Abstract
Purpose
Spermatogonial stem cells are affected by the interactions of extrinsic signals produced by components of the microenvironment niche, in addition to the chemical and physical properties of the extracellular matrix. Therefore, this study was initiated to assess the interaction of these cells on a synthetic nanofibrillar extracellular matrix that mimicked the geometry and nanotopography of the basement membrane for cellular growth.
Methods
This study has used a variety of experimental approaches to investigate the interaction of mouse neonatal-derived spermatogonial stem-like cells on a synthetic random oriented three-dimensional nanofibrillar matrix composed of electrospun polyamide nanofibers (Ultra-Web™).
Results
Spermatogonial stem-like cell colonies were characterized by their ability to express α6-integrin, Thy-1, PLZF, and β1-integrin. After culture of cells on the nanofibrillar surfaces for 7 days, the number of colonies, the number of cells in each colony, and the average area of colonies were increased (P < 0.05). However, the expression difference of related markers in both groups was not significant. A significantly higher proliferation and survival was observed in the nanofibrillar group (P < 0.05). After transplantation into the testes of busulfan-treated adult mice, spermatogonial stem-like cell colonies that were cultured on the nanofibrillar surface demonstrated functionality, as verified by their ability to migrate to the seminiferous basal membrane, where they produced additional colonies.
Conclusions
These results have suggested that electrospun nanofibrillar surfaces could provide a more favorable microenvironment for in vitro short term culture of spermatogonial stem-like cell colonies.
Keywords: Nanofibrillar matrix, Proliferation, Spermatogonial stem-like cells, Survival, Transplantation
Introduction
In mammals, spermatogonial stem cells (SSCs) are unique since they are the only adult stem cells that can contribute to the next generation [35]. The numbers of these stem cells in testes is approximately 0.03 % of the total testicular cell population in the adult mouse [28, 36]. Additionally, the establishment and maintenance of a niche’s microenvironment in the seminiferous tubules of the testis is important to regulate the SSC population and its function [4]. These cells are located close to several supporting somatic cells and the basement membrane or extracellular matrix (ECM) of seminiferous tubules, which may contribute to the formation of the SSC niche. This niche plays important roles in the regulation of SSC self-renewal as well as in different stages of spermatogenesis [9, 30].
The SSC niche is significant in fertility treatment of oncology patients [12, 21] and livestock; therefore, developing efficient methods that mimic the structure of the niche’s microenvironment are necessary to provide a suitable condition for ex vivo cell growth [8]. The ECM is a complex structure built to meet tissue-and organ-specific requirements, which primarily consist of nanometer diameter fibrils [8]. Synthetic ECMs are often designed to exploit the interaction with cell surface receptors, which directly participate in promoting cell adhesion, migration, growth, differentiation, and apoptosis [15]. Electrospun nanofiber matrices exhibit morphological similarities to the natural ECM, as characterized by ultrafine continuous fibers, a high surface-to-volume ratio, high porosity and variable pore-size distribution [22]. To date, the influences of nanofibrillar surface topography on expansion of testicular SSC have yet to be reported.
In the present study we cultured mouse spermatogonial stem-like cells on a synthetic nanofibrillar three-dimensional (3D) matrix composed of electrospun polyamide nanofibers (Ultra-Web™). We evaluated their interactions (e.g., colony formation, proliferation, survival, stemness, and function) by a variety of experimental approaches. This synthetic nanofibrillar ECM mimicked the geometry and nanotopography of the basement membrane for cellular growth [27, 33].
Materials and methods
Animals
All animal experiments were approved and undertaken according to regulations provided by the Royan Institutional Review Board and the Institutional Ethical Committee. Male mouse NMRI adults (10–12 weeks, n = 15) and pups (6-day-old, n = 80) were purchased from Pasteur Institute (Tehran, Iran) and 6-day-old male C57BL/6 mouse pups (n = 20) that expressed the green fluorescent protein (GFP) gene were provided by Royan Institute’s Animal Laboratory (Tehran, Iran).
Isolation and culture of testicular cells
The testes of mouse pups were collected in phosphate buffered saline (PBS; Invitrogen). After decapsulation, the testes were minced into small pieces in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen). To achieve a single cell suspension, we performed a two-step enzymatic digestion as described previously [17] with slight modification.
In brief, testicular tissue were transferred into a digestion medium that contained collagenase type IV (1 mg/ml), dispase (0.5 mg/ml), hyaluronidase type 1-S (1 mg/ml) and kept at 37 °C for 10 min, followed by pipetting for 5 min. The resultant suspension after the first digestion step was centrifuged at 30 × g for 2 min. Single cells were isolated by a second enzymatic digestion with collagenase IV (1 mg/ml), dispase (0.5 mg/ml), hyaluronidase type 1 (1 mg/ml) and DNase I (5 μg/ml), under the same conditions. All enzymes were purchased from Sigma-Aldrich. To prepare a cell suspension, cells were passed through a 70-μm nylon filter. Cell viability was determined by trypan blue exclusion.
After enzymatic dissociation, 1 × 106 testicular cells were cultured onto nanofibrillar surfaces (+Nano group, Ultra-Web™ nanofibrillar matrix, TC02-06, Surmodics Inc., www.Synthetic-ECM.com) in six-well tissue culture plates (TPP, TP92006, Switzerland) and expanded for 7 days in expansion medium. Expansion medium consisted of DMEM supplemented with 13.5 g/l NaHCO3 (Sigma-Aldrich), non-essential amino acids, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 40 μg/ml gentamycin with 1 % fetal bovine serum (FBS) in the presence of 40 ng/ml of glial-derived neurotrophic factor (GDNF), 20 ng/ml mouse epidermal growth factor (EGF) (all from Invitrogen) and 10 ng/ml of human basic fibroblast growth factor (bFGF, Royan institute). The same tissue culture plates with no nanofibers were used as the control group (−Nano). Prior to cell seeding, both surfaces, -Nano and +Nano were coated with similar volume (2 ml) of 1 % gelatin in PBS for 60 min at 37 °C.
The isolated cells were maintained at 32 °C in an atmosphere of 5 % CO2 in air for 7 days. The culture medium was changed every 3 days.
Analysis of spermatogonial stem-like cell colonies
The numbers of colonies per seeded cells were counted under inverted microscope. Colony surface area was measured by Image J software (National Institutes of Health).
To evaluate the number of cells per colony, 100–150 colonies were mechanically separated from culture plates, 7 days after primary culture of the testicular cells. Colonies were dissociated by an enzyme solution that included trypsin-EDTA (0.05 %) and collagenase IV (1 mg/ml). Subsequently they were counted with a hemocytometer.
Immunofluorescence staining and flow cytometry analysis
The cultured spermatogonial stem-like cell colonies were fixed in 4 % paraformaldehyde in PBS (pH 7.4) for 20 min. Cells were washed twice with 0.1 % Tween 20 in PBS prior to blocking in 10 % normal goat serum (Vector, Burlingame, CA) in PBS for 15 min, followed by incubation with primary antibody solution overnight at 4 °C. Primary antibodies were: rat polyclonal anti-α6-integrin (1:100; Sigma-Aldrich), rat polyclonal anti-β1-integrin (1:100; Sigma-Aldrich), rat polyclonal anti-Plzf (1:100; Sigma-Aldrich), and mouse polycolonal anti-Thy-1 antibody (1:100, Santa Cruz, CA). The following day, cells were washed twice with 0.1 % Tween-20 in PBS for 5 min and incubated with the appropriate secondary antibody [goat anti-rat and goat anti-mouse labeled with fluorescein isothiocyanate (FITC); 1:200; Sigma-Aldrich] for 1 h and subsequently washed with 0.1 % Tween 20 in PBS for 5 min. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) in PBS. Labeled cells were examined with a fluorescent microscope (BX51; Olympus) and images acquired with an Olympus D70 camera.
For flow cytometry analysis, single cells were obtained from picked up spermatogonial stem-like cell colonies and processed as described above. All steps were performed on ice. After washing, flow cytometric analysis was performed with a BD-FACS Calibur flow cytometer. The experiments were replicated three times and the acquired data analyzed with WinMDI (version 2.9) software.
Survival and proliferation of mouse testicular cells
We measured the amount of apoptosis by utilizing a combined staining with FITC-conjugated annexin V (IQP-116 F) and propidium iodide. Initially, mechanically harvested colonies were trypsinized into single cells. Then, according to the manufacturer’s instructions, approximately 1 × 106 cells were washed once with Ca2+-binding buffer [10 mM HEPES (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2] and resuspended in 100 μl of the same buffer that consisted of FITC-conjugated annexin V. After 20 min of incubation in the dark at 4 °C, cells were diluted with 400 μl of binding buffer and propidium iodide was added before flow cytometric analysis.
We determined the proliferative activity of cultured colonies by adding anti-BrdU (1:100, Roche) into the culture medium for 4 days prior to mechanical harvesting. Cultured, harvested colonies were processed for flow cytometry according to the manufacturer’s protocol.
Scanning electron microscopy (SEM)
Spermatogonial stem-like cell colonies grown in the presence and absence of the nanofibrillar surfaces were washed with PBS, pre-fixed with 2.5 % glutaraldehyde in 0.1 M PBS for 2 h and post-fixed with 1 % osmium tetroxide for 1.5 h. After dehydration in an ethanol series, samples were air-dried, mounted on stab and gold-coated using a sputter coater (EM/TECH, K 350, England). The samples were examined with a scanning electron microscope (VEGA\TESCAN, Czech Republic).
Rete-testis micro injection and analysis of recipient mice
Recipient NMRI mice were treated with busulfan, 40 mg/kg intraperitoneally, at 4–6 weeks of age to deplete endogenous spermatogenesis [3, 21]. Additionally, recipient mice were administrated with cyclosporine solution (sandimmune; Novartis Pharmaceuticals, Switzerland) intraperitoneally at a dose of 20 mg/kg/24 h.
We mechanically isolated and trypsinized cultured spermatogonial stem-like cell colonies to determine their functionality. The dissociated cells were transplanted into the left testes of each recipient male mouse. Before transplantation, the cells from the nano+ and nano-groups were labeled with BrdU for 48 h. In some cases, we grew spermatogonial stem-like cells which were derived from GFP transgenic mice on nano+ and nano-surfaces for 7 days. The dissociated cells were suspended at a concentration of 10 × 106/ml in DMEM that contained 0.04 % trypan blue for visualization, then only injected into the left testes of different recipient mice (approximately 10 μl/testis) as previously described [29]. Four weeks after busulfan treatment the transplanted testes of the recipient mice were fixed in Bouin’s solution, dehydrated and embedded in paraffin. The sections were immunostained with a primary anti-BrdU to identify donor cells. In case of GFP transgenic transplanted cells we used cryosections and the donor cells were visualized by fluorescence microscope (BX51; Olympus). The numbers of spermatogonial stem-like cells that migrated to the seminiferous basal membrane and donor-derived colonies of spermatogenesis in each recipient testis were counted using a fluorescent microscope (30 seminiferous tubules were randomly selected). Because each colony was derived from a single SSC [19, 24], we counted the donor-derived colonies of spermatogenesis from the 30 randomly selected seminiferous tubules and compared them in two different treatments. This provided a relative quantification of spermatogonial stem-like cell number in an injected cell suspension.
Statistical analysis
The results were expressed as mean ± SD. All experiments were repeated at least three times. Normality of all variables was assessed by the Kolmogorov Smirnov test. All statistical analysis was conducted using the Statistical Analysis System (SAS) software, v.15. The difference between the means was examined using the general linear model (GLM) procedure and the Duncan test. P < 0.05 was considered significant.
Results
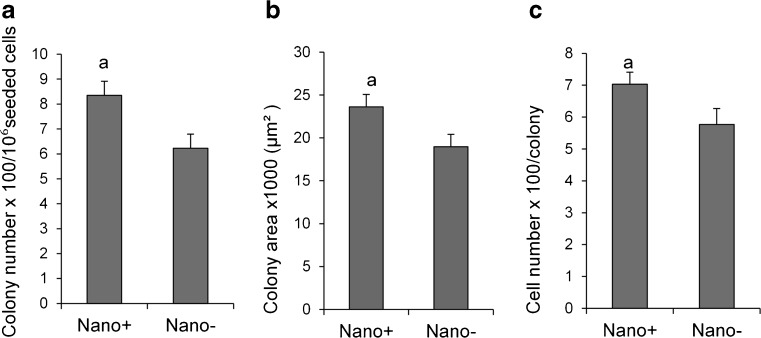
To evaluate the topographic effect on the proliferation and function of spermatogonial stem-like cells, 1 × 106 testicular cells from NMRI strain pups were cultured in the absence or presence of randomly orientated electrospun polyamide nanofibers in six-well tissue culture plates for 7 days in DMEM supplemented with 1 % FBS, bFGF, EGF, and GDNF. Spermatogonial stem-like cell colonies appeared around 3 to 5 days after initial plating of the testicular cells. Most colonies were round with individually detectable cells and consisted of a dense core of cells that included spermatogonial stem-like cells, which were intermingled with somatic cells. After 7 days, the numbers of colonies in the +Nano group was more than the −Nano, or control group (Fig. 1a, P < 0.05). The numbers of cells and surface area per colony were higher in the +Nano group (Fig. 1b and c, P < 0.05).
Fig. 1.
Primary culture of spermatogonial stem-like cell colonies for 7 days in the presence and absence of a nanofiber surface. a: P < 0.05
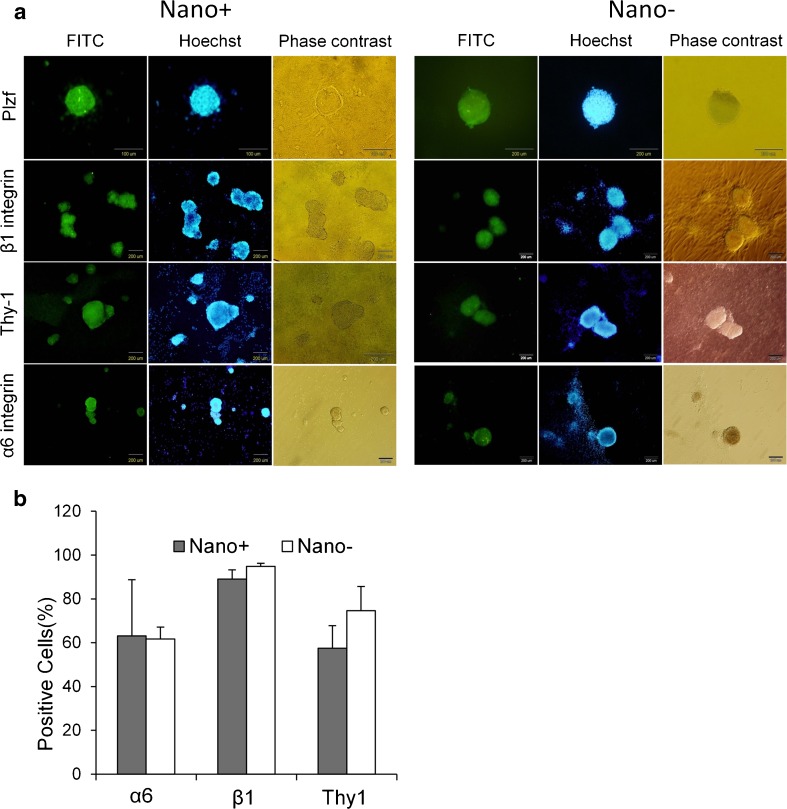
Spermatogonial stem-like cells were characterized by immunofluorescence staining (Fig. 2a). The colonies were positive for Plzf, β1-integrin, Thy-1, and α6-integrin, which showed the presence of spermatogonial stem-like cells. Flow cytometry analysis of cells 7 days after culture indicated that the percentages of positive cells in isolated colonies for α6-integrin, β1-integrin, and Thy-1 were similar in both groups (Fig. 2b).
Fig. 2.
Characterization of spermatogonial stem-like cell colonies. (a) Immunofluorescence staining of spermatogonial stem-like cell colonies for Plzf as a nuclear marker and α6-integrin, β1-integrin and Thy-1 as membrane markers. (b) Flow cytometry analysis of the percentage of positive cells
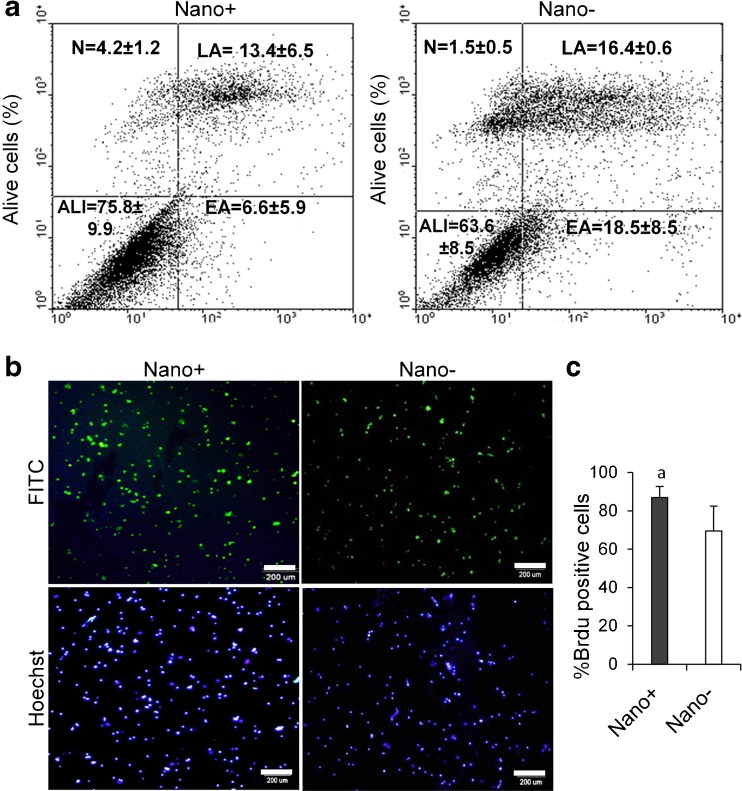
Flow cytometric quantification using annexinV revealed a greater number of surviving cells in the +Nano group (75.8 ± 9.9 % vs. 63.6 ± 8.5, P < 0.05) compared with the −Nano group. In addition, there were fewer apoptotic cells [(late apoptotic: 13.4 ± 6.5)+(early apoptotic: 6.6 ± 5.9) vs. (late apoptotic: 16.4 ± 0.6)+(early apoptotic: 18.5 ± 8.5)] in the +Nano group compared with the -Nano group (Fig. 3a).
Fig. 3.
Viability and proliferation in spermatogonial stem-like cell colonies which cultured in presence and absence of nanofibrillar surfaces. (a) Comparison of flow cytometry for surviving and appototic cells by the annexin test. ALI: Alive, EA: Early apoptosis, LA: Late apoptosis, N: Necrosis. (b) Immunostaining with BrdU-FITC. Nuclei were counterstained with Hoechst. (c) Flow cytometry analysis for isolated spermatogonial stem-like cells labeled with BrdU. a: P < 0.05
To verify whether nanofiber matrix has a role in the proliferation of cells in colonies, the BrdU cell proliferation assay was performed 7 days after cell seeding. A noticeably significant higher proliferation was observed in cells cultured on the nanofibrillar matrix compared to the control group (Fig. 3b and c, P < 0.05). BrdU is a synthetic nucleoside that can be incorporated into cell chromosomes during the “S” phase of the cell cycle [6].
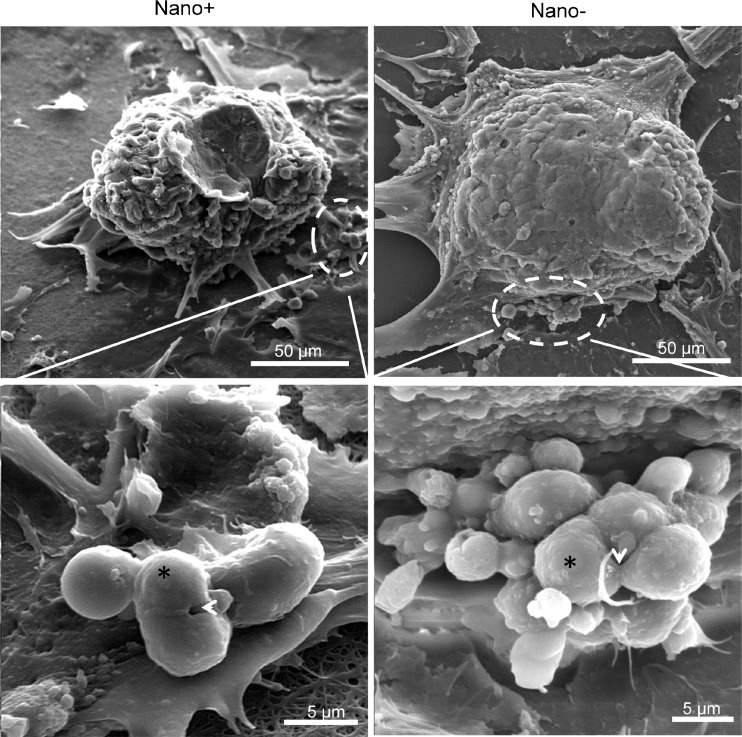
Analysis of SEM micrographs also showed dividing cells in colonies and the presence of apoptotic cells with surface protrusions and bulbs in both groups (Fig. 4).
Fig. 4.
SEM of spermatogonial stem-like cell colonies. Note the dividing cells (*) in both groups along with midbody/interbody (arrow head)
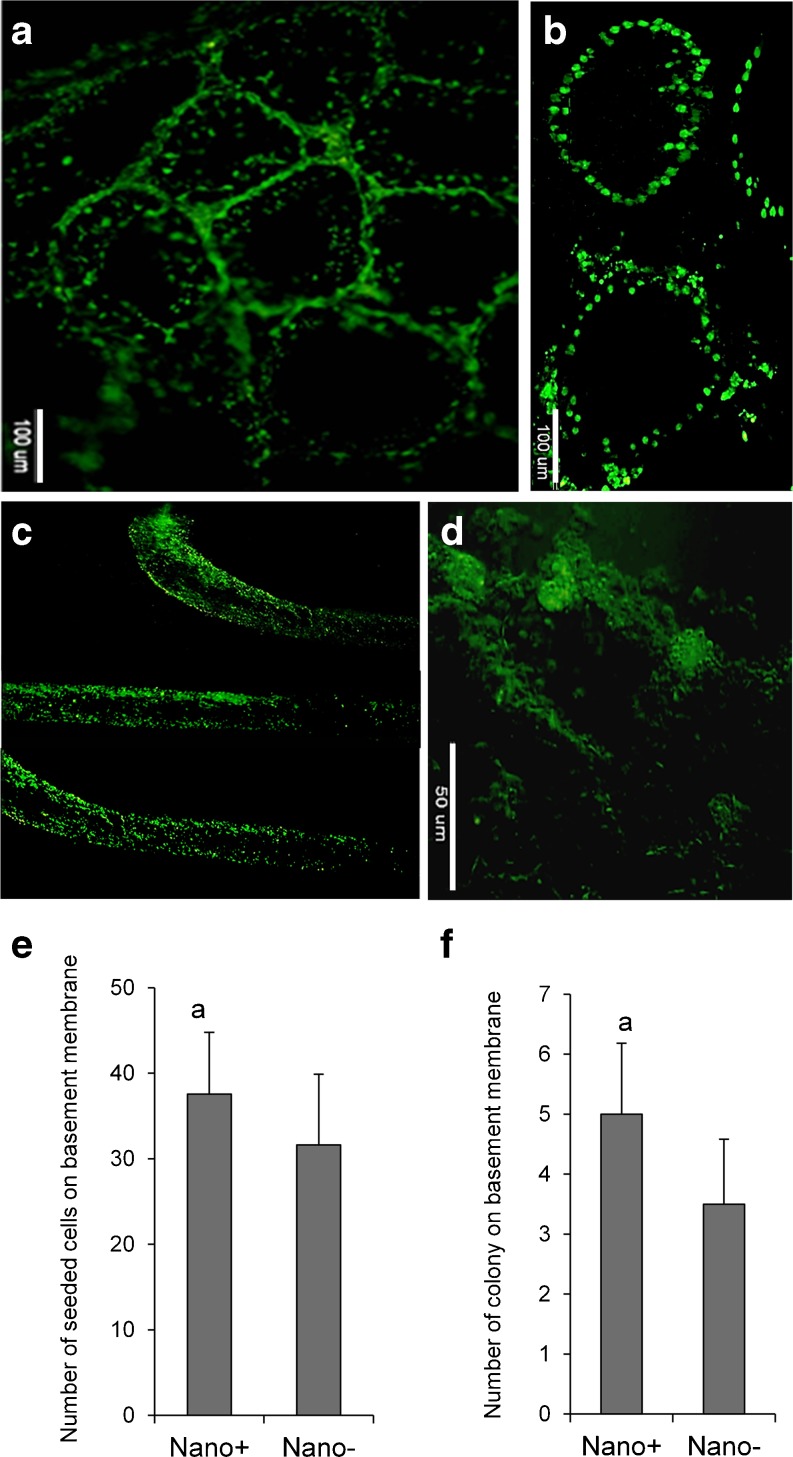
In order to evaluate their function, cultured cells were transplanted into the seminiferous tubules of busulfan-treated adult NMRI mice, which had been depleted of germ cells before transplantation. Before transplantation, the cultured cells in presence or absence of nanofibrillar surfaces were labeled with BrdU. Moreover, in some cases we used spermatogonial stem-like cells which were derived from GFP transgenic mice and cultured on nano+ and nano-surfaces for 7 days. Four and sixteen weeks post-implantation we looked at the transplanted BrdU or GFP-labeled mice, respectively. We observed fluorescent signal in the seminiferous basement membrane indicating that the cells retained their undifferentiated germ cell properties (Fig. 5a and b). Some of the cells generated colonies 16 weeks after transplantation (Fig. 5c and d).
Fig. 5.
Functional analysis of spermatogonial stem-like cells. Spermatogonial stem-like cells were labeled with BrdU and transplanted into the seminiferous tubules of busulfan-induced infertile adult mice. Transplanted BrdU positive cells were detected in the base of the seminiferous tubules at 2 weeks (a) and 4 weeks (b) after transplantation. These figures represent cells in the +Nano group. Long term (16 week) follow-up of GFP-positive transplanted spermatogonial stem cells showed positive cells in the seminiferous tubules (c and d). The transplanted spermatogonial stem-like cells created colonies in the seminiferous tubules after transplantation (d). (e) Quantification of the number of seeded cells (e) and colonies (f) on basement membrane 4 and 16 weeks after transplantation of spermatogonial stem-like cells, respectively. a: P < 0.05
The number of transplanted seeded cells, (4 weeks after transplantation) on the basement membrane and spermatogonial stem-like cell colonies (16 weeks after transplantation) in the seminiferous tubules of the +Nano group were significantly higher than the control group (Fig. 5e and f, P < 0.05). In the case of long-term follow up of transplanted cells we used GFP-positive transplanted spermatogonial stem-like cells which derived from 6-day-old male C57BL/6 mouse pups. The results showed positive cells and colonies in the seminiferous tubules (Figs. 5c and d).
The number of transplanted cells decreased over time, which was possibly related to stimulation of the animal's immune system by the transplanted cells or inappropriate reception of the signaling necessary for efficient integration of transplanted cells into the seminiferous tubules.
Discussion
In this study, we report the positive influence of nanofibrillar surface spermatogonial stem-like cell colonies in terms of colony numbers, cell numbers per colony, colony area, survival, proliferation, and implantation in seminiferous tubules of mice. These phenomena could be a result of the difference in the niche’s microenvironment, such that the nanofibrillar surface creates a 3D surface for in vitro culture of spermatogonial stem-like cells, which is important for protecting and perpetuating self-renewal [14].
It has been demonstrated that ECM-cell interaction directs cellular behaviors such as cell shape changes and cell migration [20]. We have created our nano ECM from an electrospun nonwoven matrix of polyamide nanofibers with an average fiber diameter of 280 nm [33], which was employed as a model to mimic the geometry and nanotopography of the ECM/basement membrane for cell growth [10]. The beneficial influence of this nanofibrillar surface has been reported in cultures of different somatic cells [1, 2, 25, 27] and the differentiation of human embryonic stem cells into a hepatic lineage as well as mouse mesenchymal stem cells [13, 31]. Recently, we demonstrated enhanced migration, proliferation, and morphology of neural progenitors plated on this nano-structure in comparison with their maintenance on tissue-culture plates [32]. Furthermore, human embryonic stem cell-derived neuronal cells and their function improved on this surface [34]. The morphology, proliferation, differentiation, and function of cells were influenced by topographical features of the ECM [11, 18]. Nano surfaces have been shown to create a high surface area to volume ratio, which provides a wide area for cells to adhere [22].
As spermatogonial stem-like cells were isolated along with Sertoli cells from neonatal mice, another interpretation is that there are more suitable culture conditions on the nano surface for better functionality of Sertoli cells in paracrine secretions.
In ECM-cell interaction, focal adhesion contacts are formed in cells [7] and interact with ECM molecules using integrins, ECM-cell specific surface receptors [5, 7, 16]. This leads to signal transduction into the cells, possibly affecting cell behavior through the activation of the small GTPase Rac, or the phosphoinositide 3-kinase (PI3K) pathways [23, 26, 27].
These early data have shown that spermatogonial stem-like cells exhibit better behaviors in terms of expansion and function when cultured onto electrospun nanofiber surfaces, which emphasizes the importance of topographic cues. Therefore, the combination of SSCs and engineering of nanomaterials may be a better approach for the expansion and function of SSCs to treat infertility.
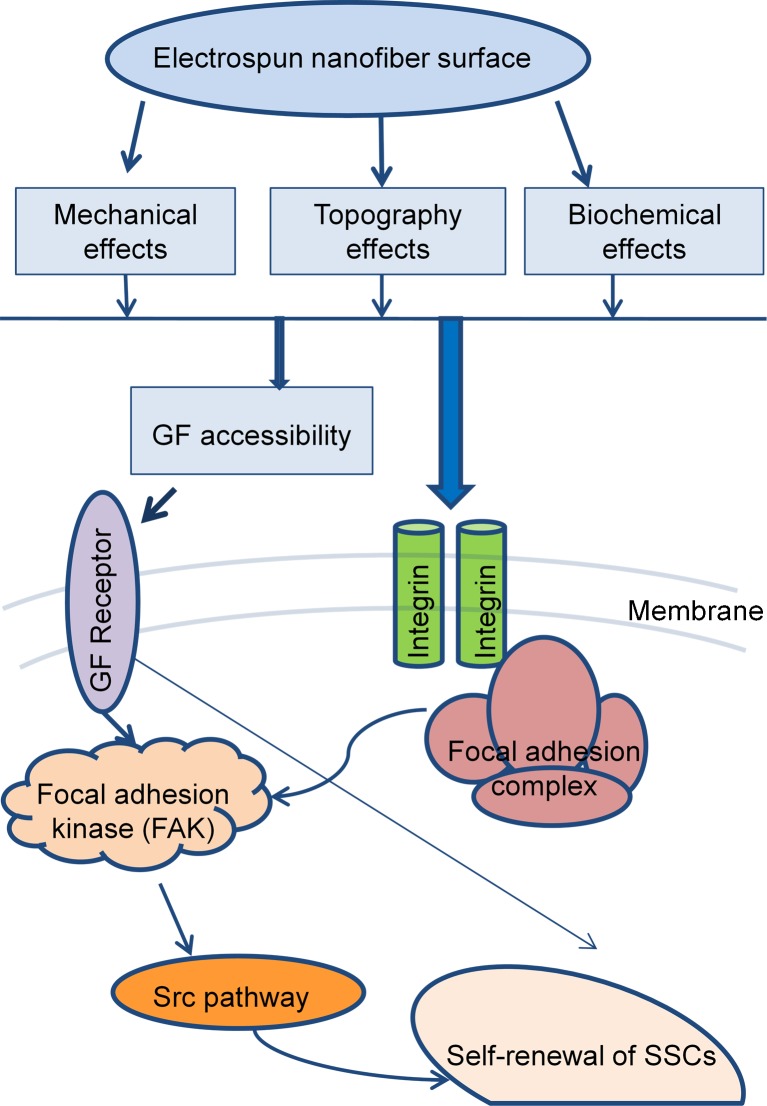
These results indicated that the use of nanofibers influenced spermatogonial stem-like cells proliferation and maintenance of the pup mouse in short-term culture by affecting the gene expression of genes associated with formation of the ECM. However, irrespective of the chemical composition of the fibers, the mechanics, geometry and nanotopography impacted cell fate and function by improving the condition of the involved signaling molecules (Fig. 6). Ultimately these results can be regarded as the stepping stone for the development of more functional SSCs from in vitro cultured stem cells.
Fig. 6.
A proposed model for the effects of nanofiber matrix on molecular signaling pathways in spermatogonial stem-like cell proliferation and maintenance. GF: Growth factor
Acknowledgments
This study was funded by a grant provided from Royan Institute.
Disclosure
None of the authors have any conflicts of interest to disclose and all authors support submission to this journal.
Footnotes
Capsule
Electrospun nanofibrillar surfaces could provide a more favorable microenvironment for in vitro short term maintain of mouse neonatal-derived spermatogonial stem-like cell colonies.
References
- 1.Ahmed I, Liu HY, Mamiya PC, Ponery AS, Babu AN, Weik T, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces covalently modified with tenascin-C-derived peptides enhance neuronal growth in vitro. J Biomed Mater Res A. 2006;76(4):851–60. doi: 10.1002/jbm.a.30587. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed I, Ponery AS, Nur EKA, Kamal J, Meshel AS, Sheetz MP, Schindler M, Meiners S. Morphology, cytoskeletal organization, and myosin dynamics of mouse embryonic fibroblasts cultured on nanofibrillar surfaces. Mol Cell Biochem. 2007;301(1–2):241–9. doi: 10.1007/s11010-007-9417-6. [DOI] [PubMed] [Google Scholar]
- 3.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caires K, Broady J, McLean D. Maintaining the male germline: regulation of spermatogonial stem cells. J Endocrinol. 2010;205(2):133–45. doi: 10.1677/JOE-09-0275. [DOI] [PubMed] [Google Scholar]
- 5.Cary LA, Han DC, Guan JL. Integrin-mediated signal transduction pathways. Histol Histopathol. 1999;14(3):1001–9. doi: 10.14670/HH-14.1001. [DOI] [PubMed] [Google Scholar]
- 6.Choudhary RK, Daniels KM, Evock-Clover CM, Garrett W, Capuco AV. Technical note: A rapid method for 5-bromo-2′-deoxyuridine (BrdU) immunostaining in bovine mammary cryosections that retains RNA quality. J Dairy Sci. 2010;93(6):2574–9. doi: 10.3168/jds.2009-2837. [DOI] [PubMed] [Google Scholar]
- 7.Dalby MJ, Riehle MO, Johnstone HJ, Affrossman S, Curtis AS. Polymer-demixed nanotopography: control of fibroblast spreading and proliferation. Tissue Eng. 2002;8(6):1099–108. doi: 10.1089/107632702320934191. [DOI] [PubMed] [Google Scholar]
- 8.Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, Fausto N. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci U S A. 2006;103(26):9912–7. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121(3):347–54. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- 10.Delgado-Rivera R, Harris SL, Ahmed I, Babu AN, Patel RP, Ayres V, Flowers D, Meiners S. Increased FGF-2 secretion and ability to support neurite outgrowth by astrocytes cultured on polyamide nanofibrillar matrices. Matrix Biol. 2009;28(3):137–47. doi: 10.1016/j.matbio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehmcke J, Hubner K, Scholer HR, Schlatt S. Spermatogonia: origin, physiology and prospects for conservation and manipulation of the male germ line. Reprod Fertil Dev. 2006;18(1–2):7–12. doi: 10.1071/RD05119. [DOI] [PubMed] [Google Scholar]
- 13.Farzaneh Z, Pournasr B, Ebrahimi M, Aghdami N, Baharvand H. Enhanced functions of human embryonic stem cell-derived hepatocyte-like cells on three-dimensional nanofibrillar surfaces. Stem Cell Rev. 2010;6(4):601–10. doi: 10.1007/s12015-010-9179-5. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116(6):769–78. doi: 10.1016/S0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 15.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9(6):518–26. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 17.Izadyar F, Matthijs-Rijsenbilt JJ, den Ouden K, Creemers LB, Woelders H, de Rooij DG. Development of a cryopreservation protocol for type A spermatogonia. J Androl. 2002;23(4):537–45. [PubMed] [Google Scholar]
- 18.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanatsu-Shinohara M, Ikawa M, Takehashi M, Ogonuki N, Miki H, Inoue K, Kazuki Y, Lee J, Toyokuni S, Oshimura M, Ogura A, Shinohara T. Production of knockout mice by random or targeted mutagenesis in spermatogonial stem cells. Proc Natl Acad Sci U S A. 2006;103(21):8018–23. doi: 10.1073/pnas.0601139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103(8):2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubota H, Brinster RL. Technology insight: In vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat Clin Pract Endocrinol Metab. 2006;2(2):99–108. doi: 10.1038/ncpendmet0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3(3):034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 23.Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23(4):397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 24.Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod. 1999;60(6):1429–36. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nur EKA, Ahmed I, Kamal J, Babu AN, Schindler M, Meiners S. Covalently attached FGF-2 to three-dimensional polyamide nanofibrillar surfaces demonstrates enhanced biological stability and activity. Mol Cell Biochem. 2008;309(1–2):157–66. doi: 10.1007/s11010-007-9654-8. [DOI] [PubMed] [Google Scholar]
- 26.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three dimensional nanofibrillar surfaces induce activation of Rac. Biochem Biophys Res Commun. 2005;331(2):428–34. doi: 10.1016/j.bbrc.2005.03.195. [DOI] [PubMed] [Google Scholar]
- 27.Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24(2):426–33. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 28.Oatley JM, Brinster RL. Spermatogonial stem cells. Methods Enzymol. 2006;419:259–82. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41(1):111–22. [PubMed] [Google Scholar]
- 30.Olie RA, Looijenga LH, Dekker MC, de Jong FH, van Dissel-Emiliani FM, de Rooij DG, van der Holt B, Oosterhuis JW. Heterogeneity in the in vitro survival and proliferation of human seminoma cells. Br J Cancer. 1995;71(1):13–7. doi: 10.1038/bjc.1995.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev. 2011;7(1):103–18. doi: 10.1007/s12015-010-9126-5. [DOI] [PubMed] [Google Scholar]
- 32.Rahjouei A, Kiani S, Zahabi A, Mehrjardi NZ, Hashemi M, Baharvand H. Interactions of human embryonic stem cell-derived neural progenitors with an electrospun nanofibrillar surface in vitro. Int J Artif Organs. 2011;34(7):559–70. doi: 10.5301/IJAO.2011.8511. [DOI] [PubMed] [Google Scholar]
- 33.Schindler M, Ahmed I, Kamal J, Nur EKA, Grafe TH, Young Chung H, Meiners S. A synthetic nanofibrillar matrix promotes in vivo-like organization and morphogenesis for cells in culture. Biomaterials. 2005;26(28):5624–31. doi: 10.1016/j.biomaterials.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Shahbazi E, Kiani S, Gourabi H, Baharvand H. Electrospun nanofibrillar surfaces promote neuronal differentiation and function from human embryonic stem cells. Tissue Eng Part A. 2011;17(23–24):3021–31. doi: 10.1089/ten.tea.2011.0121. [DOI] [PubMed] [Google Scholar]
- 35.Singh SR, Burnicka-Turek O, Chauhan C, Hou SX. Spermatogonial stem cells, infertility and testicular cancer. J Cell Mol Med. 2011;15(3):468–83. doi: 10.1111/j.1582-4934.2010.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290(2):193–200. doi: 10.1016/0027-5107(93)90159-D. [DOI] [PubMed] [Google Scholar]