Abstract
Purpose
To investigate the stability and repeatability of electrochemiluminescence immunoassay (ECLIA) for beta-hCG detection in embryo spent culture media. To evaluate the correlation between the viability of preimplantation embryo and beta-hCG profile by the new assay.
Methods
In a retrospective study, a total of 357 spent culture media from day1 to day5 were individually collected and quantified by ECLIA. The blank controls and reliability test were performed with normal saline/pure culture media.
Results
1) There was no detectable amount of beta-hCG in blank controls. A high degree of linearity (R2 = 0.995) was found in this study; intra-assay and inter-assay coefficient of variation were 4.87 % and 6.25 %. 2) A significantly higher concentration of beta-hCG was found at day5 group than it at day3 group, both in total samples (1.47 ± 0.68mIU/ml vs 0.55 ± 0.32mIU/ml) and in homologous embryo samples (1.43 ± 0.91mIU/ml vs 0.52 ± 0.23mIU/ml). 3) There was a positive correlation between beta-hCG concentration and implantation rate (r = 0.559 at day3 and 0.535 at day5) or blastocyst morphological grading (r = 0.411).
Conclusions
ECLIA may be an optimal choice for detecting beta-hCG in spent culture media to assess embryo viability, indicating secreted beta-hCG as a useful biomarker for embryo selection in IVF-ET procedure.
Keywords: beta-hCG, Electrochemiluminescence immunoassay, Spent embryonic culture media, Morphological grading, Implantation, IVF-ET
Introduction
In recent years, assisted reproductive technology (ART) has made great progress; the grading systems based on stage of embryo development have been the primary criteria to identify embryos with high competence for transfer. However, it is not always in accordance with embryo implantation potential, resulting in multiple embryos transferred as well as multiple pregnancies. As a result, other non-invasive methods such as evaluating the metabolomic profile of the embryonic spent culture media are necessary and urgent for embryo assessment.
Embryos resulted in pregnancy are reported to be different in their metabolomic profile compared with embryos that do not [1]. Moreover, beta-hCG was the first reported bio-marker embryonic secretion [2], which is primarily produced by the embryo and later by the syncytiotrophoblast. The hormone was detected at various levels in embryonic culture media from day2 to blastocyst stage by different assays [2–4], however, they were only used in research now. An electrochemiluminescence immunoassay (ECLIA) procedure was a method based on solid-phase sandwich immunoassay with advantages of the superior sensitivity of beta-hCG at 0.100mIU/ml in plasma or urine sample and every cycle only took 20 min, which was very popularly employed for diagnosis of conception and trophoblastic disease.
In this study, we modified the ECLIA for detecting and quantifying beta-hCG in spent embryo culture media from day1 to day5 after fertilization. We tried to explore whether the new method could be used for embryo competence assessment with beta-hCG as a biomarker in clinical application.
Materials and methods
Patients and embryo culture media
A total of 357 spent embryo culture media were individually collected for analyzing of beta-hCG, including day1 (9 samples), day3 (183 samples) and day5 (165 samples, in which 66 were used for the stability test) from 107 women undergoing IVF/ICSI treatment from Nov. 2011 to Jun. 2012. All patients were informed consent. Hospital Institutional Review Board approval was obtained for the research.
Embryos with 2 pronucleis on day1 were transferred to individual 25 μl micro-droplets of Vitrolife G1 (Vitrolife AB, Goteburg, Sweden) or Sage Cleavage (Quinn’s) for culture to the cleavage stage. The day3 embryos without transferring into the uterus were replaced into another 25 μl Vitrolife G2 or Sage Blastocyst for culture to blastocyst stage (day5). Embryo quality was assessed morphologically on day3 by a modified grading system [5] and the blastocyst morphology was graded according to Gardner and Schoolcraft [6]. These samples were collected individually and stored at −80 °C until beta-hCG detection. Another 24 samples of pure culture media (G1, G2, Cleavage and Blastocyst, respectively) incubated under the same conditions were kept frozen for analysis as well.
beta-hCG detection by electrochemiluminescence immunoassay
Each sample was diluted 1:2 with 40 μl normal saline (NS) because the minimal detected volume was 30 μl and normal saline served as the blank control. Roche cobas-e601 fully automated electrochemiluminescence immunoassay analyzer (Germany) whose sensitivity was at 0.100mIU/ml was employed to measure beta-hCG and every cycle took 20 min around. The original reagents were as followed.
Reagent M
Streptavidin-coated microparticles, 1bottle, 6.5 ml: Streptavidin-coated microparticles 0.72 mg/ml; preservative (Roche, Germany).
Reagent R1
Anti-hCG-Ab ~ biotin, 1 bottle, 9 ml: Biotinylated monoclonal anti-hCG antibodies (mouse) 2.6 mg/ml; phosphate buffer 40 mm/l, pH 7.5; preservative (Roche, Germany).
Reagent R2
Anti-hCG-Ab ~ Ru(bpy)32+, 1 bottle, 10 ml: Monoclonal anti-hCG antibody (mouse) labeled with ruthenium complex 4.6 mg/ml; phosphate buffer 40 mmol/l, pH 6.5; preservative (Roche, Germany).
For the monoclonal antibodies used, the following cross-reactivities were found: TSH: not detectable, LH 0.12 %, FSH <0.1 %. The antibody we employed was directly against beta-hCG rather than the whole CG molecule, however, it did not recognize hyperglycosylated CG.
Standards
Human chorionic gonadotropin (hCG) was obtained from Lizhu pharmaceutical factory, China. Standards were diluted in normal saline and ranged from 0 to 3 mIU/ml, stored at −80 °C.
Statistical analysis
Quantitative data were expressed as mean and 95 % confidence interval (CI) for mean. Differences between groups were assessed by Student’s t-test; paired t-test and ANOVA where appropriate. Spearman correlation between beta-hCG concentration and embryo implantation rate was employed. All analyses were performed using the SPSS13.0 package. P < 0.05 was considered statistically significant.
Results
Demographic characteristic
The mean age of women is 34.7 ± 5.8 years; the average number of oocytes retrieved, embryos at day3 and day5 are 11.7 ± 3.4, 6.2 ± 2.9 and 3.0 ± 1.1, respectively. A total of 61 blastocysts were transferred into the uterus cavity of 33 patients.
Reproducibility of the standard curve
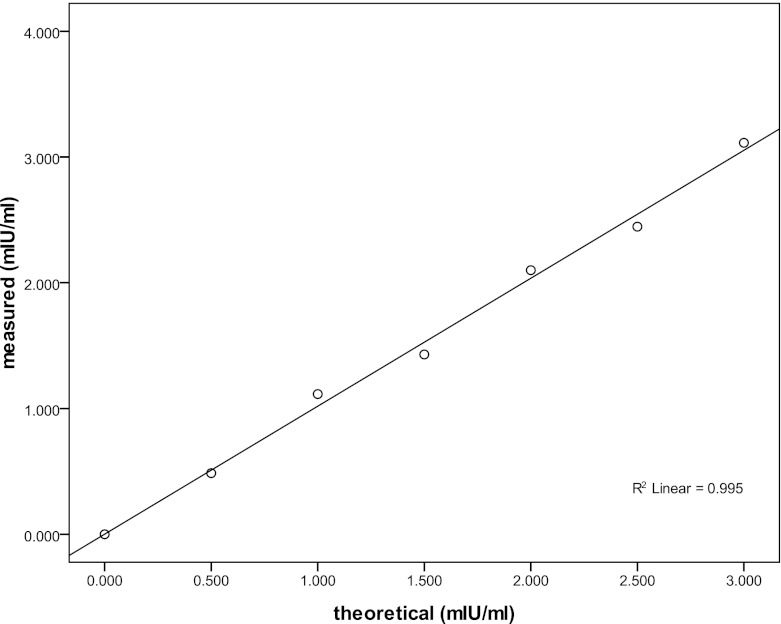
Standards were prepared ranging from 0 to 3mIU/ml. A polynomial regression study was performed to verify the linearity of ECLIA. Figure 1 presented the standard curve in the range of 0–3 mIU/ml by expressing the counts obtained from each calibrant, resulting in a regression line, which showed a high degree of linearity (R2 = 0.995).
Fig. 1.
Reproducibility of standard curve. (R2 = 0.995)
The detection of beta-hCG in blank controls and culture medium
There was no detectable amount of beta-hCG (<0.100mIU/ml) in NS, fresh pure G1,G2 medium and fresh pure Cleavage, Blastocyst medium, so as in pure G1, Cleavage medium incubated for 3 days and pure G2, Blastocyst medium incubated for 2 days.
Precision of beta-hCG ECLIA procedure
Reproducibility (intra-assay variability) and repeatability (inter-assay variability) of the assay was evaluated with culture medium spiked with beta-hCG in 15 consecutive assays on independent days. The intra- and inter-assay CVs at different levels of the standard curve were measured by the automated ECILA. Intra-assay CVs were 4.37 %, 5.21 %, 5.01 % and 4.89 % (average: 4.87 %). Inter-assay CVs were 6.26 %, 6.47 %, 5.83 % and 6.44 % (average: 6.25 %).
Stability of beta-hCG ECLIA procedure
In order to test the stability of beta-hCG in embryonic culture media after storing, 3 samples on day5 from the same women (n = 22) were mixed, then divide it into two new samples (one stored at RT and another at −80 °C) for analyzing the stability of beta-hCG in culture media after storing. There was no significant difference between the sample storing at RT and −80 °C, 1.528 ± 0.260 mIU/ml vs 1.544 ± 0.381mIU/ml, p = 0.420.
The profile of beta-hCG in embryonic spent culture media at different stage
Excluding the embryos which were group cultured, 357 spent single embryo culture media (in which 66 samples for the stability test) were collected individually for detecting beta-hCG by ECLIA procedure. Beta-hCG was found in culture media at day1(33.3 %, 3/9), day3(85.8 %, 157/183) and day5(99.0 %, 98/99). However, the beta-hCG was positive in 7 homologous samples at day5 culture media while it’s negative at day3 media from the same embryo (Table 1).
Table 1.
7 homologous samples whose beta-hCG was positive at day5 culture media while negative at day 3 media from the same embryo
| Samples No. | D3 embryo grade | D5 embryo grade | D5 β-hCG (mIU/ml) |
|---|---|---|---|
| 1 | 2.3/8 | compact | 0.516 |
| 2 | 2.3/6 | 4 cc | 1.270 |
| 3 | 2.2/8 | morula | 0.855 |
| 4 | 2.3/8 | 3 cc | 1.506 |
| 5 | 2.2/6 | 3cb | 2.001 |
| 6 | 2.3/6 | I | 1.101 |
| 7 | 2.2/7 | morula | 1.422 |
The average concentrations of beta-hCG in day1, day3 and day5 culture media were 0.22 ± 0.15mIU/ml, 0.55 ± 0.32mIU/ml, and 1.47 ± 0.68mIU/ml, respectively. The concentration of embryonic beta-hCG in homologous samples (n = 33 pairs) at day5 group was significantly higher than it in day3 group, 1.43 ± 0.91mIU/ml vs 0.52 ± 0.23mIU/ml, p < 0.001.
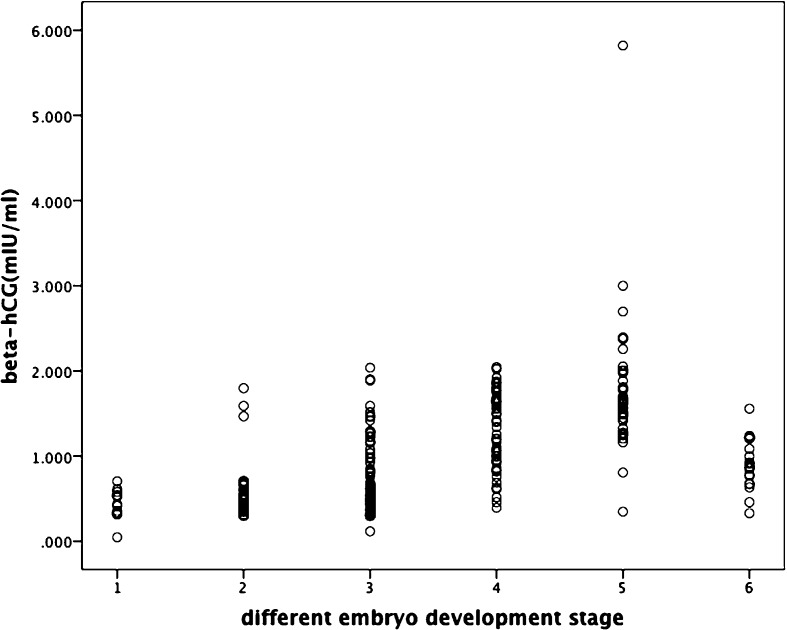
Figure 2 showed the details of beta-hCG in spent culture media of preimplantation embryos at different stage in vitro. The concentration of beta-hCG in spent culture media increased gradually along with the increasing number of blastomeres, but it decreased remarkably when embryos suffered from arresting.
Fig. 2.
The concentration of beta-hCG in spent embryo culture media at different stage 1:day1(2PN), 2:day3(5–8 cells), 3:day5(compact/morula), 4:day5(unexpanded blastocyst),5:day5 (expanded blastocyst), 6:day5(arrest)
Correlation between beta-hCG in spent culture media and the morphology assessment of pre-implantation embryo
The level of beta-hCG correlated positively with blastocyst morphological grading(r = 0.411, p < 0.001). A significantly higher beta-hCG concentration was detected in subgroups of blastocysts with expanded cavity or high trophoblast grading (A OR B) (p = 0.007). However, there was no statistical difference between blastocysts with high inner cell mass (ICM) grading (A OR B) and poor ICM (C or lower than C) (p = 0.101) (Table 2), as well as among embryos based on the number of blastomeres, fragmentation grading and blastomere size grading on day3 (Table 3).
Table 2.
The concentration of beta-hCG in day 5 spent culture medium in different subgroupsa
| Subgroup | No. of samples | beta-hCG (mIU/ml) | P value | |
|---|---|---|---|---|
| Blastocyst cavity grade of inner cell mass | Cavity-expanded | 46 | 1.66 [1.41, 1.90] | 0.007 |
| Cavity-unexpanded | 52 | 1.29 [1.16,1.42] | ||
| A OR B | 31 | 1.63 [1.27,1.99] | NSb | |
| C OR < C | 67 | 1.39 [1.27,1.50] | ||
| A OR B | 33 | 1.72 [1.39,2.06] | 0.007 | |
| C OR < C | 65 | 1.34 [1.22,1.45] | ||
a1 negative sample was not counted in Table 2; the data were expressed as mean and 95%CI grade of trophoblast
bNS, not significant
Table 3.
The concentration of beta-hCG in day 3 spent culture medium in different subgroupsa
| Subgroup | No. of samples | Beta-hCG (mIU/ml) | P value | |
|---|---|---|---|---|
| Number of blastomeres | 6-cell | 44 | 0.50 [0.42,0.59] | NSb |
| 7-cell | 50 | 0.58 [0.48,0.68] | ||
| 8-cell | 63 | 0.55 [0.47,0.63] | ||
| Fragment grading | Grade 1 | 62 | 0.51 [0.44,0.58] | NS |
| Grade 2 | 66 | 0.62 [0.53,0.72] | ||
| Grade 3 | 29 | 0.46 [0.41,0.50] | ||
| Morphology grading | Grade 2 | 153 | 0.55 [0.50,0.61] | ---c |
| Grade 3 | 4 | 0.39 [0.29,0.49] | ||
a26 negative samples were not counted in supplemental Table 1; the data were expressed as mean and 95%CI
bNS, not significant
cThe statistical analysis could not be performed because of the small sample size in subgroup “morphology Grade 3”
Correlation between beta-hCG in spent culture media and implantation
A total of 77 patients underwent embryo-transfer procedure, 44 patients on day3 and 33 patients on day5. A significantly higher beta-hCG level emerged in pregnant and high implantation rate (IR) women both on day3 and day5. The amount of beta-hCG correlated positively with implantation rate; the correlation coefficients were 0.559 (p < 0.001) in day3 and 0.535 in day5 (p = 0.001). (Table 4 and Table 5)
Table 4.
The average concentration of beta-hCG in pregnant group and non-pregnant groupa
| Day 3 | Day 5 | |||
|---|---|---|---|---|
| Pregnant (n = 18) | Non-pregnant (n = 26) | Pregnant (n = 15) | Non-pregnant (n = 18) | |
| beta-hCG (mIU/ml) | 0.58[0.45,0.72] | 0.39[0.36,0.42] | 1.92[1.63,2.22] | 1.32[1.03,1.60] |
| P value | 0.041 | 0.004 | ||
aThe data were expressed as mean and 95%CI
Table 5.
The average concentration of beta-hCG in different implantation rate groupa
| Implantation rate | beta-hCG (mIU/ml) | P value | |
|---|---|---|---|
| Day 3 | 0 % (n = 27) | 0.40 [0.36, 0.44] | <0.001b |
| 33.3 % (n = 6) | 0.45 [0.31, 0.59] | ||
| 50.0 % (n = 4) | 0.48 [0.37, 0.58] | ||
| 66.7 % (n = 3) | 0.57 [0.28, 0.87] | ||
| 100.0 % (n = 4) | 0.89 [0.65, 1.63] | ||
| Day 5 | 0 % (n = 18) | 1.32 [1.03, 1.61] | 0.046c |
| 33.3 % (n = 2) | 1.81 [1.27, 3.49] | ||
| 50.0 % (n = 4) | 1.76 [1.43, 2.08] | ||
| 66.7 % (n = 4) | 1.81 [1.33, 2.29] | ||
| 100.0 % (n = 5) | 2.20 [1.14, 3.27] | ||
aThe data were expressed as mean and 95%CI
bThe average concentration of beta-hCG in day3 samples differed significantly between group 0 % and 100 %, 33.3 % and 100 %, so as 50 % and 100 % when Bonferroni test was employed
cThe average concentration of beta-hCG in day5 samples differed significantly between group 0 % and 100 % when Bonferroni test was employed
Discussion
The assessment of embryo quality is fundamental to improve the pregnancy rate in IVF-ET treatment. It is known that the non-invasive morphological grading system is insufficient for choosing embryos with high competence [7], because it is not always in accordance with embryo development and implantation potential. At the same time, multiple pregnancies appear due to the transfer of more than one embryo, and this will cause socio-economic setbacks as well as numerous complications of mothers and babies. Moreover, the visual scoring of embryos is subjective; the intra-and inter-investigators agreement on the grading could be fair to poor [8]. As a result, other methods or techniques are in need for assessing embryo viability. Meanwhile, the biomarker detection of preimplantation embryos served as a useful and potential method in IVF-ET clinical procedure.
Beta-hCG was the first reported biomarker produced only by an embryo in the spent embryo culture media by Fishel and Edward et al. in 1983 [2]. The secretion was found by radioimmunoassay in Earle’s culture medium, in which the embryos had been cultured for 170–216 hours after fertilization, ranging from 25mIU/ml to 50mIU/ml, and the concentration rise up to 6033 mIU/ml in medium surrounding trophoblast outgrowth embryo. In 2011, beta-hCG was detected in day2 spent embryo culture media by ELISA [4], whose lowest detectable concentration was 1.4 pg/50 μl (0.255mIU/ml), which is within the range from day1 (0.22mIU/ml) to day 3 (0.55mIU/ml) groups in our study. However, these detection methods could only be employed in research due to the limitations such as more sample volume requirement, suboptimal sensitivity and dynamic range.
Electrochemiluminescence (or electrogenerated chemiluminescence, ECL) is a kind of means to emit measurable luminescent signals by converting electrochemical energy into radiative energy via an electrochemical reaction [9]. The reaction mixture is aspirated into the measuring cell where the microparticles are magnetically captured onto the surface of the electrode. Unbound substances are then removed with ProCell. Application of a voltage to the electrode then induces chemiluminescent emission, which is measured by a photomultiplier. Results are determined via a calibration and a master curve provided via the reagent barcode.
With ECLIA, a micro-amount of beta-hCG secreted by embryos was detected in spent culture media of day1, day3 and day5. Compared with other assays reported before [2–4], ECLIA could be automated and did not cause radioactive contamination, besides, its analytical precision (mean intra-assay CV: 4.87 %, mean inter-assay CV: 6.25 %) had been improved considerably, which indicated the high dependability in our study. In addition, there was no significant difference between samples in room temperature and stored at −80 °C, further supporting the stability of samples and ECLIA. Moreover, another powerful advantage that each cycle cost 20 min only made the beta-hCG detection in culture media in vitro become possible to be employed in clinical application.
A. Lopata et al. [3] discovered the heterogeneity of hCG released by blastocyst tissues 11–14 days following fertilization by a chromatofocusing method, which separates the isoforms of the gonadotrophin based on differences in their isoelectric points. This biochemical profile of beta-hCG molecules changed along with the differentiation of trophectoderm, which in turn might influence the biological activity of this molecule. In this study, 85.8 % of day3 samples and 99.0 % of day5 samples were detected embryonic beta-hCG secretion by ECL based immunoassay. Interestingly, there were 7 negative samples at day3, but positive when they reached further development at day5, which clarified that the undetectable value was owing to the stage of embryo development or the delayed secretion rather than the problem of ECILA itself. These together suggested the secretion of beta-hCG was significantly enhanced with the increasing number of human blastomeres and embryo development stage, which kept in accordance with what Woodward BJ reported [10]. Since embryonic hCG had different isomers in different development stages and current detecting methods could not reach the consistent experimental outcome, it lead to a lack of analysis about the relationship between embryo grading and beta hCG level. According to Sivakumar et al. [4], beta-hCG level from day 2 culture media was independent of the embryo grading in day3 and blastocyst formation. Woodward revealed that only 52 % of the blastocysts could secret hCG, and only 24 % of the original bipronucleate pre-embryos in vitro could be considered anatomically and biochemically competent [10]. Similarly, we did not find difference among subgroups based on the blastomere number, fragmentation grading and blastomere size of day3 samples, however, the blastocyst morphological grading was positively correlated with the beta-hCG concentration of day5 samples. Blastocysts with better morphology such as cavity or trophoblast had a higher beta-hCG concentration, but the difference could not occur between blastocysts with better and worse ICM; the explanation for this phenomenon might be that beta-hCG was secreted by trophectoderm and it had less relation with ICM. Furthermore, these results together illustrated that the embryonic beta-hCG secretion was consistent with morphological grading in blastocyst stage, and the detection of beta-hCG in culture media by ECLIA may become one of the biomarkers of embryonic development as well.
The metabolomic profile was different between embryos resulted in pregnancy and embryos that did not [1, 11]. Sivakumar R reported that the concentration of beta-hCG on day2 embryos did not rely on embryo development when they were transferred at blastocyst stage [4]. However, in this study, more than 1 embryos were transferred in the same cycle, so we had to calculate the average concentration of beta-hCG per embryo in each woman. It is meaningful that the embryos lead to successfully implantation and/or pregnancy showed higher beta-hCG levels in the culture medium, especially between embryos resulted in 100 % IR and those resulted in 0%IR, no matter transferred on day3 or day5. A positive correlation between beta-hCG and implantation rate further supported the view that embryonic beta-hCG could be a predictor of embryos that would implant.
Conclusion
Based on our knowledge, this study first presented a robust and highly sensitive ECILA to measure beta-hCG in embryo culture media, which had more strengths including the small sample requirement, speediness, high validity and reliability. Embryonic beta-hCG may be beneficial to identify embryos with higher strong implantation potential both on day3 and day5. Combined with morphological scoring systems and embryonic beta-hCG concentration may be a reasonable way for selecting competent human embryos in clinical IVF-ET procedure. But prospective and randomized large sample size studies are in demand, particularly in the single embryo transfer procedure.
Acknowledgments
Conflict of interest
The authors declared no conflict of interest.
Financial support
This study was supported by the Science Foundation of Guangdong Province (2009B030801022) and Nature Science Foundation of China (2008; no.30872762).
Footnotes
Capsule The concentration of beta-hCG detected by highly sensitive ECLIA in culture media positively correlated with blastocyst morphological grading and implantation ability, which may make it as a useful biomarker for assessing embryo viability in clinical procedure.
References
- 1.Seli E, Sakkas D, Scott R, Kwok SC, Rosendahl SM, Burns DH. Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil Steril. 2007;88:1350–7. doi: 10.1016/j.fertnstert.2007.07.1390. [DOI] [PubMed] [Google Scholar]
- 2.Fishel SB, Edwards RG, Evans CJ. Human chorionic gonadotropin secreted by preimplantation embryos cultured in vitro. Science. 1984;223:816–8. doi: 10.1126/science.6546453. [DOI] [PubMed] [Google Scholar]
- 3.Lopata A, Oliva K, Stanton PG, Robertson DM. Analysis of chorionic gonadotrophin secreted by cultured human blastocysts. Mol Hum Reprod. 1997;3:517–21. doi: 10.1093/molehr/3.6.517. [DOI] [PubMed] [Google Scholar]
- 4.Ramu S, Acacio B, Adamowicz M, et al. Human chorionic gonadotropin from day 2 spent embryo culture media and its relationship to embryo development. Ferti Steril. 2011;96(3):615–7. doi: 10.1016/j.fertnstert.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Veeck LL. An atlas of human gametes and conceptuses: An illustrated reference for assisted reproductive technology. New York: Parthenon Publishing; 1999. [Google Scholar]
- 6.Gardner DK, Schoolcraft WB, Jansen R, et al., editors. In vitro culture of human blastocyst. Toward reproductive certainty: Fertility and genetics beyond 1999: The plenary proceedings of the 11th worth congress on in vitro fertilization and human reproductive genetics. Pearl River, NY: Parthenon; 1999. pp. 378–88. [Google Scholar]
- 7.Macas E. Metabolic status of oocyte and IVF success- is there a relationship? J Fertil Reprod. 2006;4:16–8. [Google Scholar]
- 8.Paternet G, Wetzels AM, Thonon F, Vansteenbrugge A, Willemen D. Intra-and inter-observers analysis in the morphological assessment of early stage embryos during an IVF procedure: A multicentre study. Reprod Biol Endocrinol. 2011;9:127. doi: 10.1186/1477-7827-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bard AJ, editor. Electrogenerated chemiluminescence. New York: Marcel Dekker; 2004. [Google Scholar]
- 10.Woodward BJ, Lenton EA, Turner K, Grace WF. Embryonic human chorionic gonadotrophin secretion and hatching: Poor coorelation with cleavage rate and morphological assessment during preimplantation development in vitro. Hum Reprod. 1994;9(10):1909–14. doi: 10.1093/oxfordjournals.humrep.a138357. [DOI] [PubMed] [Google Scholar]
- 11.Botros L, Sakkas D, Seli E. Metabolomics and its application for non-invasive embryo assessment in IVF. Mol Hum Reprod. 2008;14:679–90. doi: 10.1093/molehr/gan066. [DOI] [PMC free article] [PubMed] [Google Scholar]