Abstract
Purpose
To avoid inducing a state of oxidative stress (OS), assisted reproductive technologies (ART) must maintain a balance of reactive oxygen species (ROS) and antioxidants during the in vitro culture of oocytes. However, oocyte requirements and tolerance thresholds for ROS during in vivo development are still unclear. Previous studies have examined ROS levels in follicular fluid (FF) using pooled samples or according to follicle size. This study sought to examine two OS markers, lipid hydroperoxides (LPO) and hydrogen peroxide (H2O2), in FF of individually sampled follicles from bovine ovary pairs according to follicle size, atresia, and dominance status.
Methods
TUNEL and cleaved Caspase-3 labeling were used to identify apoptotic granulosa cells and determine follicle atresia status. LPO were measured directly for the first time in FF.
Results
Non-atretic follicles and dominant follicles contained more FF H2O2 than atretic follicles and corresponding subordinate follicles, respectively. FF LPO did not vary in relation to atretic status, and no difference existed between dominant and subordinate follicles. However, FF LPO was significantly lower in first subordinate follicles than in the second subordinate follicles from each ovary pair. Neither H2O2 nor LPO levels correlated with follicle size.
Conclusions
These data provide clear evidence that the events of antral folliculogenesis are relevant to ROS dynamics in vivo. Furthermore, such studies will help to optimize in vitro conditions for oocyte culture protocols, particularly when combined with a comparison of oocyte quality with respect to source follicle characteristics.
Keywords: Follicle atresia, Follicle dominance, Reactive oxygen species, Lipid hydroperoxides, Follicular fluid, Hydrogen peroxide
Introduction
The capacity of an oocyte to be fertilized and create a viable embryo, also called oocyte quality, is achieved largely through developmental processes that occur prior to ovulation. While the oocyte progresses towards meiotic and developmental competence inside an antral follicle, the follicle itself changes dynamically throughout antral folliculogenesis. These processes are interdependent; for instance, the dominance and atresia statuses of an antral follicle are tied to oocyte quality (reviewed by [1]). Thus, to generate or identify high quality oocytes in assisted reproductive technologies (ART), the follicular milieu merits attention.
In species such as humans and cows, each menstrual or estrous cycle is characterized by two or three waves of antral follicle growth and regression, with the final wave producing an ovulatory follicle and oocyte. In each wave of follicle growth, a dominant follicle is selected from a group of growing follicles, while all other subordinate follicles in the group eventually regress through atresia [2]. The antral follicular milieu is especially relevant to in vitro maturation (IVM), an emerging technology for fertility treatment and preservation. For IVM, oocytes are retrieved during the protracted period of antral follicle growth and differentiation, during which time they would normally complete a pre-maturation developmental phase [1]. Despite attempts to enable the process ex vivo, the success of in vitro pre-maturation strategies and IVM are hindered by a scarcity of information regarding the specific biochemical requirements of oocytes during the relevant developmental windows. Indeed, implantation rates for IVM remain below those of standard in vitro fertilization (IVF) treatment [3]. Data characterizing the biochemical dynamics of antral follicles throughout antral folliculogenesis may help to tailor in vitro environments for oocyte development and to close the gap between IVM and IVF.
Reactive oxygen species (ROS) may contribute to the relative inferiority of oocyte quality during in vitro ART procedures as compared to natural in vivo development [4]. ROS are oxygen-containing molecules with unpaired electrons, and include superoxide anion, hydrogen peroxide (H2O2), and the hydroxyl radical. They can be generated by a variety of factors unique to in vitro environments, and can cause cellular damage if present above vital threshold levels (i.e. oxidative stress or OS) (reviewed by [5]). The oxidation of lipids by ROS can produce excessive levels of lipid hydroperoxides (LPO), a highly reactive, dysfunctional lipid with a tendency to degrade into cytotoxic molecules [6, 7].
No consensus exists regarding the ROS or LPO levels conducive to producing a high quality oocyte in vitro or in vivo. Although an upper limit of acceptable ROS concentration in human follicular fluid (FF) has been proposed [8], studies of FF collected during IVF cycles have conflicting results, reporting both positive and negative correlations between pre-ovulatory FF ROS levels and pregnancy outcomes [9, 10].
The relationship between LPO levels in pre-ovulatory FF collected from IVF cycles and oocyte maturation, fertilization, or pregnancy rates is similarly convoluted [8, 9, 11, 12]. Furthermore, these studies measure LPO concentration indirectly, by measuring levels of LPO degradation products; primarily the aldehydes malondialdehyde (MDA) and 4-hydroxy-2-nonenal (HNE). As MDA and HNE are neither produced exclusively by LPOs, nor are they the only LPO products possible, inferring LPO concentrations by this method is potentially unreliable and may be a cause of conflicting data [7].
To our knowledge, no studies thus far have presented a profile of LPO levels in FF with respect to individual follicle size, atresia, or dominance status. Prior studies suggest that ROS may be subject to regulation, or serve a regulatory purpose, during antral folliculogenesis. For instance, FF H2O2 concentration decreases as follicle size increases in the bovine [13] and pig [14]. But prior evaluations of H2O2 are limited to follicle size. Further, the human samples from previous studies possess limitations, including sample pooling and failure to consider relevant follicle characteristics. Our work thus takes advantage of the bovine as a well-established model for studying human reproduction, since both species share several significant characteristics in antral folliculogenesis and oocyte development [15]. This report provides the first research documenting LPO and H2O2 levels in bovine FF corresponding to individual follicle size, atresia, and dominance status.
Materials and methods
All reagents were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A) unless specified otherwise.
Collection of bovine antral follicles, follicular fluid, and granulosa cells
Ovaries of naturally cycling Holstein-Friesian heifers (approximately 30 months of age) were collected from a slaughterhouse (Champlain Beef Co., Whitehall, NY, U.S.A.) with a permit issued by the United States Department of Agriculture. Ovaries were transported in medium containing 0.9 % NaCl and 1X antibiotic/antimycotic at approximately 4 °C, with a transit time of 45 minutes from slaughterhouse to laboratory. Antral follicles were dissected from ovaries with an active corpus luteum. The three largest follicles within an ovary pair were individually dissected and placed in dissection medium (Hank’s balanced salt solution minimal essential medium, supplemented with 25 mM HEPES, 1 mM sodium pyruvate, 2 mM L-glutamine, 4 μg/ml bovine serum albumin, 100 μg/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml heparin). Diameters of each follicle were recorded to the nearest tenth of a millimeter.
FF was aspirated from each follicle individually and immediately placed on ice. All FF samples were centrifuged for 4.5 min at 5,400 × g to remove blood and cells. Supernatants of each sample were examined under light microscopy for absence of contaminating cells. All FF samples were analyzed immediately without freezing.
After FF collection, each follicle shell was bisected and the contents flushed with gentle agitation. Granulosa cells (GCs) were fixed immediately after collection in 2 % paraformaldehyde in phosphate buffered saline (PBS) for 15 minutes, followed by extraction in 0.2 % Triton in PBS for 30 minutes, and then blocked in WASH solution (0.2 % Azide, 0.2 % nonfat powdered milk, 2 % normal donkey serum, 1 % BSA, 0.1 M glycine). Samples were stored at 4 °C.
Hydrogen peroxide (H2O2) assay
H2O2 concentrations in FF were measured using the Colorimetric Hydrogen Peroxide Kit (Assay Designs; Ann Arbor, MI, U.S.A.). In this assay, a reaction involving a color reagent produces a color whose intensity is directly proportional to the concentration of H2O2 in the sample. All reagents were allowed to warm to room temperature for at least 30 minutes. Standards (106.25, 212.5, 425, 850, 1,700, and 3,400 ng/ml H2O2) were prepared using the kit’s 100,000 ng/ml standard and a sample diluent (50 mM phosphate, pH 6.0). FF from follicles greater than 6 mm in diameter were diluted 1:4 in sample diluent, while FF from smaller follicles were diluted between 1:8 and 1:15 depending on the volume available (with all readings falling within the dynamic range of the standard curve). Standards and diluted samples were run in duplicate. The samples were read at an optical density of 550 nm. H2O2 concentrations were calculated from the linear regression equation of the standard curve.
Lipid hydroperoxide (LPO) assay
The LPO assay was adapted from an LPO assay kit supplied by Cayman Chemical Company (Ann Arbor, MI, U.S.A.) to accommodate small sample volumes of FF. Adaptations to the original kit protocol will be noted as such, and were instituted only after consultation with Cayman Chemical Company. This assay directly measures LPO concentrations in fluid samples. Only FF samples greater than 75 μl were analyzed due to volume requirements of this assay. All assay preparations occurred in glass tubes with polypropylene caps, and efforts were made to limit exposure of chloroform (septum-sealed anhydrous) to air at all stages of the assay. To a known volume of FF from a single follicle, an equal volume of Extract-R saturated methanol (100 mg Extract R/15 ml methanol) and a double volume of cold chloroform (cooled on ice) were added. If there was less than 200 μl of FF in a tube, more chloroform was added so that the chloroform and FF totaled 800 μl (assay adaptation). After centrifugation at 0 °C, a water layer, a protein layer, and a chloroform layer (from top to bottom) were present in each tube. The bottom chloroform layer was collected without contamination from other layers.
To each sample of chloroform extract, a 2:1 chloroform-methanol solution was added such that the total volume for each sample was 950 μl (assay adaptation). Standards were prepared to 0, 0.5, 1.5, and 2.0 nmol LPO from an LPO standard (50 μM ethanolic solution of 13-hydroperoxyoctadecadienoic acid). Chromagen was prepared by combining and vortexing equal volumes of FTS Reagent 1 (4.5 mM FeSO4 in 0.2 M HCl) and FTS Reagent 2 (3 % methanolic solution of ammonium thiocyanate). To each assay tube, 50 μl of chromagen was added and vortexed. Capped tubes were left at room temperature for 5 minutes. Samples were read at an optical density of 500 nm using a glass well plate. Each sample was run in duplicate. Concentration of LPO was determined using the linear regression equation of the standard curve.
Immunocytochemistry: cleaved Caspase-3 and TUNEL dual-labeling
Two markers were used to detect apoptosis in granulosa cells of antral follicles: terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), and activated cleaved Caspase-3 as an earlier marker of apoptosis. Granulosa cells from individual antral follicles were incubated with 2.5 μg/ml anti-cleaved Caspase-3 antibody (rabbit polyclonal; Cell Signaling; Danvers, MA, U.S.A.) diluted in WASH-blocking solution (undiluted WASH-blocking solution was used for controls) for 12–14 hours at 4 °C. Cells were then incubated in 5 μg/ml secondary Alexa-Fluor 594 donkey anti-rabbit antibody (Life Technologies; Carlsbad, CA, U.S.A.) diluted in WASH-blocking solution for 1.5 hours at 37 °C. A positive control was created with 50 U/ml DNase I (Promega; Madison, WI, U.S.A.) diluted in WASH-blocking solution. All samples save for the negative control were then transferred to a TUNEL solution (1:100 solution of terminal deoxynucleotidyl transferase in fluorescein-dUTP) from an in situ cell death detection kit (Fluorescein; Roche; Indianapolis, IN, U.S.A.). The negative control was treated with undiluted fluorescein-dUTP instead. Samples were incubated at 37 °C for 60 min, prior to labeling with 0.5 μg/ml DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) for 1 hour at 37 °C. Cells from each sample were mounted in mounting medium (50 % glycerol, 50 % PBS, 25 mg/mL Na-Azide), sealed, and stored at −20 °C.
Fluorescence microscopy
TUNEL, cleaved Caspase-3, and DAPI labels were visualized under fluorescence microscopy (Fig. 1). A quadrant of the field of view was haphazardly selected with DAPI and two hundred cells were counted. The cells staining positive for Caspase-3 and TUNEL were then counted. GC samples containing >10 % signal-positive cells for either Caspase-3 or TUNEL were characterized as belonging to an atretic follicle while those containing <10 % signal-positive cells for either activated Caspase-3 or TUNEL were characterized as belonging to non-atretic follicles. Images were then taken to confirm counts. There was an 88.5 % concordance between markers (n = 130).
Fig. 1.
Detection of total DNA (blue), TUNEL (green), and cleaved Caspase-3 (red) in granulosa cells from three different antral follicles (a–c). These three examples show an increased proportion of apoptotic cells from panels a through c, with no detectable apoptosis in a. Scale bar: 10 μm
Follicle dominance classification
The atretic status of each follicle was compared to that of the other follicles from its ovary pair. If the largest of the three follicles collected from each ovary pair was atretic followed by two non-atretic follicles, or if all follicles in a set were non-atretic, the largest non-atretic follicle was determined to be partially dominant (i.e., the future dominant follicle; selection has not yet occurred). If the largest follicle was non-atretic while the smaller two were atretic, the largest follicle was determined to be fully dominant. If all follicles were atretic, the set was classified as regressing. In each set, the follicles that were smaller than the dominant or partially dominant follicle were categorized as subordinate follicles. These criteria are based upon an approach applied by Irving-Rodgers et al. [16–18].
Statistical analysis
Two-tailed Pearson’s correlation tests were used to examine the relationships between follicle diameter and H2O2 or LPO concentration. When comparing means, Mann–Whitney non-parametric tests were used. Box plots were used to graphically represent the data, with boxes indicating the 25th to 75th percentile of data and the line within the box indicating the median. Whiskers represent the range of data.
To assess variation in H2O2 or LPO across follicle dominance status for the three largest follicles from each cow, a repeated-measure design was chosen; potential variability among cows could thus be accounted for. The data were log-transformed to achieve a normal distribution and a repeated-measures analysis of variance (ANOVA) was used. P values of less than 0.05 were considered significant and all analyses were performed with PASW Statistics 18 (SPSS Inc., Chicago, IL, U.S.A.)
Results
H2O2 and LPO concentrations with respect to follicle size and atresia
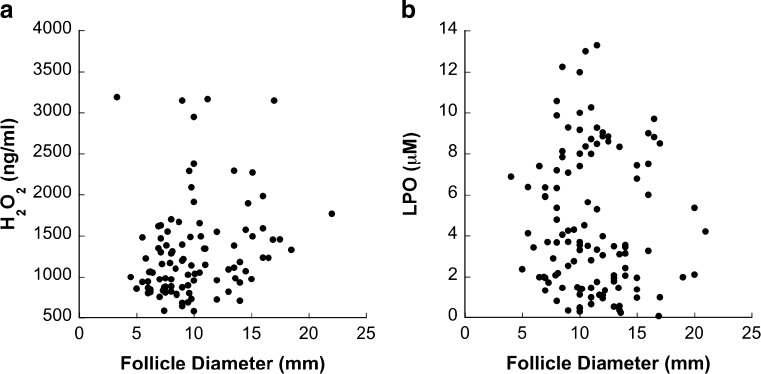
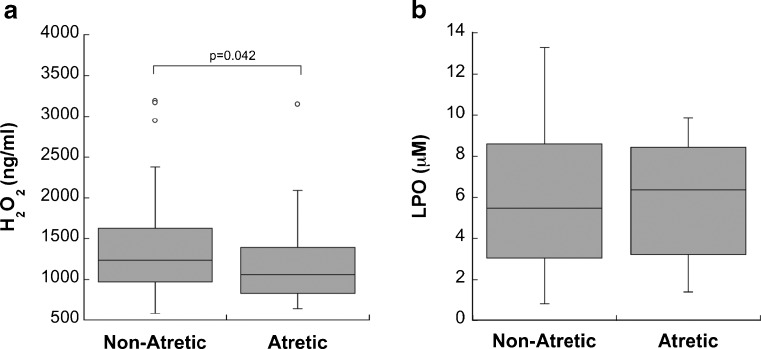
While there was no significant correlation between follicle size and FF H2O2 concentration (n = 103 follicles, diameter range 3.3–22 mm) (Fig. 2a), non-atretic follicles contained significantly more FF H2O2 than did atretic follicles (p = 0.042, n = 53 non-atretic follicles, n = 50 atretic follicles) (Fig. 3a).
Fig. 2.
H2O2 (a) and LPO (b) concentrations in follicular fluid across antral follicle growth (n = 103 and 117, respectively). There was no significant correlation between follicle size and H2O2 or LPO concentration
Fig. 3.
A comparison of H2O2 (a) and LPO (b) concentrations in non-atretic and atretic follicles. H2O2 concentration was significantly higher in non-atretic follicles than in atretic follicles (p = 0.042, n = 53 non-atretic follicles, n = 50 atretic follicles). There was no significant difference in LPO concentration between non-atretic and atretic follicles (n = 54 non-atretic follicles, n = 24 atretic follicles)
There was no significant correlation between FF LPO concentration and follicle size (n = 117 follicles, diameter range 4–21 mm) (Fig. 2b). There was also no difference in FF LPO concentration between non-atretic and atretic follicles (n = 54 non-atretic follicles, n = 24 atretic follicles) (Fig. 3b).
H2O2 and LPO concentrations with respect to follicle dominance status
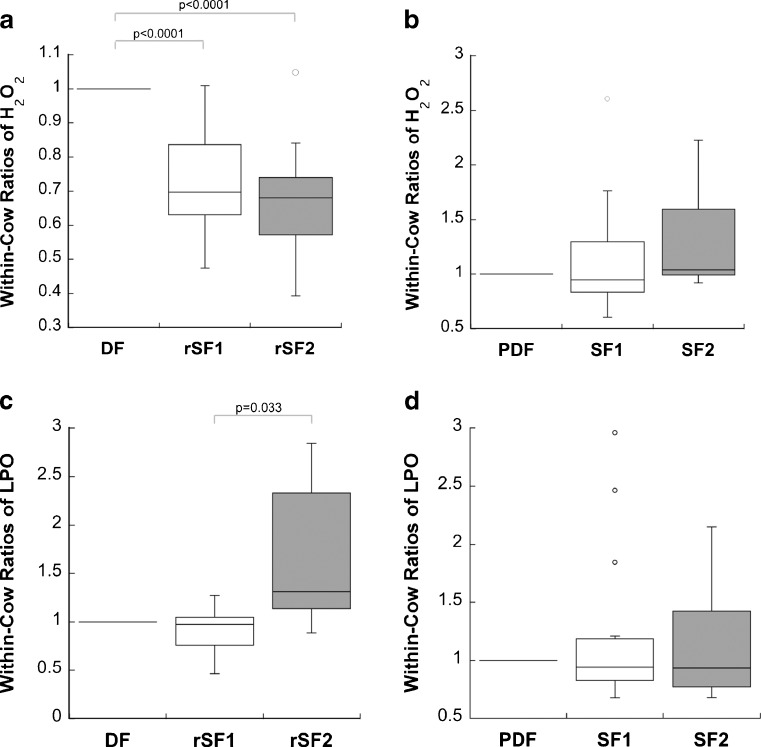
Dominant follicles contained significantly higher levels of FF H2O2 than did their corresponding subordinate follicles (p < 0.0001 for both subordinate follicles, n = 14 cows) (Fig. 4a). Partially dominant follicles and their subordinate follicles did not significantly differ in FF H2O2 concentrations (n = 15 cows) (Fig. 4b).
Fig. 4.
Proportional within-cow comparisons of H2O2 or LPO concentrations with respect to follicle dominance status. Ratios were derived for each follicle trio using the H2O2 or LPO concentration in the largest follicle as the base value. Dominant follicles (DF) are followed by regressing subordinate follicles (rSF1, rSF2) in (A) and (C), while partially dominant follicles (PDF) are followed by subordinate follicles (SF1, SF2) in (B) and (D). H2O2 concentrations were significantly higher in dominant follicles compared to their corresponding subordinate follicles (p < 0.0001 for both subordinate follicles, n = 14 cows) (a), whereas H2O2 concentrations in partially dominant follicles did not significantly differ from that in their subordinate follicles (n = 15 cows) (b). No significant difference existed in LPO concentrations between dominant follicles and the corresponding subordinate follicles (n = 7 cows). However, a significant difference in LPO concentration was found in these follicle sets between the largest subordinate follicles and the smaller subordinate follicles (p = 0.033) (c). No difference existed between partially dominant follicles and their subordinates (n = 15 cows) (d)
Repeated-measures ANOVA tests showed no significant difference in FF LPO concentrations between dominant follicles and their corresponding subordinate follicles. However, a significant difference existed between the FF LPO concentrations of the larger subordinate follicle (first subordinate) and the smaller subordinate follicle (second subordinate) in each fully dominant follicle set (p = 0.033, n = 7 cows) (Fig. 4c). There were no significant differences between partially dominant follicles and their subordinates (n = 15 cows) (Fig. 4d).
Discussion
Our study shows that the FF milieu of dominant follicles is characterized by elevated levels of H2O2. Yet at a time prior to the establishment of full dominance, partially dominant follicles do not exhibit differences in H2O2 from subordinate follicles—a pattern which may point to a role for H2O2 in the pre-ovulatory preparations of follicle and oocyte. Of relevance are previous studies showing that ovulation rates and ovulatory events are perturbed upon ROS reduction in the mouse and rabbit [19, 20]. Thus, an increase in H2O2 in dominant follicles may be, in part, a preparatory step for ovulation that commences upon dominant follicle selection. The full scope of regulation and the roles for H2O2 during follicle dominance remains to be elucidated through further study.
The dynamics of H2O2 during follicular atresia also merit further examination, as non-atretic follicles exhibited elevated H2O2 in FF compared to atretic follicles. This pattern is interesting given that H2O2 is known to participate in signaling caspase activation and apoptosis initiation in somatic cells [21, 22]. It is possible that ROS levels in FF differ from those within the granulosa cells themselves, for reasons including the vascular (in addition to follicular) origins of FF, and the potentially limited diffusion efficiency of ROS across cell membranes [23–25]. In particular, the progressive exchange between blood serum and FF during follicle growth and vascularization may provide a source of H2O2 that atretic follicles—which are no longer growing—lack.
It is relevant to note that some of the variation in our FF H2O2 and LPO concentration data is likely due to the cumulative effects of several factors that guide ROS production and regulation, which we did not monitor in this study. For instance, whether antioxidant concentrations vary in response to changing ROS concentrations within the follicle remains unknown. Levels of enzymatic antioxidants such as superoxide dismutase and catalase were shown to vary in FF across follicular size categories in the cow [13, 26]. The onset of atresia in bovine dominant follicles was linked to an increase in mRNA expression of superoxide dismutase and glutathione peroxidase in granulosa cells, albeit no changes in enzymatic antioxidant activities [27]. Nonetheless, relative differences in antioxidant activities between dominant and subordinate follicles were not studied, with a sole focus on antioxidant levels in dominant follicles over time. Thus, the profiling of follicular antioxidants in relation to follicular size, dominance, and atresia has not yet been reported. Additionally, although our study did not distinguish between degrees of atresia, early versus late atresia may have differing effects on the concentration of H2O2 in FF. Dominant follicles and early atretic follicles share morphological and biochemical characteristics [28–30], and contain high quality oocytes relative to non-dominant and non-atretic follicles, respectively [30, 31]. However, as atresia progresses and the follicular environment deteriorates, oocyte quality plummets. A detailed determination of ROS dynamics during the progression of follicle atresia thus deserves further attention.
This study measured LPO levels directly for the first time in FF. Although they are clearly present, LPO levels do not vary in relation to follicle size, atresia, or dominance status. This may indicate that lipid peroxidation is tightly controlled by each follicle throughout antral folliculogenesis. One probable mechanism for this control is enzymatic reduction by glutathione peroxidase. GPx-4 protein, an isoform of glutathione peroxidase largely responsible for reducing LPO in vivo [32], is present in bovine FF (unpublished findings).
There is some evidence to suggest that the lipid content of FF may vary within or between individual cows. As lipids represent the substrate from which LPO are created, FF lipid concentrations may contribute to some of the variability seen in our FF LPO concentration data. Studies thus far have demonstrated differences in FF fatty acid and lipoprotein concentrations between individual cows and follicles, respectively [33, 34]. Since oocyte lipid stores are accumulated from the follicular surroundings, FF lipid content and LPO concentrations may have a direct effect on oocyte development.
FF LPO levels were significantly lower in the first subordinate follicles (largest subordinate) than in the second subordinate follicles of fully dominant follicle sets. This trend may be a consequence of characteristics unique to the first subordinate follicle. Notably, the first subordinate follicle maintains a slightly higher concentration of estradiol and IGF-1 than the second subordinate follicle [35]. Estradiol has antioxidant capacities, and suppressing estradiol in ewes resulted in follicular tissue lipid peroxidation (inferred by MDA concentration) [36], while IGF-1 has protective effects against lipid peroxidation in various organs of the rat [37]. Although the reported estradiol and IGF-1 difference between first and second subordinate follicles is small [35], it may influence FF LPO levels to some degree. Further information regarding the biochemical and redox profiles of antral follicles with respect to dominance status is necessary to draw further conclusions from these data.
It is clear from the data collected in this study that H2O2 and LPO, although both products of oxidative reactions, vary differently during antral folliculogenesis. This is not surprising, as these molecules are distinct from one another in many ways. While H2O2 is a natural by-product of myriad metabolic processes and enzymatic reductions of other ROS, LPO is primarily a result of oxidative attack. They each play distinct roles in cellular signaling, and are reduced by different enzymatic antioxidants—thus the regulation of one is not directly related to the other. Additionally, their molecular compositions, and therefore the substrates for their creation, are entirely different [38–40]. Consequently, as antral follicles develop, the concentrations of these molecules in FF will vary according to separate, although not exclusive, pathways. The biological differences between LPO and H2O2, and the distinctions reported in this study, highlight the importance of evaluating multiple avenues of OS.
Previous studies of FF have pooled follicles from the same animal together, thus approaching their questions about FF content on a whole-body scale [8, 9, 11, 12]. However, the overall variability in FF ROS levels between individual follicles seen in this study emphasizes the importance of examining follicles individually. Thus, the evaluation of oocyte quality during ART may benefit from an examination of the follicle from whence the oocyte came. The success of in vitro development and maturation strategies will likewise be improved when the biochemical profile of dominant, non-atretic follicles is better defined. To contribute to these approaches, future studies can investigate potential links between oocyte quality and individual follicle characteristics, such as those examined in this study. In particular, the comparison of oocyte quality with the FF ROS profiles of their individual antral follicles will be an important next step in defining optimal pre-maturation and IVM culture conditions.
OS is a concern for ART. Our study provides the first FF H2O2 and LPO profiles with respect to follicle atresia and dominance status, which will help to identify H2O2 and LPO levels that correspond specifically with non-atretic dominant follicles and, in turn, competent oocytes. Our data also serve as direct evidence that the events of antral folliculogenesis, beyond changes in follicle size alone, are relevant to consider when studying ROS in vivo and when optimizing protocols for the development of oocytes in vitro.
Acknowledgments
We thank Richard Single (Department of Mathematics and Statistics, University of Vermont) and Sallie Sheldon for statistical advice; Jim Larrabee and Roger Sandwick (Department of Chemistry and Biochemistry, Middlebury College) for assistance with technical and chemical adjustments to the lipid peroxidation assay; and Tom Sheluga for laboratory support. This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2011-67016-20041 from the USDA National Institute of Food and Agriculture.
Footnotes
Capsule
The follicular fluid milieu of dominant and non-atretic follicles is characterized by elevated levels of hydrogen peroxide, while lipid hydroperoxides don’t vary according to follicle size, atresia, and dominance status.
References
- 1.Hennet ML, Combelles CMH. The antral follicle: a microenvironment for oocyte differentiation. Int J Dev Biol. 2013;In Press [DOI] [PubMed]
- 2.Scaramuzzi RJ, Baird DT, Campbell BK, Driancourt MA, Dupont J, Fortune JE, et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev. 2011;23:444–67. doi: 10.1071/RD09161. [DOI] [PubMed] [Google Scholar]
- 3.Nogueira D, Sadeu JC, Montagut J. In vitro oocyte maturation: current status. Semin Reprod Med. 2012;30:199–213. doi: 10.1055/s-0032-1311522. [DOI] [PubMed] [Google Scholar]
- 4.Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18:864–80. doi: 10.1016/S1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combelles CMH, Hennet ML. Oxidative stress in assisted reproductive technologies. In: Agarwal A, Rizk B, Aziz N, editors. Studies on women’s health: oxidative stress in applied basic research and clinical practice. New York: Humana Press, Springer Science and Business Media; 2013. pp. 205–36. [Google Scholar]
- 6.Dotan Y, Lichtenberg D, Pinchuk I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog Lipid Res. 2004;43:200–27. doi: 10.1016/j.plipres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S–24. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 8.Jana SK, K NB, Chattopadhyay R, Chakravarty B, Chaudhury K. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol. 2010;29:447–51. [DOI] [PubMed]
- 9.Das S, Chattopadhyay R, Ghosh S, Ghosh S, Goswami SK, Chakravarty BN, et al. Reactive oxygen species level in follicular fluid–embryo quality marker in IVF? Hum Reprod. 2006;21:2403–7. doi: 10.1093/humrep/del156. [DOI] [PubMed] [Google Scholar]
- 10.Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, et al. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45:314–20. [PubMed] [Google Scholar]
- 11.Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, et al. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973–6. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Jozwik M, Wolczynski S, Jozwik M, Szamatowicz M. Oxidative stress markers in preovulatory follicular fluid in humans. Mol Hum Reprod. 1999;5:409–13. doi: 10.1093/molehr/5.5.409. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Choi A, Yu HY, Czerniak SM, Holick EA, Paolella LJ, et al. Fluctuations in total antioxidant capacity, catalase activity and hydrogen peroxide levels of follicular fluid during bovine folliculogenesis. Reprod Fertil Dev. 2011;23:673–80. doi: 10.1071/RD10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basini G, Simona B, Santini SE, Grasselli F. Reactive oxygen species and anti-oxidant defences in swine follicular fluids. Reprod Fertil Dev. 2008;20:269–74. doi: 10.1071/RD07147. [DOI] [PubMed] [Google Scholar]
- 15.Aerts JM, Bols PE. Ovarian follicular dynamics. A review with emphasis on the bovine species. Part II: antral development, exogenous influence and future prospects. Reprod Domest Anim. 2010;45:180–7. doi: 10.1111/j.1439-0531.2008.01298.x. [DOI] [PubMed] [Google Scholar]
- 16.Irving-Rodgers HF, Harland ML, Sullivan TR, Rodgers RJ. Studies of granulosa cell maturation in dominant and subordinate bovine follicles: novel extracellular matrix focimatrix is co-ordinately regulated with cholesterol side-chain cleavage CYP11A1. Reproduction. 2009;137:825–34. doi: 10.1530/REP-08-0485. [DOI] [PubMed] [Google Scholar]
- 17.Irving-Rodgers HF, Krupa M, Rodgers RJ. Cholesterol side-chain cleavage cytochrome P450 and 3beta-hydroxysteroid dehydrogenase expression and the concentrations of steroid hormones in the follicular fluids of different phenotypes of healthy and atretic bovine ovarian follicles. Biol Reprod. 2003;69:2022–8. doi: 10.1095/biolreprod.103.017442. [DOI] [PubMed] [Google Scholar]
- 18.Irving-Rodgers HF, van Wezel IL, Mussard ML, Kinder JE, Rodgers RJ. Atresia revisited: two basic patterns of atresia of bovine antral follicles. Reproduction. 2001;122:761–75. doi: 10.1530/rep.0.1220761. [DOI] [PubMed] [Google Scholar]
- 19.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011;108:1462–7. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki T, Sueoka K, Dharmarajan AM, Atlas SJ, Bulkley GB, Wallach EE. Effect of inhibition of oxygen free radical on ovulation and progesterone production by the in-vitro perfused rabbit ovary. J Reprod Fertil. 1991;91:207–12. doi: 10.1530/jrf.0.0910207. [DOI] [PubMed] [Google Scholar]
- 21.Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414:552–6. doi: 10.1016/S0014-5793(97)01068-5. [DOI] [PubMed] [Google Scholar]
- 22.Stridh H, Kimland M, Jones DP, Orrenius S, Hampton MB. Cytochrome c release and caspase activation in hydrogen peroxide- and tributyltin-induced apoptosis. FEBS Lett. 1998;429:351–5. doi: 10.1016/S0014-5793(98)00630-9. [DOI] [PubMed] [Google Scholar]
- 23.Mishina NM, Tyurin-Kuzmin PA, Markvicheva KN, Vorotnikov AV, Tkachuk VA, Laketa V, et al. Does cellular hydrogen peroxide diffuse or act locally? Antioxid Redox Signal. 2011;14:1–7. doi: 10.1089/ars.2010.3539. [DOI] [PubMed] [Google Scholar]
- 24.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50:225–32. doi: 10.1095/biolreprod50.2.225. [DOI] [PubMed] [Google Scholar]
- 26.Combelles CM, Holick EA, Paolella LJ, Walker DC, Wu Q. Profiling of superoxide dismutase isoenzymes in compartments of the developing bovine antral follicles. Reproduction. 2010;139:871–81. doi: 10.1530/REP-09-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valdez KE, Cuneo SP, Turzillo AM. Regulation of apoptosis in the atresia of dominant bovine follicles of the first follicular wave following ovulation. Reproduction. 2005;130:71–81. doi: 10.1530/rep.1.00430. [DOI] [PubMed] [Google Scholar]
- 28.Kruip TA, Dieleman SJ. Intrinsic and extrinsic factors influencing steroid production in vitro by bovine follicles. Theriogenology. 1989;31:531–44. doi: 10.1016/0093-691X(89)90238-0. [DOI] [PubMed] [Google Scholar]
- 29.Assey RJ, Hyttel P, Greve T, Purwantara B. Oocyte morphology in dominant and subordinate follicles. Mol Reprod Dev. 1994;37:335–44. doi: 10.1002/mrd.1080370313. [DOI] [PubMed] [Google Scholar]
- 30.Blondin P, Sirard MA. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Dev. 1995;41:54–62. doi: 10.1002/mrd.1080410109. [DOI] [PubMed] [Google Scholar]
- 31.Salamone DF, Adams GP, Mapletoft RJ. Changes in the cumulus-oocyte complex of subordinate follicles relative to follicular wave status in cattle. Theriogenology. 1999;52:549–61. doi: 10.1016/S0093-691X(99)00151-X. [DOI] [PubMed] [Google Scholar]
- 32.Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–69. doi: 10.1016/S0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 33.Bender K, Walsh S, Evans AC, Fair T, Brennan L. Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction. 2010;139:1047–55. doi: 10.1530/REP-10-0068. [DOI] [PubMed] [Google Scholar]
- 34.Andersen MM, Kroll J, Byskov AG, Faber M. Protein composition in the fluid of individual bovine follicles. J Reprod Fertil. 1976;48:109–18. doi: 10.1530/jrf.0.0480109. [DOI] [PubMed] [Google Scholar]
- 35.Beg MA, Bergfelt DR, Kot K, Ginther OJ. Follicle selection in cattle: dynamics of follicular fluid factors during development of follicle dominance. Biol Reprod. 2002;66:120–6. doi: 10.1095/biolreprod66.1.120. [DOI] [PubMed] [Google Scholar]
- 36.Lund SA, Murdoch J, Van Kirk EA, Murdoch WJ. Mitogenic and antioxidant mechanisms of estradiol action in preovulatory ovine follicles: relevance to luteal function. Biol Reprod. 1999;61:388–92. doi: 10.1095/biolreprod61.2.388. [DOI] [PubMed] [Google Scholar]
- 37.Kokoszko A, Dabrowski J, Lewinski A, Karbownik-Lewinska M. Protective effects of GH and IGF-I against iron-induced lipid peroxidation in vivo. Exp Toxicol Pathol. 2008;60:453–8. doi: 10.1016/j.etp.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–3. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 39.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE Signal Transduct Knowl Environ. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 40.Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun. 2005;338:668–76. doi: 10.1016/j.bbrc.2005.08.072. [DOI] [PubMed] [Google Scholar]