Abstract
Purpose
To investigate the clinical characteristics of different categories of sex-reversed 46,XX individuals and their relationships with chromosomal karyotype and the SRY gene.
Methods
Chromosome karyotyping for peripheral blood culture and multi-PCR and FISH were performed.
Results
Endocrinological data showed that their endocrine hormone levels were similar to that observed for Klinefelter syndrome, with higher FSH and LH levels and lower T levels. Chromosome karyotyping for peripheral blood culture revealed 46, XX complement for 11 males. Molecular studies showed that there were locus deletions at SY84, SY86, SY127, SY134, SY254 and SY255 in AZF on chromosome Y in 9 cases, with the SRY gene present at the terminus of the X chromosome short arm. In one case, besides 6 locus deletions in AZF, there was also SRY gene deletion. In another case, there were locus deletions only at SY254 and SY255, with SY84, SY86, SY127 SY134 loci and SRY present.
Conclusions
The majority (10/11) of 46,XX males were SRY positive, with the SRY gene translocated into the terminus of the X chromosome short arm. These patients were caused mainly by an X/Y chromosomal inter-change during paternal meiosis, leading to the differentiation of primary gonads into testes. Only a single patient (1/11) was SRY-negative, in which there might be some unknown downstream genes involved in sex determination.
Keywords: 46,XX male; Sex reversal; SRY gene
Introduction
Male sex determination has not yet been completely elucidated in humans. But there is agreement that the genes of the Y chromosome, in particular, the sex-determining region Y gene (SRY), plays a major role in encoding a testis determining factor (TDF) [1]. In XY embryos, SRY induces the gonadal primordium to develop into testis. 46,XX male syndrome is rare and its incidence is about 1:20000 in newborn males [2]. As far as the sexual phenotype is concerned, three clinical categories of sex-reversed 46,XX individuals have been identified: (1) Classic XX males, infertility with normal male internal and external genitalia; (2) XX males with ambiguous genitalia, usually detected at birth by external genital ambiguities such as hypospadias, micropenis, or hyperclitoridy; (3) XX true hermaphrodites, who carry internal or external genital ambiguities detected at birth [3–5].
At the molecular level, XX males can be classified as Y-DNA positive and Y-DNA negative, depending on the presence or absence of Y-specific sequences [6].
In this paper, the clinical characteristics, endocrinology, chromosomal karyotype and SRY gene were analyzed on eleven sex reversal males discovered during routine examinations for infertility, with the aim of investigating the clinical characteristics of different categories of sex-reversed 46,XX individuals and their relationships with chromosomal karyotype and the SRY gene.
Materials and methods
Subjects and clinical data
We retrospectively analyzed 11 married men with a history of several years of infertility, who were referred to us for genetic review. Their body heights were between 160 and 165 cm. The patients had complete outward signs of masculinity, with no symptom of undervirilization. Semen analyses showed azoospermia. Their testes were small in size and final clinical features were compatible with severe testicular atrophy. Their endocrine hormone levels were similar to those in patients with a chromosomal karyotype of 47, XXY. Ultrasonography failed to identify female gonads or female internal genitalia in all patients.
Karyotype analysis of G-banding in lymphocytes
G-banded chromosomes from cultured peripheral blood lymphocytes were analyzed with conventional methods. Briefly, whole blood (0.5 ml) was cultivated in RPMI-1640 containing PHA-M for 72 h at 37 °C, and then colchicine (1 μg/ml) was added to incubate for 30 min at 37 °C. Chromosomal preparations were carried out according to standard techniques. 100 metaphases were counted in all patients in order to exclude 46, XY mosaicism, with 3–5 karyotypes analyzed.
Polymerase chain reaction (PCR)
For analysis of Y chromosome specific regions, multiplex reactions were performed for the amplification of the six loci of the azoospermia factor (AZFa SY84, SY86; AZFb SY127, SY134 and AZFc SY254, SY255) regions on the Y chromosome and the SRY genes following the manufacturer’s instructions. The multi-PCR kits were purchased from Toujing Life Technology Co., Ltd (Shanghai, China). The DNA samples of normal men, normal women and double distilled water were used as positive, negative and blank controls, respectively.
Fluorescence in situ hybridization (FISH)
FISH was performed in metaphase chromosome of peripheral blood lymphocytes with CEPX (DXZ1, Xp11.1-q11.1, Spectrum Green, Vysis), LSI SRY(Yp11.3, Spectrum Orange, Vysis) and CEP18 (D18Z1, 18p11.1-q11.1, Spectrum Aqua, Vysis) probes following the instructions provided by the manufacturer. 10–15 metaphases were analyzed in each sample using a Nikon fluorescence microscope for signals collection, with the chromosome mapping evaluated with CytoFish 5.6 software (United Scientific USA Inc).
Results
In 11 cases, the average body height was 161 cm and the average volume of testes 2.3 ml. Their endocrine hormone levels were similar to that observed in Klinefelter syndrome, with higher level of FSH and LH and lower level of T. General characteristics and endocrine hormone levels are shown in Table 1.
Table 1.
General characteristics and endocrine hormone levels
| Subjects | Height | Volume of testes | PRL | FSH | LH | E2 | T |
|---|---|---|---|---|---|---|---|
| cm | L/Rml | ng/ml | mIU/ml | mIU/ml | pmol/l | nmol/l | |
| 1 | 163 | 3/3 | 17.9 | 63.6 | 19.4 | 122 | 10.72 |
| 2 | 163 | 2/2 | 18.5 | 24.7 | 14.4 | 161 | 9.64 |
| 3 | 162 | 2/3 | 4.5 | ||||
| 4 | 161 | 2/2 | 22.9 | 81.6 | 27.7 | <73.4 | 4.78 |
| 5 | 158 | 2/4 | 9.67 | 13.1 | 3.61 | 126 | 8.49 |
| 6 | 162 | 2/2 | 10.08 | 54.7 | 19.4 | 101 | 6 |
| 7 | 162 | 3/3 | 9.88 | 37.1 | 16.5 | 105 | 11.1 |
| 8 | 161 | 3/2 | 7.28 | 43 | 33.9 | 81.1 | 7.5 |
| 9 | 160 | 2/2 | 10 | 72 | 34.6 | <73.4 | 11.7 |
| 10 | 160 | 1/2 | 15.8 | 49.2 | 26.8 | <73.4 | 6.28 |
| 11 | 161 | 2/2 | 49.6 | 87.7 | 31.4 | 112 | 18.1 |
Karyotype analysis of all patients showed apparent 46, XX chromosome complement and no evidence of mosaicism in peripheral blood cells.
Results of PCR and FISH
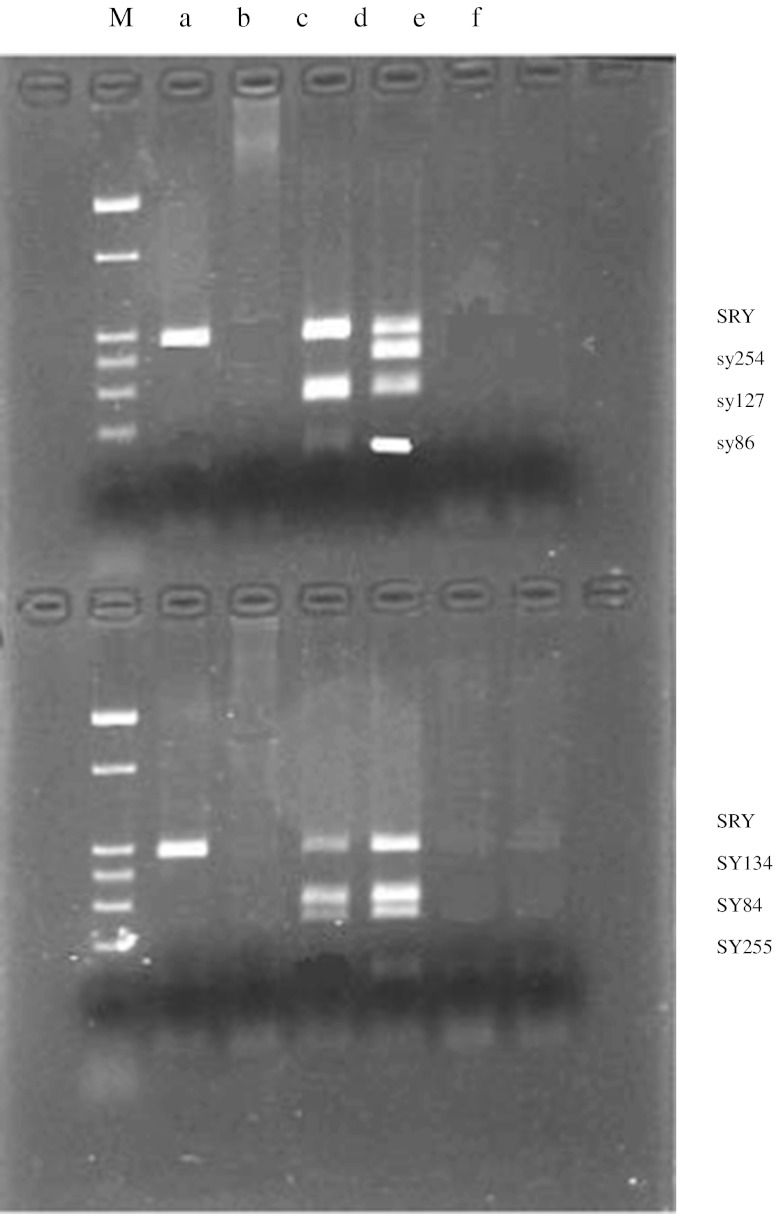
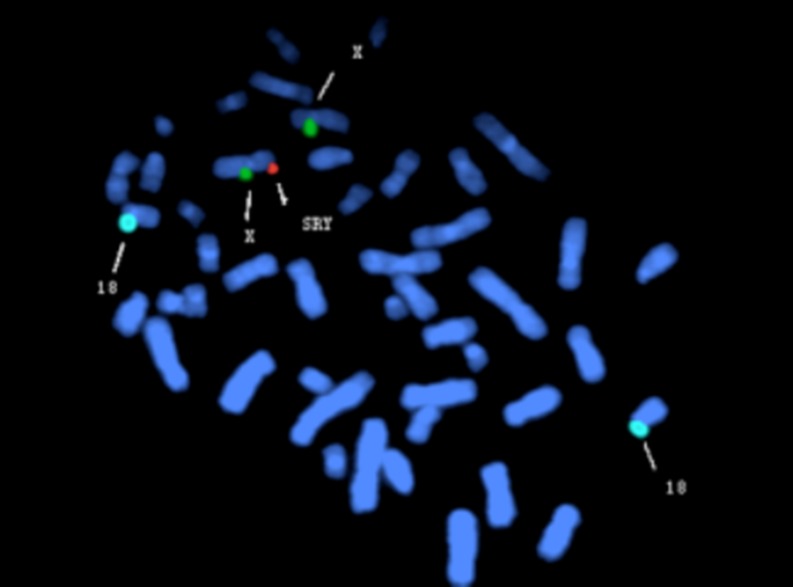
In 9 cases, there were locus deletions at SY84, SY86, SY127, SY134, SY254 and SY255 in AZF on chromosome Y, with the SRY gene present (Fig. 1a) and mapped to the distal end of the short arm of chromosome X (Fig. 2). In one case we found deletion of the SRY gene except 6 locus deletions in AZF (Fig. 1b), and another case had locus deletions only at SY254 and SY255, with SY84, SY86, SY127, SY134 loci and SRY gene all present (Fig. 1c). Details of PCR and FISH results are shown in Table 2.
Fig. 1.
PCR for SRY gene and the locus of AZF on chromosome Y. NOTE: M: marker a: 9th case b: 10th case c: 11th case d: normal male e: normal female f: blank control
Fig. 2.
The FISH on metaphase chromosomes of 9th case with LSI SRY(Yp11.3) in Orange hybridizing to the distal end of short arm of chromosome X
Table 2.
Examination of STS locus and SRY gene on Y chromosome
| SY84 | SY86 | SY134 | SY127 | SY254 | SY255 | SRY | ||
|---|---|---|---|---|---|---|---|---|
| PCR | FISH | |||||||
| 1 | − | − | − | − | − | − | + | Xp |
| 2 | − | − | − | − | − | − | + | Xp |
| 3 | − | − | − | − | − | − | + | Xp |
| 4 | − | − | − | − | − | − | + | Xp |
| 5 | − | − | − | − | − | − | + | Xp |
| 6 | − | − | − | − | − | − | + | Xp |
| 7 | − | − | − | − | − | − | + | Xp |
| 8 | − | − | − | − | − | − | + | Xp |
| 9 | − | − | − | − | − | − | + | Xp |
| 10 | − | − | − | − | − | − | − | − |
| 11 | + | + | + | + | − | − | + | Xp |
Discussion
46,XX male sex reversal syndrome was first reported by Chapelle in 1964 [7]. Most patients (85 %) have a normal male phenotype at birth and are usually diagnosed after puberty during consultation because of hypogonadism, gynecomastia, and/or infertility [8]. Approximately 15 % of XX males are observed to have hypospadias, cryptorchidism, or severe genital ambiguity [9]. In present study, no abnormalities of urinal reproductive system were found in any patient, except for infertility with severe testicular atrophy.
Furthermore, their body heights were all under 165 cm (with an average of 161 cm), which is likely due to the lack of an appropriate T-dependent pubertal growth spurt [10] and Y-specific growth gene(s) mapped in the pericentromeric region of the Y chromosome [11].
SRY is considered a genetic switch directing the identity of the bipotential gonadal primordium to testes [12]. Molecular genetics analyses have demonstrated that approximately 90 % of these patients carry a variable amount of Y material due to a Y-to-X interchange caused by an illegitimate recombination during paternal meiosis [13–15]. There was a report of an XX male caused by a translocation of an SRY gene fragment from the Y chromosome to an autosome [16].
In contrast, there are about 10 % of XX males whose testicular differentiation occurs in absence of detectable Y sequences [8, 13, 17–21]. The majority of these patients have a variable degree of ambiguous external genitalia although a complete male phenotype could also be observed [8, 15]. That would be considered as evidence supporting the theory that the SRY gene does not function alone in the determination of the male phenotype, but disruption of an unidentified autosomal or X-linked sex-determining gene may interact in the sex-determining cascade [13, 18].
Furthermore, the DAX1 and SOX9 genes might function downstream of the SRY gene in the sex-determination pathway. Any mutation leading to increased expression of either gene could lead to female-to-male sex reversal in 46,XX SRY-negative individuals [22, 23]. Huang et al. reported a 46,XX sex reversal individual with duplication of chromosome 17q including SOX9 [24], which suggested that overexpression of male determining genes could trigger testis determination in the absence of SRY. The observation of male development on SOX9 transgenic XX mice further confirmed this hypothesis and raised the possibility that some SRY-negative XX males might have a microduplication of chromosome 17q involving the SOX9 gene [25]. Other study on an individual with SRY-negative 46,XX true hermaphroditism who had a duplication of the long arm of chromosome 22 from q13.1 to qtelomere also suggested that the existence of certain genes on 22q play a fundamental role in testis determination [26]. However, Tossaporn et al. [27] investigated 13 subjects with SRY-negative 46,XX sex reversal for microduplication of chromosome 22q in the region of SOX10 gene, but failed to find any evidence for this. To explain the induction of testicular tissue in SRY-negative patients, another hypothesis suggesting the presence of hidden mosaicism containing Y-derivative material has been proposed [2]. But there has been few reports to prove it on XX-reversed patients as of yet. In this study, no mosaicism containing Y-derived material was detected in all 11 cases with 46,XX male sex reversal, at least in the lymphocytes.
In present study, the presence of the SRY gene were confirmed in 10 out of 11 cases both by PCR and FISH, with the SRY gene located at the short arm of chromosome X, but translocation of SRY to autosome was not found. In our opinion, the illegitimate X/Y chromosomal inter-change during paternal meiosis would result in the differentiation of primary gonads into testes in 46,XX SRY-positive male. 46,XX SRY-negative individuals with complete masculinization are rare [8, 13, 17–19]. Here, we reported one case of 46,XX SRY-negative male with full masculinization but infertility.
In our study, all patients showed azoospermia, which might be related to absence of the Y chromosome long arm which contains three AZF regions [28]. Furthermore, many genes related to spermatogenesis are known to be harbored in these regions, such as USPY9 and DBY in AZFa, RBMY1 in AZFb, and DAZ in AZFc.
Except for SY254 and SY255, some STS loci in AZF including SY84 and SY86 in AZFa, SY127 and SY134 in AZFb, were all present in one out of 11 cases who had normal limits of androgen levels but azoospermia. The possible explanation might be that besides the translocation of SRY gene fragment on the short arm of chromosome X, the translocations of other related genes on the long arm of chromosome Y would also interfere with the function of genes related to spermatogenesis on the area.
Acknowledgments
Dr. Douglas Chesters helped us to improve the text. We appreciate comments from reviewers and suggestions from editors on this manuscript.
Footnotes
Capsule Investigated the clinical characteristics of different categories of sex-reversed 46,XX individuals and their relationships with chromosomal karyotype and the SRY gene.
References
- 1.Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 2.Wachtel SS. XX sex reversal in the human. In: Wachtel SS, editor. Molecular genetics of sex determination. San Diego: Academic; 1994. p. 267. [Google Scholar]
- 3.Abbas NE, Toublanc JE, Boucekkine C, Toublanc M, Affara NA, Job JC, et al. A possible common origin of “Y-negative” human XX males and XX true hermaphrodites. Hum Genet. 1990;84:356–360. doi: 10.1007/BF00196234. [DOI] [PubMed] [Google Scholar]
- 4.Boucekkine C, Toublanc JE, Abbas N, Chaabouni S, Ouahid S, Sem-rouni M, et al. Clinical and anatomical spectrum in XX sex reversed patients. Relationship to the presence of Y specific DNA-sequences. Clin Endocrinol (Oxf) 1994;40:733–742. doi: 10.1111/j.1365-2265.1994.tb02506.x. [DOI] [PubMed] [Google Scholar]
- 5.McElreavey K, Rappaport R, Vilain E, Abbas N, Richaud F, Lortat-Jacob S, et al. A minority of 46, XX true hermaphrodites are positive for the Y-DNA sequence including SRY. Hum Genet. 1992;90:121–125. doi: 10.1007/BF00210754. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson-Smith MA, Cooke A, Affara NA, Boyd E, Tolmie JL. Gen- otype-phenotype correlations in XX males and their bearing on current theories of sex determination. Hum Genet. 1990;84:198–202. doi: 10.1007/BF00208942. [DOI] [PubMed] [Google Scholar]
- 7.De la Chapelle A, Hortling H, Niemi M, Wennstroem J. XX chromosomes in a human male. First case. Acta Med Scand. 1964;175(suppl412):25–28. doi: 10.1111/j.0954-6820.1964.tb04630.x. [DOI] [PubMed] [Google Scholar]
- 8.Zenteno JC, López M, Vera C, Méndez JP, Kofman-Alfaro S. Two SRY-negative XX male brothers without genital ambiguity. Hum Genet. 1997;100:606–610. doi: 10.1007/s004390050561. [DOI] [PubMed] [Google Scholar]
- 9.De la Chapelle A. Nature and origin of males with XX sex chromosomes. Am J Hum Genet. 1972;24:71–105. [PMC free article] [PubMed] [Google Scholar]
- 10.Ogata T, Matsuo N. Comparison of adult height between patients with XX and XY gonadal dysgenesis: support from a Y specific growth gene(s) J Med Genet. 1992;29:539–541. doi: 10.1136/jmg.29.8.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirsch S, Weiss B, Kleiman S, Roberts K, Pryor J, Milunsky A, et al. Localisation of the Y chromosome stature gene to a 700 kb interval in close proximity to the centromere. J Med Genet. 2002;39:507–513. doi: 10.1136/jmg.39.7.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capel B. Sex in the 90s: SRY and the switch to the male pathway. Annu Rev Physiol. 1998;60:497–523. doi: 10.1146/annurev.physiol.60.1.497. [DOI] [PubMed] [Google Scholar]
- 13.McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci USA. 1993;90:3368–3372. doi: 10.1073/pnas.90.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fechner PY, Marcantonio SM, Jaswaney V, Stetten G, Goodfellow PN, Migeon CJ, et al. The role of the sex-determining region Y gene (SRY) in the etiology of 46, XX maleness. J Clin Endocrinol Metab. 1993;76:690–695. doi: 10.1210/jc.76.3.690. [DOI] [PubMed] [Google Scholar]
- 15.Boucekkine C, Toublanc JE, Abbas N, Chaabouni S, Ouahid S, Semrouni M, et al. Clinical and anatomical spectrum in XX sex reversed patients. Rela- tionship to the presence of Y specific DNA-sequences. Clin Endocrinol. 1994;40:733–742. doi: 10.1111/j.1365-2265.1994.tb02506.x. [DOI] [PubMed] [Google Scholar]
- 16.Dauwerse JG, Hansson KB, Brouwers AA, Peters DJ, Breuning MH. An XX male with the sex-determining region Y gene inserted in the long arm of chromosome 16. Fertil Steril. 2006;86:463.e1–463.e5. doi: 10.1016/j.fertnstert.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 17.Kolon TF, Ferrer FA, McKenna PH. Clinical and molecular analysis of XX sex reversed patients. J Urol. 1998;160:1169–1172. doi: 10.1016/S0022-5347(01)62729-0. [DOI] [PubMed] [Google Scholar]
- 18.Vernole P, Terrinoni A, Didona B, De Laurenzi V, Rossi P, Melino G, et al. An SRY-negative XX male with Huriez syndrome. Clin Genet. 2000;57:61–66. doi: 10.1034/j.1399-0004.2000.570109.x. [DOI] [PubMed] [Google Scholar]
- 19.Abusheikha N, Lass A, Brinsden P. XX males without SRY gene and with infertility. Hum Reprod. 2001;16:717–718. doi: 10.1093/humrep/16.4.717. [DOI] [PubMed] [Google Scholar]
- 20.Ma S, Tang SS, Yuen BH, Bruyere H, Penaherrera M, Robinson WP. Cytogenetic and molecular study of a premature male infant with 46, XX derived from ICSI: case report. Hum Reprod. 2003;18:2298–2301. doi: 10.1093/humrep/deg462. [DOI] [PubMed] [Google Scholar]
- 21.Valetto A, Bertini V, Rapalini E, Simi P. A 46, XX SRY-negative man with complete virilization and infertility as the main anomaly. Fertil Steril. 2005;83:216–219. doi: 10.1016/j.fertnstert.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 22.Maciel-Guerra AT, de Mello MP, Coeli FB, Ribeiro ML, Miranda ML, Marques-de-Faria AP, et al. XX maleness and XX true hermaphroditism in SRY-negative monozygotic twins: additional evidence for a common origin. J Clin Endocrinol Metab. 2008;93:339–343. doi: 10.1210/jc.2007-1115. [DOI] [PubMed] [Google Scholar]
- 23.Rajender S, Rajani V, Gupta NJ, Chakravarty B, Singh L, Thangaraj K. SRY-negative 46, XX male with normal genitals, complete masculinization and infertility. Mol Hum Reprod. 2006;12:341–346. doi: 10.1093/molehr/gal030. [DOI] [PubMed] [Google Scholar]
- 24.Huang B, Wang S, Ning Y, Lamb AN, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349–353. doi: 10.1002/(SICI)1096-8628(19991203)87:4<349::AID-AJMG13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- 26.Aleck KA, Argueso L, Stone J, Hackel JG, Erickson RP. True hermaphroditism with partial duplication of chromosome 22 and with—out SRY. Am J Med Genet. 1999;85:2–4. doi: 10.1002/(SICI)1096-8628(19990702)85:1<2::AID-AJMG2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Seeherunvong T, Perera EM, Bao Y, Benke PJ, Benigno A, Donahue RP, et al. 46, XX Sex Reversal With Partial Duplication of Chromosome Arm 22q. Am J Med Genet A. 2004;127A:149–151. doi: 10.1002/ajmg.a.20630. [DOI] [PubMed] [Google Scholar]
- 28.Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, Kiesewetter F, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5:933–943. doi: 10.1093/hmg/5.7.933. [DOI] [PubMed] [Google Scholar]