic technique may be considered safe for very young patients with cancer or at high risk of POF [25, 42, 49]. In our series, the youngest patient was aged just 9 months at the time of OTC.
Our study includes 24.9 % of patients who are still +/− 25 years of age. In the future, the rate of patients undergoing transplantation (which currently stands at 2.3 %) will surely increase, as these women decide to attempt pregnancy [25].
Histology
Histological evaluation and follicle count performed on cortical tissue samples prior to cryopreservation is not a reliable method to quantify follicular density due to follicle clustering [23, 43, 53] and subsequent heterogeneity in analyzed samples [7]. Nevertheless, anatomopathological evaluation remains an essential tool, yielding an initial analysis of the presence of follicles, as well as the presence or absence of malignant cells. In order to predict ovarian follicular density and help us select the best indications for OTC, a first report on age-related distribution of basal serum AMH levels in women of reproductive age was recently published by Shebl et al. [54]. In the near future, OTC indications may be adapted to take age and serum AMH concentrations into account, even if the exact role and involvement of AMH still needs to be elucidated. Our team recently found no correlation between post-transplantation serum AMH values and pregnancy outcome in patients with grafted frozen-thawed ovarian tissue [27]. Moreover, it is not always known from the start which chemotherapy will be required, and the level of toxicity may finally be much greater than initially suspected. Indeed, disease evolution is never completely predictable. Jadoul et al. [25] described a series in which 14 % of patients initially at low or medium risk of POF changed risk category and needed more aggressive gonadotoxic treatment in the months following cryopreservation. For us, this is a crucial argument in favor of OTC, even in medium or low-risk patients. On the other hand, a large majority of girls in whom ovarian function is assessable show persistent ovarian function. However, persistent ovarian function does not exclude the risk of subsequent POF, nor does it equate with fertility.
Risk of malignant cells in cryopreserved ovarian tissue
This is the largest existing study to assess the incidence of malignant cells in ovarian tissue before cryopreservation. Indeed, according to records from our database, 1.3 % (5/391) of all cancer indications proved positive for malignant cells at light microscopy evaluation. However, more sensitive methods like PCR and xenografting are essential to exclude micrometastases in some specific diseases like leukemia. Indeed, despite normal histological evaluation, grafting of frozen-thawed ovarian tissue from leukemia patients [13, 34] carries a risk of transplanting malignant cells. In this case, isolation of primordial follicles could be carried out, as described by Dolmans et al. [12], with the ultimate goal of recreating an artificial ovary [2]. Another option to avoid the risk of malignant cell transmission is to perform in vitro maturation from primordial follicles to antral follicles, as investigated by Telfer et al. [60]. These options are always explained to leukemia patients at high risk of malignant involvement of the ovary [13]. We make it clear that approaches other than tissue transplantation need to be applied to restore their fertility. However, especially in young girls with these malignancies, we firmly believe that in the future, cryopreserved cortex will be able to be used for in vitro culture or follicle transplantation.
Breast cancer is our second most frequent indication for OTC and, so far, no malignant cells have been found in ovarian tissue from breast cancer patients by immunohistochemistry [47, 52]. Nevertheless, more sensitive approaches need to be developed to further ascertain the safety of ovarian tissue grafting in breast cancer patients.
Abir et al. also recently reported the possibility of finding malignant cells in non-hematological cancers, such as metastatic Ewing’s sarcoma [1].
In conclusion, ovarian tissue biopsy and cryopreservation constitute a safe, simple, swift and promising procedure that gives young girls and patients who cannot delay cancer treatment a chance of preserving their fertility. Autotransplantation results are also encouraging, as proved by pregnancies achieved to date, although ovarian graft lifespan still needs to be improved. Nevertheless, caution should be exercised in case of certain malignant indications.
We firmly believe it is necessary to provide general gynecologists treating patients with cancer with adequate information since, according to the existing literature, <20 % of young cancer patients are currently offered the option of ovarian tissue cryopreservation.
Acknowledgments
The present study was supported by grants from the Fonds National de la Recherche Scientifique de Belgique (grant Télévie N0 7.4507.10, grant 3.4.590.08 awarded to Marie-Madeleine Dolmans), the Fonds Spéciaux de Recherche, the Fondation Saint-Luc, the Foundation Against Cancer, and donations from Mr Pietro Ferrero, Baron Frère and Viscount Philippe de Spoelberch. We thank Mélanie Gorez and Cristina Marinescu for their help with data completion of the database.
Footnotes
Capsule
This article describes our 15 years of experience in ovarian tissue cryopreservation and cryobanking, from patient selection to postcryopreservation and autotransplantation outcomes.
References
- 1.Abir R, Feinmesser M, Yaniv I, Fisch B, Cohen IJ, Ben-Haroush A, et al. Occasional involvement of the ovary in Ewing sarcoma. Hum Reprod. 2010;25:1708–1712. doi: 10.1093/humrep/deq121. [DOI] [PubMed] [Google Scholar]
- 2.Amorim CA. Artificial ovary. In: Donnez J, Kim SS, editors. Principles and practice of fertility preservation. First Edition. Cambridge: Cambridge University Press; 2011. pp. 448–458. [Google Scholar]
- 3.Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23:160–186. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 5.Borgström B, Hreinsson J, Rasmussen C, Sheikhi M, Fried G, Keros V, et al. Fertility preservation in girls with turner syndrome: prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab. 2009;94(1):74–80. doi: 10.1210/jc.2008-0708. [DOI] [PubMed] [Google Scholar]
- 6.Brizek CL, Schlaff S, Pellegrini VA, Frank JB, Worrilow KC. Increased incidence of aberrant morphological phenotypes in human embryogenesis– an association with endometriosis. J Assist Reprod Genet. 1995;12:106–112. doi: 10.1007/BF02211378. [DOI] [PubMed] [Google Scholar]
- 7.Dath C, Van Eyck AS, Dolmans MM, Romeu L, Delle Vigne L, Donnez J, et al. Xenotransplantation of human ovarian tissue to nude mice: comparison between four grafting sites. Hum Reprod. 2010;25:1734–1743. doi: 10.1093/humrep/deq131. [DOI] [PubMed] [Google Scholar]
- 8.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 9.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15:649–665. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97:387–390. doi: 10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Dolmans MM, Demylle D, Martinez-Madrid B, Donnez J. Efficacy of in vitro fertilization after chemotherapy. Fertil Steril. 2005;83:897–901. doi: 10.1016/j.fertnstert.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Dolmans MM, Yuan WY, Camboni A, Torre A, Van Langendonckt A, Martinez-Madrid B, et al. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod Biomed Online. 2008;16:705–711. doi: 10.1016/S1472-6483(10)60485-3. [DOI] [PubMed] [Google Scholar]
- 13.Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116:2908–2914. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 14.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 15.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 16.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Restoration of ovarian function after orthotopic (intraovarian and periovarian) transplantation of cryopreserved ovarian tissue in a woman treated by bone marrrow transplantation for sickle cell anaemia: case report. Hum Reprod. 2006;21:183–188. doi: 10.1093/humrep/dei268. [DOI] [PubMed] [Google Scholar]
- 17.Donnez J, Squifflet J, Van Eyck AS, Demylle D, Jadoul P, Van Langendonckt A, et al. Restoration of ovarian function in orthotopically transplanted cryopreserved ovarian tissue: a pilot experience. Reprod Biomed Online. 2008;16:694–704. doi: 10.1016/S1472-6483(10)60484-1. [DOI] [PubMed] [Google Scholar]
- 18.Donnez J, Jadoul P, Donnez O, Squifflet J, Gilliaux S, Dolmans M-M. How to preserve fertility before chemotherapy. BJMO. 2009;3:7–15. [Google Scholar]
- 19.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43:437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 20.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98:720–725. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Ernst E, Bergholdt S, Jorgensen JS, Andersen CA. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25:1280–1281. doi: 10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- 22.Feigin E, Abir R, Fisch B, Kravarusic D, Steinberg R, Nitke S, et al. Laparoscopic ovarian tissue preservation in young patients at risk for ovarian failure as a result of chemotherapy/irradiation for primary malignancy. J Pediatr Surg. 2007;42:862–864. doi: 10.1016/j.jpedsurg.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 23.Gook DA, Edgar DH, Borg J, Archer J, McBain JC. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum Reprod. 2005;20:72–78. doi: 10.1093/humrep/deh550. [DOI] [PubMed] [Google Scholar]
- 24.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 °C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 25.Jadoul P, Dolmans MM, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. 2010;16:617–630. doi: 10.1093/humupd/dmq010. [DOI] [PubMed] [Google Scholar]
- 26.Jadoul P, Anckaert E, Dewandeleer A, Steffens M, Dolmans MM, Vermylen C, et al. Clinical and biologic evaluation of ovarian function in women treated by bone marrow transplantation for various indications during childhood or adolescence. Fertil Steril. 2011;96(1):126–133. doi: 10.1016/j.fertnstert.2011.03.108. [DOI] [PubMed] [Google Scholar]
- 27.Janse F, Donnez J, Anckaert E, de Jong FH, Fauser B, Dolmans MM. Limited value of ovarian function markers after orthotopic transplantation of ovarian tissue after gonadotoxic treatment. J Clin Endocrinol Metab. 2011;96:1136–1144. doi: 10.1210/jc.2010-2188. [DOI] [PubMed] [Google Scholar]
- 28.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 29.Kitajima M, Defrère S, Dolmans MM, Colette S, Squifflet J, Van Langendonckt A, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril. 2011;96:685–691. doi: 10.1016/j.fertnstert.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 30.Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88:5307–5314. doi: 10.1210/jc.2003-030352. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American society of clinical oncology. American society of clinical oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 32.Mayerhofer K, Ott J, Nouri K, Stoegbauer L, Fischer EM, Lipovac M, et al. Laparoscopic ovarian tissue harvesting for cryopreservation: an effective and safe procedure for fertility preservation. Eur J Obstet Gynecol Reprod Biol. 2010;152:68–72. doi: 10.1016/j.ejogrb.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Meirow D, Baum M, Rabinovici Y, Levron J, Hardan I, Schiff E, et al. Ovarian tissue cryopreservation in hematologic malignancy: 10 year’s experience. Leuk Lymphoma. 2007;48:1569–1576. doi: 10.1080/10428190701471957. [DOI] [PubMed] [Google Scholar]
- 34.Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007–1013. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- 35.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. NEJM. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 36.Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with Cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999;14:1903–1907. doi: 10.1093/humrep/14.7.1903. [DOI] [PubMed] [Google Scholar]
- 37.Michaeli J, Weintraub M, Gross E, Ginosar Y, Ravitsky V, Eizenman E, et al. Fertility preservation in girls. Obstet Gynecol Int. 2012;139193:1–10. doi: 10.1155/2012/139193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74:122–129. doi: 10.1016/S0015-0282(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 39.Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2010;93:762–768. doi: 10.1016/j.fertnstert.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Peretz NM, Goldberg H, Kuten A, Meller I, Krivoi E, Lorber A, et al. Long-term sequelae of malignant tumors in childhood: consequences of late side-effects. Harefuah. 2001;140:95–100. [PubMed] [Google Scholar]
- 41.Piver P, Amiot C, Agnani G, Pech J, Rohrlich PS, Vidal E, Aubard Y, Roux C (2009) Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue. In: 25th Annual Meeting of ESHRE, 28 June–1 July, 2009. Amsterdam, the Netherlands: Oxford University Press, Hum Reprod i15.
- 42.Poirot C, Brugières C, Genestie HM. Ovarian tissue cryopreservation for prepubertal girls: indications and feasibility. Gynécol Obstét Fertil. 2005;33:799–803. doi: 10.1016/j.gyobfe.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 43.Qu J, Godin PA, Nisolle M, Donnez J. Distribution and epidermal growth factor receptor expression of primordial follicles in human ovarian tissue before and after cryopreservation. Hum Reprod. 2000;15:302–310. doi: 10.1093/humrep/15.2.302. [DOI] [PubMed] [Google Scholar]
- 44.Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod. 2011;26:1097–10103. doi: 10.1093/humrep/der063. [DOI] [PubMed] [Google Scholar]
- 45.Revelli A, Marchino G, Dolfin E, Molinari E, Delle Piane L, Salvagno F, et al. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertil Steril. 2012;S0015–0282(12):02248. doi: 10.1016/j.fertnstert.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 46.Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG, et al. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. Reprod Biomed Online. 2011;22:162–171. doi: 10.1016/j.rbmo.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Rosendahl M, Timmermans Wielenga V, Nedergaard L, Kristensen SG, Ernst E, Rasmussen PE, et al. Cryopreservation of ovarian tissue for fertility preservation: no evidence of malignant cell contamination in ovarian tissue from patients with breast cancer. Fertil Steril. 2011;95:2158–2161. doi: 10.1016/j.fertnstert.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93:2413.e15–9. doi: 10.1016/j.fertnstert.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 49.Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358:271–276. doi: 10.1016/S0140-6736(01)05482-4. [DOI] [PubMed] [Google Scholar]
- 50.Sánchez M, Novella-Maestre E, Teruel J, Ortiz E, Pellicer A. The Valencia Programme for Fertility Preservation. Clin Transl Oncol. 2008;10:433–438. doi: 10.1007/s12094-008-0227-4. [DOI] [PubMed] [Google Scholar]
- 51.Sánchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escribá MJ, Simón C, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93:268.e11–3. doi: 10.1016/j.fertnstert.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 52.Sánchez-Serrano M, Novella-Maestre E, Rosello-Sastre E, Camarasa N, Teruel J, Pellicer A. Malignant cells are not found in ovarian cortex from breast cancer patients undergoing ovarian cortex cryopreservation. Human Reprod. 2009;24:2238–2243. doi: 10.1093/humrep/dep196. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt KL, Byskov AG, Nyboe Andersen A, Müller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–1164. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 54.Shebl O, Ebner T, Sir A, Schreier-Lechner E, Mayer RB, Tews G, et al. Age-related distribution of basal serum AMH level in women of reproductive age and a presumably healthy cohort. Fertil Steril. 2011;95:832–834. doi: 10.1016/j.fertnstert.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Shebl O, Ebner T, Sommergruber M, Sir A, Tews G. Anti muellerian hormone serum levels in women with endometriosis: a case–control study. Gynecol Endocrinol. 2009;25:713–716. doi: 10.3109/09513590903159615. [DOI] [PubMed] [Google Scholar]
- 56.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94:2191–2196. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 57.Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;23:1531–1537. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- 58.Silber SJ, Lenahan KM, Levine DJ, Pineda JA, Gorman KS, Friez MJ, et al. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. NEJM. 2005;353:58–63. doi: 10.1056/NEJMoa043157. [DOI] [PubMed] [Google Scholar]
- 59.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 60.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 61.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–744. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]