Abstract
Purpose
Histone H3 lysine 9 (H3K9) methylation plays an important role in the regulation of preimplantation embryo development. G9a has been reported to be a major H3K9mono (m1)/dimethylation(m2) methyltransferase and to contain nuclear localization signals. This study was performed to investigate the correlation between H3K9 methylation level and G9a localization when the nuclear membrane undergoes periodic reconstruction in the cell cycle during preimplantation embryo development.
Methods
The fluorescence intensity was examined via immunofluorescence. The mRNA expression of G9awas determined using real-time reverse transcriptase (RT)-PCR. Eight-cell embryos were cultured in KSOM supplemented with nocodazole (0.5 μM) for 12 h.
Results
In this study, it was observed that the fluorescence intensity of H3K9m2 and G9a began to increase significantly from the 4-cell stage and reached the peak at the morula stage (p < 0.001), but the fluorescence intensity declined to 4-cell-stage levels when it reached the blastula stage. We observed a similar pattern when we examined G9a mRNA expression. Once the nuclear membrane disintegrated, G9a and H3K9m1 were not detectable by immunofluorescence; when it was reconstructed, G9a and H3K9m1 had relocated to the cell nucleus. However, no significant change was observed in the H3K9m2 localization or in the G9a mRNA level (p > 0.05) during the whole process. JHDM2A was consistently localized in the cytoplasm irrespective of the presence or absence of a nuclear membrane.
Conclusion
These results indicate dynamic changes in the expression level of H3K9m2 and G9a as preimplantation embryogenesis progresses. G9a co-localized with H3K9 m1 in a nuclear membrane-dependent manner during mouse preimplantation embryo development.
Keywords: G9a, Histone H3 lysine 9 methylation, JHDM2A, Nuclear membrane, Mouse preimplantation embryo development
Introduction
Eukaryotic genetic information is stored in chromatin, which consists of genomic DNA, histones, and wide array of chromosomal proteins. Chromatin templates undergo various post-translational modifications to regulate many distinct biological functions [1, 2]. Methylation of histone H3 lysine 9 (H3K9) to mono(m1)-, di(m2)-, and tri(m3)-methylated, is a well-conserved epigenetic mark that has a crucial role in the early development of the embryo and of the pluripotency of embryonic stem cells [3].
In mammals, dynamic reprogramming of epigenetic events begins during gametogenesis and continues through embryogenesis [4]. DNA methylation at the symmetrical dinucleotide CpG by DNA methyltransferase are important in the epigenetics of embryonic reprogramming events [5]. Shortly after a sperm fertilizes an egg, the paternal genome rapidly undergoes genome-wide active DNA demethylation and remains demethylated following multiple rounds of cell division. During this time, the maternal genome undergoes gradual passive demethylation. Recently, new methylation patterns have been recognized during the development of the blastocyst [6]. In addition, more and more investigators have started to focus on the relationship between DNA methylation and histone modification. G9a is a histone lysine methyltransferase (HKMT) that can methylate histone H3K9 [7]. Studies have reported that G9a plays a role in the regulation of DNA methylation in the process of DNA replication [8], in developmental reprogramming [9], and in the establishment of mouse embryonic stem cells (mESCs) [10]. When extensive DNA demethylation occurred during the preimplantation stage of mouse embryos, the H3K9 methylation pattern changed, and the reason for this change is unknown. Biochemically, G9a can catalyze mono-, di-, and tri-methylation reactions in H3K9 [11]. Studies of G9a-deficient cells have demonstrated that G9a is a major H3K9me1 and H3K9me2 HKMT of euchromatin [12–14]. In addition, it has been reported that G9a has two nuclear localization signals (NLSs) and that their deletion leads diffuse nuclear distribution [15]. It is still unknown whether G9a localization changes periodically during the periodic reconstruction of the cell nuclear membrane in the process of preimplantation embryo development, and whether it influences the methylation state of H3K9. Additionally, JHDM2A, also known as Jmjd1a and Kdm3a, is as a histone lysine demethylase (HKDM) that selectively demethylates H3K9m1 and -m2, with a preference for H3K9m2 in vivo [16]. The role of JHDM2A in the regulation of H3K9 methylation during periodic reconstruction of the cell nuclear membrane also remains unknown. Thus in this study, we investigated the dynamic pattern of H3K9 methylation and G9a during mouse preimplantation embryo development. We studied nocodazole-treated 8-cell embryos to confirm the localization of G9a/JHDM2A and H3K9m1/H3K9m2 during periodic reconstruction of the nuclear membrane in the cell cycle.
Materials and methods
Animals
The specific-pathogen-free (SPF) grade CD-1 (ICR) outbred mice were housed in the Laboratory Animal Center of the Institute of Genetics and Developmental Biology, Chinese Academy of Science. The study procedures adhered to and were consistent with the Guide of the Institute of Genetics and Developmental Biology for the Care and Use of Laboratory Animals, Chinese Academy of Science.
Collection and preservation of oocytes and embryos
Metaphase II oocytes and embryos (pronuclear (PN), 2-cell, 4-cell, 8-cell, morula, and blastocyst) were collected from the oviducts or uteri of 8–12-week-old female CD-1 (ICR) mice after sequential injection of 7.5 IU pregnant mare serum gonadotrophin (PMSG) and 7.5 IU human chorionic gonadotropin (HCG). Adherent cumulus cells were removed by hyaluronidase treatment, and the cumulus-cell–free oocytes and embryos were cultured in KSOM (Millipore, Bellerica, MA, USA) augmented with amino acids as described by Doherty et al. [17].
Nocodazole treatment
A concentrated stock solution of nocodazole (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide at 5 mM and stored at 4 °C. Eight-cell embryos were cultured in KSOM supplemented with nocodazole (0.5 μM) for 12 h under liquid paraffin at 37 °C under a 5 % CO2 and 95 % air atmosphere [18]. Nocodazole-treated 8-cell embryos were further cultured in nocodazole-free KSOM medium for 30 min.
Quantitative real-time PCR
A total of 150 ~ 200 embryos were pooled for each sample of embryos developed in vivo (containing different stages) and stored at −80 °C until RNA extraction. Total RNA was extracted from each set of 150 ~ 200 embryos using TRIzol (Invitrogen, Grand Island, NY, USA) and treated with Rnase-free DNaseI (TURBO DNA-free kit; Ambion, Invitrogen). Quantitative RT-PCR (qRT-PCR) was performed with a Light Cycler thermocycler (ABI Prism 7900HT; Applied Biosystems, Foster City, CA, USA). Each reaction mixture consisted of 1.5 ~ 2 embryo equivalents of cDNA template, 500 nM of each primer, and 1x SYBR Green I Master Mix (Qiagen, Hilden, Germany) in a 20-μl reaction volume. A negative control (H2O replacing cDNA template) and a positive control (liver cDNA) were always included in each run. An initial 15 min at 95 °C was followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. At the end of the run, a melting-curve analysis was performed to confirm the PCR product. Triplicates of each sample were simultaneously amplified, and the results were averaged to constitute one independent replicate. A threshold cycle (CT) standard curve was constructed using serial 10-fold dilutions of a known concentration of template (prepared by PCR from embryo cDNA). The number of mRNA copies per embryo was determined using the absolute quantification method according to the manufacturer’s instructions. Amplicon size was confirmed by agarose gel electrophoresis. All data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primer pairs used were as follows: G9a (forward primer: 5′-TCGGGCAATCAGTCAGACAG-3′; reverse primer: 5′-TGAGGAACCCACACCATTCAC -3′), GAPDH (forward primer: 5′-AGGAGCGAGACCCCCTAACAT-3′; reverse primer: 5′-GTGAAGACACCAGTAGACTCCACG-3′).
Immunofluorescence
The embryos were compared by placing two different consecutive developmental stages under the same cover slip and photographing them in pairs (e.g., PN and 2-cell embryo, 2-cell and 4-cell embryo, 4-cell embryo and 8-cell embryo, etc.). By placing them side by side, we ensured the comparison of fluorescence intensity between different developmental stages. Furthermore, the fluorescence intensity at different stages of one embryo was standardized to correct the error between embryos.
The method used for immunofluorescence was adapted from the work of Santos and colleagues [19], with some modifications. All steps were performed at room temperature, unless otherwise mentioned. The collected oocytes/embryos were fixed with 4 % paraformaldehyde for 30 min, and then permeabilized with 0.1 % Triton X-100 for 30 min in PBS. After being washed three times, all samples were incubated overnight in a blocking solution (4 % bovine serum albumin (BSA) and 0.01 % Tween-20 in phosphate buffered saline (PBS) at 4 °C. Then oocytes/embryos were incubated overnight at 4 °C with the primary antibodies to H3K9 mono-methylation (dilution 1:250 Abcam (Cambridge, UK)), H3K9 di-methylation (dilution 1:250, Abcam), G9a (dilution 1:200, Invitrogen (Carlsbad, CA, USA)), and JHDM2A (dilution 1:200, Invitrogen). (All of the primary antibodies were generated in rabbits except G9a, which was generated in mice.) After being washed three times, the oocytes/embryos were incubated for 1 h with a goat anti-rabbit second antibody (dilution 1:1000, Alexa Fluor® 594 Dye, Invitrogen) and goat anti-mouse second antibody (dilution 1:1000, Alexa Fluor® 488 Dye). Finally, the DNA was stained with Hoechst 33258 (10 μg/ml), and all the samples were mounted in anti-fade solution. At least 10 oocytes/embryos were processed for each separate sample, and the experiments were replicated at least three times.
Confocal microscopy and statistical analyses
Stained embryos mounted on slides were observed on a LSM 510 META microscope (Zeiss, Heidenheim, Germany) using the excitation wavelengths of 488 nm and 594 nm. Each channel signal was collected sequentially. For each experiment, the same detector gain, amplifier offset, and pinhole parameters were used. The fluorescence intensity of all collected images was quantified using Adobe Photoshop software (Adobe Systems, San Jose, CA), without any adjustment of contrast and brightness to the images. All data analyses were conducted using SPSS 13.0 software (SPSS Inc, Chicago, IL). Statistical significance (P-value) was analyzed using one-way ANOVA, where P < 0.05 was considered to be statistically significant.
Results
Dynamic pattern in H3K9m2 during mouse preimplantation embryo development
To accurately quantify the H3K9m2 and G9a change during the mouse preimplantation embryo development, the embryos were compared by placing two different developmental stages under the same cover slip and photographing them in pairs (e.g., PN and 2-cell embryo, 2-cell and 4-cell embryo, 4-cell embryo and 8-cell embryo, etc.). By placing them side by side, we ensured the comparison of fluorescence intensity between different developmental stages. Furthermore, the fluorescence intensity at different stages of one embryo was standardized to correct the error between embryos. This eliminated the potential error from different batches of repeated experiments and from personal error during confocal imaging.
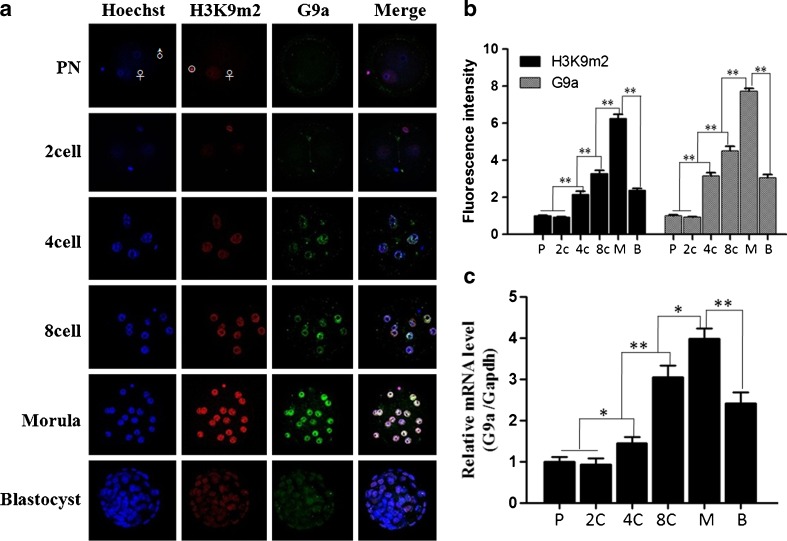
Overall, the fluorescence intensity of H3K9m2 and G9a showed a dynamic change as the embryos progressed. The fluorescence intensity of H3K9m2 and G9a was relatively weaker before the 2-cell stage, and it significantly increased from the 4-cell stage (p < 0.001), reaching its peak value at the morula stage (p < 0.001). But during the blastocyst stage, the fluorescence intensity decreased to the levels of the 4-cell stage (p = 0.12)(Fig. 1a and b). Further, we examined the G9a mRNA expression using real-time RT-PCR, which indicated that there was a similar pattern between the fluorescence intensity of H3K9m2 and G9a and G9a mRNA expression (Fig. 1c). In addition, the fluorescence intensity of H3K9m2 was significantly lower than that of the non-degraded polar body (Fig. 1, white circle) at the PN stages, which demonstrates that the decreased fluorescence intensity of H3K9m2 was specific for the embryonic cells.
Fig. 1.
Dynamic pattern of H3K9m2and G9a expression at different stages during mouse preimplantation embryo development. a. Immunofluorescence image of H3K9m2 and G9a at different stages during mouse preimplantation embryo development. b. Quantitative analysis of fluorescence intensity of H3K9m2 and G9a at different embryonic stages during mouse preimplantation embryo development. The white circle indicates the non-degraded polar body. c. G9a mRNA relative abundance was examined at different stages during mouse preimplantation embryo development by real-time-PCR. The fluorescence intensity of H3K9m2 and G9a at PN stage was defined as “1”, and the fluorescence intensity at other stages were compared with it. All values are presented as the mean ± SD of three independent experiments. Bars denote SD. *P < 0.05;**P < 0.01. Note: P: PN; 2c: 2-cell stage; 4c:4-cell stage; 8c:8-cell stage; M: morula stage; B: blastocyst
Inconsistent localization of H3K9m2 and G9a when cell nuclear membrane of egg or zygote disintegrated
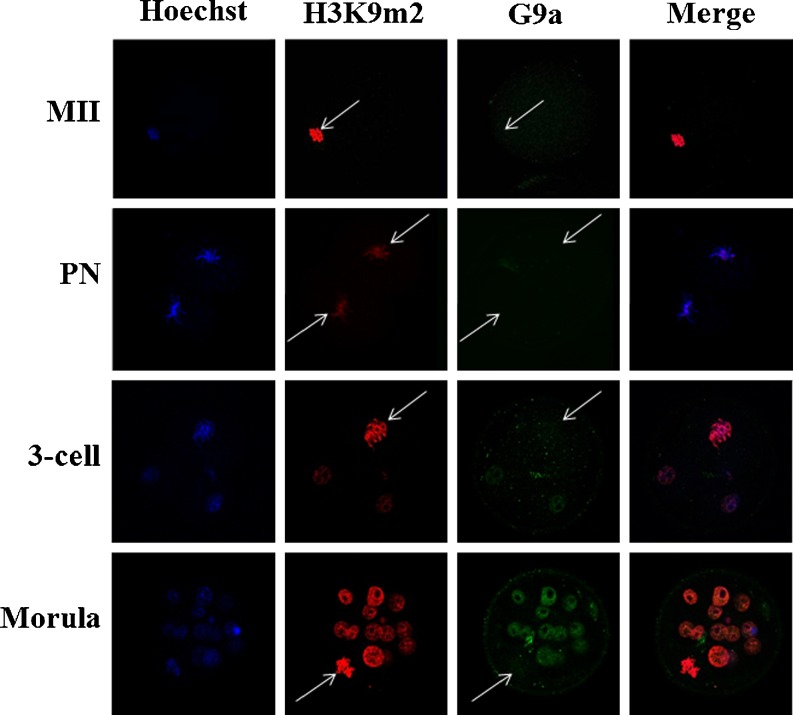
Mouse G9a is well known as a major H3K9m1/m2 methyltransferase. In this study, when the cell nuclear membrane disintegrated, the G9a was detected neither in eggs nor the zygote at different development stages (Fig. 2). However, G9a relocated in the cell nucleus as the cell cycle proceeded. To confirm that H3K9m2 had the same pattern, with G9a as the nuclear membrane during periodic reconstruction in the cell cycle, double-antibody staining of G9a and H3K9m2 were performed. Surprisingly, no periodic localization changes occurred in H3K9m2 (Fig. 2).
Fig. 2.
Co-localization of H3K9m2 and G9a in metaphase blastomere cell of a mouse embryo at different developmental stages. The white arrow indicates the metaphase chromosome
G9a specifically regulated H3K9m1 in a nuclear membrane-dependent manner
Thereafter, the state of H3K9m1 was studied when the G9a localization periodically changed as the cell cycle proceeded. Eight-cell embryos were selected for observation because of their relatively higher fluorescence intensity of H3K9 methylation (Fig. 1a) and the moderate blastomere number. In the nocodazole-treated identical 8-cell embryos, the cell cycle was not completely synchronous in different blastomeres: Some still had nuclear membrane, while the nuclear membranes of the others had disintegrated and their chromosomes had diffused into the cytoplasm. G9a was distinctly located in the cell nuclei of blastomeres with nuclear membrane and was not detected in blastomeres with disintegrated nuclear membranes. However, the localization and fluorescence intensity of H3K9m2 were not affected by the presence or absence of the nuclear membrane. We continued to culture the nocodazole-treated 8-cell embryos in KSOM solution without nocodazole for 30 min until they progressed into metaphase or anaphase, then we conducted double-antibody staining of G9a and H3K9m2. The results show that G9a was not detected in all of the blastomeres, but no significant change was observed in H3K9m2 (Fig. 3a). A similar method was applied to determine the correlation between G9a and H3K9m1 localization. The results indicate that co-localization was present between G9a and H3K9m1, both of which were nuclear membrane-dependent, as the cell cycle proceeded (Fig. 3b). Compared with the control, there was no significant difference in the G9a mRNA expression level of nocodazole-treated 8-cell embryos irrespective of the presence or absence of a nuclear membrane (Fig. 3c, p > 0.05).
Fig. 3.
Localization of G9a and H3K9m1/m2 and their correlation with the nuclear membrane. a. Immunofluorescence image of relationship between localization of G9a and H3K9m2 and the nuclear membrane. b. Immunofluorescence image of the relationship between localization of G9a and H3K9m1 and the nuclear membrane. c. G9a mRNA was examined at 8-cell embryos treated with nocodazole by real-time-PCR. Control indicates the untreated 8-cell embryos; nocodazole indicates the 8-cell embryo treated with nocodazole (0.5 μM) for 12 h; nocodazole-2 indicates 8-cell embryo treated with nocodazole (0.5 μM) for 12 h and then cultured in nocodazole-free KSOM medium for another 30 min
No correlation between H3K9m1 demethylation and JHDM2A when the nuclear membrane disintegrated
The double-antibody staining of G9a and JHDM2A in nocodazole-treated 8-cell embryos was performed to determine whether H3K9m1 demethylation was regulated by JHDM2A demethylase. The results indicate that JHDM2A was consistently located in the cytoplasm, did not enter into the cell nucleus, and had no association with H3K9m1 demethylation (Fig. 4).
Fig. 4.
Co-localization analysis between G9 and JHDM2A after the nuclear membrane disintegrated. Control shows the untreated 8-cell embryo; nocodazole represents 8-cell embryo treated with nocodazole (0.5 μM) for 12 h; nocodazole-2 indicates 8-cell embryo treated with nocodazole (0.5 μM) for 12 h and then cultured in nocodazole-free KSOM medium for another 30 min
Discussion
The results of our experiments represent the dynamic pattern of change of H3K9m2 and G9a during the mouse preimplantation embryo development. In addition, our results also reveal that G9a can periodically regulate H3K9m1 in a nuclear membrane-dependent manner.
Nocodazole has been proven to have the least side effects on mouse embryo development compared with other commonly used cell-cycle arrest agents, such as 6-dimethylaminopurine (6-DMAP) and aphidicolin [20]. The treatment concentration and time of nocodazole varies significantly between the embryo and the somatic cells [21]. Additionally, different treatment concentrations of nocodazole are effective among the early embryos of different species, such as pig (100 μM) [22], cattle (0.33 μM) [23], goat (0.3 μg/ml) [24], rabbit (0.4 μg/ml) [5], and Xenopus (10 μg/ml) [25]. In mouse embryos, 10 μM nocodazole was used in early studies [26–28]. One study has demonstrated that high concentration and long-term nocodazole treatment resulted in chromosomal abnormality or even embryo-lethal mutants [29]. A 0.05–0.5 μM nocodazole treatment blocked the cell cycle, but it did not damage embryonic development [30]. Therefore, 0.5 μM nocodazole was selected in this study to treat 8-cell mouse embryos. This treatment not only blocked the cell cycle at pre-metaphase but also allowed cells to continue to develop into the blastula stage once cultured in KSOM without nocodazole. Moreover, the developmental rate of embyros treated with nocodazole was not significantly different from that of untreated embryos cultured in vitro, which further demonstrates that treatment of nocodazole is reversible and safe to pre-implantation embryo development (nocodazole treated 8-cell embryos develop into blastocysts in 24 h when cultured in KSOM without nocodazole).
Development after fertilization mainly depends on the translation of the maternal mRNA until the later 2-cell stage, when the zygotic genome is activated and supports further embryo development. Affymetrix microarrays have been used to characterize global patterns of genes expression that accompany the development of preimplantation embryos of mice [31, 32]. The results were as follows: the expression profiles of oocytes and 1-cell embryos were very similar, presumably because the mRNA complement of the 1-cell embryo was inherited from the oocyte. A major reprogramming of gene expression occurred concomitant with zygotic genome activation (ZGA) during the 2-cell stage, and the expression profile of the 2-cell embryos differed markedly from that of oocytes/1-cell embryos and 8-cell embryos/blastocysts. In mice, ZGA was concomitant with extensive epigenetic remodeling of the parental genomes into the newly formed embryos [33]. Our results reveal that the fluorescence intensity of H3K9m2 and G9a began to significantly increase from the 4-cell stage and reached the peak value at the morula stage, but the fluorescence intensity decreased to the levels of the 4-cell stage during the blastocyst stage. An epigenetic remodeling phenomenon during mouse preimplantation embryo development may be indicated, which might be involved in ZGA regulation. Another study indicated that an epigenetic modification was associated with transcriptional repressive genes in fertilized embryos [6]. DNA methylation levels gradually declined after the 2-cell stage, reached the minimum level at morula stage, and were restored to 2-cell-stage levels during the blastocyst stage.
Gene expression is well known as an orderly and controllable process. When the zygotic gene began to translate after the 2-cell stage, the DNA methylation level significantly decreased to support gene transcription. However, if there were no other regulated mechanisms controlling zygotic genome expression at the same time, the gene expression would be out of control and, further, detrimental to embryo development. Therefore, combined with our results, it was assumed that the reverse methylation pattern of H3K9 (compared with DNA methylation) played a crucial role in the regulation of zygotic gene expression when a genomic DNA methylation was absent. Once the DNA methylation level was restored, the H3K9 methylation level subsequently declined, and DNA methylation resumed a major role in the regulation of genomic gene expression.
In addition, these results also show that, regardless of eggs or fertilized embryos, G9a was not detected by immunofluorescenceonce the nuclear membrane disintegrated. However, when the nuclear membrane reformed, the G9a re-localized into the cell nucleus. We further confirmed the phenomenon in the nocodazole-treated 8-cell embryos using immunofluorescence (Fig. 3a and b). There was no significant difference between nocodazole-treated and untreated 8-cell embryos when the G9a mRNA expression was examined using real-time RT-PCR (Fig. 3c). Therefore, we speculated that the disappearance of G9a (i.e., could not be detected by immunofluorescence) when the nuclear membrane disintegrated may have been caused by the following reasons. The first reason, the mouse G9a contained two nuclear localization signals(NLS) [15], so, it would diffuse into the whole cytoplasm since unable localize to the nuclear membrane, and thus was not detected by immunofluorescence due to low concentration. The second reason, the G9a protein maybe regulated by some post-transcriptional regulation. We prefer to support the first speculation, not only because the mouse indeed contained two NLS but also because these signals were conserved in mammals [15]. Even in Drosophila, the nuclear localization signal of G9a has been identified [34], which suggests that the cell nucleus localization of G9a is highly conserved and that its own biological function is very important. In addition, in evolutionary terms, an organism would not produce a protein and then degrade it repeatedly within the limited time frame because it would be an extreme waste of biosynthetic energy [35]. And the regulated mechanism of a protein always evolves into an economic design under the pressure of nature selection [36, 37].
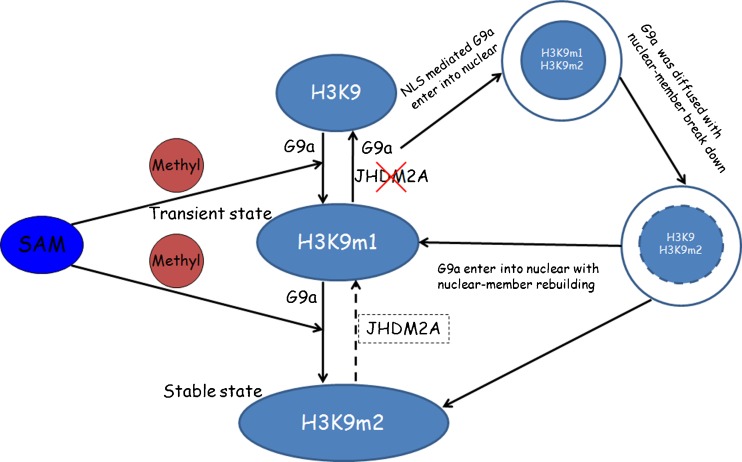
Our further studies have shown consistent localization between H3K9m1 and G9a, both of which were accompanied by periodic reconstruction of the nuclear membrane. In contrast, H3K9m2 did not display a similar trend, which is not in agreement with the previous study of G9a function [38]. Tachibana and his colleagues showed that the modification levels of both H3K9m1 and -m2 were significantly reduced in G9a-deficient germ cells. This may have resulted from the fact that G9a first catalyzed H3K9m1 and then H3K9m2. Between the two, H3K9m1 was the intermediate status, being the precondition for H3K9m2 formation, which represents a stable state. Therefore, knockout of G9a resulted in the decrease of both H3K9m1 and -m2 due to the inability to catalyze H3K9m1. In addition, one study has reported that JHDM2A is a specific demethylase for H3K9m1 and -m2 [10]. However, the results of this study show that JHDM2A was not involved in the demethylation process of H3K9m1 when the nuclear membrane disintegrated. Therefore, on the basis of this study, the following hypothesis was formulated: In the process of early embryo development, G9a periodically accumulates in the cell nucleus as the periodic reconstruction of the nuclear membrane proceeds. The H3K9m1 modification is completed using S-adenosyl methionine (SAM) as a methyl donor [39]. During this process, G9a plays a role in transferring a methyl to H3K9 and sustaining the H3K9m1; that is, G9a methylates H3K9m1, and the demethylation process of H3K9m1 occurs in the absence of G9a (not associated with JHDM2A). H3K9m1 is further methylated into H3K9m2 by G9a, which is a more stable histone modification. In early embryonic development, gene expression is co-managed by complementary methylation levels of H3K9 and DNA [6] to ensure normal embryo development (Fig. 5). To evaluate the biological function of H3K9m1 in a future study, we aim to introduce Bix-01294, a selective, reversible inhibitor of G9aHMTase that specifically suppresses G9a activity at different stages during preimplantation embryo development. Additionally, signaling pathway of G9a periodic change will also be identified.
Fig. 5.
Hypothesis for the mechanism of regulation of H3K9 methylation by G9a and JHDM2A during mouse preimplantation embryo development
Acknowledgments
This work is supported by the National Key Basic Research (973) program of China (Grant No. 2007CB948101) and Scientific and Technological Developing Scheme of Shaanxi Province (Grant No. 2012K17-02-03). We thank Dr. Lei Pan from Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for skillful technical assistance. We also appreciate the valuable comments from other members of our laboratory.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule
G9a specifically regulates histone H3 lysine 9 monomethylation in a nuclear membrane-dependent manner during mouse preimplantation embryo development.
Bo Li and Na Tang contributed equally to this work.
Contributor Information
Xiaohong Wang, Phone: +86-29-84777690, FAX: +86-29-84777690, Email: xhwang_1968@126.com.
Fangzhen Sun, Email: fzsun_1962@126.com.
References
- 1.Strahl B, Allis C. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 2.Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code? Curr Opin Genes Dev. 2005;15:163–176. doi: 10.1016/j.gde.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Loh YH, Zhang WW, Chen X, George J, Ng HH. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007;21:2545–2557. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabot R. Chromatin remodeling and embryo development. Biol Reprod. 2010;83:98. [Google Scholar]
- 5.Pickard B, Dean W, Engemann S, Bergmann K, Fuermann M, Jung M, Reis A, Allen N, Piotrowska K, Modliński JA, Korwin-Kossakowski M, Karasiewicz J. Effects of preactivation of ooplasts or synchronization of blastomere nuclei in G1 on preimplantation development of rabbit serial nuclear transfer embryos. Biol Reprod. 2000;63:677–682. doi: 10.1095/biolreprod63.3.677. [DOI] [PubMed] [Google Scholar]
- 6.Wu SC, Zhang Y. Active DNA demethylation: many roads lead to rome. Nat Rev Mol Cell Biol. 2010;9:607–620. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem. 2001;276:25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- 8.Estève PO, Chin HG, Smallwood A, Feehery GR, Gangisetty O, Karpf AR, Carey MF, Pradhan S. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Gene Dev. 2006;20:3089–3103. doi: 10.1101/gad.1463706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myant K, Termanis A, Sundaram AY, Boe T, Li C, Merusi C, Burrage J, de Las Heras JI, Stancheva I. LSH and G9a/GLP complex are required for developmentally programmed DNA methylation. Genome Res. 2011;21:83–94. doi: 10.1101/gr.108498.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Leung DC, Dong KB, Maksakova IA, Goyal P, Appanah R, Lee S, Tachibana M, Shinkai Y, Lehnertz B, Mager DL, Rossi F, Lorincz MC. Lysine methyltransferase G9a is required for de novo DNA methylation and the establishment, but not the maintenance, of proviral silencing. PNAS. 2011;108:5718–5723. doi: 10.1073/pnas.1014660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins RE, Tachibana M, Tamaru H, Smith KM, Jia D, Zhang X, Selker EU, Shinkai Y, Cheng X. In vitro and in vivo analyses of a Phe/Tyr switch controlling product specificity of histone lysine methyltransferases. J Biol Chem. 2005;280:5563–5570. doi: 10.1074/jbc.M410483200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, Fukuda M, Takeda N, Niida H, Kato H, Shinkai Y. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters AH, Kubicek S, Mechtler K, O’Sullivan RJ, Derijck AA, Perez-Burgos L, Kohlmaier A, Opravil S, Tachibana M, Shinkai Y, Martens JH, Jenuwein T. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell. 2003;12:1577–1589. doi: 10.1016/S1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 14.Yokochi T, Poduch K, Ryba T, Lu J, Hiratani I, Tachibana M, Shinkai Y, Gilbert DM. G9a selectively represses a class of late-replicating genes at the nuclear periphery. PNAS. 2009;106:19363–19368. doi: 10.1073/pnas.0906142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estève PO, Patnaik D, Chin HG, Benner J, Teitell MA, Pradhan S. Functional analysis of the N-and C-terminus of mammalian G9a histone H3 methyltransferase. Nucleic Acids Res. 2005;33:3211–3223. doi: 10.1093/nar/gki635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. JHDM2A, a JmjC-containing H3K9demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Doherty AS, Mann MRW, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- 18.Otaegui PJ, O’Neill GT, Campbell KH, Wilmut I. Transfer of nuclei from 8-cell stage mouse embryos following use of nocodazole to control the cell cycle. Mol Reprod Dev. 1994;39:147–152. doi: 10.1002/mrd.1080390205. [DOI] [PubMed] [Google Scholar]
- 19.Santos F, Peters AH, Otte AP, Reik W, Dean W. Dynamic chromatin modifications characterize the first cell cycle in mouse embryos. Dev Biol. 2005;280:225–236. doi: 10.1016/j.ydbio.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Samake S, Smith LC. Effects of cell-cycle-arrest agents on cleavage and development of mouse embryos. J Exp Zool. 1996;274:111–120. doi: 10.1002/(SICI)1097-010X(19960201)274:2<111::AID-JEZ4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Matsui Y, Nakayama Y, Okamoto M, Fukumoto Y, Yamaguchi N. Enrichment of cell populations in metaphase, anaphase, and telophase by synchronization using nocodazole and blebbistatin: a novel method suitable for examining dynamic changes in proteins during mitotic progression. Eur J Cell Biol. 2012;91:413–419. doi: 10.1016/j.ejcb.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT, Day BN, Prather RS, Schatten H. Translocation of active mitochondria during pig oocyte maturation, fertilization and early embryo development in vitro. Reproduction. 2001;122:155–163. doi: 10.1530/rep.0.1220155. [DOI] [PubMed] [Google Scholar]
- 23.Korfiatis N, Trounson A, Lacham-Kaplan O. Cell synchronization for the purposes of nuclear transfer in the bovine. Cloning Stem Cells. 2001;3:125–138. doi: 10.1089/153623001753205089. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LS, Jiang MX, Lei ZL, Li RC, Sang D, Sun QY, Chen DY. Development of goat embryos reconstituted with somatic cells: the effect of cell-cycle coordination between transferred nucleus and recipient oocytes. J Reprod Dev. 2004;50:661–666. doi: 10.1262/jrd.50.661. [DOI] [PubMed] [Google Scholar]
- 25.Danilchik MV, Bedrick SD, Brown EE, Ray K. Furrow microtubules and localized exocytosis in cleaving Xenopuslaevis embryos. J Cell Sci. 2003;116:273–283. doi: 10.1242/jcs.00217. [DOI] [PubMed] [Google Scholar]
- 26.Hoebeke JC, Van Nijen G, De Brabander M. Interaction of nocodazole(R17934), a new anti-tumoral drug, with rat brain tubulin. Biochem Biophys Res Commun. 1976;69:319–324. doi: 10.1016/0006-291X(76)90524-6. [DOI] [PubMed] [Google Scholar]
- 27.Maro B, Bornens M. The centriole-nucleus association: effects of cytochalasin B and nocodazole. Biol Cells. 1980;39:287–290. [Google Scholar]
- 28.Johnson MH, Pickering SJ, Dhiman A, Radcliffe GS, Maro B. Cytocortical organization during natural and prolonged mitosis of mouse 8-cell blastomeres. Development. 1988;102:143–155. doi: 10.1242/dev.102.1.143. [DOI] [PubMed] [Google Scholar]
- 29.Sun F, Betzendahl I, Pacchierotti F, Ranaldi R, Smitz J, Cortvrindt R, Eichenlaub-Ritter U. Aneuploidy in mouse metaphase II oocytes exposed in vivo and in vitro in preantral follicle culture to nocodazole. Mutagenesis. 2005;20:65–75. doi: 10.1093/mutage/gei010. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y, Xia P, Li S, Yan Y, Tan J. Determination and synchronisation of G1-phase of the cell cycle in 2-and 4-cell mouse embryos. Zygote. 2002;10:245–251. doi: 10.1017/s0967199402002320. [DOI] [PubMed] [Google Scholar]
- 31.Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol. 2004;272:483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol. 2005;283:40–57. doi: 10.1016/j.ydbio.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 33.Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14:47–58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 34.Kato Y, Ushijima Y, Yamaguchi M. Identification of nuclear localization signals of Drosophila G9a histone H3 methyltransferase. Cell Struct Funct. 2011;36:121–129. doi: 10.1247/csf.10027. [DOI] [PubMed] [Google Scholar]
- 35.Weiss SL, Lee EA, Diamond J. Evolutionary matches of enzyme and transporter capacities to dietary substrate loads in the intestinal brush border. PNAS. 1998;95:2117–2121. doi: 10.1073/pnas.95.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond JM. In: Logic of life: the challenge of integrative physiology. Noble D, Boyd CAR, editors. Oxford: Oxford Univ. Press; 1993. pp. 89–111. [Google Scholar]
- 37.Diamond J, Hammond K. The matches, achieved by natural selection, between biological capacities and their natural loads. Experientia. 1992;48:551–557. doi: 10.1007/BF01920238. [DOI] [PubMed] [Google Scholar]
- 38.Tachibana M, Nozaki M, Takeda N, Shinkai Y. Functional dynamics of H3K9 methylation during meiotic prophase progression. EMBO J. 2007;26:3346–3359. doi: 10.1038/sj.emboj.7601767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rydberg B, Lindahl T. Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction. EMBO J. 1982;1:211–216. doi: 10.1002/j.1460-2075.1982.tb01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]